Abstract
It has been demonstrated that vascular endothelial cell growth factor (VEGF) induction of angiogenesis requires activation of the nuclear factor of activated T cells (NFAT). We show that NFATc2 is also activated by basic fibroblast growth factor and blocked by the inhibitor of angiogenesis pigment epithelial–derived factor (PEDF). This suggests a pivotal role for this transcription factor as a convergence point between stimulatory and inhibitory signals in the regulation of angiogenesis.
We identified c-Jun NH2-terminal kinases (JNKs) as essential upstream regulators of NFAT activity in angiogenesis. We distinguished JNK-2 as responsible for NFATc2 cytoplasmic retention by PEDF and JNK-1 and JNK-2 as mediators of PEDF-driven NFAT nuclear export.
We identified a novel NFAT target, caspase-8 inhibitor cellular Fas-associated death domain–like interleukin 1β–converting enzyme inhibitory protein (c-FLIP), whose expression was coregulated by VEGF and PEDF. Chromatin immunoprecipitation showed VEGF-dependent increase of NFATc2 binding to the c-FLIP promoter in vivo, which was attenuated by PEDF. We propose that one possible mechanism of concerted angiogenesis regulation by activators and inhibitors may be modulation of the endothelial cell apoptosis via c-FLIP controlled by NFAT and its upstream regulator JNK.
Keywords: regulatory kinases, angiogenesis inhibitors, signaling cross-talk, transcription factors, pigment epithelial–derived factor
Introduction
Angiogenesis is regulated by the balance of extracellular inducers and inhibitors (1). On the intracellular level, the effects of pro- and antiangiogenic factors translate into molecular events that lead to endothelial cell (EC) survival or apoptosis (2). Situations in which the EC environment is populated exclusively by inducers or by antiangiogenic molecules are extremely rare. Thus, the cells are forced to resolve the balance between prosurvival and proapoptotic events, and the outcome determines whether existing vasculature will expand, remain the same, or regress (3). However, recent discoveries indicate that simple balance does not adequately reflect the course of events, and that the activated state of the endothelium due to angiogenic stimuli is the necessary prerequisite for the inhibitors to exert their activity (4, 5). Therefore, pro- and antiangiogenic signals are likely to engage in cross-talk with interdependent outcome.
We have recently uncovered one point where the stimulatory and inhibitory pathways overlap. Several angiogenic stimuli cause elevated surface levels of CD95/Fas, a death receptor, on the activated endothelium, whereas the pigment epithelial–derived factor (PEDF), a potent antiangiogenic factor, increases endothelial FasL. When simultaneous, these two molecular events initiate apoptosis; and, thus, the cessation of angiogenesis (5). Here, we investigated molecules other than CD95 involved in the cross-talk between angiogenic stimuli, vascular endothelial cell growth factor (VEGF), or basic fibroblast growth factor (bFGF), and inhibitory PEDF with an emphasis on the stress-activated c-Jun NH2-terminal kinases (JNKs) and NFAT, and their contribution to the outcome of angiogenic switch, the balance between EC survival and apoptosis.
JNK-1 contributes to opposing biological functions, cell proliferation, or cell death depending on the activation kinetics. Short and transient activation is usually associated with cell death, whereas long, sustained activation leads to proliferation (6, 7). In the ECs subjected to stress by serum deprivation or by ceramide, VEGF inhibits rapid and transient JNK induction typical for apoptosis (8).
Signals generated during apoptosis alter the activity of transcription factors via distinct transduction pathways. Several trans-acting, DNA-binding proteins such as members of Jun and NF-κB families are necessary for apoptosis (7, 9), whereas NFAT family members are implicated in survival (10–12). The NFAT family of transcription factors encompasses five proteins related to the Rel/NF-κB family (13–15). Cytosolic NFAT proteins are dephosphorylated upon stimulation and shuttled to the nucleus to become engaged in transcription (16, 17). This pathway is regulated by the extracellular signals using NFAT kinases (18), some of which are constitutively active, such as casein kinase 1 and glycogen synthase kinase-3, and maintain the inactive, phosphorylated NFAT state in resting cells (19, 20). The others are inducible, such as p38 and JNK. Both inducible and constitutive kinases rephosphorylate activated NFAT and cause its nuclear export (18, 21, 22).
NFAT is expressed in numerous cell types and contributes to diverse functions (23, 24). Importantly, NFATc2 activation was identified as a critical component of VEGF-induced angiogenesis and linked to the induction of cyclooxygenase-2 (25), which is also a critical player in angiogenesis (26, 27).
Our work identified NFATc2 as a crossing point for the endothelial cell survival pathways initiated by proangiogenic VEGF and bFGF and the inhibitory, proapoptotic factor PEDF. We showed that PEDF activated JNK kinases in vascular ECs. PEDF-dependent JNK activation restored NFATc2 phosphorylation and localization to the cytoplasm in ECs activated by VEGF and bFGF. Neither JNK activation nor NFAT activation blockade could be detected in nonstimulated, resting ECs. JNK activation was crucial for PEDF inhibitory activity because SP600125, a generic inhibitor of JNK kinases, reversed PEDF ability to induce EC apoptosis and to block chemotaxis, and curtailed its antiangiogenic activity in vivo. Finer dissection of NFATc2–JNK interactions induced by PEDF in the activated endothelium showed that NFATc2 retention in the cytoplasm was solely due to JNK-2, whereas NFAT nuclear export was driven by both JNK-1 and -2.
We identified a novel NFAT transcriptional target, cellular FLICE inhibitory protein (cellular Fas-associated death domain–like interleukin 1β–converting enzyme inhibitory protein [c-FLIP]), an endogenous dominant negative version of caspase-8, that mediates resistance to apoptotic signaling (28). JNK activation by PEDF lead to decrease in endothelial c-FLIP message and NFATc2 binding to c-FLIP endogenous promoter.
In summary, our findings showed that apoptosis and angiogenesis blockade by PEDF occurred in stimulated and not in quiescent ECs and required phosphorylation of NFATc2 by JNK-2 followed by sequestration to the cytoplasm, which precludes transcription of the target genes that, like c-FLIP, are critical for the EC survival and angiogenesis. This is the first mechanistic demonstration of the overlap between the signaling pathways generated by angiogenesis inhibitors and stimuli.
Materials and Methods
Cell Culture, Antibodies, and Reagents.
Human umbilical vein ECs (HUVECs; National Cancer Institute) and human microvascular ECs (Clonetics Cell Systems) were grown to 90% confluence on gelatinized surface in MCDB131 medium (Sigma-Aldrich) with growth supplements (bullet kit; BioWhittaker) in 5% CO2 at 37°C and used at passages 5–8.
We used rabbit polyclonal NFATc2 pAb 672 (J.M. Redondo, Universidad Autonoma de Madrid, Madrid, Spain) for immunoblots and NFATc2 mouse mAb G1-D10 (Santa Cruz Biotechnology, Inc.) for immunostaining. Mouse anti–c-FLIP mAb G-11 and goat antiactin pAb I-19 were obtained from Santa Cruz Biotechnology, Inc. Phospho-specific antibodies for the catalytic core peptides of JNK or p38 were obtained from Promega. Rabbit phospho-Erk1/2 pAb were obtained from Biosource International. A mouse JNK1 mAb (BD Biosciences), rabbit anti-JNK/SAPK1 pAb (Upstate Biotechnology), anti-Erk1/2 (Promega), and anti-p38 (Santa Cruz Biotechnology, Inc.) were used to ensure equal loading. For immunoprecipitation, we used rabbit phosphoserine pAb (Zymed Laboratories) and protein A/G agarose (Santa Cruz Biotechnology, Inc.). Antibodies for GM130 Golgi matrix protein were obtained from Transduction Labs. Fluorescent-labeled secondary antibodies were obtained from Jackson ImmunoResearch Laboratories.
