Abstract
An unresolved issue in the field of T helper (Th) cell development relates to the findings that low doses of antigen promote Th2 cell development in vitro, whereas several classic in vivo studies suggest the opposite. Here we resolve this paradox by studying the early immune response in mice after infection with different doses of Leishmania major. We found that low parasite doses induced a Th2 response in C57BL/6 (B6) mice, whereas high doses induced a Th1 response. However, the Th2 response in low dose–infected mice was transient and the animals healed. The appearance of a Th1 response after low dose infection was dependent upon the concomitant activation of interferon γ–producing CD8+ T cells. In the absence of CD8+ T cells, the Th2 response was maintained. However, either neutralization of interleukin (IL)-4 or administration of IL-12 promoted a Th1 response after low dose infection of CD8-deficient mice, indicating that the required role for CD8+ T cells was limited to modulation of CD4+ T cell responses. Thus, the discrepant results seen between in vivo and in vitro studies on the effects of antigen dose on Th cell differentiation may depend upon whether CD8+ T cells participate in the immune response.
Keywords: Leishmania major, infection, CD8+ T cell, Th1/Th2 immune response, cytokines
Introduction
The effect of antigen dose on the development of CD4+ Th1 and Th2 cells is controversial, as some reports suggest that high and low antigen doses promote Th1 and Th2 responses, respectively, whereas others suggest the opposite. Resolving this controversy is of importance for the development of vaccines as well as immunotherapies directed at modulating Th1 and Th2 responses. Most in vitro results indicate that lower antigen doses favor a Th2 response, whereas higher doses favor a Th1 response (1–5). For example, in vitro stimulation of TCR transgenic CD4+ T cells with low peptide doses induced an IL-4 response, whereas higher doses led to increased IFN-γ production (1, 4). On the other hand, although some in vivo studies indicate that low antigen doses favor a Th2 response, several others suggest the opposite (6–8). A classic experiment that addressed antigen dose found that daily injections of flagellin in rats was associated with the dose-dependent development of either delayed-type hypersensitivity or antibody responses, with high doses favoring antibody responses and lower doses favoring a delayed-type hypersensitivity response (6, 7). One explanation for these discrepant results might be that the in vitro studies measured the response of isolated CD4+ T cells, whereas the in vivo response was assessed in the presence of cells (such as NK cells, CD8+ T cells, etc.) that might modulate the response.
The outcome of infection with the protozoan parasite Leishmania major is dependent upon whether CD4+ Th1 or Th2 cells develop after infection, and experimental infections in mice with L. major have been used extensively to define the factors that regulate Th1 and Th2 cell development (9, 10). In resistant C57BL/6 (B6) mice, production of IFN-γ by Th1 cells leads to parasite control, whereas BALB/c mice develop a Th2 response and a progressive disease. In contrast to CD4+ T cells, CD8+ T cells have been thought to play only a secondary role in resistance to L. major (11, 12). However, it was recently found that in the absence of CD8+ T cells, C57BL/6 mice are unable to heal after low dose L. major infection (13). This result has prompted us to reexamine the influence of antigen dose on the development of Th1 and Th2 cells in vivo, and to investigate the possible regulatory role of CD8+ T cells on Th cell development. At present, the prevailing thought is that low doses of L. major promote a Th1 response, based on the finding that although BALB/c mice infected with high parasite doses develop an uncontrolled infection, a protective Th1 response is induced after low dose infection (14, 15).
Here we report that low dose L. major infection of B6 mice primes CD4+ T cells for Th2 cell development. However, L. major concomitantly activates IFN-γ–producing CD8+ T cells, which promotes a switch from the CD4+ Th2 response to a protective Th1 response. Thus, our results unveil an important role for CD8+ T cells as regulators of CD4+ Th1 cell development, suggesting that low antigen doses may preferentially promote a CD4+ Th2 response in vivo, but in situations where CD8+ T cells are concomitantly activated this Th2 response might be masked. These findings have direct implications for how vaccines or immunotherapies might be administered to preferentially develop a Th1 or Th2 response.
Materials and Methods
Mice.
6–8 wk old female C57BL/6 (B6), homozygous CD8α (B6. 129S2-cd8atmimak, CD8 KO), IFN-γ (B6.129S7-Ifngtm1Ts, γ-KO), and β-2 microglobulin (B6. 129P2-β2mtmiUnc, β2m KO)–deficient mice were purchased from The Jackson Laboratory. All mice were maintained in a specific pathogen-free environment at the University of Pennsylvania Animal Care Facilities.
Parasites and Antigen.
L. major parasites (MHOM/IL/80/Friedlin) were grown in Grace's insect medium (Life Technologies) supplemented with 20% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. For infection, 7-d stationary phase promastigotes were washed three times in PBS and counted. Mice were infected by injecting 106 (high dose) or 103 (low dose) parasites suspended in 10 μl PBS in the ear (intradermal), and the course of lesion progression was monitored weekly by measuring the diameter of ear induration with vernier calipers (Thomas). In some experiments, mice were infected with low dose parasites suspended in 10 μl PBS containing leishmanial antigen (106 freeze-thawed L. major).
In Vivo Anti–IL-4 mAb and rIL-12 Treatment.
Mice were treated intraperitoneally with 2 mg anti–IL-4 antibody (11B11) in PBS given at the time of infection and then once weekly for 3 wk. Control mice were given the same concentration of rat IgG in PBS. Recombinant IL-12 (0. 5 μg/mouse per injection; provided by J. Sypek and S. Wolf, Wyeth Discovery Research, Cambridge, MA) was given first at the time of infection (mixed with parasites) and at 3 and 6 d after infection intraperitoneally. Control mice received injections of PBS.
CD8+ T Cell Purification and Adoptive Transfer.
B6 mice and IFN-γ–deficient mice were depleted of CD4+ T cells by injection of 1 mg anti-CD4 mAb (GK1.5) 1 and 3 d before mice were killed. This treatment routinely depletes >98% of CD4+ T cells when assessed by flow cytometry. Pooled single cell suspensions of spleens and lymph nodes were depleted of red blood cells by lysing with ammonium chloride lysis buffer, and CD8+ T cells were purified using T cell columns (R&D Systems) according to the manufacturer's recommendations. 107 purified CD8+ T cells were transferred intravenously into naive CD8-deficient mice that were then infected the next day with 103 L. major.
