Abstract
In the nonobese diabetic (NOD) mouse model of type 1 diabetes, the immune system recognizes many autoantigens expressed in pancreatic islet β cells. To silence autoimmunity, we used dendritic cells (DCs) from NOD mice to expand CD25+ CD4+ suppressor T cells from BDC2.5 mice, which are specific for a single islet autoantigen. The expanded T cells were more suppressive in vitro than their freshly isolated counterparts, indicating that DCs from autoimmune mice can increase the number and function of antigen-specific, CD25+ CD4+ regulatory T cells. Importantly, only 5,000 expanded CD25+ CD4+ BDC2.5 T cells could block autoimmunity caused by diabetogenic T cells in NOD mice, whereas 105 polyclonal, CD25+ CD4+ T cells from NOD mice were inactive. When islets were examined in treated mice, insulitis development was blocked at early (3 wk) but not later (11 wk) time points. The expanded CD25+ CD4+ BDC2.5 T cells were effective even if administered 14 d after the diabetogenic T cells. Our data indicate that DCs can generate CD25+ CD4+ T cells that suppress autoimmune disease in vivo. This might be harnessed as a new avenue for immunotherapy, especially because CD25+ CD4+ regulatory cells responsive to a single autoantigen can inhibit diabetes mediated by reactivity to multiple antigens.
Keywords: insulin-dependent diabetes mellitus, dendritic cells, CD25+ CD4+ regulatory, T cells, BDC2.5, autoimmunity
Introduction
Inbred nonobese diabetic (NOD) mice develop spontaneous autoimmune diabetes that resembles human type 1 diabetes in many respects (1–3). CD4+ and CD8+ T cells specific for a number of autoantigens prove to be important in the pathogenesis of diabetes. T cells reactive to insulin, GAD65, and IGRP are found in NOD mice concurrent with development of insulitis (4–6), and T cell clones specific for these antigens can cause diabetes upon adoptive transfer. In human diabetes, autoantibodies develop against several autoantigens including insulin and GAD65 (7, 8). A fundamental issue in diabetes research is to identify mechanisms that silence these autoimmune reactions.
Tolerance mechanisms for autoreactive T cells can be of “intrinsic” and “extrinsic” varieties (9). Intrinsic mechanisms include deletion and anergy of self-reactive T cells, whereas extrinsic mechanisms include different regulatory T cells that suppress other diabetogenic T cells. One type of extrinsic suppressor is the CD25+ CD4+ T cell, with an important source being the thymus (10, 11). Suppressor or regulatory T cells maintain tolerance to self-antigens (12–15). The transcription factor, FoxP3, is important for the development of CD25+ CD4+ T regulatory cells (16–18), and children who are born with defective FoxP3 rapidly develop autoimmunity including diabetes (19, 20). In NOD mice, CD25+ CD4+ regulatory T cells inhibit diabetes development (21–23), making this extrinsic tolerance mechanism an attractive target to develop antigen-specific therapies for autoimmune disease.
In vitro, CD25+ CD4+ T cells will suppress the proliferative or cytokine responses of naive CD25− CD4+ T cells (24, 25). However, the CD25+ CD4+ T cells are themselves unable to proliferate when stimulated by most types of APCs, although proliferation does occur if the TCR is ligated in the presence of high doses of IL-2 (24, 25). Recently, it was discovered that DCs can expand antigen-specific CD25+ CD4+ T cells, and the latter have increased suppressive activity (26). The observed ability of CD25+ CD4+ T cells to expand in a number of experimental systems in mice may well be initiated by antigen-presenting DCs (27–31). In an experimental model of multiple sclerosis mediated by transgenic T cells specific to myelin basic protein, CD25+ CD4+ T cells specific for this antigen showed better suppression of disease than CD25+ CD4+ T cells with TCRs specific for other antigens (32). These findings suggest that one can use antigen-specific T cells, rather than polyclonal populations, to suppress autoimmunity. However, it has not been determined if CD25+ CD4+ T cells of one specificity can suppress autoimmunity caused by T cell responses to many autoantigens, particularly a spontaneous disease like diabetes in NOD mice.
The capacity of DCs to expand antigen-specific CD25+ CD4+ T cells now makes it possible to address these issues. We used a CD4+ TCR transgenic line that is known to be diabetogenic, the BDC2.5 T cell (33). Despite reports of defects in both CD25+ CD4+ regulatory T cells and DCs in NOD mice (21, 34–36), we find that both populations can interact productively. As a result, we have used DCs from NOD mice to expand CD25+ CD4+ BDC2.5 T cells reactive to a natural pancreatic islet β cell antigen. We will show that these antigen-specific T cells are potent inhibitors of autoimmunity mediated by autoreactive T cells of many specificities, whereas antigen-nonselected populations from NOD mice have no detectable activity at 20-fold higher doses. This suggests a relevant physiologic as well as therapeutic role for DCs in expanding CD25+ CD4+ suppression in an antigen- and disease-specific manner.
Materials and Methods
Mice.
NOD and NOD.scid (both I-Ag7) mice were purchased from The Jackson Laboratory. BDC2.5 TCR transgenic mice on the NOD genetic background were provided by D. Mathis and C. Benoist (Joslin Diabetes Center, Boston, MA). Specific pathogen-free mice of both sexes were used at 5–12 wk of age according to institutional guidelines. Protocols were approved by the Institutional Animal Care and Use Committee at Rockefeller University.
Antibodies.
mAbs for MHC class II (TIB120), B220 (TIB146), CD8 (TIB211), CD4 (GK1.5), CD3 (145-2C11), and HSA (J11d) were from American Type Culture Collection. FITC-conjugated anti-CD25 (7D4), I-Ag7 (OX-6), Gr1 (RB6-8C5), CD11c (HL3) and CD4 (H129.19), CD86 (GL1), biotinylated anti-CD25 (7D4) and mouse IgG2b, APC anti-CD11c (HL3), CD62L (MEL-14), CD25 (PC61) and CD4 (RM4-5), and PE-streptavidin were from BD Biosciences. Purified antibody to CD3 (145-2C11), CD49b/Pan NK cells (DX5), CD16/CD32 (2.4G2), and control rat IgG were also from BD Biosciences. We purchased biotin goat anti–glucocorticoid-induced TNF receptor (GITR) from R&D Systems. A hybridoma expressing the anti-clonotype antibody specific for the BDC2.5 TCR (αBDC) was provided by O. Kanagawa (Washington University, St. Louis, MO), and the antibody was purified and biotinylated.
Bone Marrow–derived DCs.
These were prepared with GM-CSF as previously described (26, 37). DCs were isolated from normoglycemic NOD males. On day 5, LPS (Sigma-Aldrich) was added at 50 ng ml−1 for ∼16 h. On day 6, cells were collected and the more mature Grl− CD86+ cells were purified with FITC and PE magnetic microbeads (Miltenyi Biotec) as previously described (26) and irradiated with 15 Gy before use as APCs.
Proliferation Assays and Expansion.