VEGF and bFGF were obtained from R&D Systems. His-tagged recombinant human PEDF was isolated from the medium conditioned by human embryonic kidney cells (29), and its activity was verified in the in vitro EC migration assay. GST-NFATc2 (1-418) cloned into pGEX2T vector was purified as described previously (30). JNK inhibitor SP600125 (1,9-pyrazoloanthrone) was obtained from Calbiochem. All other reagents were obtained from Sigma-Aldrich.
Western Blotting.
Cells were lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, and 0.5% sodium deoxycholate with protease and phosphatase inhibitors, and extracts were incubated for 20 min on ice and cleared by centrifugation. The samples were resolved by SDS-PAGE (30 μg/lane) and transferred onto nitrocellulose membranes. Membranes were blocked in Tris-buffered saline, pH 7.4, 0.1% Tween-20 with 5% blotto; incubated overnight at 4°C with primary antibodies NFATc2 (1:3,000 dilution) and c-FLIP (1:1,000 dilution) in blocking buffer; washed with Tris-buffered saline, pH 7.4, 0.1% Tween-20; and incubated with horseradish peroxidase secondary antibodies. The bands were visualized with Lumi-GLO chemiluminescent substrate (KPL). Erk1/2, JNK, and p38 were detected as recommended by Promega.
Subcellular fractionation was performed as described previously (31). In brief, 1.5 × 106 ECs washed with cold PBS were resuspended in 10 mM Hepes, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and protease/phosphatase inhibitors (200 μl/sample) and incubated for 10 min on ice. Nuclei were pelleted, resuspended in 20 mM Hepes, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT, 25% glycerol, and protease/phosphatase inhibitors (50 μl/sample), and incubated on ice for 20 min; extracts were cleared of debris. Equal cell number equivalents of cytosolic and nuclear fractions were used.
Immunoprecipitation.
Cell extracts precleared with protein A/G agarose beads (1 h) were incubated overnight at 4°C with JNK-1 or JNK-2 antibodies, and complexes were washed three times with lysis buffer and resolved by gradient SDS-PAGE. Western blot was probed with NFATc2 antibody.
Substrate Phosphorylation.
To measure kinase activity, cells were lysed in 20 mM Hepes, pH 7.5, 10 mM EGTA, 1% NP-40, 2.5 mM MgCl2, 40 mM β-glycerophosphate, 1 mM DTT, 2 mM Na3VO4, and protease inhibitors. Cells were solubilized on ice for 60 min, and extracts were cleared by centrifugation and incubated with JNK1 antibodies (Santa Cruz Biotechnology, Inc.) and protein A/G agarose beads (4 h, 4°C). JNK complexes recovered by centrifugation were washed with lysis buffer and reconstituted in reaction buffer (20 mM Hepes, pH 7.5, 20 mM MgCl2, 20 mM β-glycerophosphate, and 2 mM DTT). Reaction was held for 30 min at 30°C in 40 μl of final mix (30 μl JNK immunocomplexes, 250 μM of cold ATP, 10 μCi of γ-[32P] ATP, and 1 μg GST-NFATc2). The samples were run on 10% SDS-PAGE, and the gels were dried and analyzed by autoradiography.
Immunofluorescence Analysis.
The cells grown on gelatinized coverslips in 24-well plates were fixed and permeabilized in 3.7% paraformaldehyde, 0.2% Triton X-100 in PBS (10 min, room temperature); blocked in 1% donkey serum (30 min, room temperature); and incubated with NFATc2 mAb (1 μg/ml, 4°C overnight). The coverslips were incubated with Cy2-labeled donkey anti–mouse antibodies (30 min, room temperature), washed and mounted in DPX medium. The images were acquired by laser-scanning confocal microscopy, with a 100× objective, 488 nm excitation, and 510 nm emission.
RT-PCR.
Total RNA was extracted from ECs with TRIzol reagent (Life Technologies). 2 μg/sample was converted into the cDNA template using a kit (Amersham Biosciences) and analyzed by semi-quantitative PCR. The amplification mixture (25 μl final volume) contained 1 × Taq polymerase buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, 0.5 μM of each primer, and 2.5 U Taq (Life Technologies). cDNA was equalized in 18-22 cycle amplification reaction (1:1,000 template dilution) with β-actin primers 5′-TGTTGGCGTACAGGTCTTTGC-3′ (forward) and 5′-GCTACGAGCTGCCTGACGG-3′ (reverse), yielding a 182-bp product. For c-FLIP, the following primers were used: 5′-GATGTCTGCTGAAGTCATCCATCA-3′ (forward) and 5′-CACTACGCCCAGCCTTTTGG-3′ (reverse), yielding a 1468-bp product. The number of cycles with c-FLIP primers (denaturation, 30 s, 60°C; annealing, 30 s; and polymerization, 60 s) was chosen for the product amount to be in the linear range with the template.
Apoptosis Assay.
Cells plated on gelatinized coverslips in 24-well plates (5 × 104 cells/well) were fixed by adding buffered paraformaldehyde to the culture medium to a final concentration of 1%. Apoptotic cells were detected using TdT-mediated dUTP nick-end labeling (TUNEL)–based ApopTag kit (Serological Corp.) following the manufacturer's instructions and counterstained with 50 μg/ml propidium iodide. Quantitative analysis was performed with MetaMorph software, and the percentage of TUNEL positive cells was calculated in two to six randomly selected fields of the two different chambers (600–1,200 cells/condition).
Electrophoresis Mobility Shift Assay (EMSA).
Nuclear extracts were prepared as described previously (32). 1.5 × 106 HUVECs washed in PBS were lysed in ice-cold hypotonic buffer (10 mM NaCl). Released nuclei were incubated for 30 min on a rocking platform in hypertonic buffer (400 mM NaCl), centrifuged for 10 min at 15,000 g, and extracts were immediately frozen and stored at −80°C. 2 μg nuclear proteins were incubated for 10 min on ice with 1 μg poly dI-dC in 4 μl 5× DNA-binding buffer (10% polyvinylethanol, 12.5% glycerol, 50 mM Tris, pH 8.0, 2.5 mM EDTA, and 2.5 mM DTT). 32P-labeled double-stranded oligonucleotide (5 × 107–108 cpm/μg) was added to a final concentration 1 ng/μl, and the reaction was continued for 30 min. For the competition, labeled probes were added 30 min after the 10-fold molar excess unlabeled oligonucleotide. For supershifts, preimmune serum or NFATc2 pAb 672 were added. DNA–protein complexes were resolved on 4% nondenaturing PAAG. The following probes were used: SP1, 5′-ATTCGATCGGGGCGGGGCGAGC-3′ (33); and NFAT, 5′-ACGCCCAAAGAGGAAAATTTGTTTCATACA-3′ (34).
Chromatin Immunoprecipitation (ChIP) Assay.
The ChIP assay was performed with the kit obtained from Upstate Biotechnology using the manufacturer's protocol with minor adjustments. HUVECs were grown to confluence, and formaldehyde was added directly to culture medium to a final concentration of 1% (20 min, 37°C). The cells were washed at 4°C in PBS, lysed for 10 min in 1% SDS, 10 mM Tris HCl, pH 8.0. The lysates were sonicated (10 s, three times; Branson Sonifier 450), and the debris was removed by centrifugation. Sonication was optimized to produce average DNA fragments of 1 kb. Aliquots were taken to control DNA input, the remainder diluted 10 times in 0.01% SDS, 1% Triton X-100, 1 mM EDTA, 10 mM Tris HCl, pH 8.0, and 150 mM NaCl, phosphatase/protease inhibitors, and incubated overnight (4°C) with NFATc2 pAb 672 or control rabbit IgG (Santa Cruz Biotechnology, Inc.). DNA–protein complexes were isolated on salmon sperm DNA linked to protein A agarose beads and extracted with 1% SDS, 0.1 M NaHCO3. Cross-linking was reversed at 65°C for 5 h, and proteins were removed with proteinase K and extracted with phenol/chloroform DNA redissolved and PCR-amplified with c-FLIP promoter primers, 5′-TCACGTTTGCTATGACTCCCAGAC-3′ (forward); and 5′-TCCACGCGTTAGGAGTAAACACTG-3′ (reverse), product length of 382 bp.