In Vitro Recall Response.
At various times after infection, retromaxillary lymph nodes were harvested and made into single cell suspensions. Cells were washed, resuspended at 4 × 106/ml in complete medium (DMEM supplemented with 10% heat-inactivated FBS, 25 mM Hepes, 5 × 10−5 M 2-ME, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin), and plated at 1 ml/well in 24-well tissue culture plates (Falcon Products). Cells were stimulated with or without 50 μg/ml soluble leishmanial antigen (SLA). To facilitate the detection of IL-4, 5 μg/ml anti–IL-4R blocking mAb (M1; provided by F. Finkelman, University of Cincinnati, Cincinnati, OH, and Immunex) was added to prevent autocrine consumption of IL-4. The culture supernatant fluids were collected after 72 h and stored at −70°C until they were assayed for cytokines by ELISA.
Cellular Proliferation and Intracellular Cytokine Staining.
To assess cellular proliferation and intracellular cytokine accumulation, cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) dye and cultured in 96-well round-bottom culture plates (2 × 105/well in 200 μl aliquots) in the presence of 50 μg/ml SLA. After 5 d, cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin for 5 h. 10 μg/ml brefeldin A was added in the last 2 h to enhance intracellular cytokine protein accumulation. Cells were harvested and stained for cell surface markers and intracellular cytokines (16).
Cytokine ELISAs.
IL-4 and IFN-γ concentrations in culture supernatants were measured by sandwich ELISA as described previously (17). The levels of detection of the ELISA were 0.4 U/ml and 30 pg/ml for IL-4 and IFN-γ, respectively.
Estimation of Parasite Burden.
The parasite burden in the ear was quantified as described previously (18) with minor modifications. In brief, the dorsal and ventral layers of the ear were separated with forceps and incubated for 30 min at 37°C in complete Grace's medium containing 1 mg/ml collagenase/dispase (Sigma-Aldrich). Thereafter, the ears were minced and homogenized in 2 ml Teflon-coated tissue grinder and the homogenate was serially diluted (1:2) in 96-well plates and incubated at 26°C. The number of viable parasites in each tissue was calculated from the highest dilution at which parasites were observed after 7 d.
Statistical Analysis.
A two-tailed Student's t test was used to compare means of lesion sizes, parasite burdens, and cytokine production from different groups of mice. Differences were considered significant at P ≤ 0.05.
Results
Low Dose L. major Infection Promotes Th2 Responses.
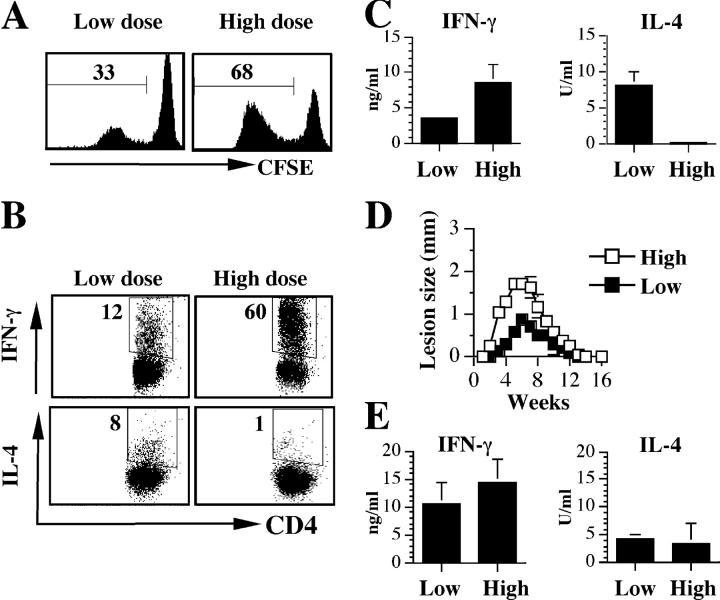
To investigate the influence of parasite dose on the development of CD4+ T cells in vivo, B6 mice were infected with either 103 or 106 L. major intradermally in the ear. After 3 wk, cells from the draining lymph nodes of infected mice were CFSE labeled to monitor proliferation and stimulated with SLA. CD4+ T cells from mice infected with a high dose of parasites exhibited substantial antigen-specific proliferation (Fig. 1 A). In addition, intracellular cytokine staining indicated that a large percentage of the CD4+ T cells produced IFN-γ, whereas few cells produced IL-4 (Fig. 1 B). In contrast, cells from low dose–infected mice proliferated less well, and a smaller percentage of cells made IFN-γ. However, a significantly higher percentage of cells from low dose–infected mice made IL-4. This pattern of response was also observed when cytokine production was measured by ELISA. Cells from low dose–infected mice produced more IL-4 and less IFN-γ, whereas those from high dose–infected mice produced no IL-4, but high levels of IFN-γ (Fig. 1 C). Thus, at this early time point, the CD4+ T cell response in B6 mice infected with low doses of L. major is strongly biased toward a Th2 response. Nevertheless, as reported previously (13, 15), low dose infections were associated with healing and effective parasite control several weeks after infection (Fig. 1 D and unpublished data). Furthermore, when the immune response was assessed in the healed mice, both low and high dose–infected mice exhibited an enhanced IFN-γ response, indicating that the dominant CD4+ Th2 response seen in low dose–infected mice was transient (Fig. 1 E).
Figure 1.
