Abstract
A rodent model of sepsis was used to establish the relationship between caspase inhibition and inhibition of apoptotic cell death in vivo. In this model, thymocyte cell death was blocked by Bcl-2 transgene, indicating that apoptosis was predominantly dependent on the mitochondrial pathway that culminates in caspase-3 activation. Caspase inhibitors, including the selective caspase-3 inhibitor M867, were able to block apoptotic manifestations both in vitro and in vivo but with strikingly different efficacy for different cell death markers. Inhibition of DNA fragmentation required substantially higher levels of caspase-3 attenuation than that required for blockade of other apoptotic events such as spectrin proteolysis and phosphatidylserine externalization. These data indicate a direct relationship between caspase inhibition and some apoptotic manifestations but that small quantities of uninhibited caspase-3 suffice to initiate genomic DNA breakdown, presumably through the escape of catalytic quantities of caspase-activated DNase. These findings suggest that putative caspase-independent apoptosis may be overestimated in some systems since blockade of spectrin proteolysis and other cell death markers does not accurately reflect the high degrees of caspase-3 inhibition needed to prevent DNA fragmentation. Furthermore, this requirement presents substantial therapeutic challenges owing to the need for persistent and complete caspase blockade.
Keywords: peritonitis, caspase-3, spectrin, ICAD, thymocytes
Introduction
Apoptosis is an evolutionary conserved process in which the caspase family of cysteine proteases plays an essential role. To date, 14 caspases have been identified in mammals, with some functioning in cytokine processing and inflammation and at least 8 others contributing to programmed cell death. The apoptotic caspases can be further divided into the initiator caspase group, which includes caspase-2, -8, -9, and -10, and the effector caspase group, to which caspase-3, -6, and -7 belong (for reviews see references 1, 2). Depletion experiments in cell-free extracts indicate that caspase-3 is the principal effector caspase (3). On the other hand, knockout studies suggest a partial redundancy in the role of caspase-3 and other caspases such as caspase-7 (4). In addition to cleaving themselves, active caspases proteolyse a set of protein substrates with the loosely conserved recognition sequence X-E-X-D. Cleavage occurs exclusively after the aspartic acid residue (1, 2). Many caspase substrates play key roles in cell function and architecture, and alteration of their normal function by caspase-mediated cleavage is thought to contribute to the characteristic morphological features of apoptotic cells (5).
A large number of pathologies exhibit either too much or too little apoptosis, and it is of considerable interest to develop agents that will modulate programmed cell death in patients (6). A potential target for antiapoptotic agents is the microbial-elicited systemic inflammatory response known as sepsis. This disease is fatal in 30–40% of cases and is the highest cause of mortality in intensive care units (7). Recent studies uncovered a depletion of B and T cells in the lymphoid organs and the presence of apoptotic markers in lymphocytes and intestinal epithelial cells of septic patients (8, 9). Loss of B and T cells was observed in the spleen and thymus of rodents in which sepsis was induced by cecal ligation and perforation (CLP; references 10, 11). Overexpression of breakpoint cluster (Bcl)-2 in B, T, or intestinal epithelial cells reduced CLP- or pneumonia-induced murine sepsis and increased survival (12–15), as did the administration of caspase inhibitors (16). Based on these studies, blockade of apoptosis using caspase inhibitors constitutes a potential treatment. Development of such inhibitors will require an accurate quantitation of caspase-dependent events during sepsis and the percentage of active caspases that must be inhibited for efficacy in vivo. To this end, we examined various manifestations of apoptosis during CLP-induced sepsis in both rats and mice. We found that monitoring of DNA fragmentation and αII-spectrin cleavage, as opposed to phosphatidylserine (PS) exposure, provides a more accurate measurement of apoptosis-related events during sepsis. Surprisingly, studies with both caspase-3–specific and polycaspase inhibitors reveal different potencies at curbing these apoptotic manifestations, both in vivo and in cell culture, illustrating the need to follow several cell death markers. From these findings, we conclude that a high degree of fractional caspase inhibition is necessary to completely block apoptotic cell death. This establishes, in an animal model of inappropriate apoptosis (CLP-induced sepsis), the required levels of caspase inhibitors needed for improved survival and the potential challenges that face the use of caspase inhibitors in human diseases.
Materials and Methods
Animals.
Female Sprague-Dawley rats (250–300 g; Charles River) and C57Bl/6-TgN(BCL-2)25 Wehi mice, heterozygous and WT siblings (20–25 g; stock no. 2320; Jackson Laboratories) were housed in a 12-h light–dark cycle with free access to food and water. All procedures were performed under appropriate Animal Care Committee approval in strict accordance to Merck and Co. animal care policies.
Surgical Procedures.
Animals were anesthetized with 2.5% isoflurane in oxygen, and body temperature was maintained by use of a thermoregulated heated blanket.
CLP was performed as follows. A midline incision was made in the abdominal wall of the animal and the cecum exteriorized. The cecum was ligated with a nylon (4–0) suture proximal to the ileocecal valve. In mice, perforation of the cecum was done using a 23-gauge needle passed through the distal portion of the cecum. Femoral vein cannulation of rats was performed by a small incision in the inguinal region, and the femoral vein was isolated. A Silicone catheter (0.02 inches × 0.037 inches; Lomir) connected to a polyurethane catheter (PU-C30, 3 French, 80 cm; Instech Solomon) was inserted into the vena cava, exteriorized at the nape of the neck, and clamped for the duration of the surgery. Cannulation was immediately followed by CLP using a 20-gauge cannula (Abbott Ireland) passed through and through the distal portion of the exteriorized cecum. Braided silk (size 0) was threaded through the cannula and secured in place to allow leakage of the cecal content into the peritoneum. Sham-operated animals had the cecum exteriorized but no ligation or puncture of the cecum. The abdominal wall was sutured with polydioxane suture (4–0) and the skin sutured with surgical glue (mice) or clips (rats). Immediately after surgery, all animals received 1 c.c. of 0.9% saline administered by subcutaneous injection. A 2 ml/kg bolus of vehicle or compound (M867) was administered via the i.v. catheter, which was then connected to a Medfusion 2010i Syringe pump (Medex Inc.) at a delivery rate of 2 ml/h/kg for 24 h.
Thymic Protein Extract Preparation.
