Abstract
The induction of antigen-specific tolerance in the mature immune system of the intact organism has met with limited success. Therefore, nonspecific immunosuppression has been the treatment of choice to prevent unwanted immunity. Here, it is shown that prolonged subcutaneous infusion of low doses of peptide by means of osmotic pumps transforms mature T cells into CD4+25+ suppressor cells that can persist for long periods of time in the absence of antigen and confer specific immunologic tolerance upon challenge with antigen. The described procedure resembles approaches of tolerance induction used decades ago, induces tolerance in the absence of immunity, and holds the promise to become an effective means of inducing antigen-specific tolerance prospectively, whereas its power to suppress already ongoing immune responses remains to be determined.
Keywords: regulatory T cells, Ag-specific tolerance, in vivo induction, subcutaneous infusion, immunity
Introduction
Phenotypically distinct subsets of suppressor (regulatory) T cells have been described previously (1–8). Of particular interest are so-called “natural” CD4+25+ suppressor T cells that are present among thymocytes and peripheral T cells and that were shown to have an essential role in the prevention of autoimmunity (9). Such cells express the Foxp3 gene at relatively high levels (10–13). Mutations of the Foxp3 gene in both mice and man are associated with severe immunopathology (14–17). Experiments designed to elucidate the origin of CD4+25+ suppressor T cells in αβTCR transgenic mice have revealed that such cells can be formed intrathymically when the relevant agonist ligand for the αβTCR is expressed on radioresistant tissue of the thymus (2, 18, 19). Furthermore, in vivo analysis has shown that CD4+25+ suppressor T cells represent a lineage of cells that have a long intermitotic lifespan in the absence of Ag but are nevertheless committed to suppressive function upon challenge with Ag. In vivo CD4+25+ suppressive T cells are capable of extensive Ag-induced proliferation during which they up-regulate CD25 and develop increased suppressive activity by inhibiting the proliferation and cytokine production of other antigenically stimulated T cells (20–23). The question whether natural suppressor cells can be generated from naive T cells has been addressed in different ways. Chronic confrontation of naive T cells with Ag has resulted predominantly in the generation of anergic T cells that are CD25− and do not express Foxp3 (24), whereas, in some experiments, a small proportion of anergic and suppressive CD4+25+ cells was also noted (2). In other experiments, there was an apparent generation of CD25+ regulatory T cells from CD25− precursors when Ag was administered either i.v. or orally, even though selective survival of some preformed suppressor cells could not be entirely excluded and Foxp3 expression could not be analyzed (25). In a more recent paper, it was shown that anti-CD3 stimulation in the presence of TGFβ in vitro can result in the polyclonal stimulation of CD25+ Foxp3-expressing suppressor T cells (26). It was our interest to develop a protocol suitable to generate CD25+ Foxp3-expressing regulatory T cells with defined Ag specificity in vivo with the goal of generating Ag-specific tolerance in the absence of concomitant immunity in the fully mature immune system. Here, it is reported that CD4+25+ T cells with the characteristics of natural suppressor cells cannot only be generated intrathymically but can be derived de novo from naive T cells after prolonged s.c. infusion of their agonist peptide ligand in the absence of proliferation of peptide-specific T cells. The peripherally generated suppressor cells were indistinguishable in cell surface phenotype, lifespan, and functional aspects from intrathymically generated Foxp3 +CD4+25+ cells and were capable of mediating tolerance in various in vivo readout systems.
Materials and Methods
Animals and Peptide Delivery Pump Treatment.
BALB/c TCR-HA,RAG-2−/− mice, Thy1.1 BALB/c congenic TCR-HA,RAG-2−/− mice, TCR-HA,Ig-HA mice, BALB/c mice, and BALB/c nude mice used in this work were described previously (2). TCR-HA,Ig-HA mice and rat insulin promoter (INS-HA) transgenic BALB/c nude mice were bred in our animal facility. Unmanipulated 7–8-wk-old BALB/c mice or 7–8-wk-old BALB/c TCR-HA,RAG-2−/− mice that had been previously thymectomized (Tx) were implanted s.c. with mini-osmotic pumps (ALZET1002; Durect Corporation) diffusing PBS or the indicated amount of influenza HA (107-119) peptide per day for 14 d. Animal care and all procedures were in accordance with the guidelines of the Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Cell Preparations, Flow Cytometry, In Vitro Proliferation Assays, Adoptive Transfer Experiments, 5-Bromodeoxyuridine (BrdU) Incorporation Assay, and Immunization.
