Abstract
In this study, we uncover a role for microRNAs in Drosophila germline stem cell (GSC) maintenance. Disruption of Dicer-1 function in GSCs during adult life results in GSC loss. Surprisingly, however, loss of Dicer-1 during development does not result in a GSC maintenance defect, although a defect is seen if both Dicer-1 and Dicer-2 function are disrupted. Loss of the bantam microRNA mimics the Dicer-1 maintenance defect when induced in adult GSCs, suggesting that bantam plays a key role in GSC self-renewal. Mad, a component of the TGF-β pathway, behaves similarly to Dicer-1: adult GSC maintenance requires Mad if it is lost during adult life, but not if it is lost during pupal development. Overall, these results show stage-specific differential sensitivity of GSC maintenance to certain perturbations, and suggest that there may be Dcr-2 dependent redundancy of GSC maintenance mechanisms during development that is lost in later life.
Introduction
The formation of embryonic tissues and the regeneration of adult tissues in the animal kingdom depend on stem cell populations. Embryonic stem cells are considered pluripotent due to their ability to differentiate into almost any cell type if placed in an appropriate context (Boiani et al., 2005). Adult stem cells are undifferentiated cells that reside in microenvironments known as niches, and they possess the ability to produce an undifferentiated stem cell and a daughter cell that can differentiate (Fuchs et al., 2004). Stem cell function has shown recently to be controlled by concerted actions of extrinsic signals from its respective regulatory niche and intrinsic factors including hyperdynamic plasticity of chromatin proteins (Li and Xie, 2005; Meshorer et al., 2006). However, not all stem cells remain in their niches continuously. For example, hematopoietic stem cells can relocate from their niche in adult animals (Li and Li, 2006). Yet, it is thought that many adult stem cells can only be fully functional in an appropriate niche. It is therefore important to understand how stem cell maintenance in the niche is regulated.
One of the most fundamental processes a developing animal needs to accomplish is to set aside and protect its precious stem cell population to replenish injured or lost tissues during adult life. At the moment little is known about the processes involved in establishing stem cells during development, though communication between stem cells and their environment is suggested to be a key regulator of the homeostasis of the process (Gilboa and Lehmann, 2006; Ward et al., 2006). Drosophila GSC niche has been extensively studied and has been an instructive model for understanding niche-stem cell communications. The GSC-niche interaction has shown to be reciprocal; stem cells communicate to niche through the Delta ligand, the niche furthermore controls the GSC maintenance via TGF-beta pathway (Chen and McKearin, 2003; Ward et al., 2006; Xie and Spradling, 1998).
Previous work has demonstrated that microRNAs, small (21-23nt) RNA molecules that can regulate gene expression, are required for normal stem cell function in mouse, Drosophila and plants [for reviews see (Hatfield, 2007; Shcherbata et al., 2006)]. Detailed analysis in Drosophila GSCs using cell cycle stage markers revealed that dcr-1 deficient GSCs were delayed in the p21/p27/Dacapo-dependent G1/S transition concomitant with increased expression of CDK-inhibitor p21/27/Dacapo, suggesting that microRNAs are required for stem cells to bypass the normal G1/S checkpoint. Hence loss of the microRNA pathway might inactivate a mechanism that makes stem cells sensitive to environmental signals that normally control the cell cycle at the G1/S transition (Hatfield et al., 2005; (Shcherbata et al., 2006)..
Here, we show that in addition to stem cell division, microRNAs are also required for stem cell maintenance. Furthermore, we identify bantam as a key microRNA required for germline stem cell maintenance in adults. Importantly, Dicer-1 activity is required for germline stem cell maintenance in adults, but, surprisingly, its activity is dispensable for maintenance if lost during development. Interestingly, we find that Dicer-2 is required for this developmental resistance of GSCs to loss of Dicer-1 function; if both dcr-1 and dcr-2 are absent in preadult GSCs, the GSCs are not maintained. Similarly, we find that Mad activity is required for GSC maintenance if lost in the adult, but not if it is lost at a younger stage. Our data therefore suggest that Drosophila ovarian GSCs have differential and stage-specific requirements for maintenance during development and in adults, and that at earlier stages Dcr-2 dependent adaptive mechanisms may exist that allow GSCs to withstand perturbations that are not tolerated in the adult.
Results
microRNAs are required for adult GSC maintenance
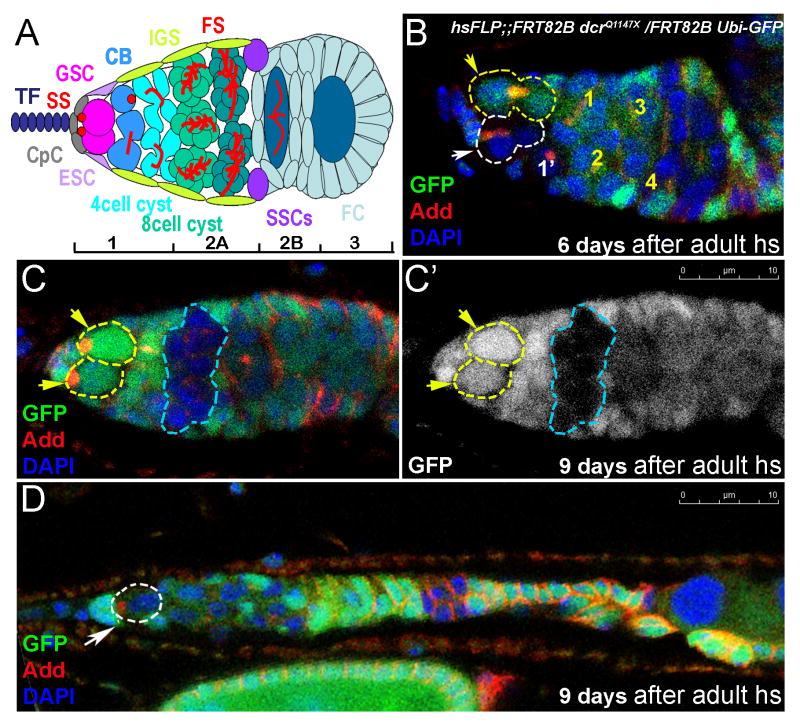
To assess the requirement for microRNAs in stem cells during different stages of development, we generated germline stem cells (GSC) that developed in normal conditions throughout larval and pupal stages, but lacked Dicer-1 during adult stages. These dicer-1 mutant germline stem cells (hsFLP;; FRT82Bdcr-1Q1147X) generated during adult life showed a defect in germline stem cell division kinetics (Figure 1B; S Table 4; S 1A-1C), similar to that shown previously for dicer-1 GSCs generated during late larval/early pupal stages (Hatfield et al., 2005). To our surprise, these mutant GSCs showed an additional phenotype, a maintenance defect (Figure 1C, S 3A). Similar findings were described recently (Jin and Xie, 2007; Park et al., 2007). Adult-induced dicer-1 mutant GSCs divide slowly and leave the niche. In many cases a wild-type GSC replaces the departed mutant GSC (Figure 1C). In other cases when there are two mutant GSCs, both GSCs may leave the niche resulting in an empty germarium (Figure 1D, S 3B). On average 12% of dicer-1 mutant GSCs were lost per day, while only 2% were lost in the control group (Figure 6A, S 1D). This is in sharp contrast to dicer-1 mutant preadult germline stem cells, which are not lost [Figure 6A, (Hatfield et al., 2005)].