As previously described (26), spleen and lymph node cell suspensions were enriched for CD4+ cells by panning and were sorted on a FACS Vantage™ (BD Biosciences) into CD25+ CD4+ and CD25− CD4+ populations (>95 and >97% pure). 104 T cells from BDC2.5 or NOD mice were cultured for 3 d with the indicated number of DCs and a mimetope peptide (termed 1040–55; 30–100 ng ml−1) having the sequence RVRPLWVRME (38), or with purified anti-CD3 antibody (0.3–1 μg ml−1). RHu IL-2 (Chiron Corp.) was added where indicated in Fig. 1 and with cultured CD25+ cells in Fig. 2 at 100 U ml−1. All CD25+ CD4+ T cell expansions for in vivo injection were performed with IL-2 in the cultures. To assess suppression by CD25+ CD4+ T cells, 5 × 104 whole NOD spleen cells irradiated with 15 Gy were used to stimulate mixtures of 104 CD25− CD4+ and the indicated number of CD25+ CD4+ T cells from BDC2.5 or NOD mice. If DC-expanded CD25+ CD4+ T cells were used, CD11c+ cells were removed using magnetic microbeads (Miltenyi Biotec) after harvesting the cells on days 5–7. [3H]thymidine uptake (1 μCi/well; PerkinElmer) by proliferating lymphocytes was measured at 60–72 h. For in vivo proliferation, CD25+ CD4+ and CD25− CD4+ cells were purified by flow cytometry and labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes). 3.3 × 105 T cells were injected i.v. into NOD recipients. 1 d later, 2 × 105 BDC peptide–pulsed or –unpulsed, LPS-matured bone marrow DCs were injected s.c. in each paw. 3 d after DCs were injected, lymph nodes were collected and cells were stained with CD4 and BDC2.5 clonotype antibody, and the level of CFSE staining was determined by flow cytometry.
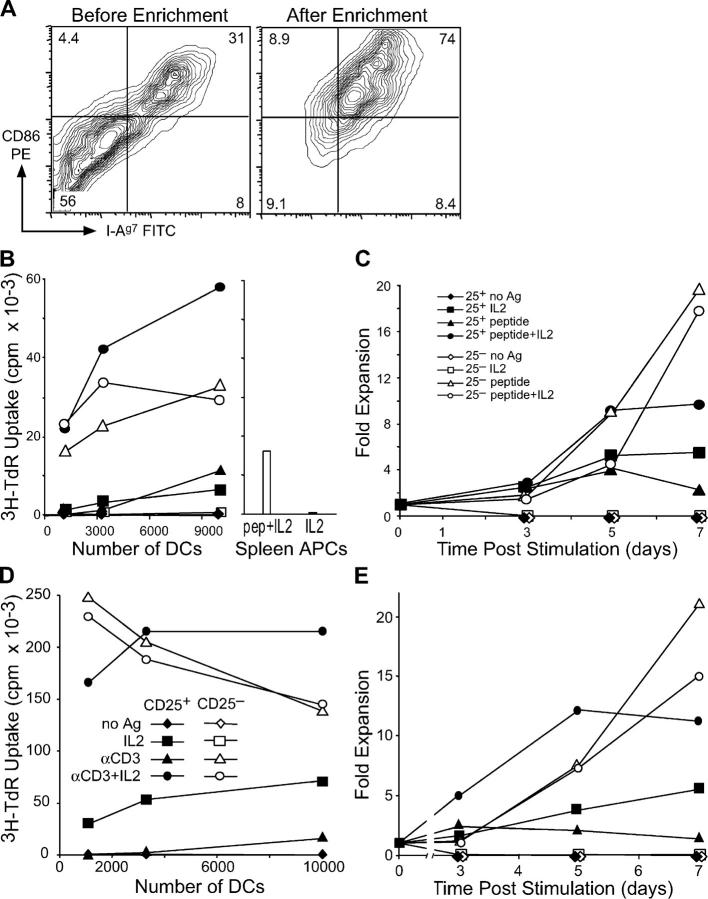
Figure 1.
NOD DCs induce growth of CD25+ CD4+ T cells from NOD.BDC2.5 or NOD mice. (A) In vitro–derived NOD DCs were stained with antibodies specific for CD86 and MHC class II before (left) and after (right) magnetic bead enrichment of CD86+ cells. (B) CD25+ CD4+ or CD25− CD4+ T cells sorted from BDC2.5 TCR transgenic mice were cultured with CD86+ NOD DCs with and without 30 ng ml−1 BDC peptide and IL-2. In the same experiment, NOD spleen cells were used with IL-2, with and without BDC peptide (right). A 12-h [3H]thymidine pulse was given on day 3. (C) Same as B, but the dose of BDC peptide was 100 ng ml−1 and the fold increase in T cell numbers was monitored by counting on days 3, 5, and 7. (D) CD25+ or CD25− CD4+ T cells were isolated from NOD mice and cultured with NOD CD86+ DCs, with and without anti-CD3 and IL-2 as indicated. Proliferation was determined by [3H]thymidine incorporation on day 3. (E) As in D, but cells were counted on days 3, 5, and 7, and the fold increase in cell numbers was calculated. One result of at least three similar experiments is shown.
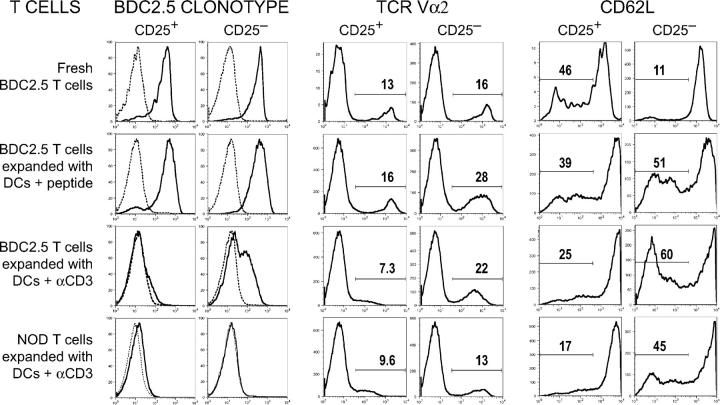
Figure 2.
Phenotype of DC-expanded T cells. Expression of BDC2.5 clonotype (left), TCR Vα2 (middle), and CD62L (right) on CD25+ or CD25− CD4+ T cells cultured under the conditions indicated. For the BDC2.5 clonotype, the isotype control peak is shown by the dashed line. For Vα2 and CD62L, the percentage of cells in the indicated gates is shown. One result of at least three similar experiments is shown.
Diabetes Experiments.
Diabetes was induced in NOD. BDC2.5 mice with one dose of cyclophosphamide (Sigma-Aldrich) at 200 μg/g in PBS. 3 d later, mice were injected with PBS or 5 × 105 CD25+ CD4+ or CD25− CD4+ T cells, which had been expanded with DCs and BDC peptide in vitro for 5–7 d. In separate experiments, diabetes was transferred to 5–9-wk-old NOD.scid mice with 3–10 × 106 spleen cells (given i.v.) from female diabetic NOD mice. At the same time (or later where indicated), the indicated numbers of purified CD25+ CD4+ or CD25− CD4+ T cells, which had been expanded with DCs, BDC peptide, and IL-2 in vitro for 5–7 d, were also given i.v. For all diabetes experiments, development of diabetes was monitored with chemstrips (Roche Applied Science), which detect urine glucose above 150 mg dL−1. A mouse was considered diabetic on the first of three consecutive readings of high urine glucose. Due to a high incidence of thymoma development in NOD.scids at ∼20 wk of age, diabetes was monitored only until the mice were 18 wk old. Statistics were calculated using the Mann-Whitney U test.
Histological Analysis.
Pancreas tissue was fixed in Bouin's solution and paraffin-embedded sections were stained with hematoxylin and eosin. Tissue cuts were made 100 microns apart to avoid counting any islets twice. Insulitis was assessed for each islet, and scored as 0, no insulitis; 1, peri-insulitis; 2, <70% infiltrated; and 3, >70% infiltrated.
Results
DCs Expand CD25+ CD4+ T Cells from Autoimmune NOD Mice.
We tested the ability of autoantigen-specific CD25+ CD4+ T cells to expand in response to DCs. We chose an autoreactive T cell that responds to a natural autoantigen and is diabetogenic. CD4+ T cells from BDC2.5 TCR transgenic NOD mice respond to a protein expressed by islet β cells (33, 39). Although the β cell autoantigen remains to be identified, a series of mimetope peptides have been uncovered, which also stimulate proliferation of BDC2.5 T cells (38). We used one of these mimetope peptides as antigen, which will be referred to as BDC peptide. This particular mimetope peptide has a high functional affinity (low EC50) and also is recognized by a small fraction of T cells from nontransgenic NOD mice (38).