Cell preparations, flow cytometry procedures, proliferation assays, and 5,6-carboxyfluorescein diacetate-succinimidyl ester (CFSE) labeling of TCR-HA cells before their transfer were performed as described previously (2, 20). BrdU incorporation assays were performed as follows. Two osmotic pumps (ALZET2001; Durect Corporation) infusing 1.2 mg BrdU per day for 7 d were implanted s.c. at 7-d intervals. BrdU detection in triple-stained cells was done with a fluorescein isothiocyanate–conjugated anti-BrdU antibody (BD Biosciences) according to the BD Biosciences BrdU flow kit instruction manual. In transfer experiments, 2 × 105 CFSE-labeled T cells and 3 × 105 HA peptide-pulsed splenic DCs, prepared as described previously (2), were coinjected intrasplenically into BALB/c mice, and cell division of splenic CFSE-labeled T cells was examined by flow cytometry 4.5 d after transfer. DC immunization of Tx and peptide-infused TCR-HA,RAG-2−/− mice was conducted by injecting 5.7 × 105 HA peptide-pulsed DCs intrasplenically. Splenic TCR-HA–expressing cells of such immunized recipients were examined for intracellular cytokine production after 4.5 d. INS-HA transgenic BALB/c nude mice were injected with the indicated T cell subset into the lateral tail vein, and their blood glucose levels were measured in 2–3-d intervals. Peptide-infused (10 μg/d of HA peptide) or noninfused BALB/c mice were immunized into each footpad with 1 μg of HA protein (Protein Science Corporation) in IFA. 13 d after immunization, poplitae (draining) as well as mesenteric LN cells from either the aforementioned mice or unmanipulated control mice were collected and cultured at 3–5 × 106 cells/well in triplicate in vitro for 2.5 d without any stimulus, with 0.05 μg/ml of HA protein, or with 10 μg/ml of HA peptide. [3H]Thymidine at 1 μCi/well was added the last 16 h of the culture.
Real-Time RT-PCR.
cDNAs from flow cytometry–sorted cells were generated as described previously (2). Expression of Foxp3 and β-actin was quantified by real-time PCR in a sequence detection system (ABI Prism 7700; Applied Biosystems) using the TaqMan® 1000 RXN gold with Buffer A Pack (Applied Biosystems) as well as the following primers and internal fluorescent probes: Foxp3, 5′-GGCCCTTCTCCAGGACAGA-3′, 5′-GCTGATCATGGCTGGGTTGT-3′, and 5′-FAM-ACTTCATGCATCAGCTCTCCACTGTGGAT-TAMRA-3′; and β-actin, 5′-AGAGGGAAATCGTGCGTGAC-3′, 5′-CAATAGTGATGACCTGGCCGT-3′, and 5′-FAM-CACTGCCGCATCCTCTTCCTCCC-TAMRA-3′. For both Foxp3 and β-actin mRNA quantitation, each sample was run in triplicate at the same time as serial dilutions of a reference cDNA sample used to generate a standard curve. Reported Foxp3 mRNA levels are normalized to the β-actin mRNA levels of each sample.
Results
Instruction of Suppressor Commitment in Naive T Cells.