Figure 1. Adult-induced dicer-1 mutant germline stem cell clones are lost from the niche.
(A) Diagram showing the germarium. Germline stem cells (GSC, pink) indicated by anterior spectrosomes (SS, red) are located at the anterior end of the germium adjacent to the niche cap cells (CpC, grey). Terminal filament (TF; dark blue), escort stem cell (ESC, lavender), differentiated cystoblast (CB, blue), inner germarial sheath cell (IGS, lime), germ cell cyst (cyst, celadon, emerald and green), marked by the presence of a branched fusomes (FS, red), somatic stem cells (SSCs, violet), follicle cells (FC, light blue).
(B) Adult induced dicer-1Q1147 mutant GSCs divide slower than control GSCs (the mutant produced one progeny, while the control produced 4 progeny) and leave the niche producing cysts that move posteriorly (C). (D) GSC loss coupled with a reduction in germline stem cell division results in smaller germaria (a germarium shown with a single dicer-1 mutant germline stem cell). Red=Adducin, Blue=DAPI, Green=GFP, mutant clones outlined with white dashed lines, control clones with yellow.
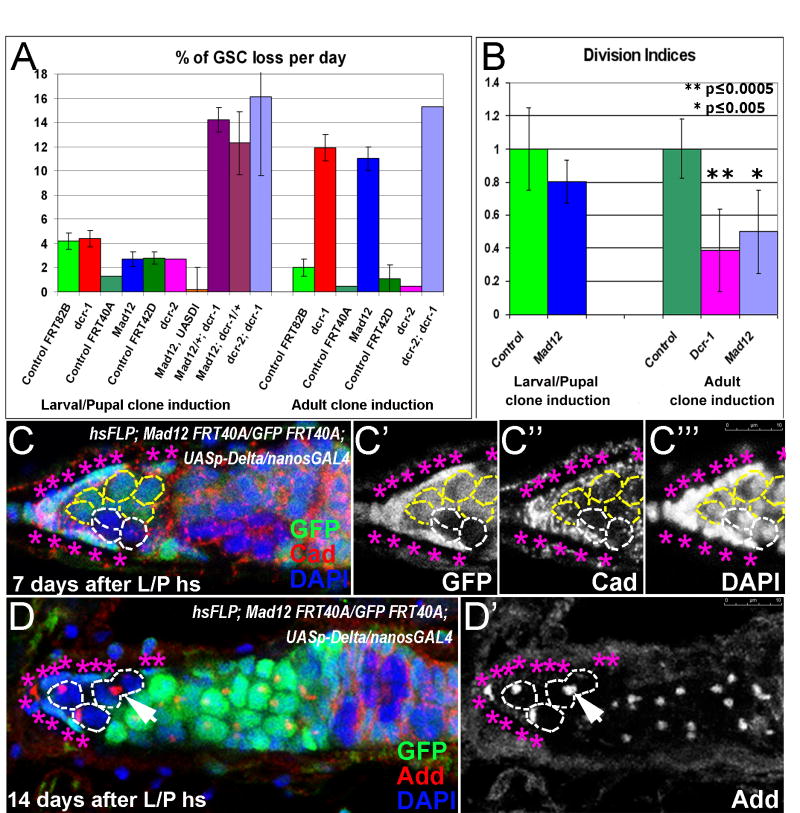
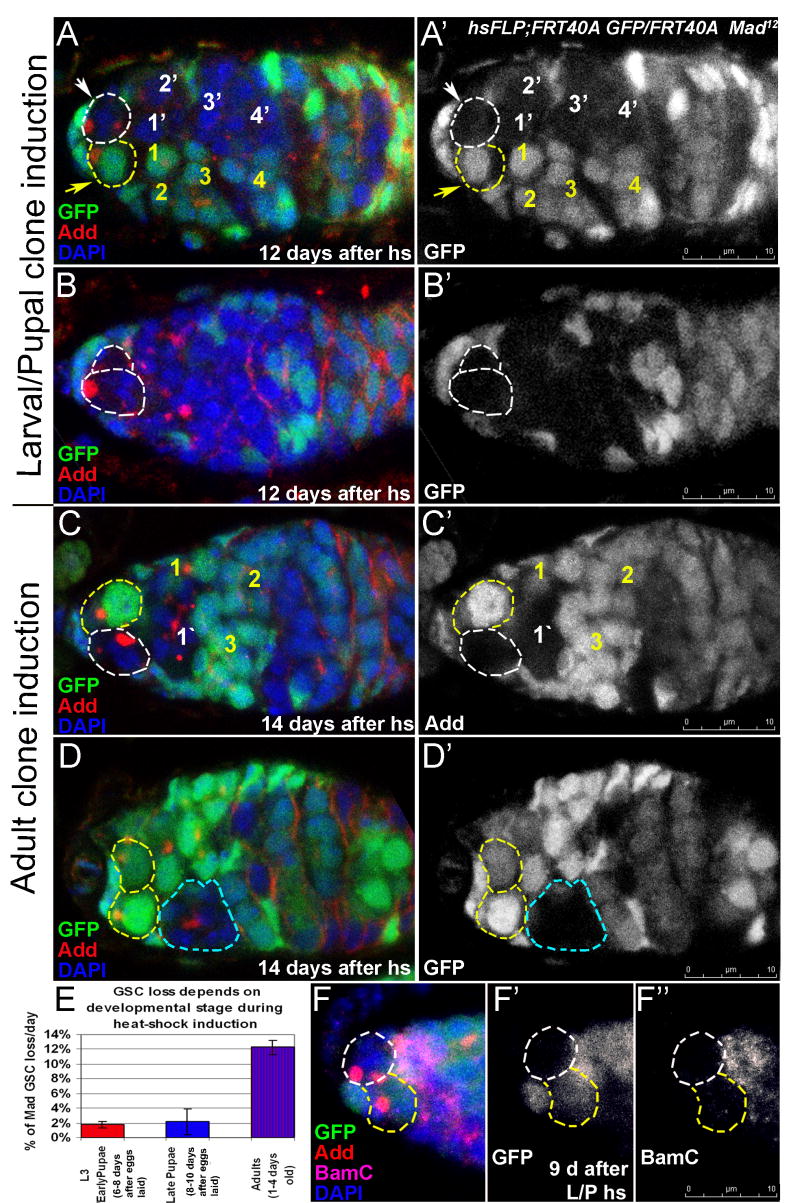
Figure 6. The GSC maintenance is governed by a robust redundant mechanism during development.