Thymi from rats or mice were recovered 24 h postsurgery and processed within 20 min of their removal. A cell suspension was obtained by grinding tissues in 50 μm Medicon and Medimachine (Dako) with 2 ml of ice cold thymocyte isolation buffer (PBS, 2 mM glucose, 2 mM l-glutamine, 1% FBS) with 2 × 15-s pulses. Cell suspensions were filtered through 50 μm nylon mesh filters (Becton Dickinson). Red blood cells were eliminated by a 10-min incubation in hypotonic buffer (17 mM Tris-Cl, pH 7.5, 140 mM NH4Cl). For some experiments, thymocytes were put in culture for 24 h at a density of 10 × 106 cells/ml in CytoSF4 (Kemp Technologies) supplemented with l-glutamine and antibiotics. Thymi fragments were lysed in cell lysis buffer (50 mM Tris-Cl, pH 7.5, 2 mM EDTA, 1% NP-40) supplemented with complete protease inhibitor (Roche), caspase inhibitor (M029), and calpain inhibitor (M638). Protein in the soluble fraction was quantitated with the BCA protein detection kit (Pierce Chemical Co.).
Enzyme-linked Immunosorbant Assays.
DNA-histone sandwich ELISA was performed using the Cell Death Detection ELISA kit (Roche) according to the manufacturer's specifications. All assays were performed on protein extracts that had not been frozen since freeze-thawing alters results. αII-spectrin ELISA was performed on rat thymus extracts only, since the neoepitope anti-p120 αII-spectrin antibody is unreactive toward mouse-cleaved αII-spectrin. The anti–αII-spectrin neoepitope antibody was raised against the NH2-terminal portion of the human caspase-3–specific p120 fragment (immunizing peptide: NH2-SVEALIKC-COOH). The assay plates were washed with Superblock/0.05% Tween-20 buffer (Pierce Chemical Co.) between each of the following steps. Goat anti–rabbit–coated 96-well plates (Pierce Chemical Co.) were incubated with 80 ng/well of anti–αII-spectrin neoepitope antibody overnight. The plates were washed, and thymus protein lysate (200 μg in a final volume of 100 μl) was added to each well for 1 h. The plates were washed and consecutively incubated for 1 h with a 1:1,000 dilution of anti–αII-spectrin antibody (mab1622; Cedarlane), a 1:6,000 dilution of anti–mouse biotin (Amersham Biosciences), and a 1:6,000 dilution of streptavidin-horseradish peroxidase (Amersham Biosciences). Color was developed with K-blue max substrate (Cedarlane) and absorbance read at 650 nm on a Spectromax photospectrometer (Molecular Devices). Units are expressed as OD values or as standard units from pooled thymus extracts with previously quantitated p120 αII-spectrin.
Western Blotting.
Western blotting was performed on 40 μg of heat-denatured thymic protein extract migrated through a 10–20% acrylamide gradient SDS-PAGE Tris-Glycine gel (Invitrogen). The following antibodies and dilutions were used: rabbit anti–caspase-3 antibody R280 (1:2,000 Merck-Frosst); goat antimurine poly(ADP-ribose) polymerase (PARP) (fragment 71–329; 1:2,000; R&D Systems); murine antispectrin (mab1622; 1:1,000; Cedarlane); and anti–rabbit IgG-HRP (1:5,000; Amersham Biosciences). Chemiluminescence was performed with Supersignal West Femto chemiluminescent reagent (Pierce Chemical Co.) and exposed to Hyperfilm ECL (Amersham Biosciences).
Flow Cytometry.
Phosphatidylserine exposure and propidium iodide (PI) permeability were measured with fluorescein-labeled annexin V (TACS; R&D Systems) according to the manufacturer's specification and two-color flow cytometric analysis. The presence of αII-spectrin neoepitope was also determined by flow cytometry. Rat thymocytes were fixed in PBS/0.1% sodium azide/0.25% paraformaldehyde on ice for 1 h and permeabilized in PBS/0.1% sodium azide/0.05% Triton X-100 for 15 min at room temperature. After a wash step, the cells were incubated in primary antibody solution (PBS/0.1% sodium azide, 1% normal goat serum [NGS], 1 ng/ml anti–αII-spectrin neoepitope antibody) for 1 h. Primary antibody binding was detected using a 1:500 dilution of goat anti–rabbit IgG-Alexa 488 (Molecular Probes) in PBS/0.1% sodium azide/1% NGS. Antibody-binding specificity was assessed by competition with the immunizing peptide. Subdiploid DNA content was determined on thymocytes fixed for 30 min on ice in 70% EtOH. Cells were washed with PBS and suspended in 500 μl of PI staining solution (PBS/0.1% Triton X-100/RNase A [Roche]/25 μg/ml PI [Sigma-Aldrich]). Flow cytometric analysis was performed on FACSCalibur (Becton Dickinson) on 20,000 events/sample, each sample prepared in duplicates. Very small cell debris was electronically gated out based on forward light scatter.
Results
Apoptotic Markers and Their Bcl-2 Dependency during Sepsis.