We recently found that CD25+ CD4+ cells from nonautoimmune mice will grow in response to antigen-bearing DCs, particularly mature CD86+ bone marrow–derived DCs (26). Therefore, we prepared >95% pure CD25+ CD4+ BDC2.5 T cells and NOD bone marrow DCs (from male normoglycemic mice). In agreement with previous data on the DCs from NOD mice (35, 40), there was a lower (approximately twofold) frequency of CD86 high DCs relative to other strains like C57Bl/6. However, we used magnetic beads to enrich the smaller subset of CD86+ NOD DCs. These expressed high levels of CD86, comparable to other strains (Fig. 1 A and not depicted).
When we cultured BDC2.5 CD25+ CD4+ T cells with NOD CD86+ DCs pulsed with BDC peptide, the T cells proliferated by day 3 (Fig. 1 B). Proliferation also took place in response to CD86− DCs pulsed with BDC peptide, but it was more limited (not depicted). CD25− CD4+ cells likewise proliferated to DCs with BDC peptide, but the addition of IL-2 did not significantly change proliferative responses. CD25+ CD4+ T cells cultured with DCs and IL-2 (but not with BDC peptide) also showed significant proliferation, as was evident with ovalbumin-specific CD25+ CD4+ T cells (26), but the combination of IL-2 and BDC peptide with DCs was more effective, resulting in higher [3H]thymidine incorporation than with CD25− CD4+ cells. Previously, groups have observed some proliferation of CD25+ CD4+ T cells cultured with spleen APCs, a TCR stimulus, and IL-2 (24, 25). We found that these conditions (BDC2.5 T cells, spleen APCs, BDC peptide, and IL-2) induced at least 3.5-fold lower proliferation than with DCs under the same conditions (Fig. 1 B, right). Using the condition in Fig. 1 B, which gave the highest level of proliferation, we tested the ability of the cells to expand during a 1-wk culture. Relative to the number of cells placed into culture, there was a 5–10-fold expansion in the number of recovered T cells from cultures of CD25+ CD4+ T cells, DCs, and BDC peptide with and without IL-2 at 5 d. CD25+ and CD25− CD4+ T cells expanded similarly up to day 5, but only the latter continued to expand up to day 7 (Fig. 1 C). Thus, CD25+ CD4+ T cells from BDC2.5 transgenic mice can grow in response to DCs in an antigen-specific manner, in much the same way as recently reported for ovalbumin-specific T cells (26).
To show that nontransgenic regulatory T cells from autoimmune NOD mice were capable of proliferation and expansion with DCs, we sorted NOD CD25+ CD4+ T cells and stimulated them with NOD CD86+ DCs and anti-CD3. With this polyclonal stimulus, DCs were able to induce DNA synthesis and expansion of NOD CD25+ CD4+ T cells (Fig. 1, D and E). The T cells also proliferated when cultured with DCs and IL-2 in the absence of a TCR stimulus, but IL-2, DCs, and anti-CD3 synergized to induce very high levels of DNA synthesis and expansion of cell numbers, >10-fold by 5 d. In contrast, NOD CD25+ CD4+ T cells cultured without DCs but with IL-2 with or without anti-CD3, gave only 2 × 103 or 7 × 103 cpm of DNA synthesis, respectively. Control NOD CD25− CD4+ T cells given DCs and anti-CD3 with or without IL-2, showed both proliferation and expansion. These results indicate that both DCs and T cells (either BDC2.5 or nontransgenic) from autoimmune NOD mice can interact to significantly expand CD25+ CD4+ regulatory T cells.
Phenotype of DC-expanded CD25+ CD4+ T Cells.
To identify BDC2.5-specific T cells after DC-mediated expansion, cultures were stained with an mAb specific for the BDC2.5 TCR. Approximately 80% of freshly isolated or DC plus IL-2–expanded BDC2.5 CD25+ CD4+ T cells expressed high levels of this clonotype (Fig. 2, left). In contrast, when BDC2.5 CD25+ CD4+ T cells were stimulated with DCs, IL-2, and anti-CD3, the level of clonotype expression was much lower than on cells expanded with DCs presenting BDC peptide. As expected, DC plus anti-CD3–stimulated NOD CD25+ CD4+ T cells did not express significant levels of BDC clonotype compared with isotype controls (Fig. 2, left). Because T cells expressing a transgenic TCR also can express endogenous TCR-α chains, we checked expression of two different endogenous TCR alphas (Vα2 and Vα8.3), and found similar percentages of cells expressing endogenous V alphas before and after DC-BDC peptide stimulation (Fig. 2, middle, and not depicted). Interestingly, the level of Vα2 expressed on CD25+ CD4+ T cells expanded with CD3 was lower than freshly isolated cells or those expanded with BDC peptide. This suggests that anti-CD3 causes a down-regulation of TCR expression that is not specific to the BDC2.5 TCR. For comparison, clonotype and Vα2 expression on CD25− CD4+ T cells expanded under the same conditions is shown, and down-regulation of either clonotype or Vα2 expression is not as severe as with CD25+ CD4+ T cells. This data indicates that in contrast to T cells expanded with DCs plus anti-CD3, BDC2.5 T cells expanded with DCs plus BDC peptide express much higher levels of TCR on their cell surfaces.
To characterize the DC-expanded regulatory cells, these cells were stained with antibodies specific for CD25, CD62L, and GITR. The cultured CD25+ CD4+ T cells maintained high levels of CD25 and GITR, and many of the cultured CD25− CD4+ T cells up-regulated both CD25 and GITR (not depicted). The freshly isolated BDC2.5 CD25+ CD4+ T cells contained ∼40% CD62L low cells, and DC expansion did not significantly change this phenotype (Fig. 2, right). In contrast, the freshly isolated CD25− CD4+ T cells contained only ∼10% CD62L low cells, and activation with DCs greatly increased the percentage of the CD62L low cells (Fig. 2, right). IL-2 was added to all cultured CD25+ CD4+ T cells in addition to the indicated culture conditions shown in Fig. 2, but similar expression of clonotype, Vα2, and CD62L was observed for CD25+ CD4+ T cells cultured without IL-2 (not depicted). Collectively, expression of these activation markers before and after DC stimulation is similar to that found in nonautoimmune strains (26), indicating that DC activation of NOD regulatory cells occurs normally.
Expansion of CD25+ CD4+ T Cells with DCs In Vivo.
To determine if DCs also can induce proliferation of CD25+ CD4+ T cells in vivo, we purified CD25+ CD4+ T cells from BDC2.5 mice, labeled them with CFSE before injection into NOD mice, and 1 d later, we s.c. injected mature marrow–derived DCs that had been pulsed (or not pulsed as control) with BDC peptide. We assessed proliferation 3 d later by progressive halving of the amount of CFSE per T cell. The CD25+ CD4+ T cells proliferated, with up to six divisions per cell, in the draining lymph nodes of mice that received BDC peptide–pulsed DCs, but not in mice that received PBS or DCs alone (Fig. 3 and not depicted). We observed similar proliferative responses with control CD25− CD4+ cells, but CFSE was not diluted in either CD25+ or CD25− CD4+ cells in the distal lymph nodes of mice receiving either pulsed or unpulsed DCs (Fig. 3). Therefore, DCs are able to induce the proliferation of CD25+ CD4+ T cells from an autoimmune strain in vivo.
Figure 3.
