Birth and early years
Protein Kinase C (PKC) was born in Japan in 1977 in the Department of Biochemistry of the University of Kobe. Inoue, Kishimoto and Takai in Nishizuka’s Laboratory, described in two papers in the Journal of Biological Chemistry a cyclic nucleotide-independent, proteolytically modified protein kinase from mammalian brain (named PKM, M for magnesium ions that were indispensable for activation) (1, 2). The full length enzyme (2), subsequently demonstrated to be activated by calcium and phospholipids (3), was named protein kinase C (C, for calcium ions, which fully activated the enzyme at low concentrations, and thus differentiate it from cyclic nucleotide-dependent kinases, protein kinase A and G). The same group observed that unsaturated diacylglycerol was an essential activator of PKC (4), linking the previously described receptor-dependent inositol phospholipid hydrolysis (5) to protein phosphorylation, thus thrusting this enzyme into intercellular signal transduction research. PKC inhibitors were characterized in 1980 as phospholipid interfering drugs (such as chlorpromazine, imipramine and dibucaine) (6) and the first direct functional assignment for PKC was made utilizing human platelets in which the thrombin-induced release of serotonin was shown to be mediated by PKC activation (7). Starting in the early eighties, the interest in PKC crossed Japan’s borders and invaded the rest of the world, making PKC one of the most studied enzymes in biology, with more than 45.000 research papers published up to now.
We thus decided to celebrate the 30th birthday of PKC with a Special Issue of Pharmacological Research, reviewing the more relevant and recent developments in its characterization in physiology and pathology, highlighting the pharmacological implications and in particular the search for isozyme-selective inhibitors and activators.
It is impossible to mention all the discoveries that attracted, and still continue to attract the attention of so many scientists to this enzyme. We will concentrate just on select break-throughts that accompained PKC trentennial research and will divide this continuing story into the developments in each of the three decades (Figure 1):
Figure 1.
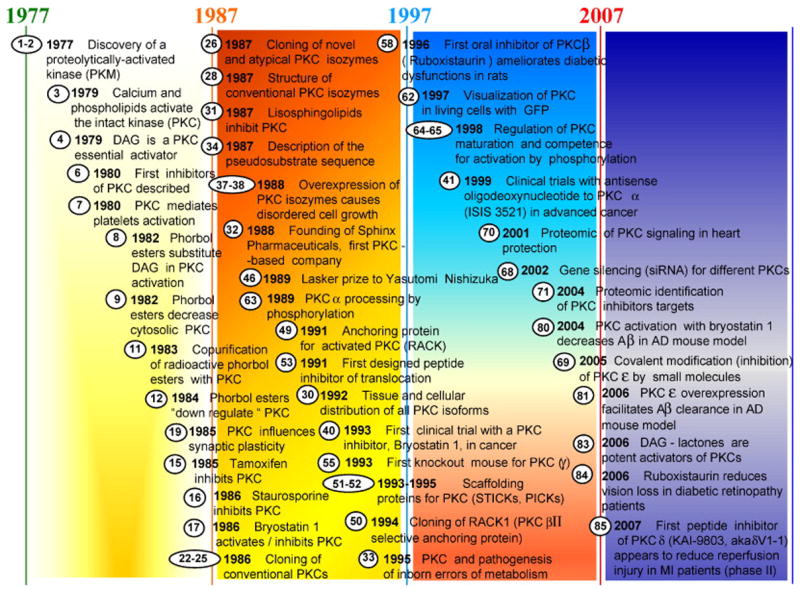
Chronological studies on protein kinases C. The principal discoveries are reported focusing on pharmacological implications. The numbers in the circles indicate the reference citation. More details is provided in the text.
