Abstract
Context:
Selective serotonin reuptake inhibitors (SSRIs) are often described as having a delayed onset of effect in the treatment of depression. However, some trials have reported clinical improvement as early as the first week of treatment.
Objective:
To test the alternative hypotheses of delayed vs early onset of antidepressant action with SSRIs in patients with unipolar depression.
Data Sources:
Trials identified by searching CENTRAL, The Cochrane Collaboration database of controlled trials (2005), and the reference lists of identified trials and other systematic reviews.
Study Selection:
Randomized controlled trials of SSRIs vs placebo for the treatment of unipolar depression in adults that reported outcomes for at least 2 time points in the first 4 weeks of treatment (50 trials from >500 citations identified). Trials were excluded if limited to participants older than 65 years or specific comorbidities.
Data Extraction:
Data were extracted on trial design, participant characteristics, and outcomes by a single reviewer.
Data Synthesis:
Pooled estimates of treatment effect on depressive symptom rating scales were calculated for weeks 1 through 6 of treatment. In the primary analysis, the pattern of response seen was tested against alternative models of onset of response. The primary analysis incorporated data from 28 randomized controlled trials (n=5872). A model of early treatment response best fit the experimental data. Treatment with SSRIs rather than placebo was associated with clinical improvement by the end of the first week of use. A secondary analysis indicated an increased chance of achieving a 50% reduction in Hamilton Depression Rating Scale scores by 1 week (relative risk, 1.64; 95% confidence interval, 1.2-2.25) with SSRI treatment compared with placebo.
Conclusions:
Treatment with SSRIs is associated with symptomatic improvement in depression by the end of the first week of use, and the improvement continues at a decreasing rate for at least 6 weeks.
Conventional wisdom suggests that the therapeutic effects of antidepressant agents, such as selective serotonin reuptake inhibitors (SSRIs), take 2 to 3 weeks or more to become evident.1 There are a variety of elegant explanations for this delay, although there is no consensus.2-4 Clinical experience shows that a proportion of patients report improvement in symptoms earlier than this, but this improvement is often ascribed to a placebo effect.5
Although the earliest studies6,7 of antidepressant drug action indicated rapid response, landmark studies5,8 in the 1980s found evidence that true drug response (as opposed to nonspecific or placebo response) was delayed for 2 weeks or more. Other research groups conversely found evidence of treatment effects as early as the first week of treatment,9,10 leading to ongoing debate as to the origin of these differences.11,12 Although many of the classic studies predate the widespread use of SSRIs, for these agents, the results are also inconsistent. Many trials13,14 do not report statistically significant benefits of SSRIs over placebo until after several weeks of treatment, but some analyses15,16 of randomized controlled trials (RCTs) comparing antidepressant agents with placebo report statistically significant benefits of SSRIs after as little as 1 week of use.
So although it is often held that significant treatment effects are not reliably demonstrated until after several weeks of treatment, there may, in fact, be early benefits.17,18 Apparent early response to active treatment may simply reflect chance, or there may be a true early effect of SSRI treatment that individual studies have usually lacked the statistical power to demonstrate consistently. If SSRIs truly have clinically important early effects, this finding will have considerable significance for physicians and patients and for our understanding of the pathogenesis of depressive disorders.
Meta-analysis can reduce uncertainty about estimates of effect by pooling the results of multiple studies.19 It is well suited to the question of early onset of SSRI effects because there are many RCTs comparing SSRIs with placebo in the treatment of depression, and these are typically of similar design, with repeated (often weekly) assessments using standardized rating scales. We conducted a systematic review and meta-analysis testing the alternative hypotheses of delayed vs early onset of antidepressant action with SSRIs.
METHODS
SEARCH STRATEGY
Published randomized trials comparing all SSRIs licensed in the United Kingdom (fluoxetine hydrochloride, fluvoxamine maleate, citalopram hydrobromide or hydrochloride, escitalopram oxalate, sertraline hydrochloride, and paroxetine hydrochloride) with placebo in the short-term treatment of unipolar depression in adults were sought by a single reviewer (M.J.T.). Trials were identified by (1) searching CENTRAL, The Cochrane Collaboration database of controlled trials (in The Cochrane Library, issue 1, 2005; using the search terms SSRI or fluoxetine or fluvoxamine or citalopram or escitalopram or sertraline or paroxetine and the key words placebo and depression); (2) the reference lists of identified RCTs; and (3) the reference lists of other systematic reviews.1,15,20,21 The CENTRAL bibliographic database includes the results of group searches of the MEDLINE, EMBASE, CINAHL, PsycLIT, PSYNDEX, and LILACS databases and controlled trials from traditionally hard-to-access sources, such as conference proceedings.22 Identification of eligible trials was further maximized by consulting previous reviews, and searches were not restricted to English language studies. Trials were excluded if they were limited to older adults (age >65 years) or specific comorbidities along with depressive episodes (eg, comorbid substance dependence). In view of the study hypothesis, trials were included only if they reported outcome measures for at least 2 time points in the first 4 weeks of treatment.
