Abstract
Diffuse brain damage following anoxia due to cardiac failure, drowning, carbon monoxide exposure or other accidents constitutes a major medical problem. We have created a novel mouse model using the breathing of pure nitrogen, followed by a recently developed assay that reflects an operational definition of generalized arousal. The operational definition is precise, complete, and leads to quantitative, physical measures in a genetically tractable animal. Exposure to pure nitrogen for controlled periods had a surprising bifurcate effect: about half the mice survived with neurological measures that were virtually normal while the other half died. The new assay detected behavioral deficits unrevealed by neurological screening. Two important features of the results were that (i) deficits were not equal across the circadian cycle, and (ii) deficits were not equal across all the measures within the operational definition of arousal. Specific voluntary motor measurements were decreased in a manner that depended on the phase of the circadian cycle. Sensory responses were also decreased, with an emphasis on vertical movement responses; but, interestingly, fear learning was not damaged. This study establishes the first useful approach to diffuse brain damage in a genetically tractable animal. The model and its outcome measurements will be useful during future attempts at amelioration of acquired neurological disabilities following hypoxic-ischemic injuries.
Keywords: anoxia, arousal, brain damage, sundowning, circadian rhythm, mice, neurological disorders, delirium, disorders of consciousness, behavioral
Introduction
There are many accidents that produce a loss of normal consciousness due to severe brain damage. Anoxic and hypoxic/ischemic brain injuries are some of the world’s leading causes of brain damage. Each year, among other causes, cardiac arrests, alone, may produce such injuries; and, there are more than 700,000 cardiac arrests per year in the United States (American Heart Assn., 2006). Following anoxia consequent to stroke and other causes such as cardiac failure, drowning, carbon monoxide exposure and various accidents, there is a complex cascade of events in neurons causing cell death and functional neurological damage (Neubauer and Sunderram, 2004; Clarkson et al., 2005; Lo et al., 2005; Rashidian et al., 2005). Altered functional states include arousal, attention, intention, memory and awareness, all observed during global disorders of consciousness (Vexler et al., 1994; Schiff and Plum, 2000; Glenn et al., 2003; Dunham et al., 2004; Laureys et al., 2004; Young et al., 2004; Caputa et al., 2005).
Fundamental to all of these problems is the concept of generalized CNS arousal. Arousing the CNS provides the most elementary support for all cognitive and emotional functions in the mammalian brain, including the human brain (Pfaff, 2006). There is a need for a precise and complete operational definition of generalized CNS arousal. The operational definition proposed states that a more aroused animal or human (i) demonstrates greater alertness to sensory stimuli in all modalities; (ii) emits more voluntary motor activity; and (iii) shows greater emotional reactivity. This operational definition leads to precise, quantitative physical measures (Garey et al., 2003; Easton et al., 2004).
To provide an assay as high-throughput as possible we constructed an apparatus (see Methods), in which all the components of the operational definition of arousal stated above can be measured 24 hours per day. Sensory stimuli from three modalities can be presented to experimental animals which, themselves, do not have to be moved or touched. Several measures of voluntary motor activity in the home cage are available to the experimenter. In terms of emotional reactivity, we have chosen fear as an emotion most convincingly present in mice and as a response pattern whose neural mechanisms have been explored successfully (LeDoux, 2000). Mice were chosen because they are genetically tractable, a property potentially useful in later attempts to achieve amelioration of brain damage.
While there are many experimental models to study the effects of TBI or stroke on certain behavioral functions in rodents (Gonzalez and Kolb, 2003; Wagner et al., 2004; Cauraugh et al., 2005; Maeda et al., 2005), we know of no comprehensive published approaches to anoxic brain damage in mice, nor have there been outcome measurements presented with adequate precision. Little has been done to treat this disease process because of a lack of basic laboratory research on mechanisms of functional loss and potential mechanisms of enhanced recovery. The present research investigates this subject in a manner important for clinical medicine, in that while dealing here with anoxia, the same methods of detection could be used with other neurological disorders. In the present study, neurological and behavioral responses were explored in mice exposed briefly to anoxic insult, using ‘generalized brain arousal’ (Pfaff, 2006) as a theoretical background, to establish a mouse model useful for future attempts to ameliorate hypoxic/ischemic brain damage. Hopefully this model will lead to a systematic testing of novel therapies useful for reversal of disease processes following hypoxia or anoxia.
Materials and Methods
Animals
Twenty four female C57BL/6 mice at six weeks old were ovariectomized by the supplier (Jackson Laboratory, Bar Harbor, ME) in order to eliminate possible variations in function and in response to anoxia across the estrous cycle. Animals were individually housed with food and water available ad libitum, and maintained on a reversed 12:12-hours light/dark cycle with lights off at 06:00 h in a temperature-controlled (20 ± 2°C) room for one week prior to experimental manipulation. All procedures and protocols were approved by The Rockefeller University’s Institutional Animal Care and Use Committee (IACUC) through The Laboratory Animal Research Center.
Procedures and experimental design
At 7.5 weeks old animals were randomly assigned to three experimental groups: one for a single exposure to anoxia (n=8), a second for multiple exposures to anoxia (n=8) and a third to pentobarbital (n=8). The order followed in the generalized arousal assay was illustrated in Figure 1. Mice were anesthetized deeply via an i.p. injection with Nembutal sodium solution (Abbott laboratories, North Chicago, IL) at a dose of 7.5 mg/100 g of body weight, and assigned to one of three experimental conditions. Animals only used for the anoxia treatment were placed inside a Plexiglas chamber for administering the N2 at a constant pressure of 100 KPa during exposure. Mice in the first condition received a single exposure to 100% of N2 for 1 minute and 50 seconds; whereas the mice in the second condition were exposed to pure nitrogen breathing five times, each 1 minute and 50 seconds, with the exposures separated by 5 minutes (‘multiple exposures’). The duration of these exposures had been pre-determined in pilot assays.
Figure 1.