1977–1986: enzymatic description and modulations of activity,
1987–1996: isozyme identifications and their functions,
1997–2006: entering the “matured” age (regulation by phosphorylation) and new technological advances
The first 10 years: Enzymatic description and modulations of activity
In 1982, the observation that in human platelets the tumor promoting phorbol derivatives directly activate PKC, mimicking but not generating DAG (i.e. not inducing phosphlipid hydrolysis) (8), opened the exciting area of research on PKC involvement in cell growth control. The same year, Kraft and coworkers reported the seminal discovery that activation with phorbol esters (9) leads to translocation (i.e. change in subcellular location) of PKC from the cell soluble to the cell particulate fraction. Radioactive phorbol 12,13 dibutyrate binding (10) was then used to localize and quantify PKC (11). Subsequently, chronic treatment with phorbol esters was shown to down-regulate PKC phosphorylating activity (12). The general interest in PKC culminated in a review paper on signal transduction and tumor promotion by Nishizuka, published in 1984, which became the most cited paper of the eighties (13). Additional inhibitors, interfering directly with PKC, were described between 1984 and 1986 and utilized extensively: the isoquinolinesulfonamide H7 (14), tamoxifen (15) and the anti-fungal alkaloid staurosporine (16) helped to clarify the implication of PKC in the regulation of several functions, including utilizing long term phorbol esters to “down regulate” the enzyme (12). Bryostatin 1, a marine bryozoan, is an unconventional PKC activator/inhibitor; it stimulates and subsequently down-regulates PKC (like phorbol esters) but it does not act as a tumor promoter, but rather can inhibit phorbol ester effects under selected conditions (17). The brain is the highest source for PKC in terms of catalytic activity and levels of expression (18), and one of the more exciting and intriguing aspects of PKC appears to be its involvement in the learning and memory phenomena. After the initial in vitro observation that synaptic plasticity is positively influenced by PKC activation (19) and that in vivo phorbol esters may antagonize scopolamine-induced amnesia (20), a variety of studies have accumulated providing biochemical, electrophysiological, behavioral, genetic and pharmacological evidence in favor of PKC as one of the relevant players in memory trace formation (for a review see 21).
The PKC field began to grow in 1986, with the cloning of the calcium-dependent PKCs (or conventional, cPKCs) (22–25), and subsequently the calcium-independent PKCs (or novel, nPKCs) followed by the atypical PKCs (aPKCs) (26). cPKCs were characterized also by chromatographic techniques, naming them as PKC-I, -II, and -III (27) (corresponding to cPKC γ β and α, respectively) and consensus followed, identifying the different isozymes with greek letters.
The second decade: isozyme identification and their functions
The PKC isozymes contain conserved and variable regions in the catalytic and regulatory subunits (28,29) and isozyme-selective antibodies were thus produced. A detailed study on tissue and cellular distribution of all the isozymes was published in 1992 by Bill Wetsel and coworkers in Y. Hannun’s laboratory (30), the same researcher who while on Bob Bell’s lab characterized sphingolipids as PKC inhibitors, thus linking PKC to sphingolipidoses (31). [The involvement of different PKC isozymes in the pathogenesis of inborn errors of metabolism (sphingolipidoses, fatty acid oxidation, bile acid and cholesterol) was proposed later, in 1995 (33).] Bob Bell, whose work into diacylglycerol and PKC regulation led characterization of how PKC signaling is turned off, founded with Carson Loomis, in 1988 the first PKC-based Company-Sphinx Pharmaceuticals (32). Spinx (in Research Triangle Park, NC, USA) was acquired in 1994 by Eli Lilly, leading to the discovery of the selective βPKC oral inhibitor, ruboxistaurin (see below).
In 1987, House and Kemp described the pseudosubstrate sequence in the PKC regulatory region that is involved in intramolecular inhibitory interactions (34). More details and updates on PKC structural composition and intramolecular regulation can be found in the opening review of this issue by Kheifets and Mochly-Rosen (35). A peptide corresponding to the pseudosubstrate sequence was found to act as a selective inhibitor of PKC (34), but its use as a pharmacological agent in cells was limited because the peptide does not cross biological membranes. The search for selective pharmacological tools to specifically inhibit PKC was prompted, in part, by the findings that in addition to binding to PKC, phorbol esters interact with other signalling molecules devoid of kinase activity such as the mammalian α– and β–chimaerins, Ras-GRP (Ras guanyl-releasing protein) and the nematode, Unc-13 (more details in 36).