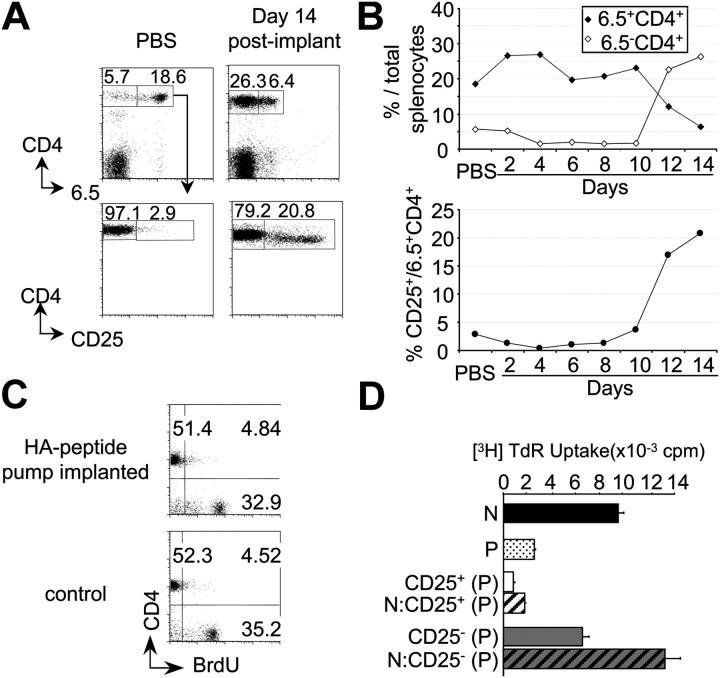
After initial hints that it might be possible to transform naive T cells into CD4+25+ cells in vivo (2), a protocol was designed to provide animals with a subimmunogenic regimen of peptide by implanting osmotic pumps that supplied relatively low doses of peptide in saline at a constant rate over a 2-wk time period. To exclude the possibility that CD4+25+ cells were generated intrathymically or that preexisting CD4+25+ suppressor T cells were simply expanded, the initial experiments were performed in adult Tx, RAG-2–deficient mice expressing a transgenic receptor specific for peptide 107-119 from influenza hemagglutinin (TCR-HA; reference 27; also referred to as TS1 mice; reference 18). When a daily dose of 10 μg of peptide was applied, two concomitant events took place after 10 d of continual infusion. CD4+25+ T cells that could be stained with the clonotypic 6.5 receptor antibody appeared with an increased frequency, whereas there was also an increase in CD4+ cells that could no longer be stained with the clonotypic reagent (Fig. 1, A and B). The latter may be due to masking and/or internalization and degradation of the idiotypic determinant. However, there was no detectable deletion as the absolute number of CD4+ T cells remained constant over this time period (unpublished data).
Figure 1.
Generation of CD25+ Ag-specific suppressor T cells by continuous peptide delivery for 14 d. (A) CD25 expression by 6.5+CD4+ splenocytes of Tx TCR-HA,RAG-2−/− mice 14 d after implantation of osmotic pumps delivering either PBS or 10 μg of HA peptide per day. (B) Percentages of 6.5+ or 6.5−CD4+ (top) and of CD25+6.5+CD4+ (bottom) T cells at various days of peptide delivery or 14 d after PBS infusion. (C) BrdU incorporation by splenocytes of either Tx TCR-HA,RAG-2−/− mice within the 14 d of peptide osmotic pump infusion or control TCR-HA,RAG-2−/− mice. (D) In vitro proliferation of flow cytometry–purified CD4+6.5+ T cells from untreated TCR-HA,RAG-2−/−(N) or from Tx TCR-HA,RAG-2−/− mice infused with 10 μg/d of HA peptide for 14 d (P; black and dotted bars, respectively), as well as CD25+ (white bar) and CD25− (gray bar) subsets of 6.5+CD4+ cells from peptide-infused mice either cultured alone (unstriped bars) or cocultured with naive 6.5+CD4+ T cells from untreated TCR-HA,RAG-2−/− mice (striped bars) in the presence of HA peptide-pulsed x-irradiated BALB/c nude splenocytes.
To examine whether this form of peptide application was accompanied by T cell activation and division as observed after i.v. injection or oral administration of Ag (25), mice were continuously infused with BrdU within the 14-d period of peptide application, and, thereafter, BrdU incorporation was determined. Fig. 1 C shows that infusion of peptide did not result in increased DNA synthesis by CD4+ T cells when compared with CD4+ T cells from control mice that were infused with BrdU in the absence of peptide over the same time period. Also, no BrdU incorporation greater than the level of unstimulated controls was detected after shorter BrdU pulses within the 14-d period, excluding cell division and subsequent death of cells (unpublished data). The absence of CD25 up-regulation on CD4+ cells at early stages (Fig. 1 B, bottom) indicated that the peptide infusion was not accompanied by T cell activation, as observed after immunogenic antigenic stimuli such as injection of peptide in IFA that resulted in transient and early CD25 up-regulation and proliferation, but not in the generation of cells stably expressing CD25 (20).
