Abstract
Antibodies specific for self-antigens mediate life-threatening pathology in several autoimmune diseases. Clearly the ability to target the plasma cells (PCs) producing the autoantibodies would be of great clinical benefit. Current immunosuppressive therapies are based on the premise that autoreactive PCs are short-lived and replenished from ongoing immune responses. However, recent results question this assumption and suggest that optimizing the treatment of severe autoimmune conditions will require a significant investment in elucidating the details of PC biology.
Conventional therapy for autoimmune conditions in which the symptoms are mediated by antibody—such as systemic lupus erythematosus (SLE)—depends on the severity of the symptoms and the circumstances of the patient. In general, mild forms of disease are first treated with steroidal and nonsteroidal antiinflammatories. More severe forms, involving organ dysfunction due to active disease, usually are treated with steroids in conjunction with strong immunosuppressive agents such as cyclophosphamide, a cytotoxic agent that targets cycling cells. Such stringent therapy is applied for the period required to induce remission. This treatment regime has been very successful in managing autoimmune diseases such as SLE with, in one study, 25% of patients achieving at least one remission of at least 1 yr duration (1). Not all patients respond to cyclophosphamide, and among these nonresponders novel therapies including B cell depletion are being tried with varying levels of success (2). The success of treating antibody-mediated disease by immunosuppression based on killing cycling cells fits well with a dogma developed during the 1980's that held PCs to be short-lived and produced continuously from cycling precursors (3). Although it may well be that a significant fraction of the PC-secreting autoantibodies in SLE fit this category and are therefore sensitive to current therapeutic approaches, patients may to varying degrees harbor autoreactive PCs that are long-lived and sessile. The presence and frequency of long-lived, disease-associated PCs may turn out to be an important indicator of therapeutic success, providing information on the likelihood of entering and remaining in remission.
Although the generation of long-lived PCs in response to foreign antigens was conclusively shown some time ago (4, 5), the first clear demonstration that such cells are produced during an autoimmune response is provided in the article of Hoyer et al. in this issue (pages 1577–1584) (6). They show in a mouse model of SLE that a large fraction of the PC-producing anti–self-antibodies (anti-DNA in this case) are long-lived, sessile cells in the bone marrow that withstand treatment with cyclophosphamide. These findings imply that developing therapies that control autoantibody production will require a detailed knowledge of the PC populations involved in the particular disease. Is the PC short- or long-lived; is it derived from cycling or quiescent precursors; where is it located; is its survival intrinsic or dependent on its environment? To identify points and methods of clinical intervention, it will first be necessary to elucidate a developmental scheme for PCs and then define variations unique to particular diseases. Here we outline our current understanding of plasma cell development and the parts of the puzzle that are missing.
PC Development in All Its (Current) Glory.
Antibody-secreting cells were at one point divided into two categories: the plasmablast and the more mature PCs (7). The PC compartment has been further divided into short- and long-lived cells (4, 5, 8), with the latter being considered more mature. Within such a simple scheme, however, precursor–product relationships have been difficult to define. For example, the plasmablasts that appear within the foci in the extrafollicular areas of lymphoid tissues shortly after immunization (9) have a limited lifespan and are considered to die in situ (10). It is unknown whether plasmablasts give rise to short- or long-lived PCs (11). Antibody production continues well after the loss of the extrafollicular foci due to PCs located in the splenic red pulp, for example, and in bone marrow (12, 13). Despite knowing for some time that plasma cells with varying life-span exist in these locations (8), it remains unclear whether one (long) is derived from the other (short). In addition to longer lifespan, PC maturation is considered to involve changes in phenotype. Certain PCs do not express MHC class II, CD19, and CD45 (10, 14), and in general loss of B cell differentiation markers correlates with increased lifespan and is considered a sign of maturity. However, it is not clear if the phenotypic changes represent distinct stages in a linear progression or are to some extent stochastic (15). In mice, PCs from all defined subsets have been shown to express CD138 (16) (although not all PCs are necessarily CD138+ nor are all CD138+ cells PC [17]) making it something of a beacon in PC biology as it is almost the only marker that allows the prospective identification of PCs rather than retrospectively measuring immunoglobulin secretion. In humans, CD138 expression is restricted to PC of the bone marrow (18), and CD38 expression provides a somewhat less robust alternative marker (19). Collectively, ample heterogeneity has been identified among PCs to satisfy the most ardent immunological subsetter, but it has yet to allow the construction of a developmental scheme whereby a particular phenotype can be associated with a particular function, lifespan, or fate. Added to this complexity is the fact that in autoimmune diseases immune reactions occurring outside lymphoid tissues (20) and PC accumulating in inflamed tissues (21) will contribute to antibody production. Both conditions may confer distinct properties on the PCs they produce.