(A) The percentage of GSC loss per day is similar to controls when either dicer-1 or Mad is removed from GSCs during larval/pupal stages. In contrast, the GSCs are lost rapidly when either dicer-1 or Mad is removed from the GSCs during adulthood. In addition, a synergetic maintenance defect is observed when both dicer-1 and Mad levels are reduced simultaneously during larval/pupal stages (Mad12/+; dcr-1 or Mad12; dcr-1/+). (B) Germline stem cells lacking Mad activity from larval/pupal stages onwards divide relatively normally compared to controls. Whereas, germline stem cell lacking either Dicer-1 or Mad activity during adulthood divide significantly slower that controls. The bar-graph shows the division indices at 14 days after larval/early pupal heatshock and 9 days after adult heatshock (see S Table 4). (C, D) Mad12 mutant GSCs in germaria over-expressing UASp-Delta in the germline (hsFLP; Mad12 FRT40A/Ubi-GFP FRT40A; UASp-Delta/nanosGAL4) are maintained in the enlarged niche. Furthermore, the number of Mad mutant GSCs in the niche is increased from 7 to 14 day timepoint after larval/pupal clonal induction (C and D respectively, a dividing Mad mutant GSC marked with an arrow. Mad mutant GSCs outlined with white dashed lines, cap cells identified with pink asterisks. Red=Cadherin (C) or Adducin (D), Blue=DAPI, Green=GFP.
Bantam is required for adult GSC maintenance
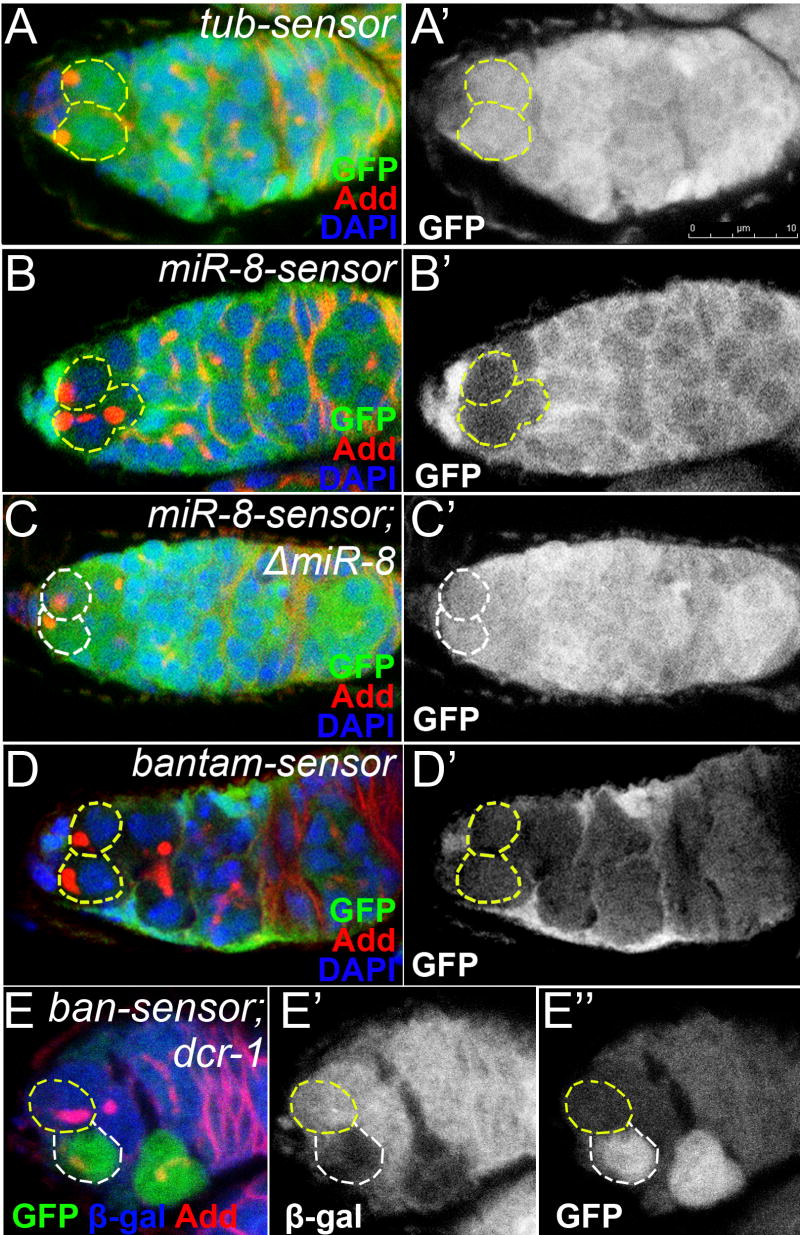
Since Dicer-1 and, therefore, microRNA function is required for adult GSC maintenance, we analyzed which microRNA(s) is/are responsible for this phenotype. By using sensor constructs for miR-8 and bantam we found that these microRNAs are expressed in GSCs (Figure 2). While the control sensor lacking microRNA binding sites shows uniform GFP expression, including the GSCs (Figure 2A), the GFP expression of miR-8- and bantam-sensors are highly reduced in the wild type GSCs (Figure 2B,D), but are up-regulated in miR-8Δ1 and dcr-1 mutant GSCs (Figure 2C,E). These data indicate that miR-8 and bantam are expressed in adult GSCs.
Figure 2. microRNA-GFP sensors are expressed in distinct subsets of cells in the germarium.
MicroRNA sensor expression patterns in (A, B and D) wild-type and (C) miR-8Δ1 mutant germaria. Sensors expression patterns determined by staining homozygous lines with anti-GFP antibodies(A-D). High GFP levels are observed in control (A), but not in miR-8- (B), or bantam-sensor GSCs (D), suggesting that miR-8 and bantam are expressed in GSCs. (C) Consistent with this, miR-8-sensor GFP levels increase substantially in homozygous miR-8Δ1 mutant germaria. (E) bantam-sensor is responsive to the dicer; in dcr-1 clones, marked by the absence of β-gal (E”), the level of GFP fluorescence is higher than that in a non-clonal neighbor (E’’). In E native GFP expression by one copy of bantam-sensor is analyzed (hs Flp; banάtub84BT:Avic/GFP-EGFP/+; FRT82B dcr-1Q1147X/FRT82B arm-lacZ). Red=Adducin, Blue=DAPI (A-D) or β-gal (E), Green=GFP, GSCs marked with white dashed lines.