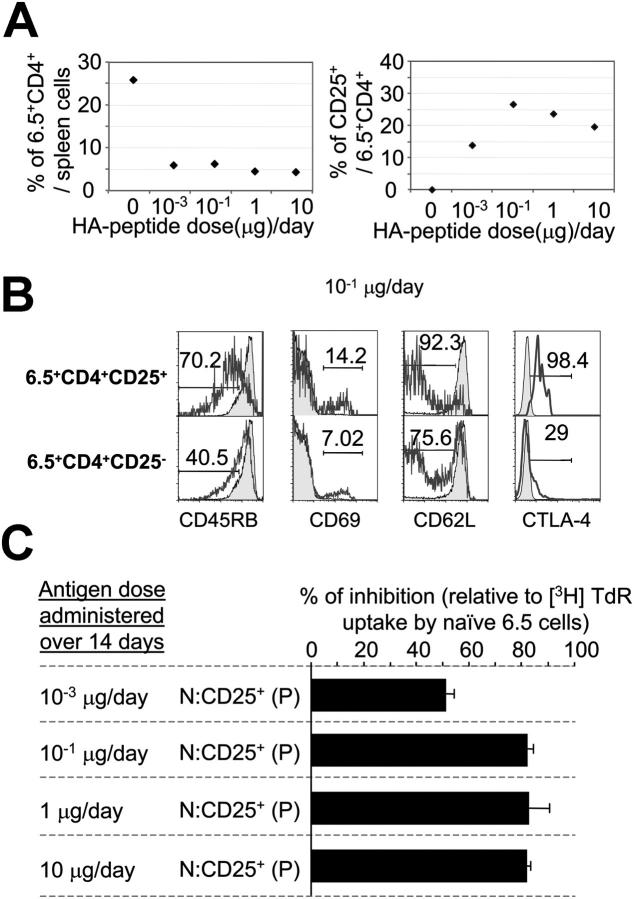
Titration of the constantly supplied peptide dose indicated that a daily dose of as little as 0.001 μg was sufficient to induce an increase in the frequency of CD4+25+ cells (Fig. 2 A) that, however, never exceeded 25% of all CD4+ 6.5+ cells. When CD25+ and CD25− CD4+ T cells from pump-implanted mice were analyzed in vitro, the former but not the latter were anergic in terms of Ag-induced proliferation (Fig. 1 D) and could suppress the proliferative response of naive T cells in the standard in vitro assay that is often used to identify suppressor T cells (28), irrespective of the inducing peptide dose (Fig. 2 C and not depicted). Thus, in this assay, the unfractionated CD4+6.5+ population of cells was unresponsive to antigenic stimulation after peptide infusion (i.e., it was composed of suppressor and suppressed cells; Fig. 1 D).
Figure 2.
Peptide dose–response relationship. (A) Percentages of 6.5+CD4+ (left) and of CD25+6.5+CD4+ (right) splenocytes in mice given either PBS (10 μg HA peptide/d) or 10−3, 10−1, 1, and 10 μg HA peptide/d for 14 d. (B) Expression of various markers by 6.5+CD4+ T cells of control mice (shaded histograms) and CD25+ and CD25− subsets of 6.5+CD4+ cells (unshaded histograms) of mice 14 d after implantation of osmotic pumps delivering 10−1 μg HA peptide/d. (C) Suppressive activity of CD25+CD4+ TCR-HA transgenic T cells from mice infused with various doses of HA peptide. Naive 6.5+CD4+ T cells from untreated TCR-HA,RAG-2−/− mice (N) were cultured alone or cocultured with sorted CD25+6.5+CD4+ T cells from Tx TCR-HA,RAG-2−/− mice (P, CD25+) given the indicated amount of peptide for 14 d in the presence of HA peptide-pulsed x-irradiated BALB/c nude splenocytes. Data are expressed as cpm ([3H]TdR incorporation) of coculture experiments over that of cultures of naive 6.5+CD4+ T cells from control TCR-HA,RAG-2−/− mice. One representative FACS® analysis of two independent experiments is shown for each peptide dosage in (A and B). All mice used in that experiment were age matched, Tx, and pump implanted at the same time.
In terms of some surface markers, CD4+25+ cells generated with all tested doses of peptide resembled intrathymically generated suppressor cells with high levels of CTLA-4 and low levels of CD45RB, whereas the CD4+25− cells with the same receptor were mostly CTLA-4 negative and CD45RB high, but in contrast with naive cells, had down-regulated the CD62L molecule (Fig. 2 B and not depicted).
Indistinguishable Phenotype of Natural and T Cell–derived Suppressors.