Parasite dose influences the development of CD4+ T cell responses in vivo. (A) Antigen-specific proliferation by CD4+ T cells is diminished in low dose–infected mice. B6 mice were infected intradermally with a low (103) or high (106) dose of L. major. 3 wk after infection, mice were killed and cells isolated from the retromaxillary draining lymph node (dLN) were CFSE labeled, stimulated for 5 d with SLA, and analyzed for proliferation by flow cytometry by gating on live CD4+ cells. (B) Low dose L. major infection induces an early CD4+ Th2 response. Cells from A above were also stained for intracellular IFN-γ and IL-4. (C) Levels of IFN-γ and IL-4 in 72-h culture supernatant fluids of lymph node cells from A above. (D and E) The early low dose–induced Th2 response in B6 mice is transient. B6 mice infected with a low or high dose of L. major were monitored weekly for the development and progression of cutaneous lesion (D). 16 wk after infection and resolution of lesion, mice were killed and cells from the dLN were stimulated with SLA for 72 h. The supernatant fluid was then assayed for IFN-γ and IL-4 by ELISA (E). Representative data from three experiments with similar results are presented.
Low Dose L. major Infection Leads to a Sustained Th2 Response in the Absence of CD8+ T Cells.
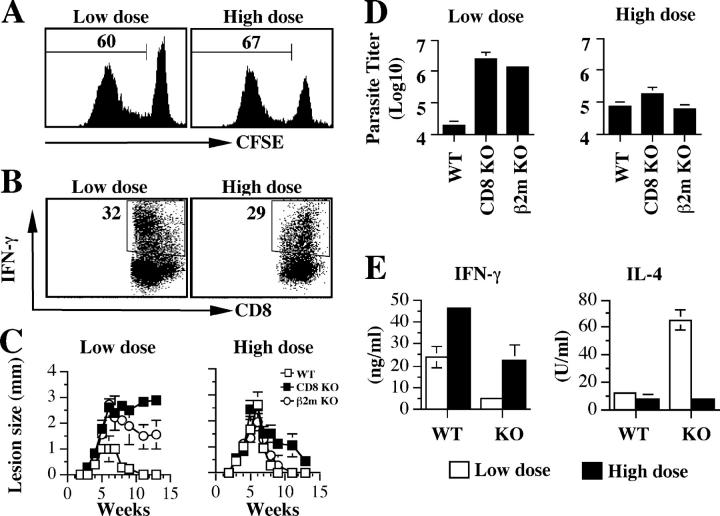
Because the Th2 response induced by low dose infection was transient, we explored the possibility that non-CD4+ T cells responding to Leishmania antigen may regulate Th cell responses. We found that both high and low dose L. major infections were associated with a robust CD8+ T cell proliferative response (Fig. 2 A), and a significant percentage of these CD8+ T cells produced IFN-γ (Fig. 2 B). Therefore, we hypothesized that CD8+ T cells might contribute to reversing the early Th2 response seen in low dose L. major infection. Consistent with this idea was a recent finding that CD8+ T cells are required for healing a low dose infection with L. major (13). However, this contrasts with several previous studies demonstrating that CD8+ T cells are not required for control of a primary infection (19–21). To determine if the discrepancy in these results was related to infection dose, and whether IFN-γ–producing CD8+ T cells were involved in modulating the initial Th2 response, we infected CD8+ T cell–deficient mice, both CD8 and β2m KO animals, with low or high doses of L. major. Similar to infected WT mice, at 3 wk after infection cells from low dose–infected CD8 T cell–deficient mice produced more IL-4 and less IFN-γ, whereas those from high dose–infected mice produced no IL-4, but high levels of IFN-γ (unpublished data). Neither CD8 nor β2m-deficient mice were able to resolve disease after low dose infections, whereas high dose infection was associated with disease resolution in both strains of mice (Fig. 2, C and D). Consistent with a role for CD8+ T cells in down-regulation of Th2 responses, at 13 wk IL-4 production by cells from low dose–infected CD8-deficient mice was significantly greater than that produced by cells from WT mice (Fig. 2 E). Correspondingly, the IFN-γ response in low dose–infected CD8-deficient animals remained low relative to either low dose–infected WT mice or either mouse strain infected with a high dose of parasites (Fig. 2 E). These results strongly implicate CD8+ T cells as important for down-regulating the early Th2 response in low dose–infected mice.
Figure 2.
CD8+ T cells are required for downmodulation of low dose–induced Th2 responses and susceptibility to L. major. (A and B) L. major infection induces proliferation and IFN-γ production by CD8+ T cells in B6 (WT) mice. The dLN cells from B6 mice infected with low and high dose L. major for 3 wk were labeled with CFSE, stimulated with SLA for 5 d, and stained for intracellular IFN-γ. (A) Proliferation (gated on live CD8+ cells) and (B) IFN-γ production by CD8+ T cells was analyzed by flow cytometry. (C and D) CD8+ T cells are required for resistance to low dose, but are dispensable for high dose L. major infection. Course of lesion progression (C) and parasite burden (D) in the ear of WT, CD8, and β2m-deficient mice infected with low and high dose L. major. (E) In the absence of CD8+ T cells, the low dose–induced Th2 response is sustained. CD8-deficient mice infected with low and high doses of L. major were killed at 13 wk, the dLN cells were stimulated with SLA for 3 d, and the production of IFN-γ and IL-4 was measured by ELISA. Representative data from three experiments with similar results are presented.
Leishmanial Antigen Overcomes the Dependence on CD8+ T Cells for Resolution of Low Dose Infections.
To determine if simply increasing the antigen dose rather than increasing the number of live parasites could promote a Th1 response, we characterized the CD4+ T cell response of B6 and CD8-deficient mice infected with a low dose of live parasites mixed with a high dose of leishmanial antigen. Because similar results were obtained from both mice, only data from CD8-deficient mice are presented. After administration of high doses of antigen in the form of killed parasites, CD4+ T cell proliferation, and the percent of those cells producing IFN-γ, was equivalent to that seen with a high dose of live L. major (Fig. 3, A and B). Similarly, IFN-γ and IL-4 production analyzed by ELISA indicated that high antigen doses promoted a Th1 response (Fig. 3 C), suggesting that the critical factor in CD4+ T cell activation is the dose of antigen and not whether the parasites are alive.
Figure 3.