Corneal Neovascularization Assay.
The assay was performed as described previously (35). Hydron-sucralfate pellets (∼1 μl) were implanted into the cornea of anesthetized C57/Bl6 mice (Jackson ImmunoResearch Laboratories) 0.5–1.0 mm from the limbus. Pellets contained 50 ng bFGF, 200 ng PEDF, and 1 μM JNK inhibitor where indicated. On day 6, the eyes were scored and photographed, and capillaries growing from the limbus and reaching the pellet were regarded as a positive response.
Statistical Analysis.
Quantitative results are presented as mean values ± SD. Differences were analyzed by paired Student's t test and p-values <0.05 were accepted as statistically significant.
Results
Angiogenesis Inducers Activate NFATc2 and Inhibitory PEDF Cause NFATc2 Phosphorylation/Deactivation.
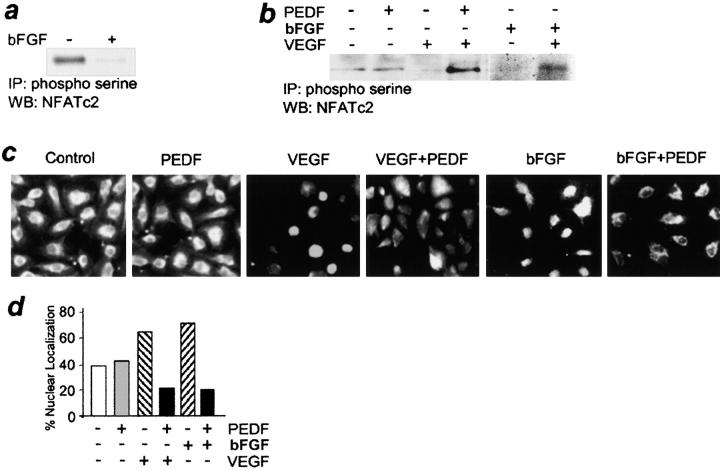
The activation and subcellular distribution of NFAT family members is determined by phosphorylation (36–38); NFAT dephosphorylation/activation was shown previously to mediate VEGF-induced angiogenesis (25). In addition to reproducing results by Hernandez et al. (Fig. 1, b and c; reference 25), we found that another inducer, bFGF, also triggered NFATc2 dephosphorylation and nuclear transfer (Fig. 1, a–d).
Figure 1.
NFAT deactivation by PEDF. (a) NFAT dephosphorylation by bFGF. Cell extracts of bFGF-induced human microvascular ECs (5 ng/ml, 15 min) were precipitated with phosphoserine antibody and analyzed by immunoblotting with NFATc2 antibody. Note the decreased NFATc2 phosphorylation in the presence of bFGF. (b) PEDF restored NFATc2 phosphorylation in activated ECs. VEGF- or bFGF-stimulated ECs were treated with PEDF (10 nM, 15 min). Note the decrease in phospho-NFATc2 by angiogenic stimuli and higher phosphorylation levels in the activated cells exposed to inhibitory PEDF. (c and d) Inhibition of NFATc2 nuclear localization by PEDF. HUVECs grown on gelatinized coverslips were treated with 200 pg/ml VEGF or 5 ng/ml bFGF and 10 nM PEDF and stained for NFATc2. Note the predominance of the cells with nuclear NFATc2 in the presence of bFGF or VEGF compared with untreated control and cytoplasmic NFATc2 localization in the presence of PEDF. The data were quantified using MetaView software package (d).
To determine if inhibitory PEDF influences NFAT activation dynamics, we analyzed NFATc2 phosphorylation by immunoprecipitation with antibodies for phosphoserine and Western blotting with NFATc2 antibody (Fig. 1 b). Subcellular localization of NFATc2 was examined by indirect immunofluorescence (Fig. 1, c and d). We found inactive NFATc2 in resting ECs, as was reflected by high phosphorylation levels, and cytoplasmic localization in the majority of the cells. The levels of phosphorylated NFATc2 dropped 4–7-fold upon VEGF stimulation and 8–20-fold with bFGF (Fig. 1, a and b). In VEGF-stimulated ECs, PEDF increased NFATc2 phosphorylation >20-fold compared with VEGF alone and ∼10-fold compared with untreated control, but failed to affect NFATc2 activation in nonstimulated cells (Fig. 1 b). In timed experiments, NFATc2 phosphorylation due to PEDF reached maximum as early as 15 min, persisted for 1 h, and subsided by 2 h of treatment (unpublished data).
Phosphorylation changes were consistent with NFATc2 redistribution; upon stimulation, the percentage of ECs with cytoplasmic NFAT localization decreased significantly, and conversely, nuclear localization increased. PEDF treatment restored NFATc2 localization to the cytoplasm in the majority of stimulated cells (Fig. 1, c and d).
NFAT Blockade by PEDF Required JNK, But Not Erk-1/2 or p38 Mitogen-activated Protein Kinases (MAPKs).
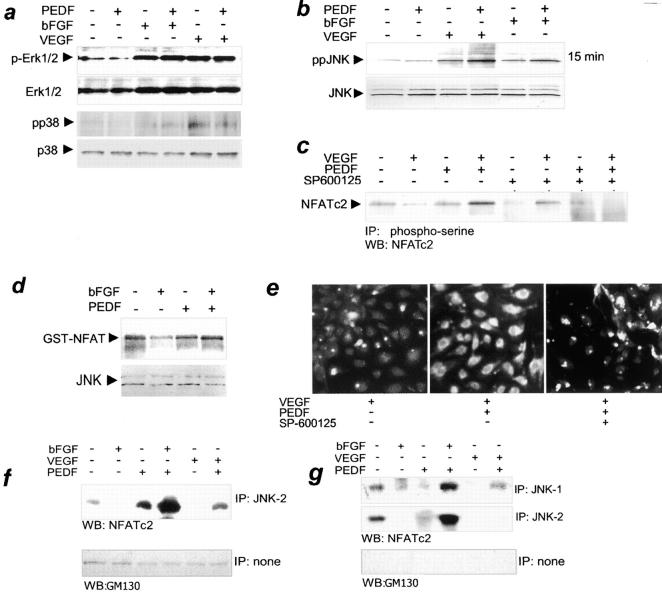
To further elucidate mechanisms of NFAT regulation by PEDF, we examined MAP kinases, a common denominator in the mitogenesis by multiple angiogenic stimuli (39, 40) as possible upstream modifiers of NFAT activity (18). Western blots of the cell extracts that probed for active phosphorylated Erk-1/2 active phosphorylated p38 showed no significant changes in their activation state due to PEDF (Fig. 2 a).
Figure 2.