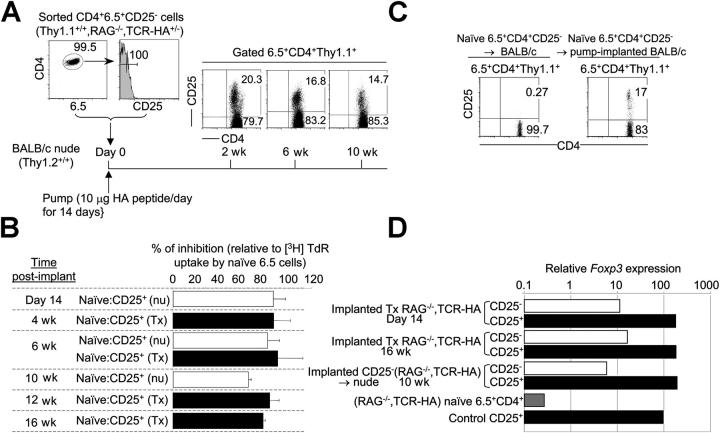
To confirm that the CD4+25+ cells were generated de novo from naive CD4+25− cells rather than representing a population of selected preexisting CD4+25+ cells, CD4+25− cells were sorted from TCR-HA,RAG-2−/− mice and injected into thymus-deficient nu/nu mice that were subsequently implanted with HA peptide–delivering osmotic pumps. The generated CD4+25+ cells could persist up to 10 wk after implantation of pumps (i.e., for 2 mo after termination of peptide delivery), while still being committed to develop suppressive activity after antigenic stimulation in vitro (Fig. 3, A and B). Similarly, CD4+25+ suppressor cells could persist for several months in Tx TCR-HA,RAG-2−/− mice after termination of peptide delivery (Fig. 3 B and not depicted). Thus, like natural suppressors, regulatory T cells obtained by peptide–MHC ligation of the TCR on naive T cells in vivo could persist for long periods of time in the absence of their agonist peptide–MHC complex in the form of CD25+ T cells committed to suppress after renewed stimulation by the TCR ligand.
Figure 3.
Long-term survival and Foxp3 expression by CD4+25+ T cells generated from naive T cells. (A) Sorted CD25 negative 6.5+CD4+ T cells from Thy1.1+/+,TCR-HA,RAG-2−/− mice were transferred into BALB/c (Thy1.2+/+) nu/nu recipients implanted with osmotic pumps delivering 10 μg/d of HA peptide for 14 d. Expression of CD25 was monitored at various time points after implantation. (B) Suppressive in vitro activity of CD4+25+ TCR-HA–expressing cells from transferred and pump-implanted nu/nu as well as implanted Tx TCR-HA,RAG-2−/− mice at various time points after delivery of peptide (10 μg/d) for 14 d. (C) Generation of CD4+25+ T cells from CD25 negative Thy1.1+/+RAG-2−/−CD4+6.5+ T cells transferred into normal BALB/c mice that were implanted with peptide-delivery osmotic pumps (10 μg/d). (D) cDNA was prepared from sorted CD25− 6.5+CD4+ and CD25+ 6.5+CD4+ subsets of either pump-implanted Tx TCR-HA,RAG-2−/− mice at day 14 and 16 wk, BALB/c nude mice adoptively transferred with naive CD25−6.5+CD4+ cells from Thy1.1+/+,TCR-HA,RAG-2−/− mice 10 wk after implantation of osmotic pumps delivering peptide for 14 d, naive 6.5+CD4+ cells from TCR-HA,RAG-2−/− mice, or control suppressor CD25+6.5+CD4+ cells of TCR-HA,Ig-HA mice. Foxp3 expression in each of the aforementioned samples was determined by real-time RT-PCR and normalized to its β-actin mRNA levels. Data are representative of at least two independent experiments.
When CD4+25− TCR-HA–expressing cells from RAG-2−/−,TCR-HA mice were transferred in low numbers to normal BALB/c mice such that they represented 0.2% of all CD4+ cells, ∼20% of such cells became CD4+25+ cells 14 d after implantation of HA peptide–delivering pumps, whereas they remained CD4+25− in untreated recipients (Fig. 3 C). Thus, the described procedure is effective in inducing suppressor T cells from naive T cells in a normal environment. Characterization of de novo peptide-induced CD4+25+ suppressor cells by quantitative real-time PCR analysis of Foxp3 expression showed that, as a rule, CD4+25+ cells in various recipients and at various time points after their induction expressed >10-fold higher levels of this gene than CD4+25− cells within the same mice as well as levels of Foxp3, which were similar to that of control CD25+CD4+ naturally generated suppressor T cells (Fig. 3 D; reference 2). However, CD4+25− cells clearly contained some significant levels of Foxp3 RNA (Fig. 3 D), which could suggest that either they had initiated, but not completed, a pathway to become suppressor T cells or that they contained a minor fraction of CD25− suppressor T cells too infrequent to suppress the proliferation of all CD25− cells. Thus, also by the criterion of Foxp3 expression, CD4+25+ suppressor T cells generated de novo from mature naive T cells resembled intrathymically generated CD4+25+ suppressor cells.