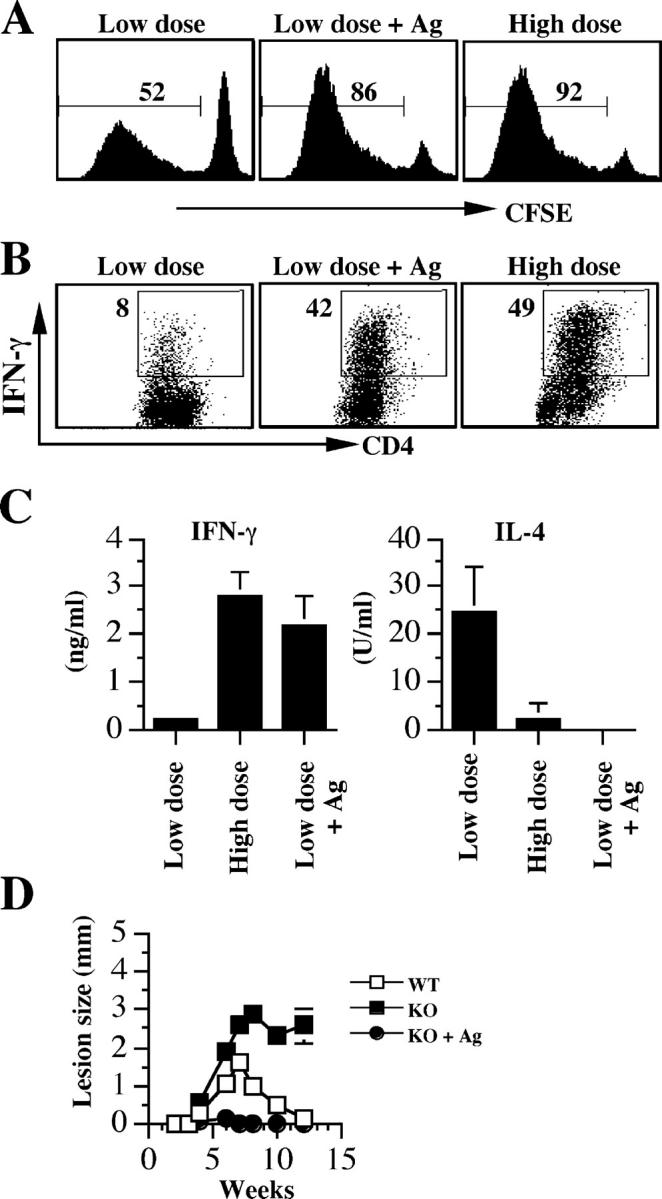
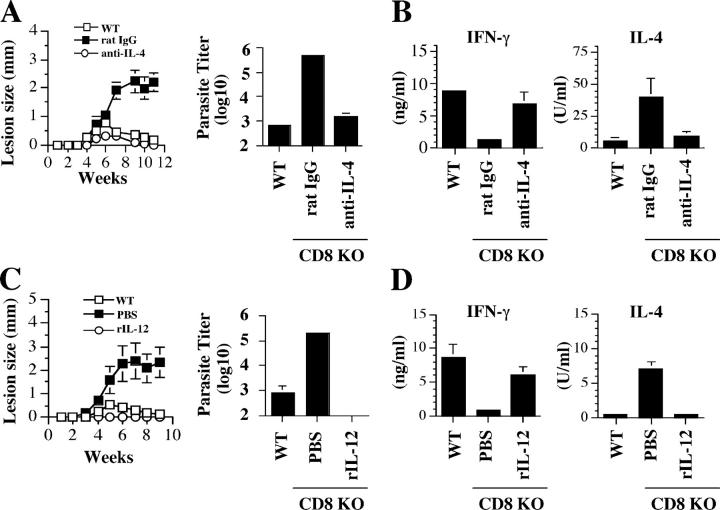
Leishmanial antigen overcomes the dependence on CD8+ T cells for resolution of low dose infections. CD8-deficient mice infected with a low dose, a high dose, or a low dose of L. major suspended in 106 freeze-thaw parasites (low dose plus antigen) were killed at 3 wk and the dLN cells were labeled with CFSE, stimulated with SLA for 5 d, and stained for intracellular IFN-γ. Proliferation (A) and intracellular IFN-γ secretion (B) by CD4+ T cells from infected mice. (C) Some cells were stimulated with SLA for 72 h and the supernatant fluids were collected and assayed for IFN-γ and IL-4 production by ELISA. (D) Enhanced resistance in CD8-deficient mice infected with low dose L. major mixed with leishmanial antigen. CD8-deficient mice were infected with a low dose of L. major or a low dose given with leishmanial antigen as described above, and lesion size was measured weekly. Some low dose–infected B6 (WT) mice were included as positive control. Data presented are a representative of two experiments with similar results.
To test if the increase in IFN-γ produced by CD4+ T cells was sufficient to overcome the requirement for CD8+ T cells in a low dose infection, we assessed the outcome of infection in low dose–infected CD8-deficient mice given leishmanial antigen. Although CD8-deficient mice given a low dose of L. major developed nonhealing lesions, the same dose of L. major given with antigen was associated with a healing infection (Fig. 3 D). In fact, control of the infection was greater than that observed in WT mice given a low dose, which would be expected if the high dose of dead parasites increased the CD4+ Th1 response. These results indicate that by increasing the antigen dose at the time of initial CD4+ T cell priming, sufficient CD4+ Th1 cells can be activated to preclude a need for CD8+ T cells.
CD8+ T Cells Are Not Required for Healing a Low Dose Infection if Th2 Cell Development Is Blocked.
If CD8+ T cells are only necessary to modulate the Th2 response induced by exposure to low doses of antigen, then blocking Th2 cell development by other means should eliminate the requirement for CD8+ T cells. To test this, we treated CD8-deficient mice with either neutralizing anti–IL-4 mAb or IL-12. In contrast to untreated animals, CD8-deficient mice depleted of IL-4 were able to heal their low dose infections with the same kinetics as that seen in B6 mice, and exhibited a reduced parasite burden (Fig. 4 A). The healing response seen in low dose CD8-deficient infected mice that were treated with anti–IL-4 was associated with enhanced IFN-γ responses and decreased IL-4 responses (Fig. 4 B). Similarly, low dose–infected CD8-deficient mice treated with IL-12 failed to develop disease, effectively controlled their parasites, and exhibited a Th1 response (Fig. 4, C and D). Taken together, these results indicate that the conditional requirement for CD8+ T cells in a low dose infection is due to their role in reversing the initial Th2 response and that if this response is blocked, CD8+ T cells are unnecessary for lesion resolution.