DATA EXTRACTION
Data were extracted for trial design, participant characteristics, and outcomes. Data for depression rating scale scores, or changes in scores, across multiple time points in the first 6 weeks of treatment were used when reported. Group numbers and means were obtained from the text or, when necessary, from figures using a computer program (Dexter; Harvard-Smithsonian Center for Astrophysics, Cambridge, Mass).23 Group standard deviations were extracted from the text or were estimated using standard formulas.24 When data from multiple depression rating scales were available for the same study, for example, the Hamilton Depression Rating Scale (HDRS)25 and the Montgomery-Asberg Depression Rating Scale (MADRS),26 then primary analyses were performed using the scale specified as the primary outcome measure. When rating scale outcomes were reported in a dichotomous manner, for example, the number of participants with a greater than 50% reduction in HDRS scores, these were also extracted. Data from individual patient meta-analyses were used when they provided information not available from primary trial reports.
PRIMARY ANALYSIS
For each study, at each weekly time point, we calculated the standardized effect size for the treatment and control groups for the treatment severity score. We used generalized estimating equations to examine the effects of trial, treatment, modeling different forms of the treatment effect, accounting for different periods within trials as repeated measures, and defining a new covariance structure for each trial by defining these as random effects. Each trial's specific estimate was weighted by the number of randomized patients in that trial. Treatment effects were described in the models as (1) a sudden-onset treatment response equating to a step function at week 4, (2) a step function at week 4 and a linearly increasing treatment effect, (3) a linearly increasing treatment effect, (4) a logarithmically increasing treatment effect defined as log(week), or (5) a treatment effect described as a function of week.1 An autoregressive variance function was used, and the best-fitting model was selected using the Akaike information criterion. Analyses were conducted using a statistical software program (SAS9.12 Proc Mixed; SAS Institute Inc, Cary, NC).
SECONDARY ANALYSES
Secondary analyses were performed for groups of trials that reported total or change scores with standard deviations for the HDRS (17 or 21 items) and the MADRS. Analyses were also performed using data for dichotomous outcomes (response or remission defined by HDRS score or improvement on the Clinical Global Impression). Pooled estimates of weighted mean difference or relative risk (RR) and 95% confidence intervals (CIs) at each time point were calculated using a software program (RevMan version 4.2 for Windows; Cochrane Collaboration, Oxford, England).
RESULTS
The search strategy identified more than 500 citations, from which 107 trial reports were retrieved for more detailed study. From these 107 trials, 50 randomized placebo-controlled trials of SSRIs in the short-term treatment of unipolar depression in adults were identified as being suitable for inclusion (Figure 1).27 In these studies, 6153 participants had been randomized to receive SSRIs and 3968 to receive placebo. Not all of these identified trials reported sufficient data on the standard deviations of results for inclusion in the specific analyses reported herein, so for each analysis, the trials contributing data are identified. The characteristics of these studies are summarized in the Table. These randomized trials were of largely uniform methodological quality, all except one38 reporting the use of a double-blind design. Data were available for intention-to-treat populations using last observation carried forward in approximately half of the cases (15 of 28 RCTs in the primary analysis). The participants in these studies were mainly female, with only a few studies recruiting mostly men.42,45,46 Recruited individuals were largely from outpatient or primary care populations but with inclusion criteria requiring that at least a moderately severe depressive illness be present at baseline.
Figure 1.
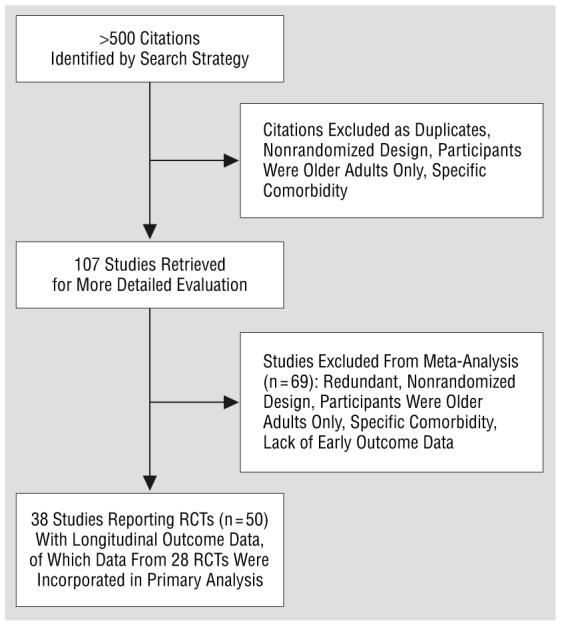
Flow diagram of the trial. RCT indicates randomized controlled trial.
Table.