Induction of Ag-specific Dominant Tolerance In Vivo.
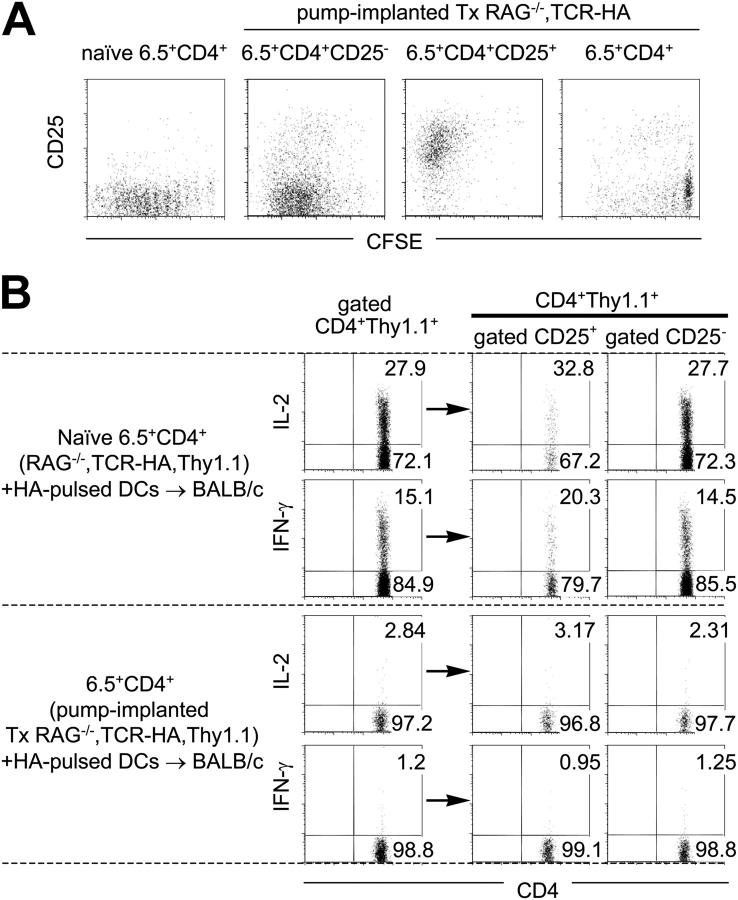
It was essential to demonstrate that the described procedure did not only result in unresponsiveness of cocultured T cells in vitro but also that the peripherally induced suppressor cells could prevent the generation of effector T cells in vivo. This was evaluated by injecting peptide-pulsed splenic DCs into previously Tx and peptide-infused TCR-HA,RAG-2−/− recipients (unpublished data) and by adoptive transfer of CD4+ T cells from peptide-infused and control mice into normal BALB/c mice that were subsequently challenged with Ag-pulsed splenic DCs. Fig. 4 A shows that flow cytometry–purified naive CD4+6.5+ as well as CD25+ and CD25−CD4+ cells from peptide-infused mice underwent seven to nine cell divisions after antigenic challenge in vivo, but that the vast majority of CD25− cells, when contained in the entire sorted CD4+ 6.5+ population (i.e., in the presence of CD4+25+ suppressive cells), exhibited much less proliferative potential. Thus, expanding Ag-specific CD4+25+ suppressor cells that appeared to expand less well in the presence of CD4+25− cells with the same antigenic specificity could very significantly suppress the in vivo proliferation of the latter as reported previously (2, 20). Furthermore, although 20–30% of naive TCR-HA–expressing T cells transferred and antigenically challenged in normal BALB/c mice produced IL-2 as well as IFN-γ (irrespective of whether the entire CD4+ or only the recently activated CD4+25+Thy1.1+ or the CD4+25−Thy1.1+ population was examined), this did not occur when the same number of CD4+ 6.5+ cells was transferred from donors implanted previously with peptide-delivering pumps (Fig. 4 B). Also, the CD4+25+ suppressor subset from peptide-infused mice failed to produce IL-2 and IFN-γ (Fig. 4 B) as well as IL-4 and IL-10 (not depicted) after in vivo stimulation, which suggests that the suppressive activity does not depend on the production of these cytokines. Thus, by the criteria of both proliferation and cytokine production, the peptide delivery had caused immunological tolerance in the entire CD4+ T cell population.