Figure 4.
CD8+ T cells are not required for healing a low dose infection if Th2 cell development is blocked. (A) Anti–IL-4 mAb promotes healing in low dose–infected CD8-deficient mice. CD8-deficient mice infected with a low dose of L. major were injected intraperitoneally with 2 mg anti–IL-4 mAb (○) or control rat IgG (▪) at the time of infection and weekly thereafter for 3 wk. Mice were monitored weekly for lesion size and killed at 11 wk to determine parasite burden. (B) Anti–IL-4 mAb treatment blocks the low dose–induced Th2 response. At the time of death, dLN cells were stimulated with SLA and the supernatant fluids were assayed for IFN-γ and IL-4 by ELISA. (C) IL-12 promotes healing in low dose–infected CD8-deficient mice. CD8-deficient mice were infected with a low dose of L. major resuspended in 0.5 μg IL-12. Infected mice received additional injections of 0.5 μg IL-12 or PBS intraperitoneally on days 3 and 6, and lesion size was monitored weekly. 11 wk after infection, mice were killed to estimate parasite burden in the ear. (D) IFN-γ and IL-4 production by dLN cells from IL-12– and PBS-treated mice after in vitro stimulation with SLA. Data presented are a representative of two experiments with similar results.
The Protective Effect of CD8+ T Cells Is Mediated by IFN-γ.
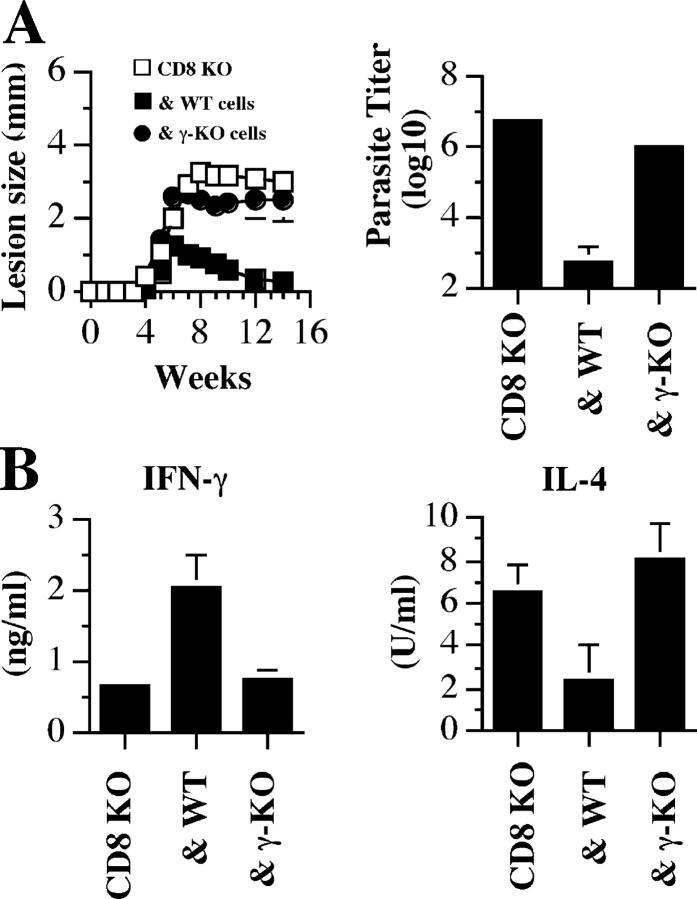
The data above suggests that CD8+ T cells are required for down-regulating the initial Th2 response by proliferating and producing IFN-γ, although it is possible that other IFN-γ–independent mechanisms might be involved. To directly address this, we adoptively transferred CD8+ T cells from B6 or IFN-γ–deficient mice into CD8-deficient mice and infected them with a low dose of L. major. As expected, recipients of WT CD8+ T cells resolved their lesions and efficiently controlled parasite replication. In contrast, mice that received cells from IFN-γ–deficient mice developed progressive disease similar to PBS controls (Fig. 5 A). Corresponding with the outcome of infection, adoptive transfer of WT CD8+ T cells promoted the development of a Th1 response, whereas mice that received IFN-γ–deficient CD8+ T cells exhibited cytokine levels similar to unreconstituted CD8-deficient mice (Fig. 5 B). These data demonstrate that the protective effect of CD8+ T cells during low dose infections is mediated by their production of IFN-γ, which down-regulates the initial Th2 response and enhances Th1 cell development.
Figure 5.
The protective effect of CD8+ T cells is mediated by IFN-γ. (A) CD8-deficient mice were given PBS (CD8 KO) or 107 purified CD8+ T cells from B6 (& WT) or IFN-γ–deficient (& γ-KO) mice, and infected with a low dose of L. major the next day. The course of lesion progression was monitored and 14 wk after infection mice were killed to determine parasite burden. (B) CD8+ T cells from WT, but not IFN-γ–deficient, mice inhibit the low dose–induced Th2 response. At the time of death, dLN cells were stimulated with SLA for 72 h and the supernatant fluids were assayed for IFN-γ and IL-4 production by ELISA. Data presented are a representative of two experiments with similar results.
CD8+ T Cells Are Not Required to Control a Low Dose Infection in Immune Mice.