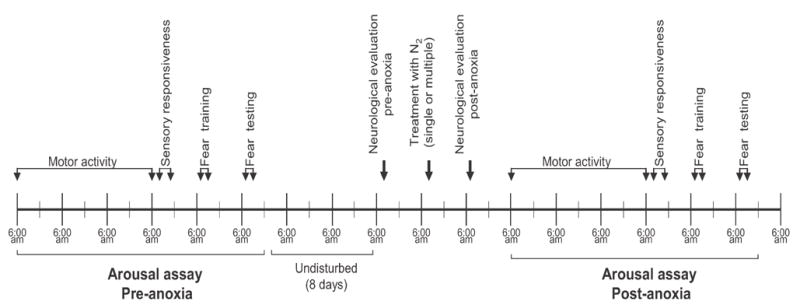
The diagram shows the timeline of behavioral assays before (pre-anoxia) and after (post-anoxia) treatment with N2. The animals were maintained on a reversed light/dark cycle, with lights off at 6:00 am and lights on at 6:00 pm. The motor activity was tested all 24 h for 3 days; the sensory responsiveness assay, fear conditioning and neurological evaluation were tested during the early hours of the dark period. See materials and methods for more details.
Neurological examination
24 hours before and after of the treatment with N2 or pentobarbital, the mice were evaluated neurologically by scoring their performance on a series of reflex tests (see Table 1), according to an established protocol entitled primary screen SHIRPA (Rogers et al., 1997) and modified with other neurological assessments (Ziporen et al., 1997). The mice were placed inside a Plexiglas cage (47 cm × 25 cm × 15cm) with four transparent walls; the order of the tests was performed as shown in table 1. The total score obtained in the first neurological evaluation was used for the baseline results and normalized to 100%.
Table 1. Neurological tests used.
Neurological examination of mice treated with N2. Twenty-eight tests were performed in each animal during the pre-anoxia and post-anoxia phases. Tests were performed in order shown in the list.
| Tests |
|---|
|
Generalized arousal assay
The mice were housed individually inside a VersaMax monitor (AccuScan Instruments Inc, Columbus, OH), consisting of an acrylic cage (19 cm × 29.5 cm × 13 cm) equipped with horizontal and vertical sensors containing a set of 48 infrared photo beams distributed side to side and front to back, spaced 1 cm apart. This monitor is connected to a VersaMax analyser (AccuScan Instruments Inc, Columbus, OH) and the disruption of a beam is recorded as an activity count. The data were collected onto a PC using VersaMax software (version 3.41, AccuScan Instruments Inc, Columbus, OH). The motor activity was measured for determining three main parameters: a) horizontal activity (HACTV) = the total number of beam interruptions in the horizontal sensor each 60 minutes; b) total distance (TOTDIST) = the distance traveled around the entire home cage in a continuous path, in cm, each 60 min.; and c) vertical activity (VACTV) = the total number of beam interruptions of the vertical sensors each 60 minutes. For sensory activity, mice were exposed to a series of three sensory stimuli in the following order: tactile, vestibular and olfactory. All stimuli were presented when mice were in a “resting state”, which was defined by the absence of horizontal activity (HACTV = 0) for 5 min. and response to each stimulus was measured by a change in home cage activity in either the horizontal or vertical direction. The stimuli consisted of brief changes in sensory input to the mouse and the response was measured until the animal reached a resting state again. For administering each stimulus the AccuScan monitors are connected to a power source controlled by Graphic State software (version 2.101-01, Coulbourn Instruments, Allentown, PA) installed in a PC for precise delivery of each stimulus. First, the tactile stimulus was a 15 second air puff (15 Psi) controlled by a PC, supplied by a compressed air tank and directed from each corner of the cage. The vestibular stimulus consisted of moving the cage in a circular motion about its vertical axis on an orbital shaker (Barnstead International, Dubuque, Iowa) for 10 seconds at 90 rpm. Finally, the olfactory stimulus was odor from 3 ml of 100% benzaldehyde (Sigma, St. Louis, MO), applied for 10 seconds duration. The sensory responsiveness data were analyzed for changes in HACTV, TOTDIST and VACTV recorded per minute using the VersaMax software and Graphic State software for sensory responsiveness. The last part of this assay consisted of measuring the emotional responsiveness. We used a fear-conditioning paradigm, carried out in one training session followed 24 hours later by the testing session, in a dark room with red light. A Habitest operant cage (30.5 cm 0215 × 28.5 cm × 26.5 cm) (Coulbourn Instruments, Allentown, PA) equipped with a metal shock grid floor connected to a programmable animal shocker (San Diego Instruments, San Diego, CA) was used with other contextual cues, including white noise and dark-colored objects placed outside the chamber near the walls. During the training session, each mouse was placed inside the test chamber and its behavior was recorded every 5 seconds for a 5 minute total period, and scored as freezing (absence of all movement), sitting immobile (only fine head movements), walking, grooming or moving. Immediately after the first 5 minutes, each mouse was exposed to a 1-sec shock (0.5 mA) per minute, three times. The animals were returned to their cages, and 24 hours later, the testing session was performed. Testing consisted of the same characteristics as the training session, without the electro-shocks.
Data and statistical analysis
The motor activity data were analyzed in bins of four hours for each 24 hour period, whereas the sensory data were analyzed for responses during the first ten minutes after the each stimulus. Fear data were analyzed as percentage of freezing or sitting immobile out of a total of 60 observations for each experimental period. Data were compiled and the statistical analysis performed using GraphPad Prism software (version 4.03, GraphPad Software, Inc, San Diego, CA). All the data were analyzed using a Two-way ANOVA (Source of variation: interaction, treatment and time) in a within-subjects design. When significant interactions were detected the Bonferroni’s post test was used to make pair-wise comparisons. Significant differences were designated *p<0.05, **p<0.01 and ***p<0.001.
Results
Neurological function secondary to anoxia
To evaluate the effect of the anoxia on the neurological function, we first determined the critical period of exposure to N2, to be sure the treatment was effective. Monitoring respiratory symptoms, we determined a critical period of exposure to N2 using non-anesthetized mice by constructing a time-of-exposure/response curve. Signs and symptoms during breathing of pure nitrogen included: hyperactivity (10 seconds), hyperventilation and tremor (20 seconds); severe breathing featured by hypoventilation or apnea (60 seconds), immobility and sphincter relaxation (80 seconds), and finally, generalized seizures (100 seconds). In most cases, death occurred between 120 seconds and 140 seconds.
After a long series of preliminary experiments it was determined that the optimum treatment time was 1 minute and 50 seconds. Using this duration, the effects of N2 exposure were evaluated. The percentage of mice surviving this treatment was 87.5 % of those that received only one exposure, whereas only 50% of the mice treated with multiple (five) exposures to N2 remained alive.