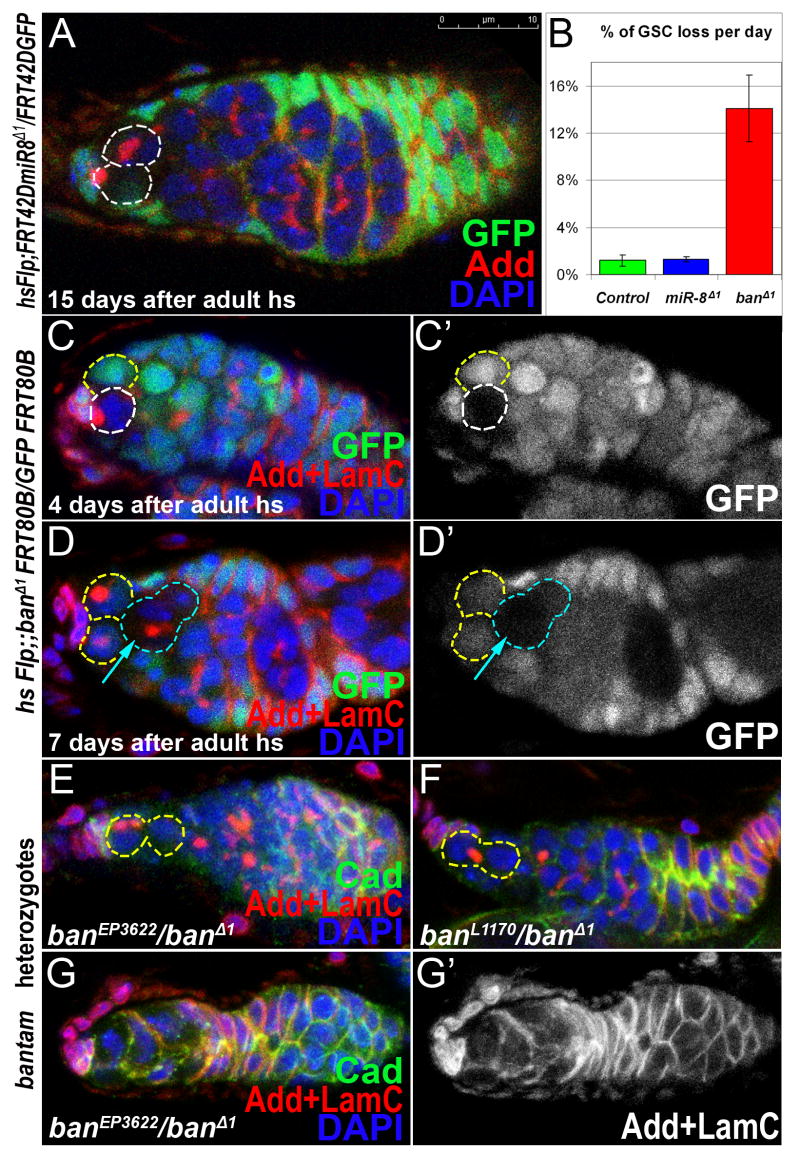
Since miR-8 and bantam are expressed in GSCs, we tested whether they are required for GSC maintenance. GSCs, mutant for miR-8 showed no obvious maintenance or cell division defects during preadult or adult stages (1.6±0.5%, 1.3±0.6% of miR-8 Δ1 mutant GSCs lost/day; Figure 3A, 3B; S Table 4). However adult-generated GSCs mutant for bantam showed maintenance and cell division defects (Figure 3C,D; S Table 1, 4). On average 14.1%±2.8% of bantam mutant GSCs (hsFLP;; banΔ1 FRT80B/Ubi-GFP FRT80B) were lost per day, while no loss was observed in the control group (hsFLP;;FRT80B/arm lacZ FRT80B; S Table 1). When bantam clones were generated in preadult stages the loss was not as dramatic (6.2±0.4% per day; S Table 1). However, given the existing evidence that all miRNA production in flies is strictly dependent on Dcr-1 function, it is surprising to note that the bantam larval/pupal clones appear to have a stronger phenotype than the dcr-1 clones. This apparent difference in phenotypic severity may be attributable to differences in gene product perdurance and/or to the inherent variability in the GSC loss assay. Heteroallelic combinations of bantam mutants (banL1170/banEP3622, banL1170/banΔ1 and banEP3622/banΔ1) exhibit similar mutant phenotypes as the banΔ1 clones (but in a lower frequency), suggesting that the defects are due to the loss of bantam function and not due to second site mutations (Figure 3E-G; S 4B-E). These data show that bantam and dicer-1 mutant defects in GSC maintenance are similar and therefore suggest that bantam is a key microRNA in assuring the maintenance of adult GSCs.
Figure 3. bantam microRNA is required for GSC maintenance in the niche.
(A) miR-8 Δ1 mutant germline stem cells are maintained in the niche and divide properly 14 days after adult heat shock. (B) Graph showing that the bantam mutant GSCs are lost 11 times faster from the niche compared to miR-8 or control GSCs. bantam mutant GSC clones (C, 4 days after clonal induction) are not maintained in the niche (D, 7days after clonal induction). (E-G) Germaria from bantam hetereoallelic mutants banEP3622/ banΔ1(E-G) and banL1170/banΔ (F) exhibit mutant phenotypes similar to bantam clones: germaria are reduced in size and have a single GSC (E,F) or no GSC (G).
Red=Adducin (A) or Adducin+LaminC (C-G), Blue=DAPI, Green=GFP (A-D) or Cadherin (E-G); mutant GSCs or cysts outlined with white dashed lines, departed or differentiated stem cells with turquoise dashes.
Mad mutant GSCs are maintained if the mutation is induced during development
The results described above present an unexpected scenario in which a mutation causes a maintenance defect when the deficiency is introduced during adult stages but not if it is introduced during late larval/pupal stages. To address the generality of the phenomenon, we tested whether a well-studied component of the GSC maintenance pathway, the transcription factor Mad, fits this paradigm. Similar to dicer-1, Mad was not essential for GSC maintenance if the defect was induced during late larval/early pupal stages (Figure 4A-4B, 6A), but was essential if the Mad mutation was introduced in GSCs during adulthood [Figure 4D, 6A; (Xie and Spradling, 1998)]. In addition, as shown before (Xie and Spradling, 1998) these adult-induced Mad-mutant GSCs were defective not only in maintenance, but also in normal cell cycle kinetics (Figure 4C, 6B; S 4). These data show a similarity between Mad and dicer-1 mutants; both maintain the adult GSCs if the mutation is introduced during pupal development. However, if the mutations are introduced during adult life, Mad and Dicer-1 are essential for normal GSC maintenance.
Figure 4. Larval/pupal-induced Mad12 mutant GSCs are maintained in the niche.
Larval/pupal-induced Mad12 mutant GSCs divide at the same frequency as control GSCs (A; S Table 4) and are maintained in the niche 12 days after clonal induction (B; S Table 1). However, as described before (Xie and Spradling, 1998), Mad12 mutant GSC clones induced during adulthood divide slower than non-clonal germline stem cells (C; S Table 4) and are not maintained in the niche (D; 14 days after adult clonal induction, the Mad mutant GSC has left the niche and produced a 8-cell cyst). A bar graph showing that GSC maintenance has a development-dependent character: when Mad12 mutation was induced during late larval/early pupal or even late pupal developmental stages, mutant GSCs were maintained, however a clear GSC loss was observed for adulthood-induced Mad12 GSCs (E). A differentiation factor Bam is not derepressed in mutant GSCs, showing that larval/pupal-induced Mad12 mutant GSCs maintain their SC identity. Red=Adducin, Blue=DAPI, Green=GFP, Purple=BamC, mutant GSCs outlined with white dashed lines, control GSCs with yellow dashes.