Characteristics of 28 RCTs Included in Primary and Secondary Analyses
| Source | SSRIs Used | Randomization, SSRI:Placebo, No. |
Setting |
Severity Criterion * |
Notes |
|---|---|---|---|---|---|
| Wade et al,16 2002 | Escitalopram | 191:189 | Primary care | MADRS 22-40 | None |
| Bech et al,28 2004 | Escitalopram, citalopram | 369:122 | Outpatient | MADRS (10) ≥22 | Secondary analysis only |
| Gorman et al,29 2002 | Escitalopram, citalopram | 923:398 | Outpatient | MADRS ≥22 | Data from 3 RCTs |
| Lepola et al,30 2003 | Escitalopram, citalopram | 315:154 | Primary care | MADRS 22-40 | Secondary analysis only |
| Andreoli et al,31 2002 | Fluoxetine | 127:128 | Inpatient and outpatient | HDRS (21) ≥22 | Secondary analysis only |
| Byerley et al,32 1988 | Fluoxetine | 32:29 | Outpatient | HDRS (21) ≥20 | None |
| Feighner et al,14 1989 | Fluoxetine | 61:59 | Outpatient | HDRS (21) ≥20 | None |
| Heiligenstein et al,33 1993 | Fluoxetine | 24:28 | Outpatient | NA | Secondary analysis only |
| Rickels et al,34 1986 | Fluoxetine | 18:24 | Outpatient | HDRS ≥20 | Secondary analysis only |
| Silverstone and Ravindran,35 1999 | Fluoxetine | 119:118 | Outpatient | HDRS(17) ≥20 | None |
| Sramek et al,36 1995 | Fluoxetine | 72:72 | Unclear | HDRS (24) ≥21 | Primary analysis only |
| Tollefson and Holman,15 1994 | Fluoxetine | 962:485 | Unclear | HDRS (21) ≥20 | Data from 6 RCTs |
| Claghorn et al,13 1996 | Fluvoxamine | 50:50 | Outpatient | NA | None |
| Dominguez et al,37 1985 | Fluvoxamine | 35:31 | Outpatient | HDRS (17) ≥15 | Primary analysis only |
| Porro et al,38 1988 | Fluvoxamine | 21:20 | Unclear | HDRS ≥18 | None |
| Roth et al,39 1990 | Fluvoxamine | 30:30 | Outpatient | HDRS(17) ≥22 | Primary analysis only |
| Walczak et al,40 1996 | Fluvoxamine | 400:200 | Outpatient | HDRS ≥20 | Secondary analysis only |
| Claghorn et al,41 1992 | Paroxetine | 170:171 | Outpatient | HDRS (17) ≥18 | Secondary analysis only |
| Claghorn,42 1992 | Paroxetine | 36:36 | Outpatient | HDRS (17) ≥18 | Primary analysis only |
| Edwards and Goldie,43 1993 | Paroxetine | 21:20 | Outpatient | HDRS (17) ≥16 | None |
| Feighner et al,44 1993 | Paroxetine | 241:244 | Outpatient | HDRS (17) ≥18 | Secondary analysis only Data from 6 RCTs |
| Katz et al,45 2004 | Paroxetine | 28:25 | Inpatient | HDRS (21) ≥18 | None |
| Kiev,46 1992 | Paroxetine | 38:40 | Outpatient | HDRS (17) ≥18 | None |
| Smith and Glaudin,47 1992 | Paroxetine | 39:38 | Unclear | HDRS (17) ≥18 | Primary analysis only |
| Trivedi et al,48 2004 | Paroxetine CR | 310:149 | Outpatient | HDRS (17) ≥20 | None |
| Fabre et al,49 1995 | Sertraline | 278:91 | Unclear | HDRS (17) ≥22 | None |
| Reimherr et al,50 1990 | Sertraline | 149:150 | Outpatient | HDRS (18) ≥18 | None |
| Trivedi et al,51 2001 | Sertraline | 237:245 | Outpatient | HDRS (21) ≥18 | Data from 2 RCTs Primary analysis only |
| Stahl,52 2000 | Sertraline, citalopram | 215:108 | Unclear | HDRS (17) ≥22 | Secondary analysis only |
Abbreviations: CR, controlled release; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; NA, not available; RCT, randomized controlled trial; SSRI, selective serotonin reuptake inhibitor.
When trials required participants to reach specified scores on the HDRS or the MADRS for inclusion, these scores are noted immediately following the scale. Numbers in parentheses refer to the number of items on the scale.
RATING SCALE SCORES
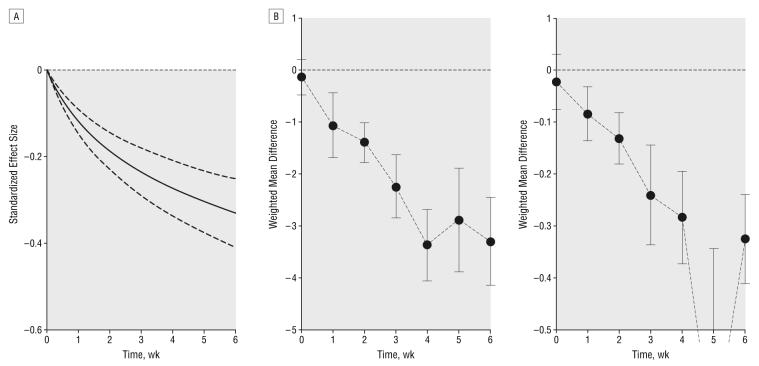
Overall, 20 articles* describing 28 trials (placebo: n=2254 and SSRI: n=3618) provided data for analysis across multiple periods for symptom score. The best-fitting model according to the Aikaike information criterion was for the logarithmically increasing treatment function (model 4), which was statistically better than the next best-fitting model, which assumed a treatment effect based on the square root of the week (model 5) . In other words, the incremental treatment effect was greatest in the first week, with a gradual decline in the magnitude of incremental benefits week by week (Figure 2A). Model 4 was substantially better than model 3 (linearly increasing treatment effect) , model 2 (step function at week 4 plus linear effect) , and model 1 (step function at week 4) (P<.001 for all). The estimate of treatment effect by log(week) from the final model was −0.17 (95% CI, −0.13 to −0.21; P<.001). The effect of treatment across time for the standardized effect size is described in Figure 2A.
Figure 2.