All the mice were examined neurologically pre-anoxia and post-anoxia treatment. The neurological function scores of the mice that were treated with a single exposure (pre 48.6 ± 1.7 vs. post 45.8 ± 1.6) or multiple exposures (pre 46.2 ± 1.2 vs. post 47.4 ± 1.3) showed no significant differences (Fig. 2 and Table 2). Thus, the results showed a surprising dichotomy: after multiple exposures about half the mice died, but among the surviving there were no observable neurological deficits.
Figure 2.
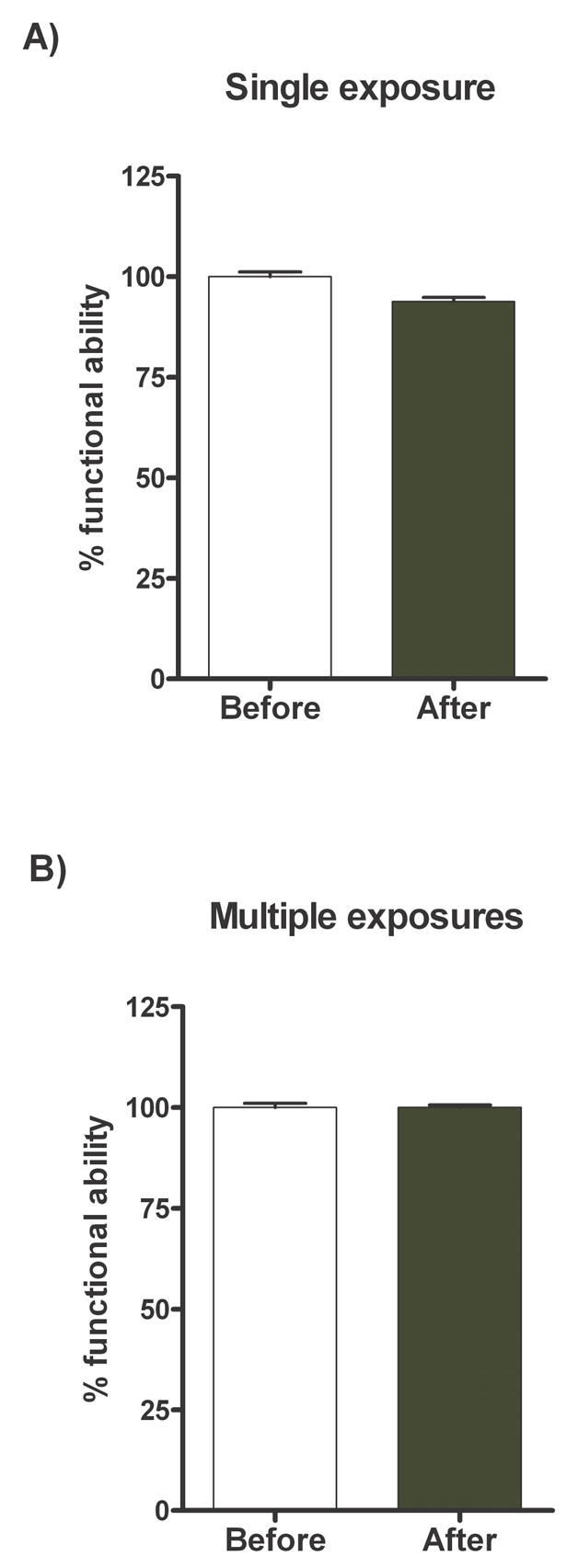
Neurological function of mice treated with N2. Mice were evaluated neurologically by scoring their performance in a series of twenty-eight tests according to established protocols. Neurological scores were determined 24 h before (pre-anoxia) and 24 h after (post-anoxia) single exposure to N2 (A) or multiple exposures to N2 (B). Values are expressed as percentages post-anoxia treatment (black bar) compared to their own pre-anoxia values (white bar) normalized at 100 %.
Table 2. Neurological function scores in mice treated with N2.
Neurological scores of mice treated with N2. These scores were determined 24 h before (pre-anoxia) and 24 h after (post-anoxia) single exposure to N2 or multiple exposures to N2. To each test was assigned a value according with the activity performed during the neurological evaluation. Scores for each test is as follows: 1. Body position (0=completely fat, 1=lying on side, 2=lying prone, 3=sitting, standing or walking, 4=rearing on hind legs, 5=repeated vertical leaping); 2. Gait (0=normal, 1=fluid but abnormal, 2=limited movement only, 3=incapacity); 3. Tremor (0=none, 1=mild, 2=marked); 4. Tail elevation (0=dragging, 1=horizontally extended, 2=elevated tail); 5. Trunk curl (0=present, 1=absent); 6. Skin colour (0=blanched, 1=blue o violet, 2=bright, deep red flush, 3=pink); 7. Palpebral closure (0=eyes closed, 1=eyes 1/2 closed, 2=eyes wide open); 8. Respiration type (0=gasping, irregular, 1=slow, shallow, 2=normal, 3= hyperventilation); 9. Urination and defecation (0=absent, 1=present); 10. Touch escape (0=no response, 1=mild (escape response to firm stroke), 2=moderate (rapid response to light stroke), 3=vigorous (escape response to approach); 11. Auditory startle (0=absent, 1=present); 12. Ear reflex (0=absent, 1=present); 13. Positional passivity (0=no struggle, 1=struggles when held by hind legs, 2=struggles when laid supine (on back), 3=struggles when held by neck (finger grip, not scruffed), 4=struggles when held by tail); 14. Postural reflex (0=absent, 1=present); 15. Front leg placing (0=absent, 1=present); 16. Hind leg placing (0=absent, 1=present); 17. Equilibrium (0=absent, 1=present); 18. Righting reflex (0=no response, 1=slow response, 2=active over its feet and not is possible to evaluate); 19. Body tone (0=Flaccid, no return of cavity to normal, 1=flaccid, return of cavity to normal, 2=slight resistance, 3=extreme resistance, board like); 20. Limb tone (0=no resistance, 1=slight resistance, 2=moderate resistance, 3=marked resistance, 4=extreme resistance) 21. Flexion reflex (0=none, 1=slight withdrawal, 2=moderate withdrawal, not brisk, 3=brisk, rapid withdrawal, 4=very brisk repeated extension and flexion); 22 and 23. Placing reactions (a or b) (0=absent, 1=present); 24. Visual placing (0=none, 1=upon nose contact, 2=upon vibrasse contact, 3=before vibrasse contact (18mm), 4=early vigorous extension (25mm); 25. Grip strength (0=none, 1=slight grip, semi-effective, 2=moderate grip, effective, 3=active grip, effective, 4=unusually effective); 26. Pinna reflex (0=none, 1=active retraction, moderately brisk flick, 2=hyperactive, repetitive flick); 27. Corneal reflex (0=none, 1=active single eye blink, 2=multiple eye blink); 28. Grip (0=absent, 1=from of 1 to 14 seconds, 2=from 15 to 30 seconds, 3=more of 30 seconds). The results are presented as mean ± S.E.M.