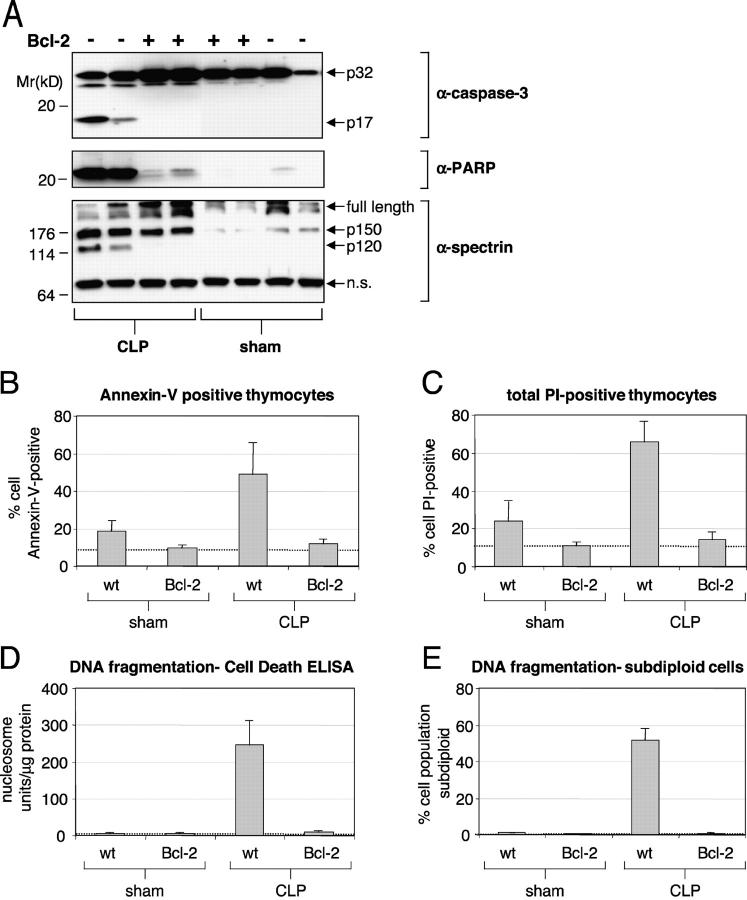
We first determined the contribution of necrosis and the relevance of various apoptotic markers during sepsis by examining several indicators of cell viability in transgenic mice that overexpress Bcl-2 in T cells. Bcl-2 is a potent antiapoptotic protein that works in part by blocking cytochrome-c release from mitochondria and the ensuing caspase activation (17, 18). CLP was used to induce sepsis and resulted in the appearance of the processed form of caspase-3 (p17 fragment) in thymocytes from WT but not of Bcl-2 transgenic animals. The p17 product was not detected in sham animals regardless of their genotype (Fig. 1 A). Hence, caspase-3 processing is induced during sepsis but can be blocked by Bcl-2 overexpression. Next, we examined whether caspase substrates were proteolytically processed and whether thymocytes exhibited characteristic signs of apoptosis. The markers chosen reflect either direct substrate cleavage (αII-spectrin and PARP) or the consequence of caspase activation on cell function (membrane permeability, PS exposure, and DNA fragmentation). PARP is a nuclear enzyme that participates in DNA repair and has been one of the first proteins identified as a caspase substrate (19). The 24-kD NH2-terminal PARP cleavage fragment was abundant in all WT mice that had undergone CLP but was absent in most Bcl-2–overexpressing animals. Some of the CLP-operated Bcl-2 transgenic and sham-operated WT animals showed slight PARP 24-kD cleavage product (Fig. 1 A). Another caspase substrate, αII-spectrin, is a major component of the cortical cytoskeleton and is proteolytically cleaved during lymphocyte apoptosis (20). Both calpain and caspase-3 cleave αII-spectrin at multiple sites, with the p120 fragment specifically generated upon caspase-3 cleavage (21–23). The p120 αII-spectrin product was prominent in WT mice but was absent in thymi from septic Bcl-2 animals. No p120 fragment was observed in sham-operated animals (Fig. 1 A). The 150-kD αII-spectrin cleavage product, which results from both calpain and caspase activity, was present in all animals but was more abundant in thymi from CLP-operated mice. Thus, both PARP and αII-spectrin cleavage at the p120 site are protected from caspase cleavage in Bcl-2–overexpressing thymocytes during sepsis. Bcl-2 overexpression did not affect significantly the αII-spectrin p150 cleavage site. Loss of membrane phospholipid asymmetry and PS externalization are detected by FITC-labeled annexin V (24). During sepsis, a higher proportion of thymocytes were annexin V positive in CLP relative to sham-operated WT animals (49 versus 18%, respectively, P < 0.001). In contrast, the number of annexin V–positive thymocytes was virtually identical in CLP or sham animals overexpressing Bcl-2 (11.8 versus 9.3%, respectively; Fig. 1 B) but was lower than in sham-treated WT animals. Similar results were obtained with membrane permeability and PI uptake (Fig. 1 C). We conclude that the loss of membrane integrity and phospholipid asymmetry is largely dependent on the mitochondrial apoptotic pathway during sepsis. Cleavage of DNA at internucleosomal spaces is a well-known apoptotic marker and is mediated by DFF40/caspase-activated DNase (CAD) (25–27). CAD activation requires cleavage of DFF45/inhibitor of caspase-activated DNase (ICAD) by caspases (25–27), principally caspase-3 (23, 28–30). We measured DNA fragmentation by the Cell Death ELISA (CDE) method and by flow cytometry using PI staining (as described in Materials and Methods). Thymocytes from mice that have undergone CLP exhibited high levels of DNA fragmentation. Overexpression of Bcl-2 in T cells abrogated DNA cleavage and brought the signal level to or near values found in sham-operated animals (Fig. 1, D and E). We conclude that DNA fragmentation, annexin V binding and αII-spectrin cleavage are dependent on the mitochondrial apoptotic pathway during sepsis (Table I).
Figure. 1.
Effect of Bcl-2 on apoptotic manifestations in the thymus of CLP-induced septic mice. Transgenic (T cell–specific Bcl-2 overexpression) and WT mice siblings underwent CLP or sham surgery, for a total of four groups (WT CLP n = 4; Bcl-2 CLP n = 7; WT sham n = 4; Bcl-2 sham n = 3). (A) Western blot from two representative animals in all four experimental groups. Detection of caspase-3, PARP, and αII-spectrin cleavage products in thymi of CLP or sham-operated mice. +, animals that carried the bcl-2 transgene. Average percentage of annexin V–positive thymocytes (B) and PI-permeable thymocytes (C) by flow cytometry. Average DNA fragmentation assessed by a DNA-histone sandwich ELISA (D) or subdiploid DNA content (E). In this figure and all subsequent figures, the error bars represent one SD. One-way analysis of variance (ANOVA) was performed with p-values < 0.001 between CLP WT and CLP Bcl-2 groups (B–E).
Table I.