TGF-β signaling within the GSC niche blocks germline stem cell differentiation by silencing Bam. In the absence of Mad, Bam is de-repressed and the GSC differentiates (Chen and McKearin, 2003; Song et al., 2004; Xie and Spradling, 1998). Since GSCs lacking the transcription factor Mad, a key component of the TGF-β pathway, from late larval/pupal developmental stages onward were maintained in the niche, we decided to test whether the differentiation factor Bam was still repressed. Interestingly, we found Bam being repressed in this case (Figure 4F; n=36 Mad12 GSCs and n=42 WT GSCs). These data suggest that larval/pupal-induced Mad12 mutant GSCs silence Bam by a mechanism other than transcriptional repression by Mad.
Period of competence of preadult stem cells extends through pupal development and ends at adulthood
Our data suggest that the miRNA pathway and Mad activity are dispensable when they are lost in young GSCs, but they are essential when they are lost in older GSCs. To identify the latest stage of development at which GSCs are able to overcome the loss of Mad activity for GSC maintenance, we introduced Mad12 mutations in GSC of 3rd instar larva/early pupae, late pupae and 1-4 days old adult flies (Figure 4E). Interestingly, Mad12 GSCs were lost only after adult clonal induction, suggesting that the period of competence of preadult GSC maintenance extends through late pupal stages, but not into adulthood. Previous studies have shown that GSCs already reside in a niche at the late pupal stage (Zhu and Xie, 2003), suggesting that this resilience is not a result of major differences in the morphological environment of GSCs during development and adulthood.
Preadult Mad and Dicer-1 interact in GSC maintenance
Since both the Mad and Dicer-1 are required during adult stages but are not required if the components are lost during preadult stages, we tested whether they interact during earlier development to maintain germline stem cells in the niche. Specifically, we reduced the level of Mad in a dicer-1 clonal background or reduced the level of Dicer-1 in a Mad clonal background (hsFLP; Mad12 FRT40A/+; FRT82B dcr-1Q1147X/FRT82B GFP and hsFLP; Mad12 FRT40A/ GFP FRT40A; FRT82B dcr-1Q1147X/+). In both cases, the clones were induced during late larval/early pupal stages. Interestingly, when both Mad and Dicer-1 activities were reduced at the same time, a clear maintenance defect was observed after preadult clone induction (Figure 6A). These data show that Dicer-1 and Mad interact genetically during developmental stages (a synthetic GSC maintenance defect).
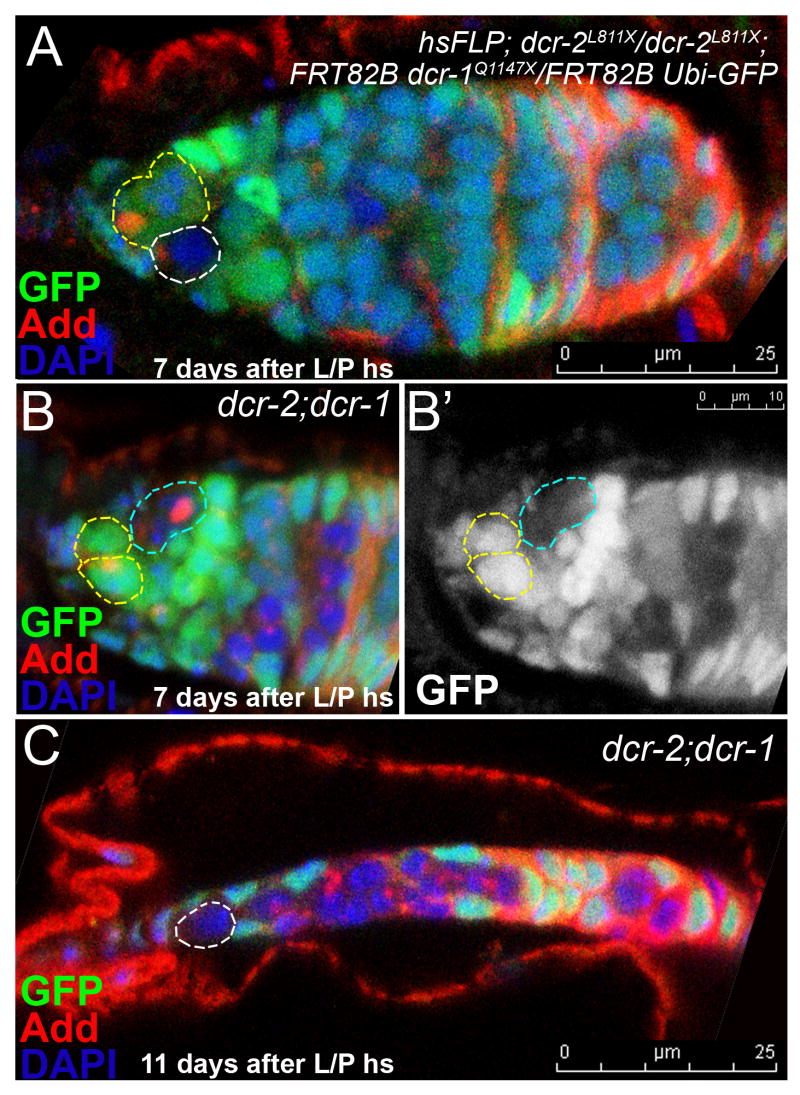
Preadult germline stem cells lacking both Dicer-1 and Dicer-2 activities are lost from the niche
To further investigate the role of small interfering RNAs in preadult germline stem cells, we tested dcr-2; dcr-1 double-mutants and observed a strong maintenance defect when the double mutants were induced during larval/pupal development (Figure 5, 6A). However the Dicer-2 pathway alone is not required for larval/pupal or adult germline stem cell maintenance (Figure 6A, S Table 3). These data indicate that dicer-1 and dicer-2 interact genetically in some manner to maintain preadult germline stem cells. The Dicer-2 contribution to the dicer-2;dicer-1 germline stem cell maintenance phenotype is not likely to be due to defective microRNA processing, as previous biochemical studies showed that Dicer-2 does not appear to process microRNAs (Lee et al., 2004; Pham et al., 2004). Furthermore, we did not observe any reduction of mature bantam levels by QPCR analysis in dcr-2 homozygous animals compared to the control animals, suggesting that Dicer-2 does not have a major role in bantam processing (data not shown). Interestingly Dicer-2 is known to act through the RNAi pathway to modify chromatin (Grimaud et al., 2006; Lee et al., 2004; Pal-Bhadra et al., 2002; Pal-Bhadra et al., 2004; Peng and Karpen, 2007; Verdel et al., 2004), raising the possibility that chromatin modification contributes to the robust maintenance behavior of preadult germline stem cells.
Figure 5. GSCs lacking Dicer-1 activity and Dicer-2 activity from pupal stages onwards are lost from the niche.
(A-C) Larval-induced dicer-1 mutant GSCs in a dicer-2 mutant background (hsFLP; dcr-2 L811X/dcr-2L811X; FRT82B dcr-1Q1147X/FRT 82B Ubi-GFP) do not divide normally and are readily lost from the niche (B). Recall, that when Dicer-2 activity is present, larval-induced dicer-1 mutant GSCs are not lost from the niche. (C) Example of a severely reduced germarium with a single mutant GSC. GSCs outlined with dashes: yellow=normal, white=mutant. GSCs departed from the niche are outlined with turquoise dashes. Red=Adducin, Green=native GFP, Blue=DAPI.