The role of JNK kinases in NFATc2 deactivation by PEDF. (a) PEDF failed to activate p38MAPK or Erk1/2 kinases. Confluent HUVECs were stimulated with 200 pg/ml VEGF or 5 ng/ml bFGF. 10 nM PEDF was added where indicated. Cell extracts were analyzed by Western blotting with antibodies for active phosphorylated forms of Erk1/2 (p-Erk) and for dually phosphorylated p38 (pp38). The blots were reprobed for total Erk and p38 to ensure equal loading. The representative result of three independent experiments is shown. (b) PEDF enhanced JNK activation in stimulated ECs. HUVECs were treated as indicated with VEGF or bFGF + PEDF for 15 min. Cell extracts were resolved by SDS-PAGE and analyzed by Western blotting with antibodies for active, dually phosphorylated JNK (top, ppJNK) or total JNK (bottom) as a loading control. Three independent experiments were performed with similar results. (c) PEDF-dependent NFATc2 phosphorylation required JNK kinases. VEGF-stimulated HUVECs were treated with PEDF alone or in combination with 100 nM of SP600125, a generic JNK inhibitor. Cell lysates were immunoprecipitated with phosphoserine antibody and analyzed by Western blotting with NFATc2 antibody. Note the increase in NFATc2 phosphorylation by PEDF in stimulated ECs and its attenuation in the presence of JNK inhibitor. (d) Increased JNK activity in PEDF-treated cells. HUVECs were treated with bFGF (15 min, 5 ng/ml) and/or PEDF, and JNK activity was assessed by immunocomplex kinase assay with exogenous recombinant GST-NFATc2 substrate. Note the decrease in JNK activity in bFGF-treated ECs and the capacity for NFATc2 phosphorylation in cells treated with bFGF + PEDF. Western blot with JNK antibodies was performed to ensure equal loading. The result is representative of three independent experiments. (e) NFATc2 redistribution by PEDF was JNK dependent. HUVECs were plated on gelatinized coverslips; treated with indicated combinations of VEGF, PEDF, and JNK inhibitor SP600125; and stained for NFATc2. JNK blockade caused persistent NFATc2 nuclear localization (active state) despite PEDF treatment. (f and g) PEDF caused physical interaction between JNK and NFATc2. Quiescent or activated HUVECs (induced with VEGF or bFGF, as indicated) were treated for 15 min with PEDF. Nuclear or cytosolic extracts were precipitated with JNK-1– or JNK-2–specific antibody and analyzed by Western blotting with NFATc2 pAb. Purity of the fractions was determined by blotting with antibodies against GM130 protein. (f) PEDF increased interaction between JNK-2 and NFATc2 in the cytosol of stimulated ECs. (g) NFATc2 interacted with both JNK-1 and JNK-2 in the nuclei; note the increased complex formation by PEDF in stimulated but not in quiescent ECs.
In contrast, PEDF changed JNK activity. Although there was no effect due to PEDF in resting ECs, in stimulated cells, it strongly augmented the increase in JNK activity caused by VEGF or bFGF alone (Fig. 2 b). Western blotting with the antibodies against active, dually phosphorylated JNK showed activation of the two JNK isoforms likely corresponding to the JNK-1 and JNK-2 (Fig. 2 b). After 15 min of stimulation with VEGF or bFGF, JNK activity was increased two- to fourfold (Fig. 2 b, top). PEDF had no effect on JNK phosphorylation in nonstimulated ECs, but augmented JNK activation by angiogenic stimuli (Fig. 2 b, top). Both JNK activation by VEGF and its PEDF-dependent increase persisted for 1 h (unpublished data). Consistent with JNK activation, the level of phospho–c-Jun in activated HUVECs underwent marked increase after PEDF treatment (unpublished data).
In vitro, JNK immunocomplexes from the extracts of resting ECs were able to phosphorylate exogenous GST-NFATc2 substrate. When isolated from bFGF-stimulated cells, similar complexes showed lower GST-NFATc2 phosphorylating activity. PEDF alone had no effect on the basal NFAT phosphorylation ability, but completely restored NFAT phosphorylation by JNK complexes in bFGF-induced cells (Fig. 2 d).
PEDF-dependent increase in the NFATc2 phosphorylation and cytoplasmic retention in the activated ECs were driven by JNK kinases because both were blocked by SP600125, an inhibitor of multiple JNK isoforms (Fig. 2, c and e). SB203580, a p38 inhibitor had no effect (unpublished data).
JNK-2–mediated NFATc2 Cytoplasmic Retention, JNK-1 and JNK-2 Contributed To Nuclear Export.
SP600125 inhibits all JNK isoforms; thus, its ability to oppose PEDF effects on NFAT phosphorylation and subcellular localization may be attributed to any of the JNK species. Western blots probed with phospho-specific JNK antibody or with total JNK antibody showed protein bands with molecular mass consistent with JNK-1 or -2, but not JNK-3 (Fig. 2 b).
To determine whether NFATc2 is a direct target for JNK kinases, we performed immunoprecipitation with antibodies, which discriminate between JNK-1 and JNK-2, using cytoplasmic or nuclear EC fractions. Resulting protein complexes were analyzed by Western blot with NFATc2 polyclonal antibodies. To confirm the purity of subcellular fractions, we reprobed the same blots with GM130 antibodies for Golgi matrix protein (Fig. 2, f and g).
We detected direct interaction of JNK-2 with NFATc2 in the cytoplasm. In resting ECs, JNK-2–NFATc2 complexes were present at low steady-state levels, which were further reduced by bFGF and VEGF. PEDF treatment caused a weak increase in JNK-2 bound NFATc2 in quiescent ECs; however, combined with VEGF or with bFGF, PEDF caused a >20-fold increase of NFATc2/JNK-2 association (Fig. 2 f). No detectable complexes between NFATc2 and JNK1 were observed in the cytoplasm (unpublished data).
In the nuclei, NFATc2 was bound at low levels to both JNK-1 and JNK-2 in resting ECs. This binding was decreased by both VEGF and bFGF (Fig. 2 g). PEDF dramatically increased JNK1–NFATc2 interaction in bFGF-stimulated cells and improved complex formation in VEGF-stimulated ones. Nuclear NFATc2–JNK-2 complexes were also increased in PEDF-dependent manner upon activation with bFGF (Fig. 2 g).
JNK Was Critical for Apoptosis and Antiangiogenesis by PEDF In Vitro and In Vivo.
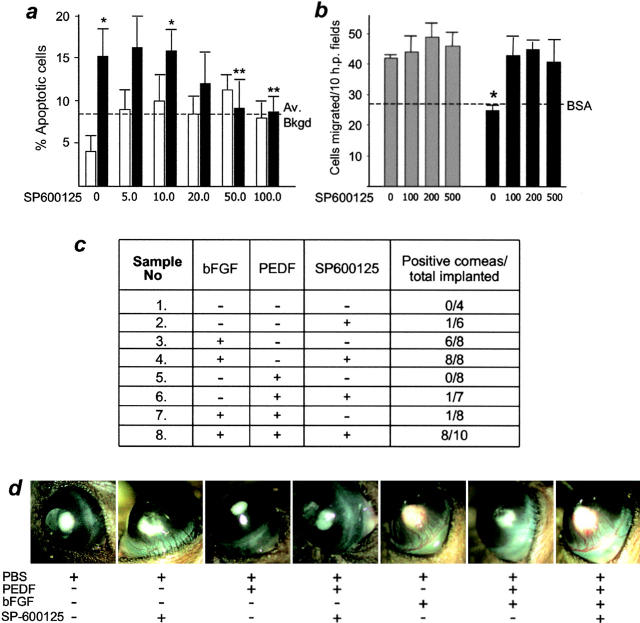
To test functional significance of JNK-dependent NFAT deactivation by PEDF, we examined the effect of SP600125 on the EC apoptosis. Increasing concentrations of SP600125 slightly increased background EC apoptosis in the presence of bFGF alone. However, in bFGF-stimulated cells, apoptosis by PEDF was decreased in a dose-dependent manner by the same inhibitor concentrations (Fig. 3 a).
Figure 3.
JNK inhibitor attenuated EC apoptosis by PEDF and interfered with its antiangiogenic activity. (a) PEDF-induced apoptosis was JNK dependent. HUVECs were treated with bFGF to maintain survival and with PEDF and/or JNK inhibitor SP600125 for 18 h. Cells positive for DNA fragmentation (apoptosis) were detected by in situ TUNEL assay and quantified using MetaView software. The data from two independent experiments are presented. (white bars) bFGF alone. (black bars) bFGF + PEDF. SEM are shown. *, Significantly different from background (P < 0.005). **, No significant difference from background (P < 0.25). (b) PEDF inhibited EC migration via JNK kinases. JNK inhibitor was used in the EC chemotaxis assay. Note that PEDF ability to block migration up the VEGF gradient was abolished by SP500125. (gray bars) VEGF alone. (black bars) VEGF + PEDF. *, Significant difference from VEGF-induced migration (P < 0.05). (c and d) JNK kinases were essential to PEDF antiangiogenic activity in vivo. Mice received corneal implants containing indicated combinations of bFGF, PEDF, and SP600125. Vascularization was examined by slit-lamp microscopy. Multiple capillaries reaching implants were scored as a positive response, and the data were presented as the number of positive corneas out of the total implanted (c). SP600125 was neutral alone and had no effect on bFGF-induced angiogenesis, but reversed PEDF inhibitory activity. Photographs of representative corneas are shown (d).