Overexpression of PKC isozymes in cell cultures opened the area of research aimed at further clarifying the involvement of selected PKC isozymes in abnormal cell growth (37,38). Further, susceptibility to transformation increased by PKC co-expression with certain oncogenes i.e, H-ras, myc and fos (39). These and other data eventually led to the first clinical studies in advanced cancer patients using bryostatin 1 [by CRC in Manchester, UK in 1993, (40)] and with anti-sense oligonucleotides to PKCα [by Isis Inc, in 1999 (41)]. Another line of research focussed on inhibiting PKC as adjuvant to improve conventional chemotherapies (42). In spite of discouraging results, additional trials are in progress with particular attention to isozyme- and tissue-selective effects (reviewed in 43). PKC involvement and future in tumor growth control is discussed here in two reviews. Martiny-Baron and Fabbro focus on conventional PKCs and the endpoints of all clinical trials with small molecular weight inhibitors and antisense compounds (44). Fields and Regala concentrate on the atypical PKCζ and ι, and how PKCι signalling is targeted to identify a novel drug for human lung cancer (45).
In the 1989, in recognition of the contribution to the advancement of the PKC field, the Albert Lasker Basic Medical Research Prize was awarded to the “father” of this enzyme family, Yasutomi Nishizuka (46).
The regulation of PKC activation due to lipid-protein interactions was revisited following the identification of protein-protein (PKC-anchoring protein) interactions for PKC activity and function. Evidence for the presence of anchoring proteins for PKC translocation was first indicated by an in vitro study of Gopalkrishna et al (47), showing that stable interaction of PKC with isolated plasma membranes is lost following pre-treatment of the membranes with proteases. That and the finding that individual isozymes are localized each to a unique subcellular site following activation (48) led to the discovery of Mochly-Rosen and coworkers in 1991 of PKC anchoring proteins, collectively named receptors for activated C kinases (RACKs) (49), and first designed peptide inhibitor of translocation. The first RACK, i.e. the βII-selective RACK1, was cloned in 1994 (50). A variety of other anchor/scaffolding proteins are known to regulate PKC homeostasis (51,52). This research led to the development of PKC isozyme-selective peptide activators and inhibitors that induce or inhibit translocation and function of individual isozymes, respectively (53). Budas, Churchill and Mochly-Rosen discuss the progress made in establishing cytoprotective mechanisms, which arise as a consequence of εPKC activation and/or δPKC inhibition, and how these may lead to protection in the setting of myocardial ischemia reperfusion (54).
The first PKC knockout mouse was produced and characterized in 1993 and lacked the neuronal-specific cPKC γ (55). Data on other knockouts and transgenic mice overexpressing various PKCs have since been established and represent an intensive area of research (for a recent review see 56). Although potential redundant roles of individual PKC isozymes complicate and limit the exact identification of isozyme-selective actions, important new information on the role of each isozyme has been described.
In addition to tumor promotion and growth, other pathologies in which abnormal PKC may be involved were recognized. These include hypertension, diabetes, atherosclerosis, to name a few, suggesting the definition of a potential “PKC syndrome” (57). The involvement of overfunctional PKCβ in diabetes was established using a staurosporine-derivative inhibitor (i.e. LY333531 or ruboxistaurin). This orally bioavailable compound inhibits PKCβ and ameliorates different vascular dysfunctions in animal models (58). In this volume, Das Evcimen and King discuss the essential role of PKC activation in diabetic cardio- and microvascular complications, the mechanisms by which hyperglycemia causes vascular damage and summarize the clinical trials with PKCβ inhibitors in diabetic complications (59). The importance of PKCs in respiratory physiology is covered by the contribution of Dempsey, Cool and Littler, who discuss the relevance of the bidirectional approach (inhibiting and activating different PKC isozymes) as they relate to lung pathology and highlight the differences in lung anatomy between animal models and humans (60). The importance of PKC theta in T cell functions (activation, proliferation, differentiation and survival), but not in anti-viral responses, is reviewed by Hayashi and Altman, who provide their perspective on the potential of this isozyme as a target for controlling allergic and autoimmune diseases (61).