Notch pathway does not require Mad activity during development
In contrast to our observations with Mad and dicer-1 clones, GSC maintenance requires Notch signaling from the GSCs to the niche throughout development. In the absence of Neuralized (required for proper processing of Delta or Serrate ligands), GSCs are not maintained in the niche. This Notch signaling requirement is observed in both late larval/early pupal and adult clones (Ward et al., 2006). Furthermore, an increase in Notch ligand production in germ line results in an enlarged niche, which in turn supports additional GSCs. This niche expansion can be induced after pupal development (Ward et al., 2006).
In order to determine whether Notch pathway function in GSC maintenance is Mad dependent during larval/pupal development we analyzed whether the additional GSCs produced by increased Notch signaling during developmental stages require Mad signaling for their maintenance. We assayed whether ectopic GSCs induced by overexpression of Delta were maintained in the niche if they were also mutant for Mad. Our clonal analysis shows that the ectopic GSCs produced during development do not require Mad for their maintenance in the niche. Similar to the Mad mutant GSCs described above, we find that the Mad, pUASP-Delta mutant GSCs are not lost from the niche following larval/pupal clonal induction (hsFLP; Mad12 FRT40A/Ubi-GFP FRT40A; pUASP-Delta/nanosGAL4, Figure 6A, 0.2±1.8% loss/day); the number of germaria containing mutant GSCs remains the same in the two timepoints analyzed. However, unlike the Mad mutant GSCs described above, in the Delta overexpression background the number of Mad GSCs increases (~2 mutant GSCs to ~3 mutant GCS/7 days; Figure 6C-6D), indicating that the Mad mutant GSCs can divide and are recruited to and maintained in the enlarged niche. Thus, the extra GSCs produced by increased Notch signaling behave similarly to normal GSCs; they do not require Mad activity for maintenance in the niche if the Mad mutation is introduced during preadult stages. Therefore, ectopic GSCs as well as wild type stem cells have a period of competence during preadult stages that ensures their maintenance within the niche even in the absence of Mad.
Discussion
We draw two important conclusions from this work. First, Dicer-1 and, more specifically, bantam microRNA are required for adult stem cell maintenance (Figure 7A). Second, preadult stem cells have a youthful resilience that is lost at adulthood. Thus, in developing animals if certain key components required for adult germline stem cell maintenance are lost, the animal can overcome this loss and maintain the stem cells throughout life (Figure 7B).
Figure 7. Model showing different mechanisms for GSC maintenance during development and adulthood.
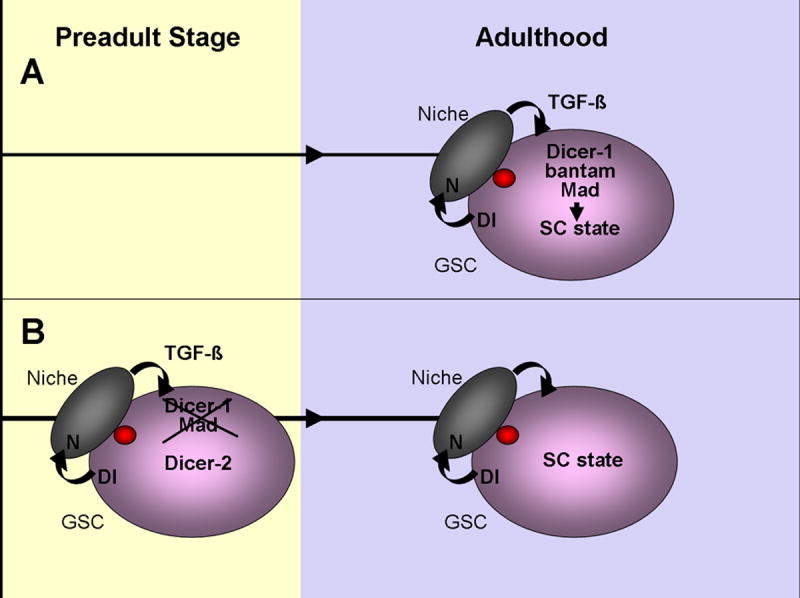
(A) TGF-β and Dicer-1 are required for GSC maintenance in adult Drosophila ovary. We have identified bantam as the key microRNA in this process. Bantam has previously been studied in cell cycle and cell death in Drosophila imaginal disc epithelial cells. However, in GSC the bantam target process that leads to GSC maintenance in the niche remains to be revealed. (B) Surprisingly, we find that Mad and Dicer-1 are not required for GSC maintenance if these components are lost in GSCs during larval/pupal development. Preadult GSCs lacking Dicer-1 are no longer maintained if they also lack Dicer-2. We propose that the Dicer-2 pathway prevents GSC loss, possibly by chromatin remodeling during developmental stages, which as a consequence promotes stem cell fate during adulthood.
Bantam function in GSCs
The microRNA bantam has been previously found to promote tissue growth in Drosophila imaginal discs (Brennecke et al., 2003). In addition, removing one copy of the endogenous bantam gene has shown to enhance, and overexpression of bantam suppress the severity of,Hid overexpression-induced apoptosis in the eye (Brennecke et al., 2003). Based on these results, a hypothesis was put forward that bantam simultaneously stimulates cell proliferation and inhibits apoptosis. Furthermore, recent studies have revealed that bantam overexpression mitigates degeneration induced by the pathogenic polyglytamine protein Ataxin-3, which is mutated in the human polyglutamine disease spinocerebellar ataxia type 3 (SCA3) (Bilen et al., 2006). These studies suggest that bantam microRNA can also suppress neuronal degeneration. The Hippo-tumor-suppressor pathway has emerged as a key regulator for bantam expression in Drosophila imaginal discs in regulating cell division (Nolo et al., 2006; Thompson and Cohen, 2006).
The present work supports a different view of bantam action in Drosophila GSCs, adding new possibilities to the repertoire of bantam’s functions. In the adult stem cell population, bantam microRNA is essential for the stem cell maintenance in the niche (Figures 2-3, S 4) and appears to be acting independently of Hippo-pathway as yorkie mutant GSCs are maintained in the niche; S Table 1. Many questions remain about this new function of bantam, such as: What biological process is defective in bantam mutant GSCs that results in their loss from the niche? What are the targets of bantam and what are the pathways that regulate bantam expression in GSCs? In theory, the biological process and the targets of bantam in GSCs might be the same as those involved with imaginal disc cell cycle control. However, cell cycle defects alone cannot account for the GSC loss as dicer-1 mutant GSCs that are generated during preadult stages show adult GSC division defects but are maintained normally in the niche [(Hatfield et al., 2005), Figure 6A]. Interestingly, the 3’ UTR of Mad is a validated target of bantam microRNA in S2 cells (Robins et al., 2005). It is therefore possible that bantam microRNA may directly regulate Mad in GSCs. However, in this scenario loss of bantam should result in Mad overexpression, yet the bantam mutant phenocopies Mad loss-of-function phenotypes. One potential explanation is that high levels of Mad are just as deleterious to germline stem cells as the lack of Mad activity. If this is the case, then Mad levels would need to be finely tuned by microRNAs in germline stem cells to ensure their maintenance in the niche. Similar fine-tuned regulation of Atrophin by miR-8 was recently reported (Karres et al., 2007). Further studies are required to test this hypothesis.