PC Precursors: It Never Rains, It Pours.
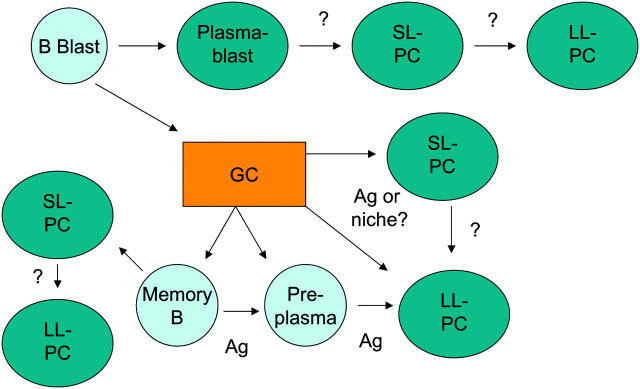
Next is the question of the cellular origin of PCs. Are particular PC phenotypes or the PCs found in particular locations associated with distinct cellular origins? Such relationships may determine the pathogenicity of the antibodies being produced. For example, PCs derived from germinal centers (GCs) may secrete antibody of a mature (IgG) isotype and of high affinity for antigen, both factors that may exacerbate tissue damage. Thus, knowing the etiology of a PC may identify points and means of clinical intervention. There are several developmental schemes for PCs that collectively encompass virtually every possible connection (Fig. 1). Although plasmablasts are potential PC precursors of long standing (7), several other populations have joined them recently. The GC is broadly accepted to produce PCs that are high affinity, long-lived, and that reside primarily in the bone marrow (13, 22). Defining the developmental processes that drive a GC B cell toward becoming a long-lived bone marrow PC may identify points at which the development of pathogenic autoantibodies can be interrupted, assuming that the long-lived PC-secreting pathogenic autoantibodies in the bone marrow are GC derived, which may not always be the case (23). If, for example, the process of PC differentiation depends on antigen-induced signaling (24), the creation of environmental niches (25), and chemokine gradients (26), then modulating any of these may be sufficient to modulate disease severity. One must include also the traditional memory B cell population as containing PC precursors since such cells rapidly differentiate into short-lived PC upon reexposure to antigen (27, 28). In mouse, two additional precursor populations have been identified: both B220− and both concentrated in the bone marrow. One of these is cycling (29) and therefore presumably susceptible to the treatment applied by Hoyer et al. (6) that failed to block anti-DNA antibody production. The role of this population in the maintenance of long-lived autoreactive PCs in the bone marrow therefore remains open. The other B220− population is derived from the GCs, lacks expression of CD138, is developmentally dependent on a transcriptional regulator of PC development, Blimp1 (30, 31), and is responsive to antigen, which long-lived PCs are not (14). Again, the role of this population in the maintenance of long-lived PCs is unresolved. In humans, PC precursor diversity is less well defined, although stages of maturation have been identified in blood ranging from a plasma cell precursor (32) to more discrete stages within committed PC (33, 34). Interestingly, one of these studies noted a significant increase in plasma cell precursor frequency in the blood of children with SLE (32), which complements other studies reporting a correlation between disease activity and circulating PCs (35). These data raise the possibility that aspects of the immune response are dysfunctional in these patients, and if the developmental origin of the PC were known, it may be possible to intervene in a relatively precise manner. The definition of relationships between precursors and PCs is going to be crucial if selective therapies are to be developed that target only the relevant PC subset.
Figure 1.
The tangled web of PCs and their precursors. Schematic representation of PC subsets and their postulated or proven precursors and products. SL, short-lived; LL, long-lived; Ag, antigen.
Solving the Problem of PC Development.
Immunologists are nothing if not adept at defining developmental pathways. What is required to elucidate PC development is relatively simple to write but more complicated to effect. First is the efficient identification of the earliest B cell committed to becoming a PC, thereby allowing its isolation and manipulation. Currently PC identification relies on the enrichment provided by various fortuitously identified markers such as CD138. Although this certainly helps, it results in considerable heterogeneity in the recovered population. The identification of early stages of PC development would allow the developmental potential and differentiation requirements of such cells to be defined in vitro and in vivo. This would allow also for the rapid accumulation of additional markers in a developmentally ordered manner, creating the schemes that have been so useful in the analysis of white blood cell development. Second is the exploitation of in vitro systems to define the impact of various factors that affect PC production. Small changes in responsiveness to differentiation stimuli such as the cytokines IL-5 and IL-10 can, over the course of an immune response, culminate in huge differences in the size and composition of the effector populations generated (15).