The third decade: entering the “matured” age (regulation by phosphorylation) and new technological advances
A diversity of functions are controlled by PKC isozymes present in the same cell. Even upon the same stimulus, individual PKCs move to different subcellular sites (membrane, organelles, cytoskeleton, nucleus) where select substrate phosphorylations leads to diverse and sometimes even opposing functions. The ability to visualize the translocation (activation) of PKC in living cells was made possible in 1997 by tagging PKC with the green fluorescent protein, GFP (62).
Although post-translational processing of PKC by phosphorylation was initially documented in tumor cells in 1989 (63), a new era in PKC regulation started in 1998, when Alexandra Newton’s and Peter Parker’s groups (64, 65) described that the mechanisms for correct maturation and catalytic activity of PKC isozymes requiring sequential serine/threonine phosphorylation reactions. The initial phosphorylation is common to all the isozymes and is mediated by phosphoinositide-dependent kinase 1 (PDK1), indicating a cross-talk between inositol phospholipid hydrolysis and phosphatidylinositol 3-kinase pathways, whereas the subsequent phosphorylation events are likely isozyme-specific autophosphorylations (66). In vivo tyrosine phosphorylation, initially documented in 1993 for PKCδ (67), also regulates PKC activity in a positive or negative manner by a mechanism specific for each isozyme.
In a search for isozyme-selective PKC inhibitors, new approaches were applied including gene silencing with anti-sense oligonucleotides (41) and short interfering RNAs (68) as well as covalent modification of PKC isozymes with cysteine-reactive peptide substrate analogs (69). One of the most frustrating aspect in the PKC field is the difficulty in identifying selective substrates of each isozyme. A recent proteomic approach has been used to study PKC signaling in cardiac protection (70) and to identify PKC targets (71). In another contribution, Agnetti et al, (72) discuss the technologies and applications of proteomics to the study of kinases, in general, and PKC-mediated phosphorylation of cytoskeletal, myofilament and mitochondrial proteins in heart failure, hypertrophy and cardioprotection, in particular.
The involvement of PKC isozymes the function and pathologies of the central nervous system is another area of intense research and therapeutic prospect. This special issue covers three potential therapeutic indications for PKC regulating drugs. These include: ethanol addiction, pain and aging. Newton and Ron summarize data on acute and chronic ethanol effects on PKC isozymes underlining the role of select isozymes as potential therapeutic targets for alcoholism (73). How PKC may function as a relevant regulator of peripheral and central sensitization that underlies many chronic pain conditions is reviewed by Velázquez, Mohammad, and Sweitzer (74). Finally, soon after PKC involvement in learning and memory had been recognized, its role in physiological and pathological brain aging has been a focus of reseach (reviewed in 75). An age-and pathology (Alzheimer’s disease)-related deficit in PKC activation and anchoring, rather than changes in isozyme levels, seem to be the most consistent finding, also in non-neuronal tissues (76). These aspects together with emerging concepts on PKC-dependent mRNA stabilization related to memory substates (77) are sumarized in the review by Pascale et al. (78), suggesting that PKC activation could be a useful approach to correct these deficits.
It is possible that the potential of PKC activators as therapeutic compounds may be limited by their tumor promoting actions. However, there are drug candidates, such as bryostatin 1, that activate PKC but are devoid of tumor promoting effects (79). Furthermore, isozyme-selective activator peptides, targeting PKC isozymes that are not related to uncontrolled growth, have also been identified (53). Such selective activators may provide benefits for patients with Alzheimer’s disease (AD), in which PKC appears to act both upstream and downstream of beta amyloid (Aβ) accumulation in brain tissues. For example, PKC activation with bryostatin 1 decreased Aβ brain deposition in mouse models of AD favoring non-amyloidogenic metabolism of the Aβ precursor (80) and PKCε activation decreased brain Aβ accumulation, favoring its clearance (81). A general outline of PKC therapeutic potential in AD can be found in a recent publication (82). In addition to isozyme-selective peptide activators (53), PKC activators belonging to the DAG-lactone chemical structure are also promising compounds for activating specific PKC isozymes (83).
The coming years: can PKC go from bench to bedside?