Robust Maintenance of Preadult Germline Stem Cell
The presented work reveals the resilience of preadult stem cells to perturbations that cause GSC maintenance defects if introduced in adults. It seems logical that developing organisms would have a means of protecting their precious stem cells during the many intricate developmental processes that occur. We have shown that stem cells are still protected late in preadult development (during pupation) but not during adulthood. What protects the germline stem cells prior to adulthood in Drosophila? As the niche has already formed by pupal stage, we suggest that the period of competency does not reflect a morphological difference in the niche at different time points. Instead, however, we found that the preadult competence requires Dcr-2. Dcr-2 activity is shown to be required for siRNA pathway. As RNAi pathway-dependent chromatin modifications have been previously observed in Drosophila (Grimaud et al., 2006; Lee et al., 2004; Pal-Bhadra et al., 2002; Pal-Bhadra et al., 2004; Peng and Karpen, 2007; Verdel et al., 2004), one possibility is that Dcr-2 acts through stem cell chromatin in preadult GSCs. Further work will help to unravel the role of Dcr-2 in this process.
Overall, our study shows that in Drosophila young germline stem cells are better able to withstand perturbations that disrupt their maintenance than adult germline stem cells. Further analysis of these findings might ultimately lead to insights into cancer stem cell resilience and even help to reveal ways to rejuvenate failing and/or aging stem cells.
Materials and Methods
Fly Strains
We used the following mutant stocks: eyFLP;;FRT82B dcr-1Q1147X/TM3Sb, eyFLP;;FRT82B, eyFLP; FRT 42D dcr-2L811X/CyO (Lee et al., 2004), Mad12 FRT40A/CyO (Xie and Spradling, 1998), FRT42D ykiB5 (Huang et al., 2005), FRT42B iswi2 (a gift from J.Tamkun), pUASP Delta (Jordan et al., 2006), w-;;Df(3L)banΔ1 FRT80B/TM6, banL1170, banEP3622, w-;banScer\UAS.T:Avic\GFP-EGFP [pUAST-bantam (Brennecke et al., 2003)], banάtub84BT:Avic/GFP-EGFP [bantam-sensor (Brennecke et al., 2003)], hsFLP;;FRT82B Ubi-GFP/TM3Sb, hsFLP; Ubi-GFP FRT40A/CyO, yw hsFLP;FRT42D Ubi-GFP/CyO, hsFLP;;Ubi-GFP FRT80B/TM3, w;NGT40/SM6a;nanosGal4/TM3Sb (Bloomington Stock Center). The miR-8Δ1 deletion line was generated by imprecise P-element excision of EP(2)2269. EP(2)2269 flies were isogenized with w1118 and balanced. Standard P element imprecise excision was carried out and 300 individual excision stocks were screened by primers 5’- ATCACACGTTAACGTAACGTAACGGCAG and 5’ – AGATTCGAAAGCCCCACACGCACAATC. The miR-8Δ1 deletion removes 1316 bp of genomic DNA, including the 23bp mature miR-8 microRNA. The deletion spans from 1057bp upstream of the mature miR-8 sequence to 236bp downstream of the mature sequence. The miR-8Δ1 deletion was recombined onto the FRT42D chromosome using standard meiotic recombination protocols (Xu and Rubin, 1993). The recombined FRT42D miR-8Δ1 lines were screened by PCR with primers 5’-AAATCTTCACCGTCACCCAGTCGT and 5’- AGAAACCAGCAGAAAGCAGCATCC.
Generation of pUASP-bantam, pUASP-miR-8 and miR-8-sensor
pUASP-bantam
A partial bantam precursor sequence (584 nt) was amplified from pUAST-EGFP-bantam construct (Brennecke et al., 2003) using the following primers:
bantam forward: 5’-ATAGCGGCCGCGTTAACTGGCAGCATATAATTTC;
bantam reverse: 5’-ATTCTAGATTATAGGCAGATTTAACATGTGG.
The amplified fragments were cloned into UASP plasmid using Not1 and Xba1.
pUASP-miR-8
A partial miR-8 precursor sequences (729 nt) were amplified from adult fly genomic DNA with the following primers:
miR-8 forward: 5’-ATAGCGGCCGCCGCGGTCACACGCACATTTCAATA;
miR-8 reverse: 5’-ATTCTAGAAATGGGAATTGGGAACGATCTCGC.
The amplified fragments were cloned into UASP plasmid with Not1 and Xba1.
miR-8-sensor
Two perfect complementary target sequences of miR-8 separated by 16 nucleotides were inserted downstream of tub-GFP plasmid into the 3’UTR of the P-element in CaSpeR4 with Not1 and Xba1. The following oligonucleotides containing the target sequences of miR-8 were used:
5’- CCGCCCTTGACATCTTTACCTGACAGTATTAACGCGAATATCCCTTTGACATC TTTACCTGACAGTATTATGAACCT;
5’-TAGAGGTTCATAATACTGTCAGGTAAAGATGTCAAAGGGATATTCGCGTTAA TACTGTCAGGTAAAGATGTCAAGGGC.
Transgenic flies were generated by injection of purified plasmid DNA into w1118 Drosophila embryos (Rainbow Transgenic Flies Inc., CA). These flies were crossed with w1118 and transformants with germline insertion of plasmid DNA were selected based on eye color. For pUASP-bantam 27 independent transgenic lines were generated and 3 analyzed. For pUASP-miR-8 34 independent transgenic lines were generated, 3 screened and showed no defects in GSC maintenance and kinetics. For miR-8-sensor 27 independent transgenic lines were generated. 6 out of 7 examined lines show similar GFP expression patterns in the germarium as shown in Figure 2.
Generation of clones, maintenance, kinetic- and statistical-analysis
Drosophila melanogaster stocks were raised on standard cornmeal-yeast-agar-medium at 25°C. Clones were induced using the hsFLP-FRT system for mitotic recombination. Larval/early pupal germline clones were produced by heat-shocking third instar larvae/early pupae (usually 6 & 7 days after crosses were set up) for 1hr at 37°C two days in a row, and dissected at different time points after the last heat shock. Late pupal germline clones were produced by heat-shocking late pupae (9 & 10 days after crosses were set up) for 1hr at 37°C two days in a row. The flies eclosed 1-2 days after the last heat shock. Adult heat-shock germline clones were induced by heat-shocking 2-4 days old F1 adult females in empty vials for 50 minutes two days in a row in a 37°C water bath.