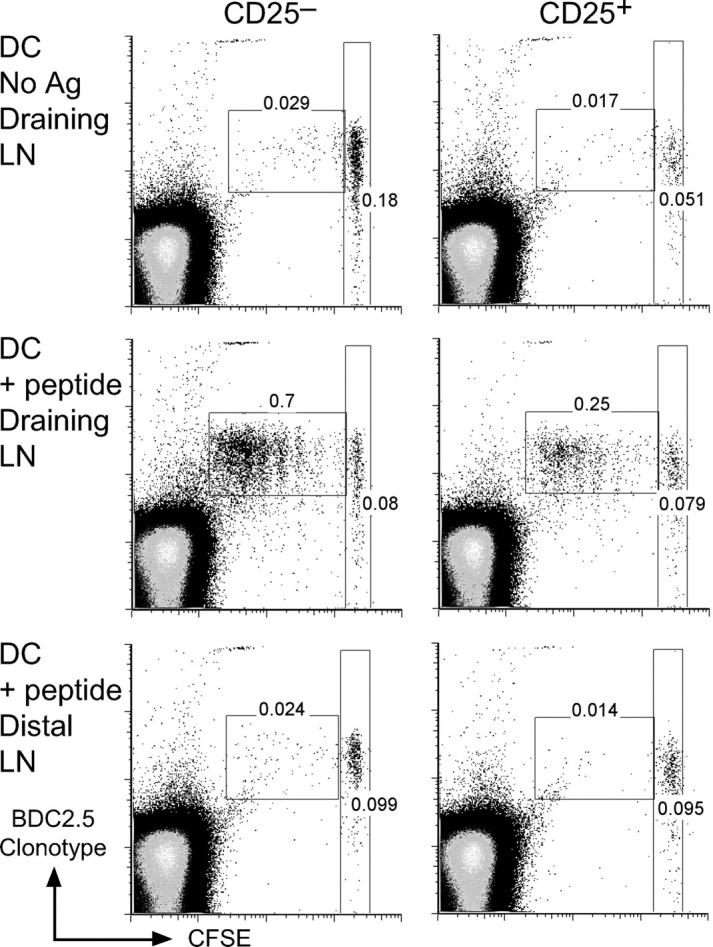
BDC2.5 CD25+ CD4+ T cells proliferate in vivo. CFSE-labeled BDC2.5 CD25− CD4+ (left) or CD25+ CD4+ (right) T cells were injected into NOD mice. 1 d later, either DCs without antigen (top) or BDC peptide–pulsed DCs (middle and bottom) were injected s.c. 3 d after antigen delivery, the injected >1,000 CFSE-labeled clonotype+ cells from draining (top and middle) or distal (bottom) lymph nodes were assessed for proliferation by flow cytometry, gating on CD4+ lymphocytes. The percentages in the dividing and nondivding populations of clonotype+ cells is shown.
Enhanced In Vitro Suppressive Function of DC-expanded, CD25+ CD4+ T Cells.
To verify that the expanded CD25+ CD4+ T cells from BDC2.5 mice retained suppressive function, we used a standard in vitro suppression assay. We removed CD11c+ DCs from 7-d expansion cultures and added the T cells in different ratios to responder CD25− CD4+ T cells to measure the inhibition of CD25− CD4+ proliferation in response to BDC peptide presented by spleen APCs. Freshly isolated CD25+ CD4+ T cells, as well as CD25+ CD4+ T cells expanded with DCs and IL-2, were able to suppress, but only partially and at high doses, i.e., when mixed at a 1:2 ratio with CD25− CD4+ cells. In contrast, CD25+ CD4+ T cells expanded with BDC peptide (without or with IL-2) had stronger activity, showing suppression even at a ratio of 1 CD25+ CD4+ T cell for every 8 CD25− CD4+ cells (Fig. 4 A). We also tested the suppressive function of NOD CD25+ CD4+ T cells expanded with DCs and anti-CD3. Again, the T cells expanded with DCs and TCR stimulus suppressed proliferation by NOD CD25− CD4+ T cells approximately fourfold more efficiently than freshly isolated CD25+ CD4+ T cells (Fig. 4 B). Although freshly purified NOD CD25+ CD4+ T cells showed ∼75% suppression at a ratio of 8 responder cells for 1 CD25+ CD4+ T cell, NOD CD25+ CD4+ T cells expanded with DCs and anti-CD3 (with or without IL-2) showed similar suppression at a ratio of 32:1. Therefore, either polyclonal or monospecific CD25+ CD4+ T cells from NOD mice can be expanded with DCs and anti-CD3 or antigen, and they show approximately fourfold enhancement in suppressive function.
Figure 4.
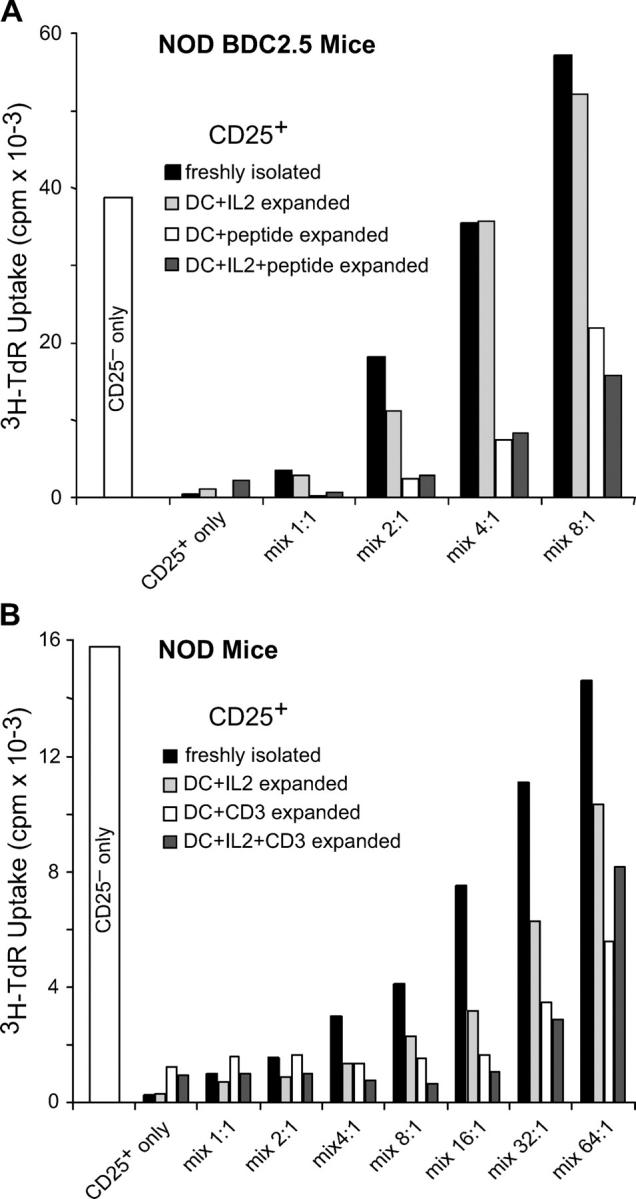
DC-expanded CD25+ CD4+ T cells suppress proliferation better than unexpanded CD25+ CD4+ T cells. (A) CD25+ CD4+ T cells from NOD.BDC2.5 mice were expanded for 7 d with irradiated NOD DCs and BDC peptide and IL-2 as indicated. 104 freshly isolated, sorted CD25− CD4+ T cells from BDC2.5 mice were cultured with NOD spleen cells, 30 ng/ml BDC peptide, and either freshly sorted CD25+ CD4+ or the indicated DC-expanded CD25+ CD4+ populations, at the ratios indicated. After 72 h, proliferation was assessed by [3H]thymidine incorporation during a 12-h pulse. One representative result from at least three is shown. (B) Same as A, but both CD25+ and CD25− CD4+ T cells were isolated from NOD mice, and anti-CD3 was used as TCR stimulus instead of BDC peptide in both expansion and suppression cultures. One representative result from at least three is shown.