Apoptotic Markers during Experimental Peritonitis in Bcl-2 or WT Mice
| Group | Percentage of subdiploid cells (±SE) |
CDE U/mg protein (±SE) |
Percentage of annexin V+ cells (±SE) |
Percentage of PI-positive cells (±SE) |
|---|---|---|---|---|
| WT CLP (n = 4) | 52.00 ± 2.90 | 246.35 ± 32.23 | 49.00 ± 8.07 | 66.12 ± 4.94 |
| Blc-2 CLP (n = 7) | 0.67 ± 0.12 | 9.78 ± 1.00 | 11.79 ± 0.96 | 13.98 ± 1.43 |
| WT sham (n = 4) | 0.96 ± 0.16 | 5.16 ± 1.47 | 18.44 ± 2.67 | 23.77 ± 5.14 |
| Blc-2 sham (n = 3) | 0.47 ± 0.09 | 5.66 ± 0.26 | 9.33 ± 0.95 | 10.98 ± 1.01 |
A Caspase-3–specific Inhibitor Reduces Apoptosis during Sepsis.
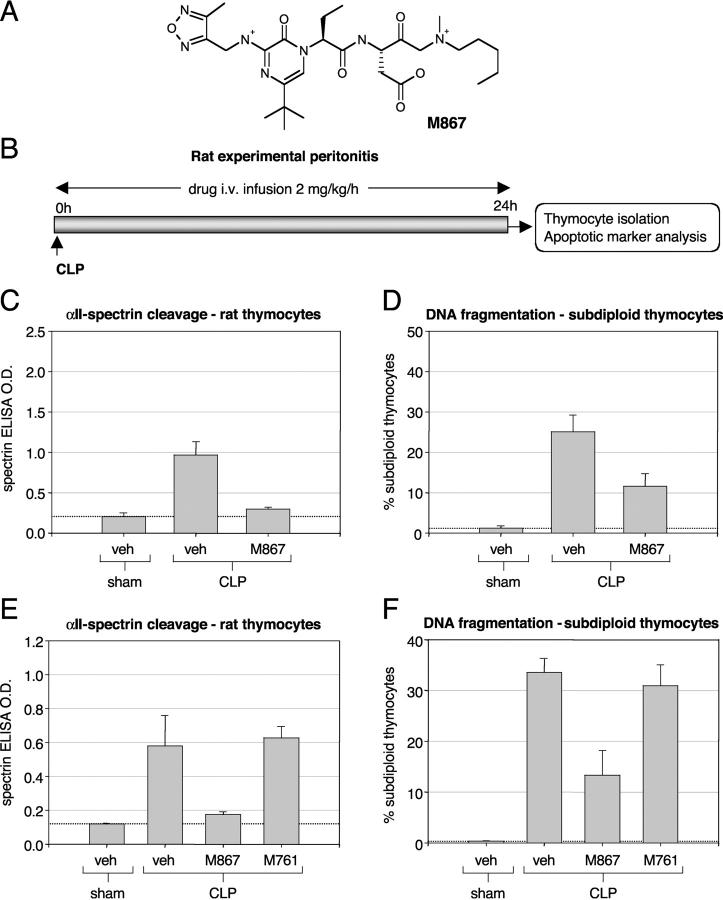
Bcl-2 blocked or reduced all markers of apoptosis examined during sepsis, including caspase-3 processing itself. It was of interest to determine if the apoptosis-specific markers were also dependent on caspase activity in CLP-induced sepsis. M867 is a highly potent reversible caspase inhibitor with a fivefold preference for caspase-3 over caspase-7 and little activity toward other caspases (Fig. 2 A and Table II). We induced sepsis in rats and allowed for continuous i.v. delivery of drug (Fig. 2 B). M867 infused over the 24 h after CLP reduced αII-spectrin cleavage in thymocytes by 88% (P < 0.001) and DNA fragmentation by 57% (P = 0.01; Fig. 2, C and D). To confirm that the reduction in apoptotic activity was specifically due to caspase inhibition, the experiment was also performed with M761, an inactive enantiomer of M867. Here, M867 inhibited αII-spectrin and DNA cleavage by 88 and 60%, respectively (P < 0.01). In contrast, M761 could not prevent cleavage of these two apoptotic markers (Fig. 2, E and F, and Table III). The average M867 concentration in plasma and spleen, with an infusion of rate 2 mg/kg/h, was 0.65 ± 0.18 and 0.37 ± 0.10 μM, respectively. M761 delivered under similar conditions gave plasma and spleen drug levels of 0.57 ± 0.05 and 0.42 ± 0.07 μM, respectively. With M867 and its inactive enantiomer M761 used under very similar pharmacokinetic conditions, we conclude that M867 specifically blocked apoptosis by inhibiting caspase-3 activity and was more effective at inhibiting substrate proteolysis relative to DNA fragmentation.
Figure 2.
Effect of caspase-3 inhibitors on DNA fragmentation and αII-spectrin cleavage in the thymus of CLP-induced septic rats. (A) Structure of the selective caspase-3 inhibitor M867 ((3S)-3-({(2S)-2-[5-tert-butyl-3-{[(4-methyl-1,2,5-oxadiazol-3-yl)methyl]amino}-2-oxopyrazin-1(2H)-yl]butanoyl}amino)-5-[methyl(pentyl)amino]-4-oxopentanoic acid). Structures and potencies for M826, M920, and M791 can be found in references 16 and 34. (B) Schematic representation of the experimental design. Average αII-spectrin cleavage index (C; as described in Materials and Methods) and DNA fragmentation (D; percentage of subdiploid thymocytes) in CLP rats treated with M867 (n = 10) or vehicle (veh; n = 10) relative to vehicle-treated sham animals (n = 3). Average αII-spectrin cleavage index (D) and DNA fragmentation (E; percentage of subdiploid thymocytes) in CLP rats treated with vehicle (n = 4), M867 (n = 5), or inactive enantiomer compound (M761; n = 4) relative to vehicle-treated sham animals (n = 3). Statistical analysis (one-way ANOVA) indicates a significant difference (P < 0.01) between vehicle- and M867-treated CLP rats. M867 had no effect on basal apoptotic parameters in sham-operated animals (unpublished data).
Table II.
M867 Potency against Caspases
| Recombinant human caspase | M867 Ki (nM) |
|---|---|
| Caspase-1 | 107 |
| Caspase-2 | >100,000 |
| Caspase-3 | 1.4 |
| Caspase-4 | 495 |
| Caspase-5 | 805 |
| Caspase-6 | 3442 |
| Caspase-7 | 8.9 |
| Caspase-8 | 4032 |
| Caspase-9 | ND |
| Caspase-10 | 29490 |
Table III.