While research on PKC continues to attract interest in the basic research community, applications of PKC regulating drugs has met limited success. PKCα and PKCε inhibitors may be useful to inhibit tumor growth and multi-drug resistance. The cardiovascular field is concentrating on β- and δ-PKC inhibitors and clinical studies have given some hope for approaching diabetic complications such as retinopathy with ruboxistaurin (84) and with a PKCδ inhibitor peptide for acute myocardial infarction; data from a phase IIa safety and dose escalation clinical trial of KAI Pharmaceuticals indicates that the peptide inhibitor of δPKC appears safe and may provide protection from reperfusion injury when given for patients immediately after acute myocardial infarction (85). Meanwhile, the concept of “PKC activation” has recently found preclinical support in terms of limiting cardiac and brain degeneration. In this case, PKCε activation could be a good candidate for clinical trials. Finally, promising preclinical work regarding regulation of immune response, alcoholism and possibly other addicion, pain sensation and other clinically relevant conditions suggest that we will see a new surge in drug development that focuses on PKC in the coming years. We hope that this Special Issue will encourage further research in the PKC field that will yield novel drugs for treatment of human diseases.
Acknowledgments
FB is supported by the grant from Italian Ministero Sanità/Regione Lazio (Progetto Alzheimer); DM-R is supported in part by NIG grants HL 52141, AA 11147, HL 76674 and NS 44350.
Disclosure: DM-R is the founder and member of the board of KAI Pharmaceuticals. However, none of the work in her lab is supported by or is in collaboration with the company.
Abbreviations
- PKM
catalytic fragment of Protein Kinase C (PKC)
- DAG
diacylglycerol
- RACK
Receptor for Activated C kinase
- STICKs
Substrates That Interact with C Kinases
- PICKs
Protein that Interact with C Kinases
- GFP
Green Fluorescent Protein
- siRNA
small interfering RNA
- Aβ
amyloid beta peptide
- AD
Alzheimer’s Disease. MI, Myocadial Infarction
Footnotes
FB dedicates this issue to the memory of his parents, Giuseppina Rossi and Gaetano Battaini, for their encouragement to pursue the exciting career in science.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J Biol Chem. 1977;252:7603–9. [PubMed] [Google Scholar]
- 2.Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977;252:7610–6. [PubMed] [Google Scholar]
- 3.Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979;254:3692–5. [PubMed] [Google Scholar]
- 4.Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979;91:1218–24. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- 5.Hokin MR, Hokin LE. Enzyme secretion and the incorporation of P32 into phospholipids of pancreas slices. J Biol Chem. 1953;203:967–77. [PubMed] [Google Scholar]
- 6.Mori T, Takai Y, Minakuchi R, Yu B, Nishizuka Y. Inhibitory action of chlorpromazine, dibucaine, and other phospholipid-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1980;255:8378–80. [PubMed] [Google Scholar]
- 7.Kawahara Y, Takai Y, Minakuchi R, Sano K, Nishizuka Y. Phospholipid turnover as a possible transmembrane signal for protein phosphorylation during human platelet activation by thrombin. Biochem Biophys Res Commun. 1980;97:309–17. doi: 10.1016/s0006-291x(80)80169-0. [DOI] [PubMed] [Google Scholar]
- 8.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–51. [PubMed] [Google Scholar]
- 9.Kraft AS, Anderson WB, Cooper HL, Sando JJ. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982;257:13193–6. [PubMed] [Google Scholar]
- 10.Driedger PE, Blumberg PM. Specific binding of phorbol ester tumor promoters. Proc Natl Acad Sci USA. 1980;77:567–71. doi: 10.1073/pnas.77.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niedel JE, Kuhn LJ, Vandenbark GR. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci USA. 1983;80:36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Pena A, Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984;120:1053–9. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- 13.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–8. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 14.Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–41. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- 15.O’Brian CA, Liskamp RM, Solomon DH, Weinstein IB. Inhibition of protein kinase C by tamoxifen. Cancer Res. 1985;45:2462–5. [PubMed] [Google Scholar]