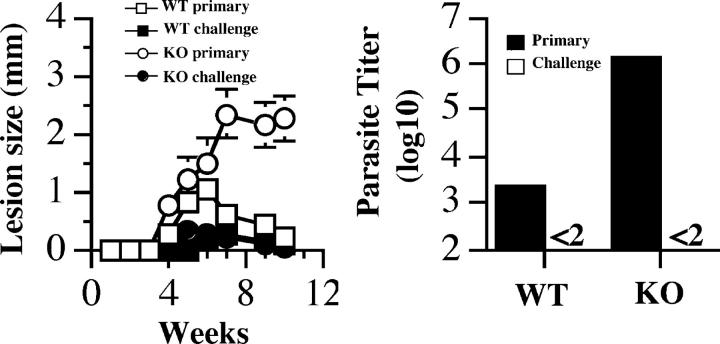
The observation that CD8+ T cells are required for resistance against low dose L. major infection has implications for vaccination strategies against leishmaniasis. Natural infections are initiated by inoculation of low numbers of parasites by the sandfly, which raises the question whether a vaccine targeting CD4+ T cells would be sufficient to mediate resistance to L. major. Indeed, several vaccine studies have found that CD8+ T cells are important for optimal resistance to L. major (22–24). However, whether CD8+ T cells are an obligatory requirement or are necessary due to suboptimal CD4+ T cell induction is unclear. To address this issue, we asked whether CD8-deficient mice that controlled a high dose L. major infection could mount a protective secondary response to low dose challenge. Therefore, B6 and CD8-deficient mice were infected with a high dose of L. major. 6 mo after the primary infection, these healed mice and their age-matched naive controls were challenged with a low dose of L. major. As expected, naive CD8-deficient mice challenged with low dose L. major developed progressive disease, whereas naive B6 mice developed small lesions that eventually resolved. In contrast, both healed B6 and CD8-deficient mice developed minimal lesions after challenge and the lesions contained few parasites (Fig. 6). These results indicate that if sufficient CD4+ T cell immunity is generated, its efficacy and durability are independent of CD8+ T cells. However, whether this only applies to mice that maintain persistent parasites, which would be the case here, has yet to be tested.
Figure 6.
CD8+ T cells are not required to control a low dose infection in immune mice. B6 (WT) and CD8-deficient (KO) mice (five mice per group) were infected with 106 (high dose) L. major and allowed to heal. 6 mo after primary infection, healed mice together with some naive controls (five mice each) were challenged with 103 L. major and the course of lesion progression was monitored. 10 wk after challenge mice were killed to estimate parasite burden. Data presented are a representative of two experiments with similar results.
Discussion
Understanding how antigen dose influences the development of Th1 and Th2 cells is important for designing vaccines and to date, experiments that have addressed this issue have had conflicting results. Several in vitro studies using TCR transgenic T cells show that low doses of antigen favor the generation of Th2 cells, whereas high doses favor Th1 cells (1–4). In contrast, the effect of antigen dose on the development of CD4+ Th cells in vivo is less clear. Although some studies indicate that low and high antigen doses favor Th1 and Th2 cell development, respectively (6, 7, 14, 15), others show the reverse (5, 8). In this study, we provide an explanation for these conflicting results. We find that low doses of L. major parasites promote the expansion of Th2 cells, but that due to the concomitant production of IFN-γ by CD8+ T cells, the Th2 response is transient. Thus, in the absence of CD8+ T cells, or in cases where CD8+ T cells are not stimulated, low doses of antigen might promote a Th2 response, not only in vitro, but also in vivo. Our observation that low dose infection induces a Th2 response is also consistent with several reports that show that the development of allergic airway disease in the murine model of atopic asthma is associated with low dose allergen sensitization resulting in high levels of IL-4 and IgE production, and can be blocked by high dose antigen sensitization (25–27).
A role for CD8+ T cells in resistance to leishmaniasis has been described, but not linked with the effects of parasite dose on Th cell development. For example, CD8+ T cells contribute to optimal resistance in a secondary infection (11, 28), to vaccine-induced immunity (22–24), and more recently were reported to be required for resistance to a primary infection (13). The function of CD8+ T cells in these systems could involve cytotoxicity because Leishmania-specific cytotoxic T cells capable of directly lysing infected macrophages have recently been cloned from mice infected or vaccinated with Leishmania amazonensis (29, 30). However, in our studies it is more likely that the ability of CD8+ T cells to make IFN-γ contributes to the resistance observed because CD8+ T cells from IFN-γ–deficient mice were unable to promote healing. CD8+ T cell–derived IFN-γ might be important for activating macrophages to kill L. major, particularly because CD8+ T cells accumulate in lesions of mice infected with L. major (13). However, we would argue that the critical function for CD8+ T cell–derived IFN-γ is to modulate the Th2 response because blockade of Th2 responses in CD8-deficient mice by other means promoted resistance in low dose–infected mice. Similarly, the role of CD8+ T cells in vaccine-induced immunity has been shown to involve IFN-γ production (23). These results are consistent with findings where it was found that CD8+ T cells switched an immune response from a humoral to a cell-mediated mode (31), contributed to maintaining a CD4+ Th1 response in an allograft-rejection model (32), inhibited IL-4 production by CD4+ T cells (33), and in viral infections promoted CD4+ Th1 cell development in an IFN-γ–dependent manner (34, 35). Taken together, the data strongly favor a role for CD8+ T cell–derived IFN-γ in promoting a Th1 response and resistance after low dose infection.
CD8+ T cell–derived IFN-γ could influence Th1 cell development indirectly via enhancement of IL-12 production by DCs (36) or through the production of proinflammatory chemokines (37, 38). Another effect of CD8+ T cell–derived IFN-γ in augmenting the Th1 response could involve increased STAT-1 activation in CD4+ T cells, leading to increased T-bet, IFN-γ, and IL-12 receptor expression. (39, 40). Consistent with this role for IFN-γ, were findings in an immunization model suggesting that CD8+-derived IFN-γ was important in maintaining IL-12 receptor expression (23). We favor this role for IFN-γ because increased IL-12 signaling would not only enhance the IFN-γ response, but might lead to the preferential expansion of Th1 cells in response to IL-12, contributing to the shift in the Th cell response from a Th2 to a Th1 response over time.