| Test | Single exposure | Multiple exposures | ||
|---|---|---|---|---|
| Pre-anoxia | Post-anoxia | Pre-anoxia | Post-anoxia | |
| 1. Body position | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| 2. Gait | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 3. Tremor | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 4. Tail elevation | 1.4 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.2 ± 0.2 |
| 5. Trunk curl | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 6. Skin colour | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| 7. Palpebral closure | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 |
| 8. Respiration type | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 |
| 9. Urination and defecation | 0.7 ± 0.1 | 0.7 ± 0.1 | 1.0 ± 0.3 | 1.0 ± 0.0 |
| 10. Touch escape | 2.7 ± 0.2 | 2.4 ± 0.2 | 2.0 ± 0.0 | 2.3 ± 0.3 |
| 11. Auditory startle | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 12. Ear reflex | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 13. Positional passivity | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 | 4.0 ± 0.0 |
| 14. Postural reflex | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 15. Front leg placing | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 16. Hind leg placing | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 17. Equilibrium | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 18. Righting reflex | 1.9 ± 0.1 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 |
| 19. Body tone | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 |
| 20. Limb tone | 2.9 ± 0.3 | 2.0 ± 0.2 | 2.0 ± 0.0 | 2.0 ± 0.0 |
| 21. Flexion reflex | 2.7 ± 0.2 | 2.4 ± 0.2 | 3.0 ± 0.0 | 3.0 ± 0.3 |
| 22. Placing reactions (a) | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 23. Placing reactions (b) | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 24. Visual placing | 4.0 ± 0.0 | 3.7 ± 0.2 | 3.5 ± 0.3 | 4.0 ± 0.0 |
| 25. Grip strength | 2.7 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.3 | 2.7 ± 0.3 |
| 26. Pinna reflex | 1.6 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.3 | 1.2 ± 0.2 |
| 27. Corneal reflex | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 28. Grip | 2.9 ± 0.1 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
|
| ||||
| Total score | 48.6 ± 1.7 | 45.8 ± 1.6 | 46.2 ± 1.2 | 47.4 ± 1.3 |
Alterations in generalized arousal after anoxia
Looking for measures that are (i) important according to the theory of generalized CNS arousal, and (ii) more sensitive measures of deficits following anoxia than traditional neurological testing, we used our generalized arousal assay for mice, as described in Methods. With respect to voluntary motor activity, a single exposure to N2 significantly decreased the HACTV, the TOTDIST and the VACTV of the mice; but, the greatest differences in activity following anoxia were limited to the first half of the dark period in the daily light/dark cycle (Fig. 3, A–C). For the mice treated with multiple exposures to N2, the decreases of HACTV, TOTDIST and VACTV were still more evident, with the largest decreases in activity again appearing during the first half of the dark period (Fig. 3, D–F). Thus, the generalized arousal assay detected differences that neurological testing did not, and there was a circadian component in the results.
Figure 3.
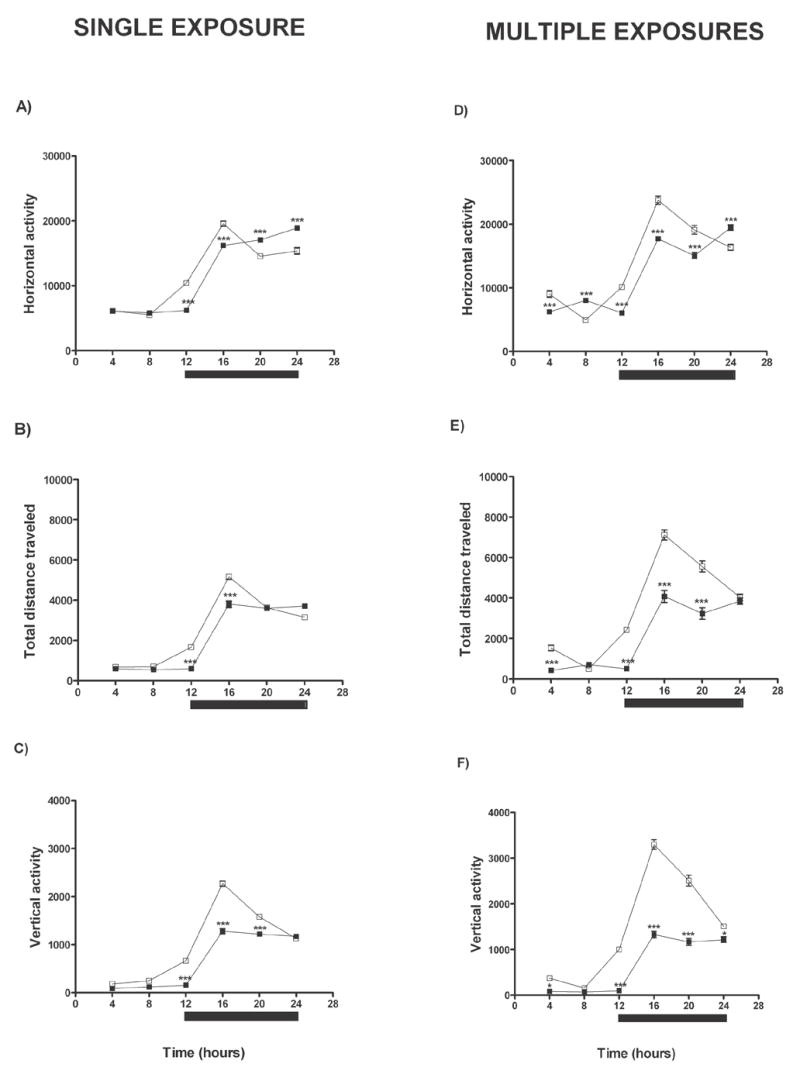
Changes in motor activity of mice treated with N2. Left panel shows the HACTV (A), the TOTDIST (B) and the VACTV (C) of the mice that received a single exposure to N2. Results were compared between the same animals pre-anoxia (□) versus post-anoxia (■). Right panel shows the HACTV (D), the TOTDIST (E) and the VACTV (F) of mice that received multiple exposures to N2; the comparisons were pre-anoxia (□) versus post-anoxia (■). The black bar in all the figures indicates the dark period of the daily light/dark cycle. Data were analyzed using Two-way ANOVA; significant differences are designated *p<0.05, **p<0.01 and ***p<0.001. Significant differences were confirmed by Bonferroni’s post-tests used to make pairwise comparisons (pre-anoxia vs. post-anoxia).