Implications for Treating Autoimmune Diseases
Appropriate and targeted therapy of antibody-mediated autoimmune diseases, as highlighted by Hoyer et al. (6), is going to depend on identifying the PCs involved in a disease process and targeting the critical component(s) of its development, be that the cycling precursor, the stimuli driving its proliferation, the factors promoting its maturation, or elements of its survival niche, both stromal and soluble. Potential approaches include: the cytotoxic and antiinflammatory drugs already in use; inhibiting B–T cell interactions (36); blocking factors that promote PC maturation, such as BAFF/BlyS (37); interfering with chemotaxis (38); and blocking the interaction between PC and stromal elements, such as adhesion molecules and cytokines (39).
Acknowledgments
The authors are indebted to their colleagues for helpful discussions and apoligize to any whose work has not been cited due to space constraints.
Work in the authors' labs is supported by the National Health and Medical Research Council (NHMRC) of Australia, and both D.M. Tarlinton and P.D. Hodgkin are fellows of the NHMRC.
References
- 1.Drenkard, C., A.R. Villa, C. Garcia-Padilla, M.E. Perez-Vazquez, and D. Alarcon-Segovia. 1996. Remission of systematic lupus erythematosus. Medicine (Baltimore). 75:88–98. [DOI] [PubMed] [Google Scholar]
- 2.Looney, R.J., J. Anolik, and I. Sanz. 2004. B cells as therapeutic targets for rheumatic diseases. Curr. Opin. Rheumatol. 16:180–185. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel, R.M. 1996. Immunology taught by viruses. Science. 271:173–178. [DOI] [PubMed] [Google Scholar]
- 4.Manz, R.A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature. 388:133–134. [DOI] [PubMed] [Google Scholar]
- 5.Slifka, M.K., R. Antia, J.K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity. 8:363–372. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer, B.F., K. Moser, A.E. Hauser, A. Peddinghaus, C. Voigt, D. Eilat, A. Radbruch, F. Hiepe, and R.A. Manz. 2004. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagraeus, A. 1948. The plasma cellular reaction and its relation to the formation of antibodies in vitro. J. Immunol. 58:1–13. [PubMed] [Google Scholar]
- 8.Ho, F., J.E. Lortan, I.C. MacLennan, and M. Khan. 1986. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 16:1297–1301. [DOI] [PubMed] [Google Scholar]
- 9.Van Rooijen, N., E. Claassen, and P. Eikelenboom. 1986. Is there a single differentiation pathway for all antibody-forming cells in the spleen? Immunol. Today. 7:193–195. [DOI] [PubMed] [Google Scholar]
- 10.Smith, K.G., T.D. Hewitson, G.J. Nossal, and D.M. Tarlinton. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur. J. Immunol. 26:444–448. [DOI] [PubMed] [Google Scholar]
- 11.MacLennan, I.C., K.M. Toellner, A.F. Cunningham, K. Serre, D.M. Sze, E. Zuniga, M.C. Cook, and C.G. Vinuesa. 2003. Extrafollicular antibody responses. Immunol. Rev. 194:8–18. [DOI] [PubMed] [Google Scholar]
- 12.Benner, R., W. Hijmans, and J.J. Haaijman. 1981. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Smith, K.G., A. Light, G.J. Nossal, and D.M. Tarlinton. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16:2996–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manz, R.A., M. Lohning, G. Cassese, A. Thiel, and A. Radbruch. 1998. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10:1703–1711. [DOI] [PubMed] [Google Scholar]
- 15.Hasbold, J., L.M. Corcoran, D.M. Tarlinton, S.G. Tangye, and P.D. Hodgkin. 2004. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat. Immunol. 5:55–63. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson, R.D., P. Lalor, and M. Bernfield. 1989. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Underhill, G.H., K.P. Kolli, and G.S. Kansas. 2003. Complexity within the plasma cell compartment of mice deficient in both E- and P-selectin: implications for plasma cell differentiation. Blood. 102:4076–4083. [DOI] [PubMed] [Google Scholar]
- 18.Ellyard, J.I., D.T. Avery, T.G. Phan, N.J. Hare, P.D. Hodgkin, and S.G. Tangye. 2003. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood. 103):3805–3812. [DOI] [PubMed]
- 19.Terstappen, L.W., S. Johnsen, I.M. Segers-Nolten, and M.R. Loken. 1990. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood. 76:1739–1747. [PubMed] [Google Scholar]