In vitro, increased EC chemotaxis up the gradient of angiogenic stimuli reflects in vivo angiogenic response (41). When tested in the migration assay, SP600125 had no effect on background random EC migration in serum-free medium, or on VEGF-induced migration. However, it drastically reduced the inhibitory effect of PEDF (Fig. 3 b).
In vivo, in corneal neovascularization assay mice developed an adequate angiogenic response to bFGF despite the presence of JNK inhibitor; however, SP600125 severely impaired the inhibitory activity of PEDF (Fig. 3, c and d). Combined, our results demonstrate that JNK kinases are crucial for PEDF antiangiogenic signaling.
PEDF Decreased NFAT DNA Binding Activity and Blocked c-FLIP Gene Transcription by NFAT in the Activated Endothelium.
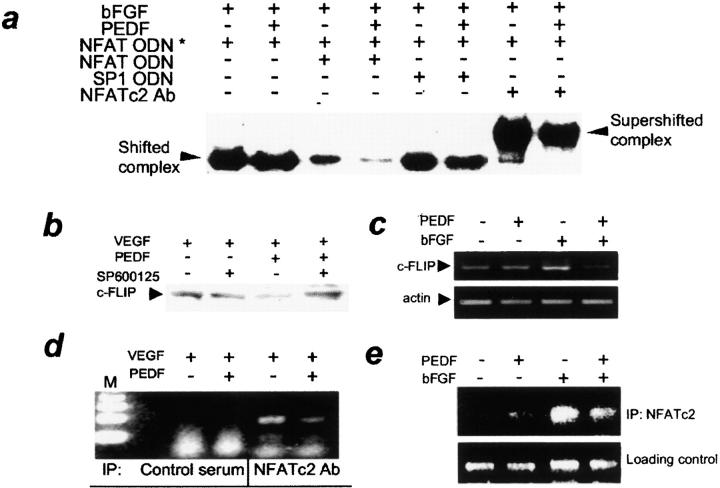
To evaluate PEDF effect on NFAT binding to the cognate DNA sequences, we used EMSA with the dual NFAT consensus binding site from the IL-2 promoter (36, 42). bFGF induced NFATc2 binding to a specific oligonucleotide, which was reduced in the presence of unlabeled specific oligonucleotide, but not in the presence of the nonspecific oligonucleotide containing a binding site for SP-1. NFAT antibody induced supershift in mobility of the DNA–NFATc2 complexes. Predictably, PEDF reversed increased NFAT DNA binding in activated ECs (Fig. 4 a).
Figure 4.
PEDF decreased NFAT binding to DNA consensus sequences and to c-FLIP promoter. (a) PEDF treatment lowered NFATc2 DNA-binding activity in stimulated ECs. HUVECs were treated for 1 h with indicated combinations of bFGF and PEDF, and nuclear extracts were examined by EMSA with NFAT consensus oligonucleotide (ODN). The same ODN (×10 excess, unlabeled) was used as a specific competitor and SP1 consensus ODN was used as a nonspecific competitor. Note the strong decrease in specific band intensity in the presence of PEDF, the supershift in the presence of NFATc2 antibody, and the decreased intensity of the supershifted band due to PEDF. (b–e) c-FLIP regulation by PEDF. VEGF- or bFGF-induced HUVECs were treated for 4 h (b and c) or 2 h (d and e) with PEDF alone or in combination with SP600125 and used for Western blotting for c-FLIP protein (b), for semi-quantitative RT-PCR for c-FLIP mRNA (c), or in chromatin ChIP assay (d and e). (b) Note the decreased c-FLIP protein due to PEDF, and the reversal by SP600125. (c) PEDF decreased c-FLIP mRNA in stimulated ECs. Three independent experiments were performed with similar results. (d and e) PEDF decreased NFAT binding to c-FLIP promoter in vivo. ChIP with NFATc2 antibody was performed on ECs activated with VEGF (d) or bFGF (e) treated with PEDF. Note the dramatic decrease in the PCR-amplified DNA fragment in the presence of PEDF.
We have shown previously that apoptosis due to angiogenesis inhibitors involves caspase-8 activation and down-regulation of c-FLIP, an endogenous caspase-8 inhibitor (5). We sought the NFATc2 role in the regulation of c-FLIP by PEDF. Decrease in c-FLIP protein in the activated ECs was detectable and reversible by the pretreatment with JNK inhibitor (Fig. 4 b). Using semi-quantitative RT-PCR, we observed significant down-regulation of c-FLIP message due to PEDF in bFGF-stimulated ECs (Fig. 4 c).
The 5′ flanking region of c-FLIP promoter contains three putative consensus NFAT sites at positions 370, 751, and 862. To determine whether c-FLIP down-regulation of PEDF occurred at the transcription initiation and to confirm the role of NFATc2, we performed ChIP assay of an ∼382 bp region of the c-FLIP promoter. ChIP revealed direct NFATc2 interaction with c-FLIP promoter chromatin of the activated ECs, which was strongly decreased by PEDF (Fig. 4, d and e).
Discussion
The growing body of evidence strongly supports the hypothesis where the balance between EC survival and apoptosis determines angiogenesis versus maintenance or regression of existing capillaries (2). Survival and apoptosis are themselves determined by the extracellular signals from angiogenesis inhibitors and stimuli (43, 44). Recent studies indicate that survival and apoptotic cascades occur simultaneously within a single cell with mutually dependent outcomes. In other words, cross-talk between the inhibitory and stimulatory signals is an integral part of angiogenic balance in which stimulated ECs become susceptible to apoptosis by inhibitors. The induction of CD95/Fas by angiogenic stimuli, to become available for ligation by the inhibitor-generated CD95L, is the first example of such interplay (5).
Here, we identified a convergence point between pro- and antiangiogenic signals. Previous studies by other labs and ours demonstrate NFATc2 requirement for the angiogenesis by VEGF (25) and possibly by insulin-like growth factor 1 (45). We added another angiogenic factor, bFGF, to this list, thereby stressing the central role of NFAT in vascular remodeling and the likelihood of its targeting by inhibitors. We showed that NFATc2 activation was disrupted by antiangiogenic PEDF exclusively in stimulated ECs, whereas basal NFATc2 phosphorylation in quiescent cells by the same inhibitors was completely unaffected. This observation indicates NFATc2 as a point of cross-regulation between inducing and inhibitory angiogenic signaling cascades.
Although NFATs are primarily involved in immune response (T cell activation), they are important in other cell types where they serve such diverse functions as survival, cell cycle progression, proliferation invasion, and transformation (10, 12, 46, 47) that could be important in angiogenic ECs. NFAT factors are involved in complex functions of cardiovascular system, pathological cardiac hypertrophy, and vascular development and patterning (48, 49). NFAT activation in immune and other tissues is calcineurin dependent with the exception of constitutively active NFAT5 (13, 23, 50), and is triggered by multiple signals including TCR ligation, CD28, and chemokine signaling. NFAT activation and nuclear localization can also be signaled via integrin α6β4 (46) and by growth factors, neurotrophic factors, and prostaglandins (45, 51–55).
Distinct NFAT family members are expressed in distinct tissues (13, 16, 23). Information regarding NFAT representation and function in the ECs is limited; NFATc2 is induced in ECs upon activation by VEGF; an unspecified NFAT family member is induced by oxidized phospholipids and insulin-like growth factor 1 (25, 32, 56, 57).