Effect of M867 on Apoptotic Markers during Experimental Peritonitis in Rat
| Group | Percentage of subdiploid cells (±SE) | Spectrin cleavage OD (±SE) |
|---|---|---|
| CLP (vehicle) (n = 10) | 25.12 ± 4.01 | 0.97 ± 0.15 |
| CLP (M867) (n = 10) | 11.61 ± 2.95 | 0.30 ± 0.01 |
| sham (vehicle) (n = 3) | 1.23 ± 0.42 | 0.21 ± 0.03 |
| CLP (vehicle) (n = 4) | 33.49 ± 2.64 | 0.58 ± 0.18 |
| CLP (M867) (n = 5) | 13.25 ± 4.70 | 0.18 ± 0.01 |
| CLP (M761) (n = 4) | 30.95 ± 3.93 | 0.63 ± 0.07 |
| sham (vehicle) (n = 3) | 0.37 ± 0.06 | 0.12 ± 0.01 |
αII-Spectrin and DNA Cleavage Are Not Equally Inhibited by Caspase Inhibitors In Vitro.
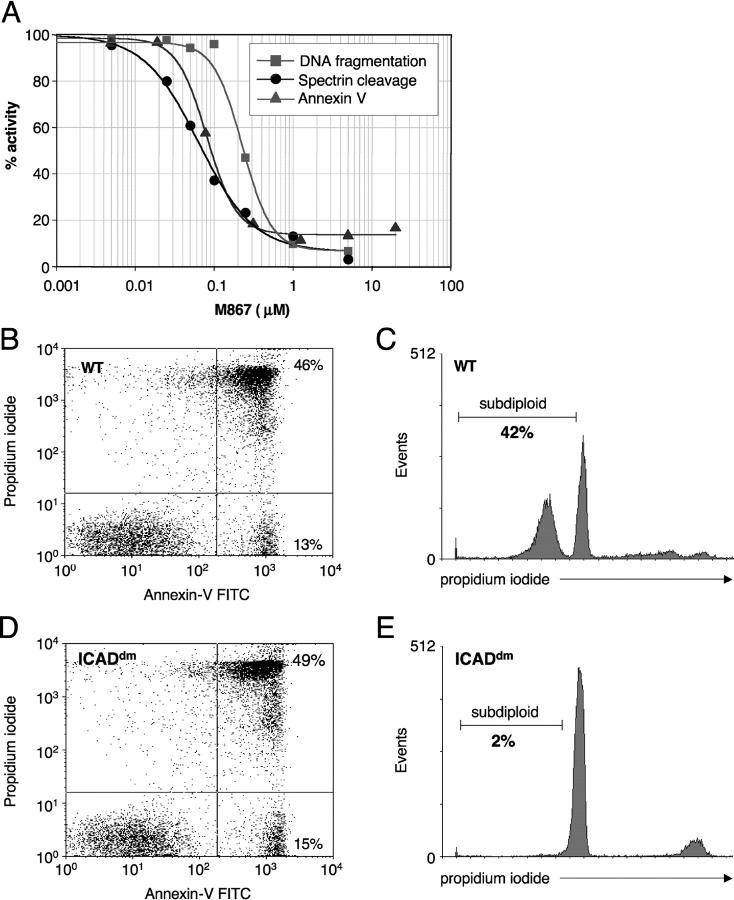
We were intrigued by the fact that in our experimental model, M867 inhibited αII-spectrin cleavage by close to 90% but reduced DNA fragmentation by only 60%. Several explanations can account for this observation: (a) DNA fragmentation could be initiated by a caspase other than caspase-3; (b) a DNase may be induced by the mitochondrial apoptotic pathway (inhibited by Bcl-2) in a caspase-independent manner; or (c) a higher fractional inhibition of caspase-3 is required to prevent the enzymatic cascade that leads to DNA fragmentation. To develop an in vitro cellular assay with good predictive value as to the ability of inhibitors to block apoptosis in sepsis, we tested the potency of several caspase inhibitors on cultured primary rat thymocytes. These cells die spontaneously by apoptosis when cultured in suspension. Additionally, thymocytes are depleted during sepsis and thus constitute relevant in vivo cellular targets. M867 (5 μM) inhibited close to 95% of both αII-spectrin cleavage and DNA fragmentation in cultured thymocytes, establishing a clear caspase dependency for both markers. However, the IC50 for the reduction αII-spectrin cleavage was 64 nM, whereas the IC50 for DNA fragmentation was 230 nM. This discrepancy is further exemplified by the fact that 0.1 μM of M867 inhibited 60% of spectrin cleavage without affecting DNA fragmentation (Fig. 3 A). Annexin V binding was blocked by M867 with an IC50 of 80 nM and profile similar to that of αII-spectrin cleavage (Fig. 3 A). To investigate the role of ICAD in this system, we compared DNA fragmentation and annexin V binding in cultured thymocytes from mice that express a caspase-resistant form of ICAD (ICADdm; reference 31) and WT controls. ICADdm thymocytes exhibited similar levels of spontaneous apoptosis (annexin V staining) but showed minimal DNA fragmentation (2 versus 42% subdiploid population for WT thymocytes; Fig. 3, B–E). Thus, cleavage of ICAD is required for DNA fragmentation and accounts for the vast majority of the activity. To evaluate whether caspases other than caspase-3 could be affecting DNA cleavage, broad specificity caspase inhibitors (M920, zVAD-fluoromethyl ketone [fmk], M808, and M310) or a strictly caspase-3–specific inhibitor (M791) were tested (Table IV). All broad range inhibitors exhibited an even greater potency shift between αII-spectrin and DNA cleavage, whereas M791 was shifted fourfold, similarly to M867. Thus, it seems unlikely that residual DNA fragmentation activity is due to a caspase that is blocked by none of the inhibitors used. Together, the results demonstrate that, in general, a higher dose of caspase inhibitor is required to block DNA fragmentation compared with the inhibition of other apoptotic markers.
Figure 3.
Potencies of caspase-3 inhibitor M867 and ICADdm on apoptotic manifestations in cultured thymocytes. (A) Effect of increasing concentrations of M867 on DNA fragmentation (squares), spectrin cleavage (circles), and annexin V labeling (triangles) in cultured rat thymocytes. IC50s were determined by 4-parameter curve fits and are as follows: DNA fragmentation, 0.23 μM; αII-spectrin cleavage, 0.064 μM; annexin V, 0.08 μM. Annexin V–PI labeling (B and D) and DNA fragmentation (C and E) in WT (B and C) and ICADdm (D and E) mouse thymocytes cultured for 24 h. The experiment was performed with thymocytes from three WT and three ICADdm mice. Shown here are representative results from one animal of each genotype.