- 16.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 17.Kraft AS, Smith JB, Berkow RL. Bryostatin, an activator of the calcium phospholipid-dependent protein kinase, blocks phorbol ester-induced differentiation of human promyelocytic leukemia cells HL-60. Proc Natl Acad Sci USA. 1986;83:1334–1338. doi: 10.1073/pnas.83.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–5. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 19.Routtenberg A. Phosphoprotein regulation of memory formation: enhancement and control of synaptic plasticity by protein kinase C and protein F1. Ann N Y Acad Sci. 1985;444:203–1. doi: 10.1111/j.1749-6632.1985.tb37590.x. [DOI] [PubMed] [Google Scholar]
- 20.Laborit H, Zerbib R. Action of PMA (phorbol myristate acetate), scopolamine, propranolol, and oxotremorine on memorization of an active or passive avoidance test. Encephale. 1989;15:29–35. [PubMed] [Google Scholar]
- 21.Micheau J, Riedel G. Protein kinases: which one is the memory molecule? Cell Mol Life Sci. 1999;55:534–48. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coussens L, Parker PJ, Rhee L, Yang-Feng TL, Chen E, Waterfield MD, Francke U, Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986;233:859–66. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- 23.Parker PJ, Coussens L, Totty N, Rhee L, Young S, Chen E, Stabel S, Waterfield MD, Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986;233:853–9. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- 24.Knopf JL, Lee MH, Sultzman LA, Kriz RW, Loomis CR, Hewick RM, Bell RM. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986;46:491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- 25.Ono Y, Kurokawa T, Fujii T, Kawahara K, Igarashi K, Kikkawa U, Ogita K, Nishizuka Y. Two types of complementary DNAs of rat brain protein kinase C. Heterogeneity determined by alternative splicing. FEBS Lett. 1986;206:347–52. doi: 10.1016/0014-5793(86)81010-9. [DOI] [PubMed] [Google Scholar]
- 26.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Identification of three additional members of rat protein kinase C family: delta-, epsilon- and zeta-subspecies. FEBS Lett. 1987;226:125–8. doi: 10.1016/0014-5793(87)80564-1. [DOI] [PubMed] [Google Scholar]
- 27.Huang KP, Nakabayashi H, Huang FL. Isozymic forms of rat brain Ca2+-activated and phospholipid-dependent protein kinase. Proc Natl Acad Sci USA. 1986;83:8535–9. doi: 10.1073/pnas.83.22.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikkawa U, Ono Y, Ogita K, Fujii T, Asaoka Y, Sekiguchi K, Kosaka Y, Igarashi K, Nishizuka Y. Identification of the structures of multiple subspecies of protein kinase C expressed in rat brain. FEBS Lett. 1987;217:227–31. doi: 10.1016/0014-5793(87)80668-3. [DOI] [PubMed] [Google Scholar]
- 29.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988;263:6927–32. [PubMed] [Google Scholar]
- 30.Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992;117:121–33. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannun YA, Bell RM. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987;235:70–4. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- 32.http://www.intersouth.com/success/sphinx.aspx
- 33.Boneh A. Possible role for protein kinase C in the pathogenesis of inborn errors of metabolism. J Cell Biochem. 1995;59:27–32. doi: 10.1002/jcb.240590104. [DOI] [PubMed] [Google Scholar]
- 34.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–8. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 35.Kheifets V, Mochly-Rosen D. Insight into intra- and inter-molecular interactions of PKC: design of specific modulators of kinase function. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13:1658–1676. [PubMed] [Google Scholar]