Differences in depression symptom rating scale scores across time between groups treated with selective serotonin reuptake inhibitors and placebo. A, Best-fit model (logarithmically increasing treatment response) for the difference in standardized effect size between groups (placebo: n=2254 and selective serotonin reuptake inhibitor: n=3618) Dotted lines represent 95% confidence intervals. B, Weighted mean difference in scores using the Hamilton Depression Rating Scale (left) (n=1893-3433) and the Montgomery-Asberg Depression Rating Scale (right) (n=133-3159). Error bars represent 95% confidence intervals.
In the secondary analyses of rating scale scores, 16 articles† reporting 25 RCTs (placebo: n=1623 and SSRI: n=2260) provided data for estimates of weighted mean difference on the 17- or 21-item HDRS and 10 articles‡ describing 16 trials (placebo: n=1382 and SSRI: n=2058) for the MADRS (Figure 2B). There was also evidence of a difference favoring SSRIs by week 1 and increasing thereafter, with a difference in mean HDRS score of −1.07 (95% CI, −1.69 to −0.44) at week 1 (n=1893), increasing to −3.3 (95% CI, −4.14 to −2.45) at week 6 (n=3432), and similarly a difference in mean MADRS score of −0.84 (95% CI, −1.36 to −0.32) at week 1 (n=2062), increasing to −3.25 (95% CI, −4.11 to −2.39) at week 6 (n=3159).
DICHOTOMOUS OUTCOMES
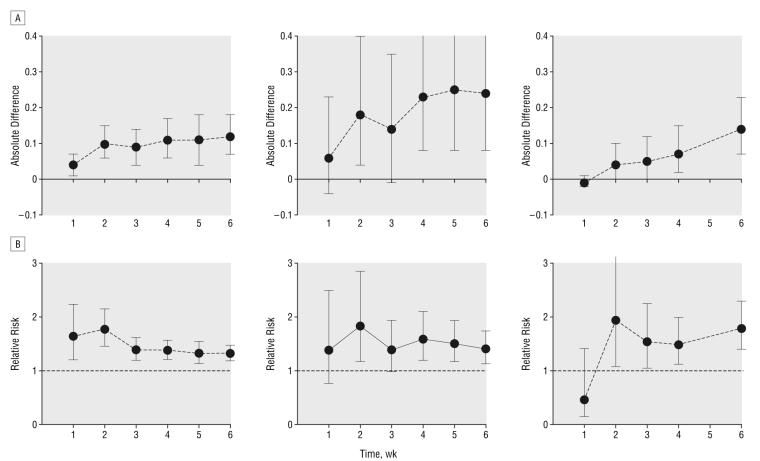
A few studies provided data on dichotomous outcomes at multiple time points (Figure 3). Five RCTs31,35,40,45,52 (placebo: n=574 and SSRI: n=791) provided data on achieving a 50% reduction in HDRS score (Figure 3). A small absolute difference in the event rates favoring SSRIs is seen by the end of week 1 (0.04; 95% CI, 0.01-0.07) (n=1428), with a greater difference seen in later weeks (0.12; 95% CI, 0.07-0.18) (n=1203 at week 6). This is equivalent to a number needed to treat with SSRIs rather than placebo for 1 additional person to achieve a 50% reduction in HDRS score of 25 (95% CI, 15 to 100) at week 1 and reducing to 9 (95% CI, 6 to 15) at week 6. However, when the difference between groups is expressed as an RR, the CIs for weeks 1 to 6 overlap (week 1: RR, 1.64; 95% CI, 1.2 to 2.25; and week 6: RR, 1.32; 95% CI, 1.18 to 1.47).
Figure 3.
Absolute difference in rate (A) and relative risk (B) of response between groups treated with selective serotonin reuptake inhibitors and placebo across time: 50% reduction in Hamilton Depression Rating Scale (HDRS) score (n=688-1428) (left); Clinical Global Impression moderately or much improved (n=166-203) (center); and HDRS score less than 7 or less than 8 (n=684) (right). Error bars represent 95% confidence intervals.
On another measure of treatment response, a Clinical Global Impression rating of moderately or much improved, only 2 RCTs43,50 (placebo: n=170 and SSRI: n=170) provided data (Figure 3). Consistent with the smaller numbers in the analyses, the CIs around estimates of effect are wider here than for the 50% reduction in HDRS. However, a qualitatively similar pattern can be observed with a greater absolute difference between groups later in the study but a largely constant RR throughout.
Two RCTs35,48 (placebo: n=267 and SSRI: n=429) provided data on numbers achieving remission, defined as an HDRS score of less than 7 or 8. The pattern of results appears different, with a trend in favor of placebo in week 1 (RR, 0.46; 95% CI, 0.15 to 1.41) (n=684) but a statistically significant benefit of SSRIs from weeks 2 to 6 (week 6: RR, 1.79; 95% CI, 1.4 to 2.29) (n=684).
COMMENT
This analysis supports the hypothesis that SSRIs begin to have observable beneficial effects in depression during the first week of treatment. The early treatment effect was seen on the primary outcome of differences in depressive symptom rating scale scores and on a secondary outcome of increased likelihood of achieving a 50% reduction in the HDRS score. The best-fitting model is described using a response variable in which the greatest absolute effect is observed in the first week and incremental responses by week diminish. This model was significantly better than all the other models, apparently excluding the possibility that treatment response from antidepressant drugs is subject to a period of delay.