In terms of responsiveness to sensory stimuli, even a single exposure to N2 significantly reduced the amplitudes of responses to the tactile, the vestibular and the olfactory stimuli as measured by HACTV, TOTDIST and VACTV (Fig. 4, A–C). In the mice given multiple exposures of N2, we also detected significantly decreased responses post-anoxia for all three sensory modalities, according to the response measured (Fig. 4, D–F). Across the response measures, responses requiring a series of organized movements (TOTDIST and VACTV) (Fig. 4, B, C, E, F) were more sensitive than HACTV (Fig. 4, A, D) in finding deficits following anoxia. In general, although there was exceptions, the larger the initial response to a sensory stimulus, the easier it was to show a highly significant decrease. In general, sensory responsiveness was reduced following anoxia treatment. Once again, the generalized arousal assay revealed deficits missed by the neurological screen.
Figure 4.

Changes in sensory responses of mice were treated with N2. Left panel shows responses to three sensory modalities (first, the tactile stimulus, second, the vestibular stimulus, and third, the olfactory stimulus) measured by HACTV (A), TOTDIST (B) and VACTV (C) of mice that received a single exposure to N2. They were compared pre-anoxia (white bar) versus post-anoxia (black bar). Right panel shows responses to each of the three sensory modalities measured by the HACTV (D), the TOTDIST (E) and the VACTV (F) of mice that received multiple exposures to N2. Comparisons were pre-anoxia (white bar) versus post-anoxia (black bar). Data were analyzed using Two-way ANOVA, and significant differences were designated *p<0.05, **p<0.01 and ***p<0.001. Significant differences were confirmed by Bonferroni’s post-tests used to make pairwise comparisons (pre-anoxia vs. post-anoxia).
With respect to emotional reactivity, as part of the operational definition of generalized arousal (see Introduction), we expected a decrease in learned fear in all mice post-anoxia compared with pre-anoxia treatments. This did not occur (Fig. 5, A–D). Interestingly, the only significant results went in the opposite direction. That is, when measured by the % of mice sitting immobile (Fig. 5, B, D) either a single exposure or a multiple exposure to N2 significantly enhanced fear. This change was not reflected in the strictest measure of immobility, namely, freezing (Fig. 5, A, C). Thus, the generalized arousal assay proved useful, but not for the predicted reason.
Figure 5.

Changes in the emotional reactivity of mice treated with N2. Left panel shows the freezing behavior (A) and the sitting immobile behavior (B) of mice that received a single exposure to N2. They were compared pre-anoxia (white bars) versus post-anoxia phases (black bars) in two different sessions (one day training session and other day testing session). Right panel shows the freezing behavior (C) and the sitting immobile behavior (D) of mice that received multiple exposures to N2. These animals were compared pre-anoxia (white bars) versus post-anoxia phases (black bars). Values are expressed as percentages of a total of 60 observations recorded in each 5 min session (training or testing). Data were analyzed using Two-way ANOVA, significant differences were designated *p<0.05, **p<0.01 and ***p<0.001. Significant differences were confirmed by Bonferroni’s post-tests were used to make pairwise comparisons (pre-anoxia vs. post-anoxia).
Could these results following anoxia have been due to the pentobarbital itself that we administered simply to spare the animals pain? To answer this question we created a separate control experiment in which only pentobarbital was administered. No changes were observed, either in measures of voluntary motor activity (Fig. 6, A, top three panels), measures of sensory responsiveness (Fig. 6, B, middle three panels), or in emotional reactivity (Fig. 6, C, bottom three panels). The data in this control experiment show that our main results were not due to the pentobarbital.
Figure 6.

Arousal assay in mice treated with pentobarbital. Top panels (A) show measurements of motor activity (HACTV, TOTDIST and VACTV). The animals were compared pre-pentobarbital (□) versus post-pentobarbital (■). The black bar indicates the dark period of the daily light/dark cycle. Middle panels (B) show the sensory responses in three sensory modalities (first, the tactile stimulus, second, the vestibular stimulus, and third, the olfactory stimulus) measured by HACTV, TOTDIST and VACTV. Mice were compared pre-pentobarbital (white bar) versus post-pentobarbital (black bar). Bottom panels (C) show the emotional reactivity measured by freezing behavior and by sitting immobility; the animals were compared pre-pentobarbital (white bars) versus post-pentobarbital (black bars) in two different sessions (one day training session and other day testing session). Values are expressed as percentages of a total of 60 observations recorded in each 5 min session (training or testing). All of the data were analyzed using Two-way ANOVA, no significant differences were found pre-pentobarbital vs. post-pentobarbital conditions.
Discussion
Major findings included (i) a surprising bifurcation in the responses of mice to anoxia, and (ii) the ability of our new generalized CNS arousal assay to measure deficits undetectable in a standard neurological screening. Part of the virtue of the assay may have derived from the assay’s monitoring of mice through the entire circadian cycle (iii) that in turn yielded a potential model for a syndrome of delirium in elderly patients. The fact that all sensory modalities were tested (iv) has logical implications; and the exaggeration of fear responses (v) following anoxia might reflect a loss of frontal cortical inhibition of fear. Thus, we have established a mouse model of anoxia in an animal which, though small and awkward for some physiological measurements, is genetically tractable. Future work can include functional genomic investigations of protection against anoxic brain damage at both the neuropathological and behavioral levels. All of these findings are discussed in relation to attempts at amelioration or reversal of deficits following anoxia.