- 37.Housey GM, Johnson MD, Hsiao WL, O’Brian CA, Murphy JP, Kirschmeier P, Weinstein IB. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988;52:343–54. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- 38.Persons DA, Wilkison WO, Bell RM, Finn OJ. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988;52:447–58. doi: 10.1016/s0092-8674(88)80037-0. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein IB. Nonmutagenic mechanisms in carcinogenesis: role of protein kinase C in signal transduction and growth control. Environ Health Perspect. 1991;93:175–9. doi: 10.1289/ehp.9193175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prendiville J, Crowther D, Thatcher N, Woll PJ, Fox BW, McGown A, Testa N, Stern P, McDermott R, Potter M, et al. A phase I study of intravenous bryostatin 1 in patients with advanced cancer. Br J Cancer. 1993;68:418–24. doi: 10.1038/bjc.1993.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nemunaitis J, Holmlund JT, Kraynak M, Richards D, Bruce J, Ognoskie N, Kwoh TJ, Geary R, Dorr A, Von Hoff D, Eckhardt SG. Phase I evaluation of ISIS 3521, an antisense oligodeoxynucleotide to protein kinase C-alpha, in patients with advanced cancer. J Clin Oncol. 1999;17:3586–95. doi: 10.1200/JCO.1999.17.11.3586. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzo PS, Dennis PA. Modulating protein kinase C (PKC) to increase the efficacy of chemotherapy: stepping into darkness. Drug Resist Updat. 2003;6:329–39. doi: 10.1016/j.drup.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann J. Protein kinase C isozymes as potential targets for anticancer therapy. Curr Cancer Drug Targets. 2004;4:125–46. doi: 10.2174/1568009043481579. [DOI] [PubMed] [Google Scholar]
- 44.Martiny-Baron G, Fabbro D. Classical PKC isoforms in cancer. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Fields AP, Regala RP. Protein Kinase C iota : human oncogene, prognostic marker and therapeutic target. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishizuka Y. The Albert Lasker Medical Awards. The family of protein kinase C for signal transduction. JAMA. 1989;262:1826–33. [PubMed] [Google Scholar]
- 47.Gopalakrishna R, Barsky SH, Thomas TP, Anderson WB. Factors influencing chelator-stable, detergent-extractable, phorbol diester-induced membrane association of protein kinase C. Differences between Ca2+-induced and phorbol ester-stabilized membrane bindings of protein kinase C. J Biol Chem. 1986;261:16438–45. [PubMed] [Google Scholar]
- 48.Mochly-Rosen D, Henrich CJ, Cheever L, Khaner H, Simpson PC. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990;1:693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–43. doi: 10.1073/pnas.91.3.839. Erratum in: Proc Natl Acad Sci U S A 1995; 92:2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapline C, Ramsay K, Klauck T, Jaken S. Interaction cloning of protein kinase C substrates. J Biol Chem. 1993;268:6858–61. [PubMed] [Google Scholar]
- 52.Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN. PICK1: a perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol. 1995;128:263–71. doi: 10.1083/jcb.128.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nat Biotechnol. 1998;16:919–24. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 54.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC-isozyme selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–62. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 56.Choi DS, Messing RO. Animal models in the study of protein kinase C isozymes. Methods Mol Biol. 2003;233:455–73. doi: 10.1385/1-59259-397-6:455. [DOI] [PubMed] [Google Scholar]
- 57.McCarty MF. Up-regulation of intracellular signalling pathways may play a central pathogenic role in hypertension, atherogenesis, insulin resistance, and cancer promotion: the ‘PKC syndrome’. Med Hypotheses. 1996;46:191–221. doi: 10.1016/s0306-9877(96)90243-1. [DOI] [PubMed] [Google Scholar]
- 58.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–31. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 59.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Dempsey EC, Littler CM. Lung disease and PKCs. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi K, Altman A. Protein Kinase C Theta (PKCθ): A key player in T Cell life and death. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakai N, Sasaki K, Ikegaki N, Shirai Y, Ono Y, Saito N. Direct visualization of the translocation of the gamma-subspecies of protein kinase C in living cells using fusion proteins with green fluorescent protein. J Cell Biol. 1997;139:1465–76. doi: 10.1083/jcb.139.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borner C, Filipuzzi I, Wartmann M, Eppenberger U, Fabbro D. Biosynthesis and posttranslational modifications of protein kinase C in human breast cancer cells. J Biol Chem. 1989;264:13902–9. [PubMed] [Google Scholar]