The underlying placebo response was taken into account by analyzing the difference between the SSRI and placebo groups, and so the differences seen reflect drug-attributable effects. An early simple numerical difference in score on symptom rating scales may reflect an effect on particular symptoms rather than a true antidepressant drug effect. For example, an antidepressant agent with hypnotic effects could improve symptoms of insomnia before other effects were apparent. If this was the case, then it might be expected that a more global measure, such as the Clinical Global Impression ratings of improvement, would not show the same early benefits of treatment compared with placebo, and rating scale scores might show a stepped pattern of improvement after a period of delay. Neither of these patterns are apparent in this analysis, indicating that the benefits of SSRI treatment seen by the end of week 1 are true antidepressant drug effects.
A key question for physicians and patients is whether the early effects of SSRIs are clinically observable. This may indeed be the case. Of the improvement in symptom ratings attributable to treatment, that is, that seen in addition to placebo, approximately one third of the total effect after 6 weeks of treatment is seen in the first week (eg, HDRS: −1.07/−3.30=0.32). One week of treatment is also associated with an increased probability of achieving treatment response (RR, 1.64; 95% CI, 1.2 to 2.25). The absolute benefits of treatment for individuals depend on baseline risk; however, these benefits increase further across time, with, at a best estimate, a number needed to treat for 1 additional person to respond to SSRIs rather than placebo by week 6 of approximately one third of that by week 1. Therefore, individuals involved in treatment decisions will continue to need to wait several weeks for key treatment goals, such as remission,53 to be met.
Next, one has to consider the reliability of the estimates of effect calculated. Some sources of potential bias related to incomplete data may be of particular note in meta-analyses, including publication bias, failure to identify all available trials, and incomplete reporting of trial data.54 The search strategy used herein was comprehensive, identifying 6153 patients randomized to receive SSRIs and including data from 3618 patients compared with only 1549 in a recent analysis.55 However, there are likely to be eligible unpublished trials that could not be included in the analysis. Not all studies that took repeated outcome measurements presented the results for all time points. The effect of all these factors may be to overestimate the true treatment effect.56 However, there does not seem to be any compelling reason to expect the pattern of the response across time to differ in a systematic way between included and excluded trials.
A key issue is whether it is biologically plausible for SSRIs to produce early symptomatic response. Inhibition of serotonin transporters occurs rapidly in vitro and in vivo. A delayed response to antidepressant drug treatment is often linked with the time taken for a variety of adaptive neurobiologic changes to occur, for example, desensitization of serotonin 1A receptors and expression of neurotrophic factors such as brain-derived neurotrophic factor.57,58 However, there is evidence in human volunteers of downstream neurochemical59 and psychological60,61 effects of SSRI treatment in the first week of use that are associated in clinical populations with improvement.62,63
It is possible that the shape of the response curve for SSRIs across time is in some way determined by the design of the trials that contributed data. In particular, the use of last-observation-carried-forward analysis to account for missing data, which has become standard in trials of pharmaceutical treatments for depression and is used by approximately half of the trials analyzed herein, could make a constant effect appear log linear. This is because participants who leave a trial early would otherwise on average have been expected to improve regardless of their treatment allocation. Thus, last observation carried forward could add a negative bias to the results across time. An alternative and attractive hypothesis is that the effect of treatment may be constant on a relative scale, and as improvement occurs across time the absolute benefits achieved from each additional week decrease proportionately. Thus, the changes in rating scale scores (Figure 2) would be analogous to the results found for improvement in Clinical Global Impression score (Figure 3), with the absolute benefit changing week to week while the relative benefit is constant across time. However, if either hypothesized effect applies to the present data, neither would indicate that the effects of treatment are delayed for a period, and, thus, they do not undermine the overall conclusion.
Why have many previous analyses not found evidence of antidepressant drug effects as early as the end of week 1? It is not surprising that statistically significant early differences are infrequently seen in individual RCTs powered to demonstrate treatment effects at a trial end point. There is an approximately inverse square law relationship between sample size and effect size such that to have equal power to find an effect of one third the magnitude requires a 9-fold increase in sample size.64,65 A variety of approaches can be taken in trial design to maximize sensitivity to early antidepressant drug effects, including more frequent assessments early in treatment and the use of pattern analysis or survival analytic approaches.8,18,66 The classic studies of Quitkin et al5,8 establishing the delayed-onset hypothesis differed in several respects from those analyzed herein. The participants included some with depressive illnesses of milder severity than those included herein; for example, in the 1987 study, the minimum score on the 21-item HDRS required for inclusion was 10. They also used a range of non-SSRI antidepressant agents, and as is often seen in clinical practice, these were titrated up to therapeutic doses during the first 2 weeks of treatment. This dose titration period, which is much less typical with SSRIs, may be the key difference, particularly because some other analyses using non-SSRIs with more rapid dose titration have found that effects begin to emerge more rapidly.9,12,45 One of these studies45 included a paroxetine treatment arm, finding a time of onset of its effects during the second week of treatment; one issue here may have been its relatively small size, with 82 patients randomized to 3 treatments.
The present analysis does not readily provide answers to some important related questions, such as whether there are particular patient characteristics, such as sex or disease severity at baseline, or features early in treatment that predict better eventual outcome. Studies66 specifically designed to address these questions can provide some answers; however, it may be that new, more sensitive measures of treatment response will have to be developed before we can reliably identify early response in smaller samples. It is intriguing to speculate that the types of investigation that have already revealed subtle early effects of SSRIs in the laboratory60 might be adapted in due course to the clinic. In summary, treatment with SSRIs is associated with symptomatic improvement in depression by the end of the first week of use. An early response is not necessarily a placebo response.