An important experiment showing the influence of antigen dose on CD4+ Th cell development in an infectious disease model came from the work of Bretscher et al. (14), who found that low dose L. major infection induces Th1 cell development and resistance in the highly susceptible BALB/c mouse. We were unable to investigate the role of CD8+ T cells in low dose healing in BALB/c mice because in our laboratory, and some others (41, 42), BALB/c mice infected with low doses of L. major failed to heal (unpublished data). The use of different L. major strains may account for these divergent results (43, 44). Many factors contribute to the development of Th cell subsets in leishmaniasis (9, 10), and we would argue that the influence of dose is subordinate to one of these factors in BALB/c mice. Nevertheless, although we were unable to directly test the role of CD8+ T cells in the resistance seen to low dose infection, other studies support the idea that CD8+ T cells are important to resistance in BALB/c mice. Thus, the ability of BALB/c mice to develop a Th1 response and resistance to L. major after vaccination with LACK DNA, which arguably would constitute a low dose of antigen, is completely dependent upon CD8+ T cells (22, 23).
The requirement for CD8+ T cells to resolve a low dose infection, as well as other reports that showed that immunity after vaccination with LACK DNA (22, 23) or heat-killed L. major in CpG adjuvant (24) depends on CD8+ T cells, has implications for vaccination against leishmaniasis. Our data indicates that one way CD8+ T cells promote resistance is by blocking the development of a CD4+ Th2 response. Whether this is the case in vaccine models is unclear, but studies using CpG as an adjuvant indicate that IFN-γ production from CD8+ T cells is required. Interestingly, the requirement for CD8+ T cells was observed both at the vaccination stage and the challenge stage (23). In contrast, we found that if immunity was induced by a high dose infection, CD8+ T cells were not required at the time of challenge. This may indicate that if a large CD4+ T cell response is generated, CD8+ T cells may not be required to maintain immunity. On the other hand, the durability of infection-induced resistance may differ from vaccine models because L. major persists after resolution of disease and therefore can contribute to the continuous activation of effector CD4+ T cells (45, 46). In any event, it is likely that optimal protection will depend on the activation of both CD4+ and CD8+ T cells, and our data elucidates one of the mechanisms by which CD8+ T cells contribute to immunity.
In summary, we have shown that low doses of L. major induce a transient Th2 response, which is modulated by CD8+ T cells over several weeks of infection. These findings may in part explain why under natural conditions, where sand flies inoculate low numbers of parasites, the development of immunity can take several weeks (18). More importantly, they indicate that the discrepancy between in vivo and in vitro findings on the role of antigen dose in Th cell development can be explained by whether CD8+ T cells are concomitantly activated. Antigen dose is an important component in Th cell development and our results provide additional insights into how dose might influence the efficacy of vaccines and immunotherapies.
Acknowledgments
We thank Drs. Jay Farrell and Chris Hunter for critically reading the manuscript and members of the Scott Lab for their insightful discussions.
This work was supported by National Institutes of Health grant number AI-35914 (to P. Scott).
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate succinimidyl ester; dLN, draining lymph node; SLA, soluble leishmanial antigen.
References
- 1.Constant, S., C. Pfeiffer, A. Woodard, T. Pasqualini, and K. Bottomly. 1995. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 182:1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constant, S.L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297–322. [DOI] [PubMed] [Google Scholar]
- 3.Hosken, N.A., K. Shibuya, A.W. Heath, K.M. Murphy, and A. O'Garra. 1995. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J. Exp. Med. 182:1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y.J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, K., J. van der Bosch, R. Fliegert, and S. Gehring. 2002. TSST-1 induces Th1 or Th2 differentiation in naive CD4+ T cells in a dose- and APC-dependent manner. Scand. J. Immunol. 56:572–579. [DOI] [PubMed] [Google Scholar]
- 6.Parish, C.R. 1971. Immune response to chemically modified flagellin. II. Evidence for a fundamental relationship between humoral and cell-mediated immunity. J. Exp. Med. 134:21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parish, C.R. 1972. The relationship between humoral and cell-mediated immunity. Transplant. Rev. 13:35–66. [DOI] [PubMed] [Google Scholar]
- 8.Guery, J.C., F. Galbiati, S. Smiroldo, and L. Adorini. 1996. Selective development of T helper (Th)2 cells induced by continuous administration of low dose soluble proteins to normal and beta(2)-microglobulin-deficient BALB/c mice. J. Exp. Med. 183:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845–858. [DOI] [PubMed] [Google Scholar]
- 10.Reiner, S.L., and R.M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151–177. [DOI] [PubMed] [Google Scholar]
- 11.Farrell, J.P., I. Muller, and J.A. Louis. 1989. A role for Lyt2+ T cells in resistance to cutaneous leishmaniasis in immunized mice. J. Immunol. 142:2052–2056. [PubMed] [Google Scholar]
- 12.Muller, I., P. Kropf, R.J. Etges, and J.A. Louis. 1993. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect. Immun. 61:3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkaid, Y., E. Von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M.C. Udey, and D. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992–4000. [DOI] [PubMed] [Google Scholar]
- 14.Bretscher, P.A., G. Wei, J.N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 257:539–542. [DOI] [PubMed] [Google Scholar]
- 15.Menon, J.N., and P.A. Bretscher. 1998. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur. J. Immunol. 28:4020–4028. [DOI] [PubMed] [Google Scholar]