Adult-induced bantam clones were generated by heat-shocking 2-4 day old hsFLP;;banΔ1 FRT80B/Ubi-GFP FRT80B flies at 37°C for 50 minutes twice daily 2 days in a row, with a five hour recovery period between daily heat shocks. Flies for this were collected for 2 days after they began eclosing and then kept on wet yeast at 25°C until dissected. They were turned over into fresh vials with wet yeast every other day.
The germline stem cell loss per day (S Table 1-3) was determined by comparison of the percentage of germaria with clonal GSCs between two different time points after clonal induction:
GSC loss per day = (% of clonal GSC at timepoint1 - % of clonal GSC at timepoint2) ×100%/% of clonal GSC at timepoint 1/elapsed time
The relative division index (S Table 4) for a marked GSC is determined by the number of cysts generated by a marked GSC divided by the number of unmarked cysts generated by an unmarked GSC in the same germaria (Hatfield et al., 2005). Division frequencies were measured using germaria containing one GFP positive GSC and one clonal (GFP negative) GSC. The total number of cysts from a GSC that are produced in a given time window provides a measurement of GSC division frequency. In our case, the time window spanned from the first heat-shock treatment to the time of harvesting the adults. Therefore, we limited our counts to the region of the germarium that was anterior to the easily-identifiable GFP+/+ cyst. This cyst developed from the first daughter cell of the clonal GSC (GFP-) after heat-shock induced mitotic recombination. Cyst production from homozygous clonal GSCs was divided by the cyst production from heterozygous non-clonal GSCs in the same germarium to obtain the division index. The student t-test was used to determine the statistical significance.
Staining Procedures
Antibody stainings and confocal microscopy were performed as described previously (Shcherbata et al., 2004). GFP was detected either by analyzing the native GFP (Figures 1, 4-6) or by using anti-GFP-directly conjugated with Alexa 488 (Figures 2-3). A confocal laser-scanning microscope (Leica SPE5) was used in this study. We used the following mouse monoclonal antibodies: Engrailed (1:20), Armadillo (1:40); Adducin (1:20) and anti-DE-Cadherin (1:50) and Lamin C (1:20) from the Developmental Studies Hybridoma Bank, and anti-p-Mad (1:500, P.ten Dijke), guinea pig anti-CycE (1:500, T.Orr-Weaver), rat anti-Bam-C (1:1000, D.McKearin), rabbit anti-GFP-directly conjugated with Alexa 488 (1:3000, Invitrogen). Secondary antibodies were Alexa 488, 568, 633 or 647 goat anti-mouse, anti-rabbit and anti-guinea pig (1:500, Molecular Probes), goat-anti-rat Cy5 (1:250, Jackson Immunoresearch).
Supplementary Material
Acknowledgments
We thank Drs. A.Spradling, D.Pan, B.Calvi, S.Cohen, T.T.Su, T.Orr-Wearver, T.Xie, J.Tamkun, and R. Carthew for suggestions, flies and reagents, Christian Nelson, Louise von Stechow, Andriy Yatsenko for help with experiments, Dr. Bradford Stadler for help with QPCR analysis. This work was supported by AHA fellowships for H.R.S. and S.H.R., and MOD and NIH grants for H.R-B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006;24:157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Boiani M, Gentile L, Gambles VV, Cavaleri F, Redi CA, Scholer HR. Variable reprogramming of the pluripotent stem cell marker Oct4 in mouse clones: distinct developmental potentials in different culture environments. Stem Cells. 2005;23:1089–1104. doi: 10.1634/stemcells.2004-0352. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 2006;443:97–100. doi: 10.1038/nature05068. [DOI] [PubMed] [Google Scholar]
- Grimaud C, Bantignies F, Pal-Bhadra M, Ghana P, Bhadra U, Cavalli G. RNAi components are required for nuclear clustering of Polycomb group response elements. Cell. 2006;124:957–971. doi: 10.1016/j.cell.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Hatfield S, Shcherbata HR, Ward EJ, Reynolds S, Ruohola-Baker H. microRNAs and their involvement in Stem Cell division. In: Clarke N, Sanseau P, editors. microRNAs; Biology, Function and Expression. DNA Press; 2007. [Google Scholar]
- Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jin Z, Xie T. Dcr-1 maintains Drosophila ovarian stem cells. Curr Biol. 2007;17:539–544. doi: 10.1016/j.cub.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Jordan KC, Clegg NJ, Blasi JA, Morimoto AM, Sen J, Stein D, McNeill H, Deng WM, Tworoger M, Ruohola-Baker H. The homeobox gene mirror links EGF signalling to embryonic dorso-ventral axis formation through notch activation. Nat Genet. 2000;24:429–433. doi: 10.1038/74294. [DOI] [PubMed] [Google Scholar]
- Jordan KC, Schaeffer V, Fischer KA, Gray EE, Ruohola-Baker H. Notch signaling through tramtrack bypasses the mitosis promoting activity of the JNK pathway in the mitotic-to-endocycle transition of Drosophila follicle cells. BMC Dev Biol. 2006;6:16. doi: 10.1186/1471-213X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The Conserved microRNA MiR-8 Tunes Atrophin Levels to Prevent Neurodegeneration in Drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31:589–595. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–116. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolo R, Morrison CM, Tao C, Zhang X, Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16:1895–1904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Pal-Bhadra M, Bhadra U, Birchler JA. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol Cell. 2002;9:315–327. doi: 10.1016/s1097-2765(02)00440-9. [DOI] [PubMed] [Google Scholar]
- Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, Birchler JA, Elgin SC. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- Park JK, Liu X, Strauss TJ, McKearin DM, Liu Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr Biol. 2007;17:533–538. doi: 10.1016/j.cub.2007.01.060. [DOI] [PubMed] [Google Scholar]
- Peng JC, Karpen GH. H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat Cell Biol. 2007;9:25–35. doi: 10.1038/ncb1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham JW, Pellino JL, Lee YS, Carthew RW, Sontheimer EJ. A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell. 2004;117:83–94. doi: 10.1016/s0092-8674(04)00258-2. [DOI] [PubMed] [Google Scholar]
- Robins H, Li Y, Padgett RW. Incorporating structure to predict microRNA targets. Proc Natl Acad Sci U S A. 2005;102:4006–4009. doi: 10.1073/pnas.0500775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbata HR, Althauser C, Findley SD, Ruohola-Baker H. The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development. 2004;131:3169–3181. doi: 10.1242/dev.01172. [DOI] [PubMed] [Google Scholar]
- Shcherbata HR, Hatfield S, Ward EJ, Reynolds S, Fischer KA, Ruohola-Baker H. The MicroRNA pathway plays a regulatory role in stem cell division. Cell Cycle. 2006;5:172–175. doi: 10.4161/cc.5.2.2343. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward EJ, Shcherbata HR, Reynolds SH, Fischer KA, Hatfield SD, Ruohola-Baker H. Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr Biol. 2006;16:2352–2358. doi: 10.1016/j.cub.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zhu CH, Xie T. Clonal expansion of ovarian germline stem cells during niche formation in Drosophila. Development. 2003;130:2579–2588. doi: 10.1242/dev.00499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.