Bifurcate nature of responses to anoxia
An unexpected feature of the results was the extreme reaction or lack of reaction by subpopulations of animals to repeated applications of anoxia. About half the animals died. Yet, among the survivors, a standard neurological screening did not reveal significant deficits. CNS mechanisms for this bifurcate response require further investigation. One possibility derives from the variable responses of animals to our dose of pentobarbital anesthesia; such variation could have contributed to dichotomous nature of the animals’ eventual response to anoxia. In addition, we note that some of the most important recent studies on anoxia and the mouse CNS have used much longer periods of anoxia followed by electrophysiological measurements of synaptic function (Tekkok and Ransom, 2004). While a substantial fraction of axons remained functional after periods of anoxia as long as 2 hours, Tekkok and Ransom (2004) concluded that ‘CNS structures vary greatly in their ability to function and survive anoxia’. Presumably the balance of activities among these structures helped to determine survival in our animals. Indicated for the future will be histochemical comparisons among extremes in our population– mice that died compared to mice with no abnormality following anoxia– using both the immunocytochemical endpoints used successfully with deep brain stimulation experiments following traumatic brain injury (Shirvalkar et al., 2006) and molecular endpoints derived from microarrays making the same comparisons among mice as have been made in the current project. We hypothesize that differences among the individual animals in diffuse brain damage caused by the anoxia will help to account (i) for the bifurcate nature of the results, and (ii) for the loss of performance in the CNS arousal assay. This hypothesis will have to be tested in the future with histochemical and electrophysiological methods.
Arousal assay more sensitive than neurologic screen
The excellent neurological scores in the mice surviving multiple anoxic exposures rule out simple diminishment of muscle tone or fatigue as reasons for the deficits in the arousal assay, post-anoxia. How, then, does one explain the ability of the arousal assay to detect deficits unobtainable by the neurological screen? The arousal assay is intended to tap the concept of generalized arousal which depends not only on principal components analysis (Garey et al., 2003) but also on two other lines of evidence: (i) The fact that its neuroanatomical, neurophysiological and genomic mechanisms are already partially understood; and (ii) The fact that we can breed for high vs. low general arousal (Zhang et al., unpublished data). The assay is not perfect --- not all of the measures are statistically independent of each other. However, in the present case of anoxia, it was able to reveal deficits. How? One possibility derives from the multiplicity of measures and large amount of data available as a result of how the automated arousal assay has been designed. Working with experimental animals it is acknowledged that generalized arousal determinations must depend on measurements of motor activity, but because of a lack of correlations among different aspects of motor activity we do not think we are simply measuring tendencies toward ‘ctivity per se’ We also note that some of the biggest differences between post-anoxia and pre-anoxia animals had to do with vertical movement. One hypothesis is that the requirement for good balance on the animals’ hind feet was responsible for this result. For example, even small changes in the animals’ vestibular systems, as a result of anoxia, could cause a deficit in VACTV, as demonstrated by the responses to vestibular stimuli in Fig. 4. Finally, it appears that with the sensitivity of this assay, the subtle problems of cognitive slowing and mild/moderate cognitive impairments could be modeled and then subsequently be approached therapeutically.
Circadian variations in magnitude of deficit
Abnormalities in the voluntary motor activity of the animals that had been subjected to anoxia were limited to the period of time in anticipation of lights-off and during the first few hours of dark, in each 24 hour period. The circadian dependence of these results are reminiscent of a phenomenon common in elderly patients, a state of confusion and delirium that occurs in the late afternoon and early evening. Its common medical name is ‘sundowning’ (Volicer et al., 2001), a form of dementia that occurs specifically during the transition of light intensity from day to night. It occurs in about 13% of the general nursing home population (Bachman and Rabins, 2006), including not only the elderly, but also others with diffuse brain impairment post-anoxia or with degenerative disorders. Sundowning carries a considerable mortality risk (about 20%).
A good model for sundowning has been lacking and is required. Because of the circadian aspect of this form of delirium, first consideration goes to the suprachiasmatic nucleus (SCN) of the hypothalamus, the neuroanatomy of which has been reported to change during aging (Tsukahara et al., 2005; Wu and Swaab, 2005; Hofman and Swaab, 2006). However, sundowning is unlikely to be limited to abnormalities in the SCN itself, because other histochemical observations (Miller et al., 2005) and in vitro molecular studies of rhythmicity (Yamazaki et al., 2002) in the SCN have revealed no age-related changes. At the behavioral level, evidence indicates declines in function with aging both in the loss of responsiveness to light signals in animals (Turek et al., 1995) and in late afternoon/early evening sleepiness in normal human volunteers (Munch et al., 2005). Munch et al., (2005) inferred age-related changes in the evening oscillator. The evening oscillator, which tracks the change from the daily period of high light intensity toward darkness, depends on the relative activities of two clock genes (Steinlechner et al., 2002; Weinert et al., 2005), and is altered in senile mice (Weinert and Weinert, 2003). It appears to depend on a different group of neurons than the morning oscillator (de la Iglesia et al., 2000; Stoleru et al., 2005). Since the SCN itself cannot clearly be held responsible, there must be changes in the neural circuitry (Ribeiro et al., in preparation) between the SCN’s outputs and the preoptic or midbrain locomotor control regions. The anoxia caused by our methodological approach may have affected such neural circuitry.
Sensory responsiveness
In tests of sensory responsiveness, post-anoxic results showed declines in all three sensory modalities, which themselves depend on completely different neuroanatomical trajectories. Sensory processing for olfactory stimuli would be dependent on the basal forebrain, for tactile stimuli on the integrity of spinal pathways, and for vestibular stimuli on their own specific nerves and brainstem nuclei. Therefore, the deficits reported here are not likely due just to declines in the efficiency of sensory processing by one type of sensory surface. Since anoxia effects on arousal-related behaviors are also not caused simply by diminished muscle tone, as discussed above, we infer that deficits in sensory responsiveness during these studies was likely due to changes in arousal systems themselves. Future work will address mechanisms for those changes.