- 16.Openshaw, P., E.E. Murphy, N.A. Hosken, V. Maino, K. Davis, K. Murphy, and A. O'Garra. 1995. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 182:1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann, T.R., and T.A.T. Fong. 1989. Specific assays for cytokine production by T cells. J. Immunol. Methods. 116:151–155. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D.L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, Z.E., S.L. Reiner, F. Hatam, F.P. Heinzel, J. Bouvier, C.W. Turck, and R.M. Locksley. 1993. Targeted activation of CD8 cells and infection of beta 2-microglobulin-deficient mice fail to confirm a primary protective role for CD8 cells in experimental leishmaniasis. J. Immunol. 151:2077–2086. [PubMed] [Google Scholar]
- 20.Erb, K., C. Blank, U. Ritter, H. Bluethmann, and H. Moll. 1996. Leishmania major infection in major histocompatibility complex class II-deficient mice: CD8+ T cells do not mediate a protective immune response. Immunobiology. 195:243–260. [DOI] [PubMed] [Google Scholar]
- 21.Huber, M., E. Timms, T.W. Mak, M. Rollinghoff, and M. Lohoff. 1998. Effective and long-lasting immunity against the parasite Leishmania major in CD8-deficient mice. Infect. Immun. 66:3968–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurunathan, S., C. Prussin, D.L. Sacks, and R.A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409–1415. [DOI] [PubMed] [Google Scholar]
- 23.Gurunathan, S., L. Stobie, C. Prussin, D.L. Sacks, N. Glaichenhaus, D.J. Fowell, R.M. Locksley, J.T. Chang, C.Y. Wu, and R.A. Seder. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915–924. [DOI] [PubMed] [Google Scholar]
- 24.Rhee, E.G., S. Mendez, J.A. Shah, C.Y. Wu, J.R. Kirman, T.N. Turon, D.F. Davey, H. Davis, D.M. Klinman, R.N. Coler, et al. 2002. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Secrist, H., R.H. DeKruyff, and D.T. Umetsu. 1995. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J. Exp. Med. 181:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogan, S.P., P.S. Foster, B. Charlton, and R.M. Slattery. 1998. Prevention of Th2-mediated murine allergic airways disease by soluble antigen administration in the neonate. Proc. Natl. Acad. Sci. USA. 95:2441–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai, K., A. Yokoyama, N. Kohno, and K. Hiwada. 1999. Effect of different sensitizing doses of antigen in a murine model of atopic asthma. Clin. Exp. Immunol. 118:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller, I., P. Kropf, J.A. Louis, and G. Milon. 1994. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect. Immun. 62:2575–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kima, P.E., N.H. Ruddle, and D. McMahon-Pratt. 1997. Presentation via the class I pathway by Leishmania amazonensis-infected macrophages of an endogenous leishmanial antigen to CD8+ T cells. J. Immunol. 159:1828–1834. [PubMed] [Google Scholar]
- 30.Colmenares, M., P.E. Kima, E. Samoff, L. Soong, and D. McMahon-Pratt. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun. 71:3172–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuttosi, S., and P.A. Bretscher. 1992. Antigen-specific CD8+ T cells switch the immune response induced by antigen from an IgG to a cell-mediated mode. J. Immunol. 148:397–403. [PubMed] [Google Scholar]
- 32.Chan, S.Y., L.A. DeBruyne, R.E. Goodman, E.J. Eichwald, and D.K. Bishop. 1995. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 59:1155–1161. [PubMed] [Google Scholar]
- 33.Holmes, B.J., P.A. MacAry, and D.M. Kemeny. 1997. Depletion of CD8+ T cells following primary immunization with ovalbumin results in a high and persistent IgE response. Int. Arch. Allergy Immunol. 113:160–162. [DOI] [PubMed] [Google Scholar]
- 34.Dittmer, U., B. Race, K.E. Peterson, I.M. Stromnes, R.J. Messer, and K.J. Hasenkrug. 2002. Essential roles for CD8+ T cells and gamma interferon in protection of mice against retrovirus-induced immunosuppression. J. Virol. 76:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson, K.E., I. Stromnes, R. Messer, K. Hasenkrug, and B. Chesebro. 2002. Novel role of CD8(+) T cells and major histocompatibility complex class I genes in the generation of protective CD4(+) Th1 responses during retrovirus infection in mice. J. Virol. 76:7942–7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas, M.J., A. Noble, E. Sawicka, P.W. Askenase, and D.M. Kemeny. 2002. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J. Immunol. 168:216–223. [DOI] [PubMed] [Google Scholar]
- 37.Kim, J.J., L.K. Nottingham, J.I. Sin, A. Tsai, L. Morrison, J. Oh, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, et al. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Invest. 102:1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook, D.N., O. Smithies, R.M. Strieter, J.A. Frelinger, and J.S. Serody. 1999. CD8+ T cells are a biologically relevant source of macrophage inflammatory protein-1 alpha in vivo. J. Immunol. 162:5423–5428. [PubMed] [Google Scholar]
- 39.Mullen, A.C., F.A. High, A.S. Hutchins, H.W. Lee, A.V. Villarino, D.M. Livingston, A.L. Kung, N. Cereb, T.P. Yao, S.Y. Yang, et al. 2001. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 292:1907–1910. [DOI] [PubMed] [Google Scholar]
- 40.Lighvani, A.A., D.M. Frucht, D. Jankovic, H. Yamane, J. Aliberti, B.D. Hissong, B.V. Nguyen, M. Gadina, A. Sher, W.E. Paul, et al. 2001. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA. 98:15137–15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Compton, H.L., and J.P. Farrell. 2002. CD28 costimulation and parasite dose combine to influence the susceptibility of BALB/c mice to infection with Leishmania major. J. Immunol. 168:1302–1308. [DOI] [PubMed] [Google Scholar]
- 42.Courret, N., T. Lang, G. Milon, and J.C. Antoine. 2003. Intradermal inoculations of low doses of Leishmania major and Leishmania amazonensis metacyclic promastigotes induce different immunoparasitic processes and status of protection in BALB/c mice. Int. J. Parasitol. 33:1373–1383. [DOI] [PubMed] [Google Scholar]
- 43.Hondowicz, B., and P. Scott. 1999. Influence of host and parasite factors on the innate immune response and Th2 stability following infection with Leishmania major. Microbes Infect. 1:65–71. [DOI] [PubMed] [Google Scholar]
- 44.Kopf, M., F. Brombacher, G. Kohler, G. Kienzle, K.H. Widmann, K. Lefrang, C. Humborg, B. Ledermann, and W. Solbach. 1996. IL-4–deficient BALB/c mice resist infection with Leishmania major. J. Exp. Med. 184:1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uzonna, J.E., G. Wei, D. Yurkowski, and P. Bretscher. 2001. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J. Immunol. 167:6967–6974. [DOI] [PubMed] [Google Scholar]
- 46.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]