Acknowledgments
Funding/Support: This study was supported by a Wellcome Trust Research Training Fellowship (Dr Taylor) and a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (Dr Bhagawar).
Footnotes
Financial Disclosure: Dr Taylor has received honoraria from AstraZeneca and Recip for educational services and has attended meetings sponsored by Janssen-Cilag and AstraZeneca. Dr Freemantle has received funding for research and consulting, speakers fees, and travel expenses from a variety of companies that manufacture antidepressant drugs and has also received funding for research from the Department of Health in England, the Medical Research Council, and other research charities. Dr Geddes has received research funding and support from GlaxoSmithKline, Sanofi-Aventis, the UK Government Department of Health, the Medical Research Council, and the Stanley Medical Research Institute. Dr Bhagwagar is on the speaker's panel for Bristol-Myers Squibb and AstraZeneca USA; has been on advisory boards for Bristol-Myers Squibb, Janssen, and Lilly; and has also received funding from the Medical Research Council, the Stanley Medical Research Institute, and the National Alliance for Research on Schizophrenia and Depression.
REFERENCES
- 1.National Collaborating Centre for Mental Health . Depression: Management of Depression in Primary and Secondary Care. London, England: National Institute for Clinical Excellence; 2004. (Clinical Guideline 23). [Google Scholar]
- 2.Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 3.Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee SP, Kung LS, Riggi SJ, Chanda SK. Development of β-adrenergic receptor subsensitivity by antidepressants. Nature. 1977;268:455–456. doi: 10.1038/268455a0. [DOI] [PubMed] [Google Scholar]
- 5.Quitkin FM, Rabkin JD, Markowitz JM, Stewart JW, McGrath PJ, Harrison W. Use of pattern analysis to identify true drug response: a replication. Arch Gen Psychiatry. 1987;44:259–264. doi: 10.1001/archpsyc.1987.01800150071009. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn R. The treatment of depressive states with G 22355 (imipramine hydrochloride) Am J Psychiatry. 1958;115:459–464. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- 7.Angst J. Mechanisms of action of imipramine and its therapeutic use. In: Angst J, editor. Tofranil. Bern, Switzerland: Stampflu & Cie; 1970. pp. 36–54. [Google Scholar]
- 8.Quitkin FM, Rabkin JG, Ross D, Stewart JW. Identification of true drug response to antidepressants. Arch Gen Psychiatry. 1984;41:782–786. doi: 10.1001/archpsyc.1984.01790190056007. [DOI] [PubMed] [Google Scholar]
- 9.Stassen HH, Angst J, Delini-Stula A. Delayed onset of action of antidepressant drugs? Pharmacopsychiatry. 1996;29:87–96. doi: 10.1055/s-2007-979551. [DOI] [PubMed] [Google Scholar]
- 10.Katz MM, Koslow SH, Maas JW, Frazer A, Bowden CL, Casper R, Croughan J, Kocsis J, Redmond E., Jr The timing, specificity and clinical prediction of tricyclic drug effects in depression. Psychol Med. 1987;17:297–309. doi: 10.1017/s0033291700024831. [DOI] [PubMed] [Google Scholar]
- 11.Quitkin FM, McGrath PJ, Stewart JW, Taylor BP, Klein DF. Can the effects of antidepressants be observed in the first two weeks of treatment? Neuropsychopharmacology. 1996;15:390–394. doi: 10.1016/0893-133X(95)00272-F. [DOI] [PubMed] [Google Scholar]
- 12.Katz MM, Bowden C, Stokes P, Casper R, Frazer A, Koslow SH, Kocsis J, Secunda S, Swann A, Berman N. Can the effects of antidepressants be observed in the first two weeks of treatment? Neuropsychopharmacology. 1997;17:110–115. doi: 10.1016/S0893-133X(97)00038-9. [DOI] [PubMed] [Google Scholar]
- 13.Claghorn JL, Earl CQ, Walczak DD, Stoner KA, Wong LF, Kanter D, Houser VP. Fluvoxamine maleate in the treatment of depression. J Clin Psychopharmacol. 1996;16:113–120. doi: 10.1097/00004714-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Feighner JP, Boyer WF, Merideth CH, Hendrickson GG. A double-blind comparison of fluoxetine, imipramine and placebo in outpatients with major depression. Int Clin Psychopharmacol. 1989;4:127–134. doi: 10.1097/00004850-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Tollefson GD, Holman SL. How long to onset of antidepressant action. Int Clin Psychopharmacol. 1994;9:245–250. doi: 10.1097/00004850-199400940-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wade A, Michael Lemming O, Bang Hedegaard K. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery SA, Bech P, Blier P, Moller HJ, Nierenberg AA, Pinder RM, Quitkin FM, Reimitz PE, Rosenbaum JF, Rush AJ, Stassen HH, Thase ME. Selecting methodologies for the evaluation of differences in time to response between antidepressants. J Clin Psychiatry. 2002;63:694–699. doi: 10.4088/jcp.v63n0806. [DOI] [PubMed] [Google Scholar]