Emotional responsiveness
We note that capacity for emotional reactivity as measured by fear conditioning was not reduced by anoxia. Within the scope of detection by our generalized arousal assay, therefore, not all emotional and cognitive capacities are damaged. This fact, in addition to the normal neurological scores, suggests that the mice are still fundamentally healthy. Our fear conditioning data are consistent with the report of Caputa et al., (2005) who stated that perinatal anoxia in rats resulted in reduced attention to potential dangers as adults. More interesting is the result in Fig. 5 (B and D) that revealed unpredicted increases in fear conditioning in the animals that were post-anoxic. This fact may also reveal a cortical deficit, using the following reasoning. It has been established that fear conditioning depends on outputs from the basolateral and cortical nuclei of the amygdala (Vazdarjanova and McGaugh, 1999; Fanselow and Gale, 2003; Ledoux, 2003), whereas suppression of fearful responses depends on the frontal cortex (Gewirtz and Davis, 1998; Morgan et al., 2003). Therefore, since fear responses were altered following diffuse brain damage due to anoxia in the present work, our experimental treatment might have reduced the effectiveness of outputs from the frontal cortex. If so, we would anticipate reduced frontal cortical modulation of limbic structures, particularly the amygdala. In turn, less frontal inhibition of amygdaloid output would be consistent with increased fear responsiveness as seen in Fig. 5 (B and D).
Having made this point, it must be acknowledged that our results could also be explained by the effect of anoxia upon attention to the cue itself or on the learning process itself.
Amelioration
Several hormones whose levels are important for normal attention, arousal and awareness have marked circadian rhythms and thus could be useful in patients’ confusional states that show a circadian pattern of incidence. Thyroid stimulating hormone provides one example. Its abnormalities in circadian pattern of secretion as a result of aging are similar to abnormalities seen in sleep-deprived patients and in depressed patients (Spiegel et al, 2003). Gene expression for thyroid hormone receptors shows a marked diurnal variation (Zandieh Doulabi et al., 2002, 2004). In the brain, effectiveness of thyroid hormones depends on their metabolism, particularly in the activity of Type II deiodinase that catalyzes the conversion of T4 to T3. The activity of this enzyme and concentrations of various T4 metabolites, likewise, vary throughout the day (Pinna et al., 2002; Kalsbeek et al., 2005). Because of the considerable importance of thyroid hormone action in the brain for normal awareness and attention, manipulations of this hormonal axis might help during recovery from anoxia. While the expected foci of thyroid manipulations on behavioral recovery would have to do with the CNS, other routes of action are possible, notably those involved in the control of daily rhythms in body temperature (Kim et al., 2002; Mazzoccoli et al., 2004). Other therapeutic approaches that have been suggested include anti-inflammatory medications (Lazovic et al., 2005), blockade of gap junctions (de Pina-Benabou et al., 2005) and the administration of melatonin (Olde-Rikkert et al., 2001; Cardinali et al., 2005). Future experiments in our lab will focus on the use of cholinesterase inhibitors and deep brain stimulation, among other approaches, to ameliorate the behavioral deficits induced by anoxia.
Acknowledgments
We are pleased to acknowledge much helpful advice from Nicholas Schiff, M.D. and Daniel Herrera, M.D. Susan Strider helped with the illustrations and Carolyn Johnson for her technical assistance. This work was supported by grants from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Heart Association. Heart Disease and Stroke Statistics. 2006 Update; Dallas, Texas: 2006. [Google Scholar]
- Bachman D, Rabins P. “Sundowning” and other temporally associated agitation states in dementia patients. Annu Rev Med. 2006;57:499–511. doi: 10.1146/annurev.med.57.071604.141451. [DOI] [PubMed] [Google Scholar]
- Caputa M, Rogalska J, Wentowska K, Nowakowska A. Perinatal asphyxia, hyperthermia and hyperferremia as factors inducing behavioural disturbances in adulthood: a rat model. Behav Brain Res. 2005;163:246–256. doi: 10.1016/j.bbr.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Cardinali DP, Furio AM, Reyes MP. Clinical perspectives for the use of melatonin as a chronobiotic and cytoprotective agent. Ann N Y Acad Sci. 2005;1057:327–336. doi: 10.1196/annals.1356.025. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Sutherland BA, Appleton I. The biology and pathology of hypoxia-ischemia: an update. Arch Immunol Ther Exp (Warsz) 2005;53:213–225. [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- de Pina-Benabou MH, Szostak V, Kyrozis A, Rempe D, Uziel D, Urban-Maldonado M, Benabou S, Spray DC, Federoff HJ, Stanton PK, Rozental R. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–2237. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- Dunham CM, Ransom KJ, Flowers LL, Siegal JD, Kohli CM. Cerebral hypoxia in severely brain-injured patients is associated with admission Glasgow Coma Scale score, computed tomographic severity, cerebral perfusion pressure, and survival. J Trauma. 2004;56:482–489. doi: 10.1097/01.ta.0000114537.52540.95. [DOI] [PubMed] [Google Scholar]
- Easton A, Norton J, Goodwillie A, Pfaff DW. Sex differences in mouse behavior following pyrilamine treatment: role of histamine 1 receptors in arousal. Pharmacol Biochem Behav. 2004;79:563–572. doi: 10.1016/j.pbb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Garey J, Goodwillie A, Frohlich J, Morgan M, Gustafsson JA, Smithies O, Korach KS, Ogawa S, Pfaff DW. Genetic contributions to generalized arousal of brain and behavior. Proc Natl Acad Sci USA. 2003;100:11019–11022. doi: 10.1073/pnas.1633773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Application of Pavlovian higher-order conditioning to the analysis of the neural substrates of fear conditioning. Neuropharmacology. 1998;37:453–459. doi: 10.1016/s0028-3908(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, Hovda DA, Bergsneider M, Hillered L, Martin NA. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cereb Blood Flow Metab. 2003;23:1239–1250. doi: 10.1097/01.WCB.0000089833.23606.7F. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Kolb B. A comparison of different models of stroke on behaviour and brain morphology. Eur J Neurosci. 2003;18:1950–1962. doi: 10.1046/j.1460-9568.2003.02928.x. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, van SR, Kaptein E, Visser TJ, Doulabi BZ, Fliers E. Daily variations in type II iodothyronine deiodinase activity in the rat brain as controlled by the biological clock. Endocrinology. 2005;146:1418–1427. doi: 10.1210/en.2004-0763. [DOI] [PubMed] [Google Scholar]