DC-expanded CD25+ CD4+ T Cells Efficiently Suppress Diabetes In Vivo.
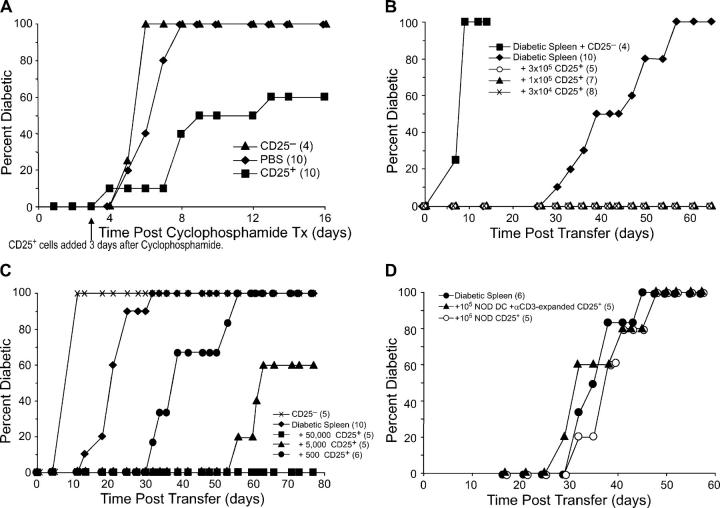
A critical in vivo function for CD25+ CD4+ T cells is the prevention of autoimmunity. Therefore, we wanted to determine if BDC2.5 CD25+ CD4+ T cells expanded in vitro with DCs and antigen could inhibit the development of diabetes. The first model of diabetes we used was one in which the pathogenic T cells to be suppressed were of the same BDC2.5 specificity. As expected from previous work (41), most BDC2.5 mice on the NOD background did not develop diabetes, but when young BDC2.5 NOD mice were given one injection of cyclophosphamide, diabetes developed 4–7 d later in 100% of the mice. To suppress this diabetes induction, 3 d after cyclophosphamide treatment, we injected BDC2.5.NOD mice with DC-expanded CD25+ CD4+ T cells from BDC2.5 mice. In two experiments, this resulted in a delay of diabetes onset and a reduced diabetes incidence. In contrast, injection of DC-expanded CD25− CD4+ from BDC2.5 mice had little effect on diabetes development (Fig. 5 A). These results show that the DC-expanded suppressor T cells are able to suppress autoimmunity even when the disease is developing rapidly.
Figure 5.
Expanded CD25+ CD4+ T cells function in vivo to suppress development of diabetes. (A) 4–6-wk-old NOD.BDC2.5 mice were given cyclophosphamide i.p. 3 d later, either 5 × 105 DC-expanded CD25+ CD4+ T cells or CD25− CD4+ cells were injected i.v. (B) NOD.scid females were injected with 3 × 106 spleen cells from a diabetic NOD female and either nothing or the indicated numbers of DC-expanded CD25+ CD4+ T cells or 3 × 105 CD25− CD4+ cells from BDC2.5 mice. (C) NOD.scid females were injected with either 4 × 105 CD25− CD4+ cells from BDC2.5 mice, or 8 × 106 spleen cells from a diabetic NOD female and either nothing or the indicated numbers of DC-expanded CD25+ CD4+ T cells from BDC2.5 mice. The difference between diabetic spleen alone to diabetic spleen plus 500 CD25+ CD4+ cells was significant (P = 0.002), as was diabetic spleen to diabetic spleen plus 5,000 CD25+ CD4+ cells (P = 0.002). One representative result from two experiments is shown. (D) NOD.scid females were injected with 8 × 106 diabetic spleen cells alone or with 105 freshly isolated or DC/αCD3-expanded CD25+ CD4+ T cells from NOD mice. The number of mice in each group is indicated in parentheses.
We then tested a second model, injection of spleen cells from diabetic NOD mice into NOD.scid females, because this model is mediated by pathogenic T cells with a diverse repertoire of TCR specificities. We injected different doses of DC-expanded CD25+ CD4+ T cells from BDC2.5 mice with 3–10 × 106 spleen cells from diabetic mice into NOD.scid females. The mice receiving diabetic spleen cells alone developed diabetes starting at 3–4 wk after injection as expected (42, 43). In the first dose-response study, the addition of 3 × 105, 105, or 3 × 104 expanded BDC2.5 CD25+ CD4+ T cells to 3 × 106 diabetic spleen cells completely prevented diabetes development (Fig. 5 B). In contrast, when we injected 3 × 105 DC-expanded CD25− CD4+ cells together with diabetic spleen cells, there was a marked acceleration of diabetes onset when compared with diabetic spleen cells alone. In a second dose-response experiment, we increased the number of diabetic spleen cells to 8 × 106, and the number of expanded CD25+ CD4+ T cells was titrated down further. Again 50,000 DC-expanded BDC2.5 CD25+ CD4+ T cells completely prevented diabetes development up to 80 d after transfer. The addition of 5,000 of these regulatory cells also gave a large delay in diabetes, and even 500 DC-expanded BDC2.5 CD25+ CD4+ T cells showed a significant delay in diabetes compared with those receiving spleen cells from diabetic mice alone (Fig. 5 C). In both experiments, unexpanded BDC2.5 CD25+ T cells showed a similar ability to block or delay diabetes development (not depicted). Three similar experiments with both DC-expanded and -unexpanded regulatory cells have now been performed with similar results.
The numbers of DC-expanded autoantigen-specific CD25+ CD4+ T cells necessary to delay or block diabetes development here were much lower than the numbers of bulk (polyclonal) NOD CD25+ CD4+ T cells used in other transfer studies, i.e., at least 2–5 × 105 cells were necessary to see a significant delay in diabetes development (22, 23, 44). To establish the need for antigen-specific T cells in disease suppression, and to confirm in our system that DC stimulation alone was not sufficient for in vivo suppression, NOD CD25+ CD4+ T cells expanded with DCs plus anti-CD3 were transferred to NOD.scid mice along with spleen cells from diabetic mice. Even 105 polyclonal NOD CD25+ CD4+ T cells, either freshly isolated or anti-CD3/DC expanded, were unable to delay diabetes (Fig. 5 D). In addition, freshly isolated BDC2.5 regulatory T cells could also block diabetes development with similar cell numbers as the DC-expanded T cells (not depicted), suggesting that antigen specificity, rather than expansion with BDC peptide stimulation, is the most critical variable for suppression of diabetes in vivo. Therefore, autoantigen-specific DC-expanded CD25+ CD4+ T cells function efficiently in vivo to suppress autoimmunity mediated by autoreactive T cells.
Mice Receiving Autoantigen-specific CD25+ CD4+ T Cells Can Develop Insulitis.
To determine at which stage disease was blocked in NOD.scid mice protected from diabetes by small numbers of BDC2.5-specific CD25+ CD4+ T cells, pancreata were isolated from those mice that still had normal glucose levels at the end of the experiment shown in Fig. 5 C (80 d after transfer). Insulitis was scored from hematoxylin and eosin–stained sections. The mice from the groups that received 5,000 or 50,000 BDC2.5-specific CD25+ CD4+ T cells (the latter group were all diabetes free), had lymphocytic infiltrates in half of the islets scored (Fig. 6 A). A representative field from both protected groups is shown (Fig. 6 B). In a separate transfer experiment, pancreata were isolated from mice earlier on, at 23 or 28 d after transfer of the diabetogenic and regulatory cells. At this earlier time point, the mice that had received only the diabetogenic cells had some insulitis, but those that had also received BDC2.5 regulatory cells lacked lymphocytic infiltrate in the islets (Fig. 6 C). This indicates that protected mice can progress past the initiation of islet inflammation, checkpoint I, but the kinetics of insulitis is slower than in the absence of regulatory cells.
Figure 6.