- 18.Gelenberg AJ, Chesen CL. How fast are antidepressants? J Clin Psychiatry. 2000;61:712–721. doi: 10.4088/jcp.v61n1002. [DOI] [PubMed] [Google Scholar]
- 19.Mulrow CD. Rationale for systematic reviews. BMJ. 1994;309:597–599. doi: 10.1136/bmj.309.6954.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joffe R, Sokolov S, Streiner D. Antidepressant treatment of depression: a metaanalysis. Can J Psychiatry. 1996;41:613–616. doi: 10.1177/070674379604101002. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Walker JR, Jakul L, Sexton K. Does elimination of placebo responders in a placebo run-in increase the treatment effect in randomized clinical trials? a metaanalytic evaluation. Depress Anxiety. 2004;19:10–19. doi: 10.1002/da.10134. [DOI] [PubMed] [Google Scholar]
- 22.Dickersin K, Manheimer E, Wieland S, Robinson KA, Lefebvre C, McDonald S. Development of the Cochrane Collaboration's CENTRAL register of controlled clinical trials. Eval Health Prof. 2002;25:38–64. doi: 10.1177/016327870202500104. [DOI] [PubMed] [Google Scholar]
- 23.Demleitner M, Accomazzi A, Eichhorn G, Grant CS, Kurtz MJ, Murray SS. ADS's Dexter data extraction applet. In: Harnden FR Jr, Primini FA, Payne HE, editors. Astronomical Data Analysis Software and Systems X: ASP Conference Proceedings; San Francisco, Calif: ASP; 2001. p. 321. [Google Scholar]
- 24.Alderson P, Green S, Higgins JPT. Cochrane Reviewers' Handbook 4.2.2. Chichester, England: John Wiley & Sons Ltd; 2004. [Google Scholar]
- 25.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement: quality of reporting of meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 28.Bech P, Tanghoj P, Cialdella P, Andersen HF, Pedersen AG. Escitalopram doseresponse revisited: an alternative psychometric approach to evaluate clinical effects of escitalopram compared to citalopram and placebo in patients with major depression. Int J Neuropsychopharmacol. 2004;7:283–290. doi: 10.1017/S1461145704004365. [DOI] [PubMed] [Google Scholar]
- 29.Gorman JM, Korotzer A, Su G. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials. CNS Spectr. 2002;7(suppl 1):40–44. doi: 10.1017/s1092852900028595. [DOI] [PubMed] [Google Scholar]
- 30.Lepola UM, Loft H, Reines EH. Escitalopram (10-20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2003;18:211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Andreoli V, Caillard V, Deo RS, Rybakowski JK, Versiani M. Reboxetine, a new noradrenaline selective antidepressant, is at least as effective as fluoxetine in the treatment of depression. J Clin Psychopharmacol. 2002;22:393–399. doi: 10.1097/00004714-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Byerley WF, Reimherr FW, Wood DR, Grosser BI. Fluoxetine, a selective serotonin uptake inhibitor, for the treatment of outpatients with major depression. J Clin Psychopharmacol. 1988;8:112–115. [PubMed] [Google Scholar]
- 33.Heiligenstein JH, Tollefson GD, Faries DE. A double-blind trial of fluoxetine, 20 mg, and placebo in out-patients with DSM-III-R major depression and melancholia. Int Clin Psychopharmacol. 1993;8:247–251. doi: 10.1097/00004850-199300840-00007. [DOI] [PubMed] [Google Scholar]
- 34.Rickels K, Amsterdam J, Avallone MF. Fluoxetine in major depression: a controlled study. Curr Ther Res. 1986;39:559–563. [Google Scholar]
- 35.Silverstone PH, Ravindran A. Venlafaxine XR 360 Study Group. Once-daily venlafaxine extended release (XR) compared with fluoxetine in outpatients with depression and anxiety. J Clin Psychiatry. 1999;60:22–28. doi: 10.4088/jcp.v60n0105. [DOI] [PubMed] [Google Scholar]
- 36.Sramek JJ, Kashkin K, Jasinsky O, Kardatzke D, Kennedy S, Cutler NR. Placebo-controlled study of ABT-200 versus fluoxetine in the treatment of major depressive disorder. Depression. 1995;3:199–203. [Google Scholar]
- 37.Dominguez RA, Goldstein BJ, Jacobson AF, Steinbook RM. A double-blind placebo-controlled study of fluvoxamine and imipramine in depression. J Clin Psychiatry. 1985;46:84–87. [PubMed] [Google Scholar]
- 38.Porro V, Fiorenzioni S, Menga C, de Cristofaro A, Bertolino A. Single-blind comparison of the efficacy of fluvoxamine versus placebo in patients with depressive syndrome. Curr Ther Res. 1988;43:621–629. [Google Scholar]
- 39.Roth D, Mattes J, Sheehan KH, Sheehan DV. A double-blind comparison of fluvoxamine, desipramine and placebo in outpatients with depression. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:929–939. doi: 10.1016/0278-5846(90)90078-u. [DOI] [PubMed] [Google Scholar]
- 40.Walczak DD, Apter JT, Halikas JA, Borison RL, Carman JS, Post GL, Patrick R, Cohn JB, Cunningham LA, Rittberg B, Preskorn SH, Kang JS, Wilcox CS. The oral dose-effect relationship for fluvoxamine: a fixed-dose comparison against placebo in depressed outpatients. Ann Clin Psychiatry. 1996;8:139–151. doi: 10.3109/10401239609147751. [DOI] [PubMed] [Google Scholar]