- 20.de Boer, B.A., I. Voigt, H.J. Kim, S.A. Camacho, M. Lipp, R. Forster, and C. Berek. 2000. Affinity maturation in ectopic germinal centers. Curr. Top. Microbiol. Immunol. 251:191–195. [DOI] [PubMed] [Google Scholar]
- 21.Cassese, G., S. Lindenau, B. de Boer, S. Arce, A. Hauser, G. Riemekasten, C. Berek, F. Hiepe, V. Krenn, A. Radbruch, and R.A. Manz. 2001. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 31:2726–2732. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi, Y., P.R. Dutta, D.M. Cerasoli, and G. Kelsoe. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. V. Affinity maturation develops in two stages of clonal selection. J. Exp. Med. 187:885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.William, J., C. Euler, S. Christensen, and M.J. Shlomchik. 2002. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 297:2066–2070. [DOI] [PubMed] [Google Scholar]
- 24.Tarlinton, D.M., and K.G. Smith. 2000. Dissecting affinity maturation: a model explaining selection of antibody-forming cells and memory B cells in the germinal centre. Immunol. Today. 21:436–441. [DOI] [PubMed] [Google Scholar]
- 25.Cassese, G., S. Arce, A.E. Hauser, K. Lehnert, B. Moewes, M. Mostarac, G. Muehlinghaus, M. Szyska, A. Radbruch, and R.A. Manz. 2003. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171:1684–1690. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves, D.C., P.L. Hyman, T.T. Lu, V.N. Ngo, A. Bidgol, G. Suzuki, Y.R. Zou, D.R. Littman, and J.G. Cyster. 2001. A coordinated change in chemokine responsiveness guides plasma cell movements. J. Exp. Med. 194:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tew, J.G., G.F. Burton, A.K. Szakal, and M.H. Kosco. 1988. A subpopulation of germinal center B cells differentiate directly into antibody forming cells upon secondary immunization. Adv. Exp. Med. Biol. 237:215–220. [DOI] [PubMed] [Google Scholar]
- 28.Tangye, S.G., D.T. Avery, and P.D. Hodgkin. 2003. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J. Immunol. 170:261–269. [DOI] [PubMed] [Google Scholar]
- 29.O'Connor, B.P., M. Cascalho, and R.J. Noelle. 2002. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 195:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McHeyzer-Williams, L.J., M. Cool, and M.G. McHeyzer-Williams. 2000. Antigen-specific B cell memory. Expression and replenishment of a novel B220(−) memory B cell compartment. J. Exp. Med. 191:1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro-Shelef, M., K.I. Lin, L.J. McHeyzer-Williams, J. Liao, M.G. McHeyzer-Williams, and K. Calame. 2003. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 19:607–620. [DOI] [PubMed] [Google Scholar]
- 32.Arce, E., D.G. Jackson, M.A. Gill, L.B. Bennett, J. Banchereau, and V. Pascual. 2001. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J. Immunol. 167:2361–2369. [DOI] [PubMed] [Google Scholar]
- 33.Medina, F., C. Segundo, A. Campos-Caro, I. Gonzalez-Garcia, and J.A. Brieva. 2002. The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood. 99:2154–2161. [DOI] [PubMed] [Google Scholar]
- 34.Arce, S., E. Luger, G. Muehlinghaus, G. Cassese, A. Hauser, A. Horst, K. Lehnert, M. Odendahl, D. Honemann, K.D. Heller, H. Kleinschmidt, C. Berek, T. Dorner, V. Krenn, F. Hiepe, R. Bargou, A. Radbruch, and R.A. Manz. 2004. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J. Leukoc. Biol. 10.1189/jlb.0603279. [DOI] [PubMed]
- 35.Jacobi, A.M., M. Odendahl, K. Reiter, A. Bruns, G.R. Burmester, A. Radbruch, G. Valet, P.E. Lipsky, and T. Dorner. 2003. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 48:1332–1342. [DOI] [PubMed] [Google Scholar]
- 36.Daikh, D.I., B.K. Finck, P.S. Linsley, D. Hollenbaugh, and D. Wofsy. 1997. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J. Immunol. 159:3104–3108. [PubMed] [Google Scholar]
- 37.Avery, D.T., S.L. Kalled, J.I. Ellyard, C. Ambrose, S.A. Bixler, M. Thien, R. Brink, F. Mackay, P.D. Hodgkin, and S.G. Tangye. 2003. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balabanian, K., J. Couderc, L. Bouchet-Delbos, A. Amara, D. Berrebi, A. Foussat, F. Baleux, A. Portier, I. Durand-Gasselin, R.L. Coffman, et al. 2003. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J. Immunol. 170:3392–3400. [DOI] [PubMed] [Google Scholar]
- 39.Minges Wols, H.A., G.H. Underhill, G.S. Kansas, and P.L. Witte. 2002. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J. Immunol. 169:4213–4221. [DOI] [PubMed] [Google Scholar]