NFAT participation in angiogenesis is little explored; more attention has been given to its role in vasculogenesis (15, 48, 58). Even less is known of NFAT transcriptional targets involved in either vascular development or angiogenesis regulation with the exception of Cox-2, an important mediator of prostaglandin production and angiogenesis (25, 27, 59). Among known NFAT targets that potentially contribute to angiogenesis are secreted factors such as cytokines IL-2, IL-4, and GM-CSF (60, 61) and tissue factor (32). NFAT also affects cell cycle regulatory molecules, which if modulated in ECs may affect capillary growth. This includes NFAT induction of cyclin expression and down-regulation of p21 and p27 (negative cell cycle regulators; reference 17).
We discovered that c-FLIP, a previously unknown NFAT target, mediates its effects on angiogenesis. c-FLIP involvement in the EC survival and angiogenesis was described previously (5, 62, 63). The attenuation of FLIP down-regulation by an inhibitor of JNK, an NFAT regulatory kinase, provided a link between NFAT and FLIP, whereas ChIP yielded direct evidence of increased NFATc2 interaction with endogenous c-FLIP promoter in activated ECs. This interaction was dramatically reduced by antiangiogenic PEDF. Interestingly, PEDF treatment had no effect on FLIP levels in quiescent endothelium, thus underscoring the importance of the cross-talk between angiogenesis inhibitors and stimuli.
To explore upstream regulatory molecules used in NFATc2 regulation by PEDF, we screened known deactivating kinases that maintain NFAT cytoplasmic localization by phosphorylation of two or more of the multiple serine clusters. In vitro NFAT activation can be blocked by Erk1 and 2, p38 (21, 64), and several JNK family members (18, 22). In vivo, two types of NFAT deactivation are seen, constitutive and inducible. Constitutive NFAT phosphorylation is mediated via glycogen synthase kinase-3 (20), casein kinase 1 (19), and JNK-2 (65, 66), resulting in its nuclear export assisted by Crm1. Inducible deactivation in response to environmental signals may be mediated by JNK1, JNK2 (67), or by p38 (68).
We focused on MAP kinases as likely mediators of NFAT activity elicited by angiogenesis inhibitors. The MAPKs participate in essential signal transduction involved in cell growth, differentiation, apoptosis, and transformation (69). The outcome of JNK activation depends on duration of the signal where sustained long-term response is associated with proliferation and survival, whereas a short, transient response is linked to apoptosis and cell cycle arrest (70). Angiogenic stimuli caused moderate induction of Erk1/2, p38, and JNK. However, neither Erk1/2 nor p38 induction were affected by angiogenesis inhibitors. On the contrary, JNK activity was subject to PEDF regulation; we observed a dramatic increase in phospho-JNK levels and JNK-dependent substrate phosphorylation by PEDF exclusively in activated ECs. SP600125, a generic JNK inhibitor, reduced NFATc2 phosphorylation and restored nuclear localization in affected ECs. The same compound had no effect on VEGF or bFGF-dependent angiogenesis, but destroyed response to PEDF in vitro and in vivo, pointing to JNK kinases and their downstream targets as critical elements of PEDF angioinhibitory signaling.
Once activated, Jun kinases trigger biological responses by phosphorylating targets that, in turn, regulate transcriptional events or are directly involved in apoptosis. In vivo gene targeting in mouse models has revealed a critical JNK role in phosphorylation of AP-1, p53, c-Myc, Bcl-2, and NFAT (71). However, the contribution of distinct JNK family members is difficult to evaluate because crossbreeding of mice null for the individual JNK kinases leads to embryonic lethality, whereas biochemical inhibitors are relatively unspecific. Some studies point out distinctions between JNK-1 and JNK-2. Remarkably, NFAT deactivation is one of the points where JNK-1 and JNK-2 functions diverge; whereas both JNK-1 and -2 are important for NFATc2 nuclear export in cardiomyocytes (67), NFATc2 nuclear export in vascular smooth muscle cells is driven by JNK-2 (65, 66).
As JNK null mice were unavailable, we sought physical interactions between respective JNK kinases and NFATc2. We demonstrated direct physical interaction of endogenous NFATc2 with both JNK-1 and JNK-2. However, only nuclear NFATc2 was associated with JNK-1, whereas NFATc2–JNK-2 complexes were both nuclear and cytoplasmic. Thus, it is likely that JNK-1 is only involved in nuclear export, whereas JNK-2 participates in both nuclear export and cytoplasmic retention of NFATc2. Our results highlight the interplay between influences that regulate angiogenesis by promoting and opposing NFATc2 nuclear accumulation in ECs.
This is one of the first examples in which transcription factors were studied downstream of the angiogenesis effector. Rather, the attention has been focused on transcriptional regulation of the production of inhibitors and stimuli by the nonECs as means to manipulate angiogenic switch (72–74). The only transcription factors known to be regulated by the effectors of angiogenesis aside from NFAT are NF-κB and GATA (75, 76). Together, our data shed new light on the fundamental aspects of angiogenesis, provide the first attempt to uncover transcriptional events elicited by angiogenesis inhibitors, and point new directions in the exploration of angiogenesis regulation in its extreme complexity.
Acknowledgments
We appreciate technical expertise, reagents, and helpful discussion provided by M.A. Alfranca and J.M. Redondo of the Universidad Autonoma de Madrid.
This work was supported by the grants from the National Heart Lung and Blood Institute of the National Institutes of Health (RO1 HL 68033-04 to O. Volpert) and the American Cancer Society (RSG-01-099-01-CSM to O. Volpert).
Abbreviations used in this paper: bFGF, basic fibroblast growth factor; c-FLIP, cellular Fas-associated death domain–like interleukin 1β–converting enzyme inhibitory protein; ChIP, chromatin immunoprecipitation; EC, endothelial cell; EMSA, electrophoresis mobility shift assay; HUVEC, human umbilical vein endothelial cell; JNK, c-Jun NH2-terminal kinase; MAPK, mitogen-activated protein kinase; PAb, polyclonal antibody; PEDF, pigment epithelial–derived factor; TUNEL, TdT-mediated dUTP nick-end labeling; VEGF, vascular endothelial cell growth factor.