- 41.Claghorn JL, Kiev A, Rickels K, Smith WT, Dunbar GC. Paroxetine versus placebo: a double-blind comparison in depressed patients. J Clin Psychiatry. 1992;53:434–438. [PubMed] [Google Scholar]
- 42.Claghorn JL. The safety and efficacy of paroxetine compared with placebo in a double-blind trial of depressed outpatients. J Clin Psychiatry. 1992;53(suppl):33–35. [PubMed] [Google Scholar]
- 43.Edwards JG, Goldie A. Placebo-controlled trial of paroxetine in depressive illness. Hum Psychopharmacol. 1993;8:203–209. [Google Scholar]
- 44.Feighner JP, Cohn JB, Fabre LF, Jr, Fieve RR, Mendels J, Shrivastava RK, Dunbar GC. A study comparing paroxetine placebo and imipramine in depressed patients. J Affect Disord. 1993;28:71–79. doi: 10.1016/0165-0327(93)90035-i. [DOI] [PubMed] [Google Scholar]
- 45.Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, Frazer A. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology. 2004;29:566–579. doi: 10.1038/sj.npp.1300341. [DOI] [PubMed] [Google Scholar]
- 46.Kiev A. A double-blind, placebo-controlled study of paroxetine in depressed outpatients. J Clin Psychiatry. 1992;53(suppl):27–29. [PubMed] [Google Scholar]
- 47.Smith WT, Glaudin V. A placebo-controlled trial of paroxetine in the treatment of major depression. J Clin Psychiatry. 1992;53(suppl):36–39. [PubMed] [Google Scholar]
- 48.Trivedi MH, Pigotti TA, Perera P, Dillingham KE, Carfagno ML, Pitts CD. Effectiveness of low doses of paroxetine controlled release in the treatment of major depressive disorder. J Clin Psychiatry. 2004;65:1356–1364. doi: 10.4088/jcp.v65n1010. [DOI] [PubMed] [Google Scholar]
- 49.Fabre LF, Abuzzahab FS, Amin M, Claghorn JL, Mendels J, Petrie WM, Dube S, Small JG. Sertraline safety and efficacy in major depression: a double-blind fixed-dose comparison with placebo. Biol Psychiatry. 1995;38:592–602. doi: 10.1016/0006-3223(95)00178-8. [DOI] [PubMed] [Google Scholar]
- 50.Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, LaPierre YD, Masco HL, Mendels J. Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. J Clin Psychiatry. 1990;51(suppl B):18–27. [PubMed] [Google Scholar]
- 51.Trivedi MH, Rush AJ, Carmody TJ, Donahue RM, Bolden-Watson C, Houser TL, Metz A. Do bupropion SR and sertraline differ in their effects on anxiety in depressed patients? J Clin Psychiatry. 2001;62:776–781. doi: 10.4088/jcp.v62n1005. [DOI] [PubMed] [Google Scholar]
- 52.Stahl SM. Placebo-controlled comparison of the selective serotonin reuptake inhibitors citalopram and sertraline. Biol Psychiatry. 2000;48:894–901. doi: 10.1016/s0006-3223(00)00957-4. [DOI] [PubMed] [Google Scholar]
- 53.McIntyre RS, O'Donovan C. The human cost of not achieving full remission in depression. Can J Psychiatry. 2004;49(suppl 1):10S–16S. [PubMed] [Google Scholar]
- 54.Davey Smith G, Egger M. Meta-analysis: unresolved issues and future developments. BMJ. 1998;316:221–225. doi: 10.1136/bmj.316.7126.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Posternak MA, Zimmerman M. Is there a delay in the antidepressant effect? a meta-analysis. J Clin Psychiatry. 2005;66:148–158. doi: 10.4088/jcp.v66n0201. [DOI] [PubMed] [Google Scholar]
- 56.Dickersin K. How important is publication bias? a synthesis of available data. AIDS Educ Prev. 1997;9((1)(suppl)):15–21. [PubMed] [Google Scholar]
- 57.Blier P, de Montigny C, Chaput Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol. 1987;7((6)(suppl)):24S–35S. [PubMed] [Google Scholar]
- 58.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 59.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161:368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 60.Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- 61.Bhagwagar Z, Cowen PJ, Goodwin GM, Harmer CJ. Normalization of enhanced fear recognition by acute SSRI treatment in subjects with a previous history of depression. Am J Psychiatry. 2004;161:166–168. doi: 10.1176/appi.ajp.161.1.166. [DOI] [PubMed] [Google Scholar]
- 62.Bouhuys AL, Geerts E, Gordijn MC. Depressed patients' perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 64.Campbell MJ, Julious SA, Altman DG. Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. BMJ. 1995;311:1145–1148. doi: 10.1136/bmj.311.7013.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lehr R. Sixteen S-squared over D-squared: a relation for crude sample size estimates. Stat Med. 1992;11:1099–1102. doi: 10.1002/sim.4780110811. [DOI] [PubMed] [Google Scholar]
- 66.Katz MM, Koslow SH, Frazer A. Onset of antidepressant activity: reexamining the structure of depression and multiple actions of drugs. Depress Anxiety. 1996;4:257–267. doi: 10.1002/(SICI)1520-6394(1996)4:6<257::AID-DA1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]