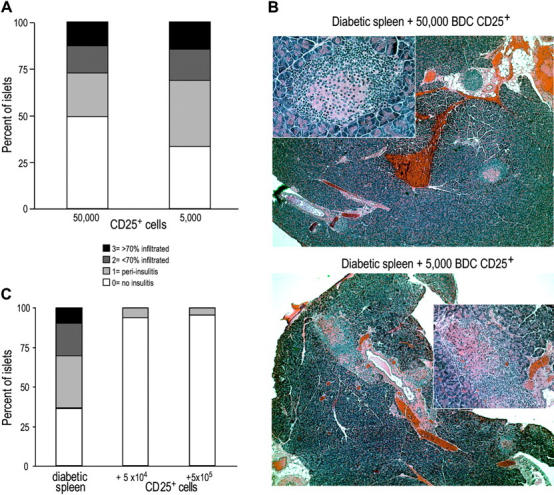
Protected mice have lymphocytic infiltrates in the pancreas. (A) Pancreata from mice that did not develop diabetes by day 80 after transfer in the experiment shown in Fig. 5 C were scored for insulitis. 150 islets from 5 mice were scored from the group that received 50,000 DC-expanded BDC CD25+ CD4+ T cells, and 48 islets from 2 mice were scored from the group that received 5,000 cells. (B) Representative fields for a mouse from the group that received 5,000 (top) or 50,000 (bottom) suppressor T cells. Large field is 5×; inset is 20×. (C) In a separate experiment, pancreata from mice 28 d after transfer were scored for insulitis. At least 50 islets from 2–3 mice were scored from each group.
Autoantigen-specific CD25+ CD4+ T Cells Can Still Regulate When Given after Diabetogenic Cells.
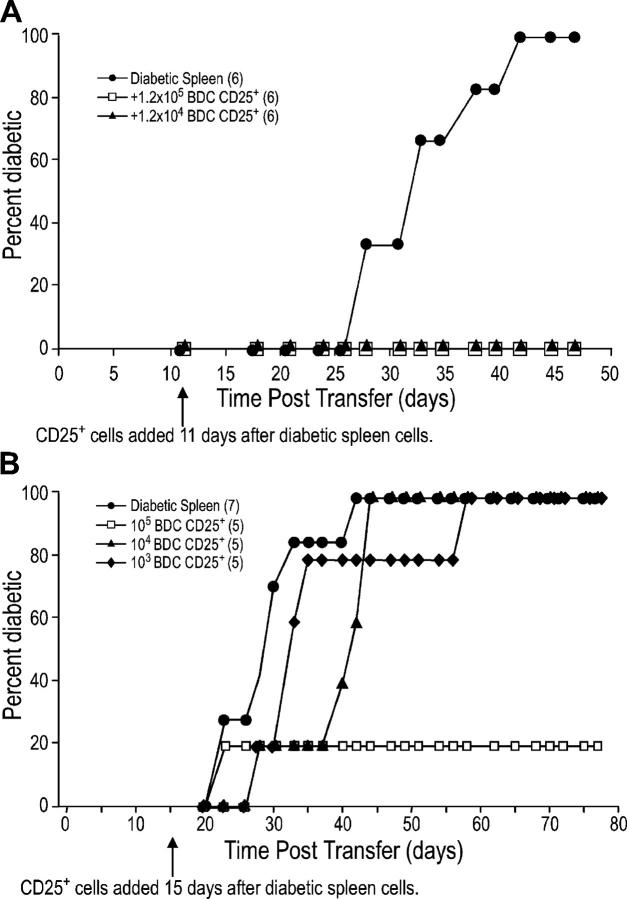
One feature of the NOD.scid system is that T cells, when injected into a lymphopenic host, undergo antigen-independent, homeostatic proliferation. To lessen the effect of such proliferation on the CD25+ CD4+ T cells, the latter were injected after the diabetogenic spleen cells. Even when given 11 d after the diabetogenic cells, as few as 12,000 DC-expanded, BDC2.5 CD25+ CD4+ T cells prevented diabetes development (Fig. 7 A). When given 15 d after diabetogenic cells, 104 cells significantly delay diabetes (Fig. 7 B). At these time points, by FACS® staining for CD4+ cells, we showed that lymphocytes from the diabetic mice had repopulated the lymphoid organs, and even entered the pancreas (not depicted). Therefore, CD25+ CD4+ T cells can block diabetes even after the diabetogenic cells have been given time to occupy the lymphoid compartments, and initiate diabetes pathogenesis. Further studies will be needed to check the capacity of DC-expanded, antigen-specific CD25+ CD4+ T cells to suppress disease under nonhomeostatic conditions.
Figure 7.
BDC2.5 CD25+ CD4+ T cells can still regulate diabetes when given after diabetogenic cells. (A) NOD.scid females were injected with 8 × 106 diabetic spleen cells and 11 d later were injected with either PBS or the indicated number of DC-expanded CD25+ CD4+ T cells from BDC2.5 mice. The difference between diabetic spleen alone to diabetic spleen plus 105 or 104 DC-expanded CD25+ CD4+ cells was significant (P = 0.002). (B) As in A, except 107 diabetic spleen cells were added 15 d before the indicated number of DC-expanded CD25+ CD4+ T cells from BDC2.5 mice. The p-value for diabetic spleen alone versus adding 105 BDC2.5 regulatory T cells is 0.055 and versus adding 104 BDC2.5 regulatory T cells is 0.0595. The number of mice in each group is indicated in parentheses.
Discussion
Despite the importance of T cell responses to islet autoantigens, currently no diabetogenic CD4+ TCR transgenic specific for a known β cell protein is available. Three islet-reactive, CD4+ transgenic lines exist. GAD65-reactive, G286 mice have nondiabetogenic T cells (45). A second 4.1 transgenic is generally stimulated with whole islets as the source of antigen (46). The third, BDC2.5, is specific for an as yet unknown antigen expressed by β cells in pancreatic islets, but this antigen can be replaced by peptide mimetopes, providing an opportunity to test the suppressive capacity of antigen-specific BDC2.5 CD25+ CD4+ T cells on the development of diabetes (38). The preparation of significant numbers of antigen-specific suppressor T cells has been held back at least in part because of a lack of information on APC requirements. This difficulty can now be addressed because DCs will expand CD25+ CD4+ regulatory T cells and if anything, increase their function per cell (26). We have now tested these systems in the NOD model of spontaneous autoimmune diabetes. Our results contribute to three issues relating to CD25+ CD4+ suppressor T cell function in autoimmune disease.
Both DCs and CD25+ CD4+ T Cells from the NOD Autoimmune Strain Have the Potential to Generate Suppressive Function.
The capacity of DCs to expand functional CD25+ CD4+ suppressor T cells was to some extent unexpected, given reports of defects in both CD25+ CD4+ T cells (21, 36) and DCs (34) in NOD mice. We find that the yield of CD86 high DCs in our bone marrow cultures from NOD mice is twofold less than that observed with bone marrow from C57BL/6 mice, suggesting that spontaneous maturation of DCs in NOD cultures is to some extent reduced. Because mature DCs are the most potent and perhaps critical APCs for expanding CD25+ CD4+ T cells (26), it is possible that DC maturation in NOD mice is less efficient in situ leading to a reduction in the number and function of regulatory CD25+ CD4+ T cells (34, 40). Further studies will address the ability of DCs isolated from NOD mice at different stages of diabetes pathogenesis to allow expansion of CD25+ CD4+ cells.