- Kim Mi-Seung, Lee Hyun J, Im Wook-Bin. Circadian rhythms of melatonin, thyroid-stimulating hormone and body temperature: Relationships among those rhythms and effect of sleep-wake cycle. Korean J Biological Sciences. 2002;6:239–245. [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Lazovic J, Basu A, Lin HW, Rothstein RP, Krady JK, Smith MB, Levison SW. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke. 2005;36:2226–2231. doi: 10.1161/01.STR.0000182255.08162.6a. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- Maeda M, Takamatsu H, Furuichi Y, Noda A, Awaga Y, Tatsumi M, Yamamoto M, Ichise R, Nishimura S, Matsuoka N. Characterization of a novel thrombotic middle cerebral artery occlusion model in monkeys that exhibits progressive hypoperfusion and robust cortical infarction. J Neurosci Methods. 2005;146:106–115. doi: 10.1016/j.jneumeth.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G, Giuliani A, Carughi S, De CA, Puzzolante F, La VM, Urbano N, Perfetto F, Tarquini R. The hypothalamic-pituitary-thyroid axis and melatonin in humans: possible interactions in the control of body temperature. Neuro Endocrinol Lett. 2004;25:368–372. [PubMed] [Google Scholar]
- Miller JP, McAuley JD, Pang KC. Spontaneous fos expression in the suprachiasmatic nucleus of young and old mice. Neurobiol Aging. 2005;26:1107–1115. doi: 10.1016/j.neurobiolaging.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behav Brain Res. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Munch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, Wirz-Justice A, Cajochen C. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26:1307–1319. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Sunderram J. Oxygen-sensing neurons in the central nervous system. J Appl Physiol. 2004;96:367–374. doi: 10.1152/japplphysiol.00831.2003. [DOI] [PubMed] [Google Scholar]
- Olde Rikkert MG, Rigaud AS. Melatonin in elderly patients with insomnia. A systematic review. Z Gerontol Geriatr. 2001;34:491–497. doi: 10.1007/s003910170025. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Brain Arousal and Information theory: Neural and Genetic mechanisms. Harvard University Press; 2006. [Google Scholar]
- Pinna G, Brodel O, Visser T, Jeitner A, Grau H, Eravci M, Meinhold H, Baumgartner A. Concentrations of seven iodothyronine metabolites in brain regions and the liver of the adult rat. Endocrinology. 2002;143:1789–1800. doi: 10.1210/endo.143.5.8770. [DOI] [PubMed] [Google Scholar]
- Rashidian J, Iyirhiaro G, Aleyasin H, Rios M, Vincent I, Callaghan S, Bland RJ, Slack RS, During MJ, Park DS. Multiple cyclin-dependent kinases signals are critical mediators of ischemia/hypoxic neuronal death in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14080–14085. doi: 10.1073/pnas.0500099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Schiff ND, Plum F. The role of arousal and “gating” systems in the neurology of impaired consciousness. J Clin Neurophysiol. 2000;17:438–452. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006;103:17007–17012. doi: 10.1073/pnas.0604811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van CE. Impact of sleep debt on physiological rhythms. Rev Neurol (Paris) 2003;159:6S11–6S20. [PubMed] [Google Scholar]
- Steinlechner S, Jacobmeier B, Scherbarth F, Dernbach H, Kruse F, Albrecht U. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J Biol Rhythms. 2002;17:202–209. doi: 10.1177/074873040201700303. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Ransom BR. Anoxia effects on CNS function and survival: regional differences. Neurochem Res. 2004;29:2163–2169. doi: 10.1007/s11064-004-6890-0. [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Tanaka S, Ishida K, Hoshi N, Kitagawa H. Age-related change and its sex differences in histoarchitecture of the hypothalamic suprachiasmatic nucleus of F344/N rats. Exp Gerontol. 2005;40:147–155. doi: 10.1016/j.exger.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Turek FW, Penev P, Zhang Y, van RO, Zee P. Effects of age on the circadian system. Neurosci Biobehav Rev. 1995;19:53–58. doi: 10.1016/0149-7634(94)00030-5. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL. Basolateral amygdala is involved in modulating consolidation of memory for classical fear conditioning. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vexler ZS, Ayus JC, Roberts TP, Fraser CL, Kucharczyk J, Arieff AI. Hypoxic and ischemic hypoxia exacerbate brain injury associated with metabolic encephalopathy in laboratory animals. J Clin Invest. 1994;93:256–264. doi: 10.1172/JCI116953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Willard LA, Kline AE, Wenger MK, Bolinger BD, Ren D, Zafonte RD, Dixon CE. Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury. Brain Res. 2004;998:113–121. doi: 10.1016/j.brainres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Weinert D, Weinert H. The relative Zeitgeber strength of lights-on and lights-off is changed in old mice. Chronobiol Int. 2003;20:405–416. doi: 10.1081/cbi-120021038. [DOI] [PubMed] [Google Scholar]
- Weinert D, Freyberg S, Touitou Y, Djeridane Y, Waterhouse JM. The phasing of circadian rhythms in mice kept under normal or short photoperiods. Physiol Behav. 2005;84:791–798. doi: 10.1016/j.physbeh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res. 2005;38:145–152. doi: 10.1111/j.1600-079X.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GB, Wang JT, Connolly JF. Prognostic determination in anoxic-ischemic and traumatic encephalopathies. J Clin Neurophysiol. 2004;21:379–390. [PubMed] [Google Scholar]
- Zandieh Doulabi B, Platvoet-ter SM, van Beeren HC, Labruyere WT, Lamers WH, Fliers E, Bakker O, Wiersinga WM. TR(beta)1 protein is preferentially expressed in the pericentral zone of rat liver and exhibits marked diurnal variation. Endocrinology. 2002;143:979–984. doi: 10.1210/endo.143.3.8706. [DOI] [PubMed] [Google Scholar]
- Zandieh Doulabi B, Platvoet-ter SM, Kalsbeek A, Fliers E, Bakker O, Wiersinga WM. Diurnal variation in rat liver thyroid hormone receptor (TR)-alpha messenger ribonucleic acid (mRNA) is dependent on the biological clock in the suprachiasmatic nucleus, whereas diurnal variation of TR beta 1 mRNA is modified by food intake. Endocrinology. 2004;145:1284–1289. doi: 10.1210/en.2003-0791. [DOI] [PubMed] [Google Scholar]
- Ziporen L, Shoenfeld Y, Levy Y, Korczyn AD. Neurological dysfunction and hyperactive behavior associated with antiphospholipid antibodies: A mouse model. J Clin Invest. 1997;100:613–619. doi: 10.1172/JCI119572. [DOI] [PMC free article] [PubMed] [Google Scholar]