References
- 1.Bouck, N., V. Stellmach, and S.C. Hsu. 1996. How tumors become angiogenic. Adv. Cancer Res. 69:135–174. [DOI] [PubMed] [Google Scholar]
- 2.Folkman, J. 2003. Angiogenesis and apoptosis. Semin. Cancer Biol. 13:159–167. [DOI] [PubMed] [Google Scholar]
- 3.Bouck, N. 2002. PEDF: anti-angiogenic guardian of ocular function. Trends Mol. Med. 8:330–334. [DOI] [PubMed] [Google Scholar]
- 4.Camphausen, K., and C. Menard. 2002. Angiogenesis inhibitors and radiotherapy of primary tumours. Expert Opin. Biol. Ther. 2:477–481. [DOI] [PubMed] [Google Scholar]
- 5.Volpert, O.V., T. Zaichuk, W. Zhou, F. Reiher, T.A. Ferguson, P.M. Stuart, M. Amin, and N.P. Bouck. 2002. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat. Med. 8:349–357. [DOI] [PubMed] [Google Scholar]
- 6.Ip, Y.T., and R.J. Davis. 1998. Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr. Opin. Cell Biol. 10:205–219. [DOI] [PubMed] [Google Scholar]
- 7.Weitzman, J.B., and M. Yaniv. 1998. Signal transduction pathways and modulation of gene activity. Clin. Chem. Lab. Med. 36:535–539. [DOI] [PubMed] [Google Scholar]
- 8.Plattner, R., S. Gupta, R. Khosravi-Far, K.Y. Sato, M. Perucho, C.J. Der, and E.J. Stanbridge. 1999. Differential contribution of the ERK and JNK mitogen-activated protein kinase cascades to Ras transformation of HT1080 fibrosarcoma and DLD-1 colon carcinoma cells. Oncogene. 18:1807–1817. [DOI] [PubMed] [Google Scholar]
- 9.Colotta, F., N. Polentarutti, M. Sironi, and A. Mantovani. 1992. Expression and involvement of c-fos and c-jun protooncogenes in programmed cell death induced by growth factor deprivation in lymphoid cell lines. J. Biol. Chem. 267:18278–18283. [PubMed] [Google Scholar]
- 10.Pu, W.T., Q. Ma, and S. Izumo. 2003. NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro. Circ. Res. 92:725–731. [DOI] [PubMed] [Google Scholar]
- 11.Pyrzynska, B., A. Lis, G. Mosieniak, and B. Kaminska. 2001. Cyclosporin A-sensitive signaling pathway involving calcineurin regulates survival of reactive astrocytes. Neurochem. Int. 38:409–415. [DOI] [PubMed] [Google Scholar]
- 12.Oukka, M., I.C. Ho, F.C. de la Brousse, T. Hoey, M.J. Grusby, and L.H. Glimcher. 1998. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 9:295–304. [DOI] [PubMed] [Google Scholar]
- 13.Rao, A., C. Luo, and P.G. Hogan. 1997. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15:707–747. [DOI] [PubMed] [Google Scholar]
- 14.Chytil, M., and G.L. Verdine. 1996. The Rel family of eukaryotic transcription factors. Curr. Opin. Struct. Biol. 6:91–100. [DOI] [PubMed] [Google Scholar]
- 15.Graef, I.A., F. Chen, and G.R. Crabtree. 2001. NFAT signaling in vertebrate development. Curr. Opin. Genet. Dev. 11:505–512. [DOI] [PubMed] [Google Scholar]
- 16.Horsley, V., and G.K. Pavlath. 2002. NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156:771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caetano, M.S., A. Vieira-de-Abreu, L.K. Teixeira, M.B. Werneck, M.A. Barcinski, and J.P. Viola. 2002. NFATC2 transcription factor regulates cell cycle progression during lymphocyte activation: evidence of its involvement in the control of cyclin gene expression. FASEB J. 16:1940–1942. [DOI] [PubMed] [Google Scholar]
- 18.Porter, C.M., M.A. Havens, and N.A. Clipstone. 2000. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J. Biol. Chem. 275:3543–3551. [DOI] [PubMed] [Google Scholar]
- 19.Porter, C.M., and N.A. Clipstone. 2002. Sustained NFAT signaling promotes a Th1-like pattern of gene expression in primary murine CD4+ T cells. J. Immunol. 168:4936–4945. [DOI] [PubMed] [Google Scholar]
- 20.Antos, C.L., T.A. McKinsey, N. Frey, W. Kutschke, J. McAnally, J.M. Shelton, J.A. Richardson, J.A. Hill, and E.N. Olson. 2002. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA. 99:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez del Arco, P., S. Martinez-Martinez, J.L. Maldonado, I. Ortega-Perez, and J.M. Redondo. 2000. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J. Biol. Chem. 275:13872–13878. [DOI] [PubMed] [Google Scholar]
- 22.Chow, C.W., C. Dong, R.A. Flavell, and R.J. Davis. 2000. c-Jun NH(2)-terminal kinase inhibits targeting of the protein phosphatase calcineurin to NFATc1. Mol. Cell. Biol. 20:5227–5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabtree, G.R., and E.N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell. 109:S67–S79. [DOI] [PubMed] [Google Scholar]
- 24.Hogan, P.G., L. Chen, J. Nardone, and A. Rao. 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17:2205–2232. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez, G.L., O.V. Volpert, M.A. Iniguez, E. Lorenzo, S. Martinez-Martinez, R. Grau, M. Fresno, and J.M. Redondo. 2001. Selective inhibition of vascular endothelial growth factor–mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J. Exp. Med. 193:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leahy, K.M., A.T. Koki, and J.L. Masferrer. 2000. Role of cyclooxygenases in angiogenesis. Curr. Med. Chem. 7:1163–1170. [DOI] [PubMed] [Google Scholar]
- 27.Iniguez, M.A., A. Rodriguez, O.V. Volpert, M. Fresno, and J.M. Redondo. 2003. Cyclooxygenase-2: a therapeutic target in angiogenesis. Trends Mol. Med. 9:73–78. [DOI] [PubMed] [Google Scholar]
- 28.Tschopp, J., M. Irmler, and M. Thome. 1998. Inhibition of fas death signals by FLIPs. Curr. Opin. Immunol. 10:552–558. [DOI] [PubMed] [Google Scholar]
- 29.Dawson, D.W., O.V. Volpert, P. Gillis, S.E. Crawford, H. Xu, W. Benedict, and N.P. Bouck. 1999. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 285:245–248. [DOI] [PubMed] [Google Scholar]
- 30.Loh, C., K.T. Shaw, J. Carew, J.P. Viola, C. Luo, B.A. Perrino, and A. Rao. 1996. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity. J. Biol. Chem. 271:10884–10891. [DOI] [PubMed] [Google Scholar]
- 31.Andrews, N.C., and D.V. Faller. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armesilla, A.L., E. Lorenzo, P. Gomez del Arco, S. Martinez-Martinez, A. Alfranca, and J.M. Redondo. 1999. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol. Cell. Biol. 19:2032–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briggs, M.R., J.T. Kadonaga, S.P. Bell, and R. Tjian. 1986. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 234:47–52. [DOI] [PubMed] [Google Scholar]
- 34.Northrop, J.P., S.N. Ho, L. Chen, D.J. Thomas, L.A. Timmerman, G.P. Nolan, A. Admon, and G.R. Crabtree. 1994. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 369:497–502. [DOI] [PubMed] [Google Scholar]
- 35.Volpert, O.V., J. Lawler, and N.P. Bouck. 1998. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastases via thrombospondin-1. Proc. Natl. Acad. Sci. USA. 95:6343–6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, J., N. Koyano-Nakagawa, Y. Amasaki, F. Saito-Ohara, T. Ikeuchi, S. Imai, T. Takano, N. Arai, T. Yokota, and K. Arai. 1997. Calcineurin-dependent nuclear translocation of a murine transcription factor NFATx: molecular cloning and functional characterization. Mol. Biol. Cell. 8:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamura, H., J. Aramburu, C. Garcia-Rodriguez, J.P. Viola, A. Raghavan, M. Tahiliani, X. Zhang, J. Qin, P.G. Hogan, and A. Rao. 2000. Concerted dephosphorylation of the transcription factor NFAT1 induces a conformational switch that regulates transcriptional activity. Mol. Cell. 6:539–550. [DOI] [PubMed] [Google Scholar]
- 38.Scott, E.S., S. Malcomber, and P. O'Hare. 2001. Nuclear translocation and activation of the transcription factor NFAT is blocked by herpes simplex virus infection. J. Virol. 75:9955–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin, E.Y., S.Y. Kim, and E.G. Kim. 2001. c-Jun N-terminal kinase is involved in motility of endothelial cell. Exp. Mol. Med. 33:276–283. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, K., M. Abe, and Y. Sato. 1999. Roles of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in the signal transduction of basic fibroblast growth factor in endothelial cells during angiogenesis. Jpn. J. Cancer Res. 90:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polverini, P.J., N.P. Bouck, and F. Rastinejad. 1991. Assay and purification of naturally occurring inhibitor of angiogenesis. Methods Enzymol. 198:440–450. [DOI] [PubMed] [Google Scholar]