Table IV.
Comparative Potencies of Various Caspase Inhibitors Using αII-Spectrin Cleavage and DNA Fragmentation as Activity Readout
| Inhibitor | Type | IC50 spectrin | IC50 subdiploid | Potency shift |
|---|---|---|---|---|
| μM | μM | |||
| M791 | C3 specific | 1.16 | 4.90 | 4 |
| M826 | C3 preference | 0.04 | 0.13 | 3 |
| M867 | C3 preference | 0.07 | 0.27 | 4 |
| M920 | Poly | 0.25 | 4.90 | 19 |
| M808 | Poly | 0.30 | 2.41 | 8 |
| M310 | Poly | 0.01 | 0.33 | 23 |
| zVAD-fmk | Poly | 0.60 | 25.00 | 42 |
Average of two to four experiments for M791, M826, M867, M920, and M310. Single experiment for zVAD-fmk and M808. C3 refers to caspase-3.
Potency Shift of Caspase Inhibitors In Vivo during Experimental Peritonitis.
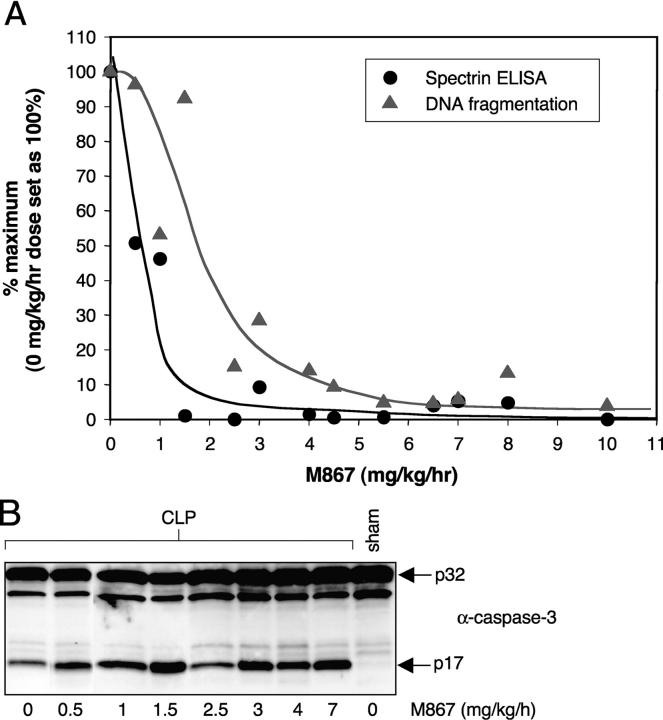
DNA fragmentation in cultured thymocytes is almost completely caspase-3 and ICAD dependent. Given the shift in potency that was observed between αII-spectrin and DNA cleavage in vitro, we asked whether a similar shift would be present in vivo during CLP-induced thymocyte apoptosis. To that end, a dose–response curve for αII-spectrin cleavage and DNA fragmentation was performed in septic rats, at M867 doses ranging from 0 to 10 mg/kg/h. M867 reduced αII-spectrin cleavage in a dose-dependent manner, with 50% inhibition at ∼0.5 mg/kg/h and an almost complete inhibition at 2 mg/kg/h (Fig. 4 A). Inhibition of DNA fragmentation was dose dependent but required larger amounts of drug (50% inhibition at ∼2 mg/kg/h; Fig. 4 A). Importantly, DNA fragmentation was almost abrogated by M867, indicating caspase-3 (and possibly caspase-7) dependency in this model. At all M867 doses, caspase-3 was processed equally well, evidence that this inhibitor did not prevent upstream events leading to caspase-3 activation (Fig. 4 B). Hence, the process of DNA fragmentation during experimental peritonitis is largely caspase-3 dependent. As is the case with cultured thymocytes, drug levels must be fourfold higher to inhibit DNA cleavage compared with αII-spectrin cleavage.
Figure 4.
Effect of M867 dose titration on caspase-3 cleavage, αII-spectrin cleavage, and DNA fragmentation in CLP-induced septic rats. Rats that underwent CLP surgery were dosed by continuous i.v. infusion of either vehicle (n = 6) or M867 (n = 1 for each dose) for 24 h. (A) Percentage of residual αII-spectrin cleavage (circles) and DNA fragmentation (triangles) in each animal after administration of various doses of M867. Percentages were calculated from the average αII-spectrin and DNA fragmentation values obtained with the vehicle-treated animals. (B) Caspase-3 Western blotting on thymus extracts of animals dosed with M867.
Discussion
The development of efficacious therapeutics requires a good understanding of the target and its site of action in animal models mimicking the human disease. CLP in rats and mice is an accepted sepsis animal model with demonstrated apoptosis and caspase contributions. Studies with genetic and pharmacological inhibitors of apoptosis in sepsis animal models suggest that caspase inhibition may improve septic patient survival (10–16).
We have made use of transgenic mice that overexpress Bcl-2 in T cells to evaluate the apoptotic component of various commonly used cell death markers in septic mice. We found that αII-spectrin cleavage at the p120 caspase site and DNA fragmentation are two markers with an almost absolute dependency on the mitochondrial apoptotic pathway. Annexin V binding, PI permeability, and PARP cleavage were also strongly associated with CLP-induced apoptosis. However, residual signal even in the presence of Bcl-2 suggests a partial nonmitochondrial contribution (Fig. 1). We also compared Bcl-2 and WT sibling septic mice to identify markers that may predict survival. Although Bcl-2 transgenic mice showed on average slightly more white blood cells and lymphocytes than their WT counterparts, the difference was not statistically significant (unpublished data). No difference in blood chemistry (glucose, urea, AST, ALT) was noted between the two groups (unpublished data).