Autoimmunity may develop as a result of defects in either the initial number of CD25+ CD4+ T cells produced in the thymus, or the homeostasis and function of these cells in the periphery. Although some groups have reported a decrease in the numbers of CD25+ CD4+ T cells in NOD mice as compared with other strains, others report normal numbers in lymphoid organs of prediabetic mice (21, 23, 47). Lower numbers of CD25+ CD4+ T cells also have been reported in patients with type I diabetes (48). Nevertheless, the addition of polyclonal populations of CD25+ CD4+ T cells can delay onset and decrease diabetes incidence, although the number of cells required is relatively high, on the order of at least 0.5 × 106 per mouse (22, 23). In our studies, 105 CD25+ CD4+ T cells from NOD mice, even when expanded by DCs and anti-CD3 in culture, are inactive in suppressing diabetes, indicating that the antigen specificity of BDC2.5 regulatory T cells is critical. We do know that after DC expansion, almost all of the BDC CD25+ CD4+ T cells express high levels of the BDC TCR. CD25+ CD4+ TCR transgenic T cells can express second TCRs due to endogenous rearrangements of TCR-α genes (49, 50), making it possible that the expanded BDC2.5 T cells have additional reactivities with pancreatic autoantigens. In any case, it is unlikely that these second TCRs are critical for the observed disease-suppressive properties of expanded BDC2.5 T cells because the polyclonal repertoire of NOD T cells has so little activity.
In normal female NOD mice, there is a 4–8-wk gap between the beginning of lymphocyte infiltration of the pancreas, which starts between 3 and 8 wk of age (called checkpoint I), and overt destruction of the islets resulting in glucose dysregulation, which starts between 12 and 20 wk of age (called checkpoint II; references 1 and 51). When CD25+ CD4+ T cells are reduced in NOD mice as a result of genetic defects, the transition from initiation of insulitis to overt diabetes is rapid (21). This suggests that CD25+ CD4+ T cells might be important for controlling checkpoint II. Consistent with this, CD25+ CD4+ T cells from 8-wk-old NOD mice have recently been shown to suppress more efficiently than those from 16-wk-old mice (44). Our data showing insulitis in mice protected from diabetes development by BDC2.5 CD25+ CD4+ T cells is consistent with regulatory cells maintaining a benign pancreatic inflammation. However, because insulitis appears later in mice that received suppressor cells, these cells also appear to delay the initial inflammation of the islets.
DCs Control the Peripheral Expansion of CD25+ CD4+ T Suppressor T Cells.
Our results introduce a potentially critical role played by DCs in controlling autoreactive suppressor T cell function in the periphery. The function of CD25+ CD4+ T cells in regulating autoimmunity is well established and dramatized by the rapid development of autoimmunity including diabetes in humans and mice that lack the FoxP3 factor, which is required for the development of these T cells and possibly not other elements of the immune system (17, 19). However, APCs have not been studied. We now find that DCs expand disease-suppressive CD25+ CD4+ T cells and that the expanded cells are more efficient suppressors of autoreactivity than their freshly isolated counterparts, at least in vitro. Expansion of these regulatory cells also occurs with DCs and IL-2 in the absence of antigen, but then, suppression in vitro is comparable to freshly isolated, nonexpanded CD25+ CD4+ T cells. Therefore, stimulation through the TCR of CD25+ CD4+ regulatory cells increases their suppressive potency in culture by approximately fourfold. When one takes into account the 5–10-fold increase in cell numbers, the total increase in overall activity is at least 20-fold. The peripheral expansion of regulatory cells with antigen and DCs described here might be important for preventing autoimmunity in normal individuals by maintaining proper levels of CD25+ CD4+ T cells. We are pursuing this suggestion by targeting autoantigens directly to DCs in situ and then observing the consequences for T cells and autoimmune disease in situ.
CD25+ CD4+ Suppressor T Cells of One Specificity Can Control Autoimmune Disease Directed to Many Specificities.
The third issue, which is a major focus of this paper, is that CD25+ CD4+ T cells expanded with DCs and a single antigen can suppress diabetes in vivo and with very low numbers of cells (Fig. 5) compared with polyclonal CD25+ CD4+ T cells from NOD mice (22, 23, 44). Adoptive transfer of 5,000 fresh or DC-expanded BDC2.5 regulatory T cells is able to suppress disease induced by a polyclonal population of T cells from diabetic mice, whereas 100,000 anti-CD3–expanded NOD CD25+ CD4+ T cells are inactive. This may occur because the regulatory T cells are specific for an autoantigen expressed in β cells, the same tissue for which the pathogenic T cells are also specific. This in turn may allow the BDC2.5 CD25+ CD4+ T cells to home to or function in the islets and/or pancreatic lymph node more efficiently than NOD CD25+ CD4+ T cells of other specificities. Because a nonislet-specific TCR transgenic restricted to I-Ag7 has not been developed, it is not possible to assess the ability of such “control” T cells to block diabetes. Antigen-nonspecific regulation has been shown in vitro by using CD25− and CD25+ CD4+ T cells from different TCR transgenic mice. As long as the CD25+ CD4+ T cells are provided with their cognate antigen, they will suppress T cells of another specificity (24, 52). In our system, because CD25+ CD4+ T cells of one specificity can block the diabetes-inducing T cells of many specificities, this suggests that in vivo, regulation by CD25+ CD4+ T cells extends to other β cell antigens, not just those specifically recognized by the CD25+ CD4+ suppressor cell. This might involve local cytokine secretion (53), or the global blocking of APC function within the islets themselves or possibly in pancreatic lymph nodes.
Our findings suggest that DCs might be harnessed to develop new antigen-specific immunotherapies for autoimmunity based on CD25+ CD4+ suppressor T cells. For example, islet autoantigen–specific regulatory cells could be expanded ex vivo by culturing CD25+ CD4+ T cells from recently diagnosed diabetics with DCs loaded with islet antigens derived either from islet tissue or peptide mixtures of identified autoantigens. New assays of T cell responses in diabetic patients are being developed, which should be helpful for designing peptide mixtures (54), especially because some evidence suggests that CD25− CD4+ effector populations have a similar TCR repertoire as CD25+ CD4+ regulatory populations (32). The advantage of directing therapy to specific antigens is the avoidance of generalized immunosuppression, but the potential disadvantage is the possibility that generating tolerance to one antigen may not block a disease mediated by T cells with many specificities, as in NOD mice (7). However, it is now evident that DC- and antigen-expanded CD25+ CD4+ T cells of one specificity are potent at preventing diabetes pathogenesis mediated by NOD T cells.
Acknowledgments
We would like to thank Klara Velinzon for expert cell sorting and Knut Wittkowski for valuable biometrics advice.
This work was supported by a postdoctoral fellowship grant number 3-2002-750 to K. Tarbell from Juvenile Diabetes Research Foundation International, and grant number AI 51573-02 to R. Steinman from the National Institutes of Health.
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate succinimidyl ester; GITR, glucocorticoid-induced TNF receptor; NOD, nonobese diabetic.