- 42.Chuvpilo, S., C. Schomberg, R. Gerwig, A. Heinfling, R. Reeves, F. Grummt, and E. Serfling. 1993. Multiple closely-linked NFAT/octamer and HMG I(Y) binding sites are part of the interleukin-4 promoter. Nucleic Acids Res. 21:5694–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpert, O.V. 2000. Modulation of endothelial cell survival by an inhibitor of angiogenesis thrombospondin-1: a dynamic balance. Cancer Metastasis Rev. 19:87–92. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez, B., and O.V. Volpert. 2001. Mechanistic insights on the inhibition of tumor angiogenesis. J. Mol. Med. 78:663–672. [DOI] [PubMed] [Google Scholar]
- 45.Rommel, C., S.C. Bodine, B.A. Clarke, R. Rossman, L. Nunez, T.N. Stitt, G.D. Yancopoulos, and D.J. Glass. 2001. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3:1009–1013. [DOI] [PubMed] [Google Scholar]
- 46.Jauliac, S., C. Lopez-Rodriguez, L.M. Shaw, L.F. Brown, A. Rao, and A. Toker. 2002. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4:540–544. [DOI] [PubMed] [Google Scholar]
- 47.Lipskaia, L., M.L. Pourci, C. Delomenie, L. Combettes, D. Goudouneche, J.L. Paul, T. Capiod, and A.M. Lompre. 2003. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ. Res. 92:1115–1122. [DOI] [PubMed] [Google Scholar]
- 48.Graef, I.A., F. Chen, L. Chen, A. Kuo, and G.R. Crabtree. 2001. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 105:863–875. [DOI] [PubMed] [Google Scholar]
- 49.Wilkins, B.J., Y.S. Dai, O.F. Bueno, S.A. Parsons, J. Xu, D.M. Plank, F. Jones, T.R. Kimball, and J.D. Molkentin. 2003. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 94:110–118. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Rodriguez, C., J. Aramburu, A.S. Rakeman, and A. Rao. 1999. NFAT5, a constitutively nuclear NFAT protein that does not cooperate with Fos and Jun. Proc. Natl. Acad. Sci. USA. 96:7214–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horsley, V., and G.K. Pavlath. 2003. Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J. Cell Biol. 161:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groth, R.D., and P.G. Mermelstein. 2003. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J. Neurosci. 23:8125–8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graef, I.A., F. Wang, F. Charron, L. Chen, J. Neilson, M. Tessier-Lavigne, and G.R. Crabtree. 2003. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 113:657–670. [DOI] [PubMed] [Google Scholar]
- 54.Minneman, K.P., D. Lee, H. Zhong, A. Berts, K.L. Abbott, and T.J. Murphy. 2000. Transcriptional responses to growth factor and G protein-coupled receptors in PC12 cells: comparison of alpha(1)-adrenergic receptor subtypes. J. Neurochem. 74:2392–2400. [DOI] [PubMed] [Google Scholar]
- 55.Faehling, M., J. Kroll, K.J. Fohr, G. Fellbrich, U. Mayr, G. Trischler, and J. Waltenberger. 2002. Essential role of calcium in vascular endothelial growth factor A-induced signaling: mechanism of the antiangiogenic effect of carboxyamidotriazole. FASEB J. 16:1805–1807. [DOI] [PubMed] [Google Scholar]
- 56.Cockerill, G.W., A.G. Bert, G.R. Ryan, J.R. Gamble, M.A. Vadas, and P.N. Cockerill. 1995. Regulation of granulocyte-macrophage colony-stimulating factor and E-selectin expression in endothelial cells by cyclosporin A and the T-cell transcription factor NFAT. Blood. 86:2689–2698. [PubMed] [Google Scholar]
- 57.Bochkov, V.N., D. Mechtcheriakova, M. Lucerna, J. Huber, R. Malli, W.F. Graier, E. Hofer, B.R. Binder, and N. Leitinger. 2002. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood. 99:199–206. [DOI] [PubMed] [Google Scholar]
- 58.Wada, H., K. Hasegawa, T. Morimoto, T. Kakita, T. Yanazume, M. Abe, and S. Sasayama. 2002. Calcineurin-GATA-6 pathway is involved in smooth muscle-specific transcription. J. Cell Biol. 156:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masferrer, J. 2001. Approach to angiogenesis inhibition based on cyclooxygenase-2. Cancer J. 7:S144–S150. [PubMed] [Google Scholar]
- 60.Shannon, M.F., S.R. Himes, and L.S. Coles. 1995. GM-CSF and IL-2 share common control mechanisms in response to costimulatory signals in T cells. J. Leukoc. Biol. 57:767–773. [DOI] [PubMed] [Google Scholar]
- 61.Kubo, M., R.L. Kincaid, and J.T. Ransom. 1994. Activation of the interleukin-4 gene is controlled by the unique calcineurin-dependent transcriptional factor NF(P). J. Biol. Chem. 269:19441–19446. [PubMed] [Google Scholar]
- 62.Aoudjit, F., and K. Vuori. 2001. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J. Cell Biol. 152:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen, Y.H., H.L. Wu, C.K. Chen, Y.H. Huang, B.C. Yang, and L.W. Wu. 2003. Angiostatin antagonizes the action of VEGF-A in human endothelial cells via two distinct pathways. Biochem. Biophys. Res. Commun. 310:804–810. [DOI] [PubMed] [Google Scholar]
- 64.Li, Y.Q., C.S. Hii, C.J. Der, and A. Ferrante. 1999. Direct evidence that ERK regulates the production/secretion of interleukin-2 in PHA/PMA-stimulated T lymphocytes. Immunology. 96:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez, M.F., L.V. Bosc, A.S. Stevenson, M.K. Wilkerson, D.C. Hill-Eubanks, and M.T. Nelson. 2003. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: role of JNK2 and Crm-1. J. Biol. Chem. 278:46847–46853. [DOI] [PubMed] [Google Scholar]
- 66.Hill-Eubanks, D.C., M.F. Gomez, A.S. Stevenson, and M.T. Nelson. 2003. NFAT regulation in smooth muscle. Trends Cardiovasc. Med. 13:56–62. [DOI] [PubMed] [Google Scholar]
- 67.Liang, Q., O.F. Bueno, B.J. Wilkins, C.Y. Kuan, Y. Xia, and J.D. Molkentin. 2003. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 22:5079–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braz, J.C., O.F. Bueno, Q. Liang, B.J. Wilkins, Y.S. Dai, S. Parsons, J. Braunwart, B.J. Glascock, R. Klevitsky, T.F. Kimball, et al. 2003. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Invest. 111:1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garrington, T.P., and G.L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211–218. [DOI] [PubMed] [Google Scholar]
- 70.Chen, Y.R., X. Wang, D. Templeton, R.J. Davis, and T.H. Tan. 1996. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271:31929–31936. [DOI] [PubMed] [Google Scholar]
- 71.Weston, C.R., and R.J. Davis. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12:14–21. [DOI] [PubMed] [Google Scholar]
- 72.Zheng, H., C. Wasylyk, A. Ayadi, J. Abecassis, J.A. Schalken, H. Rogatsch, N. Wernert, S.M. Maira, M.C. Multon, and B. Wasylyk. 2003. The transcription factor Net regulates the angiogenic switch. Genes Dev. 17:2283–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koul, D., R. Shen, A. Garyali, L.D. Ke, T.J. Liu, and W.K. Yung. 2002. MMAC/PTEN tumor suppressor gene regulates vascular endothelial growth factor-mediated angiogenesis in prostate cancer. Int. J. Oncol. 21:469–475. [PubMed] [Google Scholar]
- 74.Fang, J., L. Yan, Y. Shing, and M.A. Moses. 2001. HIF-1alpha-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 61:5731–5735. [PubMed] [Google Scholar]
- 75.Tamura, M., S. Sebastian, B. Gurates, S. Yang, Z. Fang, and S.E. Bulun. 2002. Vascular endothelial growth factor up-regulates cyclooxygenase-2 expression in human endothelial cells. J. Clin. Endocrinol. Metab. 87:3504–3507. [DOI] [PubMed] [Google Scholar]
- 76.Royds, J.A., S.K. Dower, E.E. Qwarnstrom, and C.E. Lewis. 1998. Response of tumour cells to hypoxia: role of p53 and NFkB. Mol. Pathol. 51:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]