- 64.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–5. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 65.Dutil EM, Toker A, Newton AC. Regulation of conventional protein kinase C isozymes by phosphoinositide-dependent kinase 1 (PDK-1) Curr Biol. 1998;8:1366–75. doi: 10.1016/s0960-9822(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 66.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 67.Denning MF, Dlugosz AA, Howett MK, Yuspa SH. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J Biol Chem. 1993;268:26079–81. [PubMed] [Google Scholar]
- 68.Irie N, Sakai N, Ueyama T, Kajimoto T, Shirai Y, Saito N. Subtype- and species-specific knockdown of PKC using short interfering RNA. Biochem Biophys Res Commun. 2002;298:738–43. doi: 10.1016/s0006-291x(02)02531-7. [DOI] [PubMed] [Google Scholar]
- 69.Chu F, Koomen JM, Kobayashi R, O’Brian CA. Identification of an inactivating cysteine switch in protein kinase Cepsilon, a rational target for the design of protein kinase Cepsilon-inhibitory cancer therapeutics. Cancer Res. 2005;65:10478–85. doi: 10.1158/0008-5472.CAN-05-1989. [DOI] [PubMed] [Google Scholar]
- 70.Ping P, Zhang J, Pierce WM, Jr, Bolli R. Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 71.Brehmer D, Godl K, Zech B, Wissing J, Daub H. Proteome-wide identification of cellular targets affected by bisindolylmaleimide-type protein kinase C inhibitors. Mol Cell Proteomics. 2004;3:490–500. doi: 10.1074/mcp.M300139-MCP200. [DOI] [PubMed] [Google Scholar]
- 72.Agnetti G, Kane LA, Guarnieri C, Caldarera CM, Van Eyk JE. Proteomic technologies in the study of kinases: Novel tools for the investigation of PKC in the heart. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newton PN, Ron D. PKC and Alcohol addiction. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Velázquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Battaini F, Pascale A, Paoletti R, Govoni S. The role of anchoring protein RACK1 in PKC activation in the ageing rat brain. Trends Neurosci. 1997;20:410–415. doi: 10.1016/s0166-2236(97)01084-9. [DOI] [PubMed] [Google Scholar]
- 76.Battaini F, Pascale A. Protein kinase C signal transduction regulation in physiological and pathological aging. Ann N Y Acad Sci. 2005;1057:177–92. doi: 10.1196/annals.1356.011. [DOI] [PubMed] [Google Scholar]
- 77.Amadio M, Battaini F, Pascale A. The different facets of protein kinases C: old and new players in neuronal signal transduction pathways. Pharmacol Res. 2006;54:317–2. doi: 10.1016/j.phrs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Pascale A, Amadio M, Govoni S, Battaini F. The aging brain, a target for the future: the protein kinase C involvement. Pharmacol Res. 2007;56 doi: 10.1016/j.phrs.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Sun MK, Alkon DL. Bryostatin-1: pharmacology and therapeutic potential as a CNS drug. CNS Drug Rev. 2006;12:1–8. doi: 10.1111/j.1527-3458.2006.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci U S A. 2004;101:11141–6. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi DS, Wang D, Yu GQ, Zhu G, Kharazia VN, Paredes JP, Chang WS, Deitchman JK, Mucke L, Messing RO. PKCepsilon increases endothelin converting enzyme activity and reduces amyloid plaque pathology in transgenic mice. Proc Natl Acad Sci U S A. 2006;103:8215–20. doi: 10.1073/pnas.0509725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alkon DL, Sun MK, Nelson TJ. PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol Sci. 2007;28:51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Lee J, Kang JH, Han KC, Kim Y, Kim SY, Youn HS, Mook-Jung I, Kim H, Lo Han JH, Ha HJ, Kim YH, Marquez VE, Lewin NE, Pearce LV, Lundberg DJ, Blumberg PM. Branched diacylglycerol-lactones as potent protein kinase C ligands and alpha-secretase activators. J Med Chem. 2006;49:2028. doi: 10.1021/jm0509391. [DOI] [PubMed] [Google Scholar]
- 84.PKC-DRS2 Group. Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221–30. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 85.Roe MT, et al. Targeted inhibition of delta-protein kinase C to ameliorate reperfusion injury during primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: Results from the DELTA MI trial. Am. Coll. Cardiol 56th Annual Scientific Session, i2 Summit 2007 (Innovation in Intervention). Late-Breaking Clinical Trials II, Session 2405; New Orleans. March 24–27, 2007. [Google Scholar]