References
- 1.Tisch, R., and H. McDevitt. 1996. Insulin-dependent diabetes mellitus. Cell. 85:291–297. [DOI] [PubMed] [Google Scholar]
- 2.Wong, F.S., and C.A. Janeway, Jr. 1999. Insulin-dependent diabetes mellitus and its animal models. Curr. Opin. Immunol. 11:643–647. [DOI] [PubMed] [Google Scholar]
- 3.Makino, S., K. Kunimoto, Y. Muraoka, Y. Mizushima, K. Katagiri, and Y. Tochino. 1980. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 29:1–13. [DOI] [PubMed] [Google Scholar]
- 4.Tisch, R., X.-Y. Yang, S.M. Singer, R.S. Libiau, L. Fugger, and H.O. McDevitt. 1993. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 366:72–75. [DOI] [PubMed] [Google Scholar]
- 5.Daniel, D., R.G. Gill, N. Schloot, and D. Wegmann. 1995. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 25:1056–1062. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman, S.M., A.M. Evans, B. Han, T. Takaki, Y. Vinnitskaya, J.A. Caldwell, D.V. Serreze, J. Shabanowitz, D.F. Hunt, S.G. Nathenson, et al. 2003. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 100:8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roep, B.O. 1996. T-cell responses to autoantigens in IDDM. The search for the Holy Grail. Diabetes. 45:1147–1156. [DOI] [PubMed] [Google Scholar]
- 8.Verge, C.F., R. Gianani, E. Kawasaki, L. Yu, M. Pietropaolo, H.P. Chase, and G.S. Eisenbarth. 1996. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J. Autoimmun. 9:379–383. [DOI] [PubMed] [Google Scholar]
- 9.Walker, L.S.K., and A.K. Abbas. 2002. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2:11–19. [DOI] [PubMed] [Google Scholar]
- 10.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suri-Payer, E., A.Z. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 12.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 13.Saoudi, A., B. Seddon, D. Fowell, and D. Mason. 1996. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J. Exp. Med. 184:2393–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 15.McHugh, R.S., and E.M. Shevach. 2002. Cutting edge: depletion of CD4+ CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J. Immunol. 168:5979–5983. [DOI] [PubMed] [Google Scholar]
- 16.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 17.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 19.Wildin, R.S., F. Ramsdell, J. Peake, F. Faravelli, J.L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20. [DOI] [PubMed] [Google Scholar]
- 20.Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. [DOI] [PubMed] [Google Scholar]
- 21.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 22.Szanya, V., J. Ermann, C. Taylor, C. Holness, and C.G. Fathman. 2002. The subpopulation of CD4+ CD25+ splenocytes that delays adoptive transfer of diabetes expresses l-selectin and high levels of CCR7. J. Immunol. 169:2461–2465. [DOI] [PubMed] [Google Scholar]
- 23.Wu, Q., B. Salomon, M. Chen, Y. Wang, L.M. Hoffman, J.A. Bluestone, and Y.X. Fu. 2001. Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J. Exp. Med. 193:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 25.Thornton, A.M., and E.M. Shevach. 1998. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki, S., T. Iyoda, K. Tarbell, K. Olson, K. Velinzon, K. Inaba, and R.M. Steinman. 2003. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen processing dendritic cells. J. Exp. Med. 198:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oldenhove, G., M. de Heush, G. Urbain-Vansanten, J. Urbain, C. Maliszewski, O. Leo, and M. Moser. 2003. CD4+ CD25+ regulatory T cells control T helper cell type 1 responses to foreign antigens induced by mature DCs in vivo. J. Exp. Med. 198:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker, L.S.K., A. Chodos, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisson, S., G. Darrasse-Jeze, E. Litvinova, F. Septier, D. Klatzmann, R. Liblau, and B.L. Salomon. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 198:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein, L., K. Khazaie, and H. Von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mottet, C., H.H. Uhlig, and F. Powrie. 2003. Cutting edge: cure of colitis by CD4+ CD25+ regulatory T cells. J. Immunol. 170:3939–3943. [DOI] [PubMed] [Google Scholar]
- 32.Hori, S., M. Haury, A. Coutinho, and J. Demengeot. 2002. Specificity requirements for selection and effector functions of CD25+ CD4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 99:8213–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz, J.D., B. Wang, K. Haskins, C. Benoist, and D. Mathis. 1993. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 74:1089–1100. [DOI] [PubMed] [Google Scholar]
- 34.Prasad, S.J., and C.C. Goodnow. 2002. Cell-intrinsic effects of non-MHC NOD genes on dendritic cell generation in vivo. Int. Immunol. 14:677–684. [DOI] [PubMed] [Google Scholar]
- 35.Prasad, S.J., and C.C. Goodnow. 2002. Intrinsic in vitro abnormalities in dendritic cell generation caused by non-MHC non-obese diabetic genes. Immunol. Cell Biol. 80:198–206. [DOI] [PubMed] [Google Scholar]
- 36.Wu, A.J., H. Hua, S.H. Munson, and H.O. McDevitt. 2002. Tumor necrosis factor-α regulation of CD4+ CD25+ T cell levels in NOD mice. Proc. Natl. Acad. Sci. USA. 99:12287–12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R.M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Judkowski, V., C. Pinilla, K. Schroder, L. Tucker, N. Sarvetnick, and D.B. Wilson. 2001. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J. Immunol. 166:908–917. [DOI] [PubMed] [Google Scholar]
- 39.Hoglund, P., J. Mintern, C. Waltzinger, W. Heath, C. Benoist, and D. Mathis. 1999. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 189:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feili-Hariri, M., and P.A. Morel. 2001. Phenotypic and functional characteristics of BM-derived DC from NOD and non-diabetes-prone strains. Clin. Immunol. 98:133–142. [DOI] [PubMed] [Google Scholar]
- 41.Andre-Schmutz, I., C. Hindelang, C. Benoist, and D. Mathis. 1999. Cellular and molecular changes accompanying the progression from insulitis to diabetes. Eur. J. Immunol. 29:245–255. [DOI] [PubMed] [Google Scholar]
- 42.Christianson, S.W., L.D. Shultz, and E.H. Leiter. 1993. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 42:44–55. [DOI] [PubMed] [Google Scholar]
- 43.Rohane, P.W., A. Shimada, D.T. Kim, C.T. Edwards, B. Charlton, L.D. Shultz, and C.G. Fathman. 1995. Islet-infiltrating lymphocytes from prediabetic NOD mice rapidly transfer diabetes to NOD-scid/scid mice. Diabetes. 44:550–554. [DOI] [PubMed] [Google Scholar]
- 44.Gregori, S., N. Giarratana, S. Smiroldo, and L. Adorini. 2003. Dynamics of pathogenic and suppressor T cells in autoimmune diabetes development. J. Immunol. 171:4040–4047. [DOI] [PubMed] [Google Scholar]
- 45.Tarbell, K.V., M. Lee, E. Ranheim, C.C. Chao, M. Sanna, S.K. Kim, P. Dickie, L. Teyton, M. Davis, and H. McDevitt. 2002. CD4+ T cells from glutamic acid decarboxylase (GAD)65-specific T cell receptor transgenic mice are not diabetogenic and can delay diabetes transfer. J. Exp. Med. 196:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verdaguer, J., D. Schmidt, A. Amrani, B. Anderson, N. Averill, and P. Santamaria. 1997. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186:1663–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berzins, S.P., E.S. Venanzi, C. Benoist, and D. Mathis. 2003. T-cell compartments of prediabetic NOD mice. Diabetes. 52:327–334. [DOI] [PubMed] [Google Scholar]
- 48.Kukreja, A., G. Cost, J. Marker, C. Zhang, Z. Sun, K. Lin-Su, S. Ten, M. Sanz, M. Exley, B. Wilson, et al. 2002. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 109:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. [PubMed] [Google Scholar]
- 50.Suto, A., H. Nakajima, K. Ikeda, S. Kubo, T. Nakayama, M. Taniguchi, Y. Saito, and I. Iwamoto. 2002. CD4(+)CD25(+) T-cell development is regulated by at least 2 distinct mechanisms. Blood. 99:555–560. [DOI] [PubMed] [Google Scholar]
- 51.Andre, I., A. Gonzalez, B. Wang, J. Katz, C. Benoist, and D. Mathis. 1996. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc. Natl. Acad. Sci. USA. 93:2260–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thornton, A.M., and E.M. Shevach. 2000. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183–190. [DOI] [PubMed] [Google Scholar]
- 53.Maloy, K.J., L. Salaun, R. Cahill, G. Dougan, N.J. Saunders, and F. Powrie. 2003. CD4+ CD25+ TR cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arif, S., T.I. Tree, T.P. Astill, J.M. Tremble, A.J. Bishop, C.M. Dayan, B.O. Roep, and M. Peakman. 2004. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J. Clin. Invest. 113:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]