As with Bcl-2, the caspase-3–specific inhibitor M867 reduced αII-spectrin cleavage and DNA fragmentation during CLP-induced sepsis in rats. Strikingly, whereas Bcl-2 abrogated the appearance of both markers, M867 showed a markedly different efficacy at blocking αII-spectrin cleavage relative to DNA fragmentation in vitro and in animals (Figs. 2 and 3). We systematically examined several possibilities that may account for this observation. In cultured rat thymocytes, virtually all DNA fragmentation was blocked by high doses of M867 (5 μM), indicating an absolute caspase dependency. An uncleavable form of ICAD also abrogated DNA fragmentation in this system. Thus, the reduced ability of M867 at blocking DNA fragmentation is neither caused by a noncaspase or an ICAD-independent DNase. Furthermore, several polycaspase inhibitors exhibited a strong potency shift, making it unlikely that a caspase other than caspase-3 (or in some instances caspase-7) cleave ICAD. This phenomenon was not restricted to rat thymocytes, since camptothecin-treated Jurkat cells also exhibited IC50 potency shifts between αII-spectrin and DNA cleavage with several caspase inhibitors (unpublished data).
The need for higher levels of caspase inhibitor to prevent apoptosis-induced DNA fragmentation was observed in vivo with septic rats dosed with M867. Abolition of αII-spectrin cleavage was achieved with doses below 2 mg/kg/h, and DNA fragmentation was fully inhibited with doses above 5 mg/kg/h. A crude extrapolation of the in vivo IC50 values for M867 would place αII-spectrin cleavage at 0.5 mg/kg/h and DNA fragmentation at 2 mg/kg/h (Fig. 4), a potency shift similar to that observed on cultured rat thymocytes. M867 blood levels in animals dosed with 0.5 and 1.5 mg/kg/h were 0.14 and 0.70 μM, respectively, which roughly corresponds to αII-spectrin and DNA cleavage IC50s measured in cultured rat thymocytes. The exact biochemical cause for the IC50 shift is not known. It is possible that ICAD, whose caspase-mediated cleavage is required to initiate DNA fragmentation (25–27) is a better caspase-3 substrate than the p120 site of αII-spectrin. Alternatively, since at least one enzymatic amplification step separates caspase-mediated ICAD cleavage from CAD-mediated DNA fragmentation, a small amount of ICAD cleavage may result in some CAD release and a disproportionately larger amount of DNA fragmentation. The steepness of the caspase inhibitor titration curves agrees with this possibility (Fig. 3 A). The amount of αII-spectrin cleavage activity changes from 100 to 5% over two logarithmic units of M867 concentration. In contrast, a similar change in DNA fragmentation activity takes place in only one logarithmic unit, indicating the presence of a threshold effect. A higher fractional inhibition of caspase activity may be required to pass this threshold. Alternatively, a difficulty for some caspase inhibitors at accessing the nucleus where some of the CAD–ICAD complex reside (32, 33) may leave nuclear caspase-3 partially unblocked. We are currently investigating these possibilities. In summary, our work illustrates the need to gage DNA fragmentation as an indication of success at blocking apoptosis and suggests that in acute apoptotic pathologies high and persistent levels of caspase inhibition will be needed clinically. Based on our study of potency shifts, we suggest that a caspase-3–specific reversible inhibitor would represent a potential therapeutic approach in acute apoptotic diseases such as sepsis. Finally, given the high degree of fractional inhibition of caspases required to block apoptosis low potency caspase inhibitors (such as z-VAD-fmk) cannot be used as criteria for establishing caspase-independent cell death events.
Acknowledgments
We are indebted to D. Normandin, S. Wong, C. Desroches, C. Jones, G. Castonguay, L. Bélair, M. Lavoie, R. Rasori, K. Ortega, S. Levesque, J. Rozon, D. Gendron, and N. Kelly for animal care, surgical help, clinical signs monitoring, and necropsies. We thank S. Ménard for performing CLP surgeries on mice and K. Clark for art work. We are grateful to B. Gunter for help with the statistical analysis and discussions on experimental design.
Abbreviations used in this paper: ANOVA, analysis of variance; Bcl, breakpoint cluster; CAD, caspase-activated DNase; CDE, cell death ELISA; CLP, cecal ligation and perforation; fmk, fluoromethyl ketone; ICAD, inhibitor of caspase-activated DNase; PARP, poly(ADP-ribose) polymerase; PI, propidium iodide; PS, phosphatidylserine.
References
- 1.Nicholson, D.W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028–1042. [DOI] [PubMed] [Google Scholar]
- 2.Shi, Y. 2002. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell. 9:459–470. [DOI] [PubMed] [Google Scholar]
- 3.Slee, E.A., C. Adrain, and S.J. Martin. 2001. Executioner caspase-3, -6 and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 276:7320–7326. [DOI] [PubMed] [Google Scholar]
- 4.Kuida, K., T.S. Zheng, S. Na, C. Kuan, D. Yang, H. Karasumaya, P. Rakik, and R.A. Flavell. 1996. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 384:368–372. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry, N.A., and Y. Lazbenik. 1998. Caspases: enemies within. Science. 281:1312–1316. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson, D.W. 2000. From bench to clinic with apoptosis-based therapeutic agents. Nature. 407:810–816. [DOI] [PubMed] [Google Scholar]
- 7.Angus, D.C., W.T. Linde-Zwirble, J. Lidicker, G. Clermont, J. Carcillo, and M.R. Pinsky. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated cost of care. Crit. Care Med. 29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss, R.S., P.E. Swanson, B.D. Freeman, K.W. Tinsley, J.P. Cobb, G.M. Matuschak, T.G. Buchman, and I.E. Karl. 1999. Apoptotic cell death in patients with sepsis, shock and multiple organ dysfunction. Crit. Care Med. 27:1230–1251. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss, R.S., K.W. Tinsley, P.E. Swanson, R.E. Schmieg, Jr., J.J. Hui, K.C. Chang, D.F. Osborne, B.D. Freeman, J.P. Cobb, T.G. Buchman, and I.E. Karl. 2001. Sepsis induces progressive profound depletion of B and CD4+ T lymphocytes. J. Immunol. 166:6952–6953. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss, R.S., P.E. Swanson, J.P. Cobb, A. Jacobson, T.G. Buchman, and I.E. Karl. 1997. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit. Care Med. 25:1298–1307. [DOI] [PubMed] [Google Scholar]
- 11.Ayala, A., C.D. Herdon, D.L. Lehman, C.A. Ayala, and I.H. Chaudry. 1996. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency and the nature of the mediators. Blood. 87:4261–4275. [PubMed] [Google Scholar]
- 12.Hotchkiss, R.S., P.E. Swanson, C.M. Knudson, K.C. Chang, J.P. Cobb, D.F. Osborne, K.M. Zollner, T.G. Buchman, S.J. Korsmeyer, and I.E. Karl. 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162:4148–4156. [PubMed] [Google Scholar]
- 13.Hotchkiss, R.S., K.W. Tinsley, P.E. Swanson, K.C. Chang, J.P. Cobb, T.G. Buchman, and I.E. Karl. 1999. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA. 96:14541–14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coopersmith, C.M., K.C. Chang, P.E. Swanson, K.W. Tinsley, P.E. Stromberg, T.G. Buchman, I.E. Karl, and R.S. Hotchkiss. 2002. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit. Care Med. 30:195–201. [DOI] [PubMed] [Google Scholar]
- 15.Coopersmith, C.M., P.E. Stromberg, W.M. Dunne, C.G. Davis, D.M. Amiot, II, T.G. Buchman, I.E. Karl, and R.S. Hotchkiss. 2002. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 287:1716–1721. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss, R.S., K.C. Chang, P.E. Swanson, K.W. Tinsley, J.J. Hui, P. Klender, S. Xanthoudakis, S. Roy, C. Black, E. Grimm, et al. 2000. Caspase inhibitors improve survival in sepsis: a critical role for the lymphocyte. Nat. Immunol. 1:496–501. [DOI] [PubMed] [Google Scholar]
- 17.Kluck, R.M., E. Bossy-Wetzel, D.R. Green, and D.D. Newmeyer. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 275:1129–1132. [DOI] [PubMed] [Google Scholar]
- 18.Yang, J., X. Liu, K. Bhalla, C. Naekyung Kim, A.M. Ibrado, J. Cai, T.-I. Peng, D.P. Jones, and X. Wang. 1997. Prevention of apoptosis by bcl-2: release of cytochrome c from mitochondria blocked. Science. 275:1129–1132. [DOI] [PubMed] [Google Scholar]
- 19.Lazebnik, Y.A., S.H. Kaufmann, S. Desnoyers, G.G. Poirier, and W.C. Earnshaw. 1994. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 371:346–347. [DOI] [PubMed] [Google Scholar]
- 20.Martin, S.J., G.A. O'Brien, W.K. Nishioka, A.J. McGahon, A. Mahboubi, T.C. Saido, and D.R. Green. 1995. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J. Biol. Chem. 270:6425–6428. [DOI] [PubMed] [Google Scholar]
- 21.Harris, A.S., D. Croall, and J.S. Morrow. 1988. The calmodulin-binding site in alpha-fodrin is near the calcium-dependent protease-I cleavage site. J. Biol. Chem. 263:15754–15761. [PubMed] [Google Scholar]
- 22.Wang, K.K.W., R. Posmantur, R. Nath, K. McGinnis, M. Whitton, R.V. Talanian, S.B. Glantz, and S.J. Morrow. 1998. Simultaneous degradation of αII- and βII-spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem. 273:22490–22497. [DOI] [PubMed] [Google Scholar]
- 23.Janicke, R.U., P. Ng, M.L. Sprengart, and A.G. Porter. 1998. Caspase-3 is required for α-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J. Biol. Chem. 273:15540–15545. [DOI] [PubMed] [Google Scholar]
- 24.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods. 184:39–51. [DOI] [PubMed] [Google Scholar]
- 25.Liu, X., P. Li, P. Widlak, H. Zu, X. Luo, W.T. Garrard, and X. Wang. 1998. The 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosis. Proc. Natl. Acad. Sci. USA. 95:8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enari, M., H. Sakahira, H. Yokoyama, K. Okawa, A. Iwamatsu, and S. Nagata. 1998. A caspase-activated DNase that degrades DNA during apoptosis and its inhibitor ICAD. Nature. 391:43–50. [DOI] [PubMed] [Google Scholar]
- 27.Sakahira, H., M. Enari, and S. Nagata. 1998. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 391:96–99. [DOI] [PubMed] [Google Scholar]
- 28.Wolf, B.B., M. Schuler, F. Echeverri, and D.R. Green. 1999. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 274:30651–30656. [DOI] [PubMed] [Google Scholar]
- 29.Tang, D., and V.J. Kidd. 1998. Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J. Biol. Chem. 273:28549–28552. [DOI] [PubMed] [Google Scholar]
- 30.Sharif-Askari, E., A. Alam, E. Rhéaume, P.J. Beresford, C. Scotto, K. Sharma, D. Lee, W.E. DeWolf, M.E. Nuttall, J. Lieberman, and R.-P. Sékaly. 2001. Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation. EMBO J. 20:3101–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIlroy, D., M. Tanaka, H. Sakahira, H. Fukuyama, M. Suzuki, K. Yamamura, Y. Ohsawa, Y. Uchiyama, and S. Nagata. 2000. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 14:549–558. [PMC free article] [PubMed] [Google Scholar]
- 32.Lechardeur, D.L. 2000. Drzymala, M. Sharma, D. Zylka, R. Kinach, J. Pacia, C. Hicks, N. Usmani, J.M. Rommens, and G.L. Lukacs. 2000. Determinants of the nuclear localization of the heterodimeric DNA fragmentation factor (ICAD/CAD). J. Cell Biol. 150:321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, D., R.A. Stetler, G. Cao, W. Pei, C. O'Horo, X.-M. Yin, and J. Chen. 2000. Characterization of the Rat DNA fragmentation factor 35/inhibitor of caspase-activated DNase (short form). J. Biol. Chem. 275:38508–38517. [DOI] [PubMed] [Google Scholar]
- 34.Han, B.H., D. Xu, J. Choi, Y. Han, S. Xanthoudakis, S. Roy, J. Tam, J. Vaillancourt, J. Colucci, R. Siman, A. Giroux, G. Robertson, R. Zamboni, D.W. Nicholson, D.M., and D.M. Holtzman. 2002. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic-ischemic brain injury. J. Biol. Chem. 277:30128-30136. [DOI] [PubMed] [Google Scholar]