Figure 4.
Tolerance in CD4+ cell populations from peptide-infused mice. Sorted naive 6.5+CD4+ T cells of control Thy1.1+/+,TCR-HA,RAG-2−/− mice, sorted CD4+ cells, and CD25+CD4+ and CD25−CD4+ subsets of Ag-specific 6.5+ cells of Tx Thy1.1+/+,TCR-HA,RAG-2−/− mice treated with 10 μg/ml of HA peptide for 14 d were either CFSE labeled (A) or not labeled (B) and intrasplenically coinjected with HA peptide-pulsed DCs into BALB/c mice. (A) 4.5 d later, splenocytes of recipient mice were stained with CD4, CD25, and Thy1.1 mAbs. Dot plots show CD25 versus CFSE profiles of gated CD4+ Thy1.1+ cells. (B) Splenocytes harvested at day 4.5 were stimulated in vitro with HA peptide and stained with CD4, CD25, and Thy1.1 antibodies as well as antibodies directed against cytokines. Staining for intracellular IL-2 and IFN-γ is shown for gated CD4+Thy1.1+ cells as well as their respective CD25+ and CD25− subsets.
Finally, although naive CD4+6.5+ cells from TCR-HA,RAG-2−/− mice, when transferred into nu/nu mice that express HA under control of INS-HA, caused diabetes within 9 d, INS-HA–expressing mice receiving the same number of CD4+6.5+ cells from peptide-treated mice remained diabetes free and exhibited normoglycemia for up to 15, 24, or 33 wk when analysis was terminated (Table I). It is worth noting that INS-HA recipient mice that received peptide delivery by osmotic pumps 12 h before being injected with naive CD4+6.5+ T cells did not develop diabetes, suggesting that the described procedure might be suited to interfere with ongoing immune responses (Table I).
Table I.
Peptide Infusion Interferes with Diabetogenic Potential of T Cells
| Recipient mouse | Donor mouse | Transferred cells (day 0) |
No. of transferred cells |
Diabetic recipients |
Days of diabetes onseta |
|---|---|---|---|---|---|
| INS-HA nude | RAG-2−/−, TCR-HA | 6.5+CD4+ | 5 × 104 | 4/4 | 9.25 ± 0.5 |
| INS-HA nude | implanted Tx RAG-2−/−, TCR-HA | 6.5+CD4+ | 5 × 104 | 0/3b | – |
| INS-HA nudec | RAG-2−/−, TCR-HA | 6.5+CD4+ | 5 × 104 | 0/4d | – |
Mean ± SD.
One mouse showed normoglycemia for up to 33 wk of follow up, another one for up to 24 wk of follow up, and the last one for up to 15 wk of follow up.
INS-HA nude recipients were pump implanted −0.5 d before cell transfer.
Two recipients showed normoglycemia for up to 24 wk and two others for up to 15 wk after the examination was terminated.
Induction of Ag-specific Tolerance in Nontransgenic Mice.
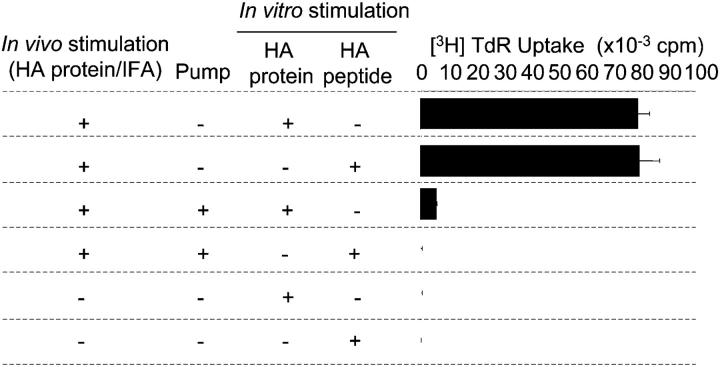
It was important to test whether tolerance could not only be induced in cells expressing transgenic TCRs but also in nonmanipulated CD4+ cells by the described protocol. Therefore, BALB/c mice or HA peptide-infused BALB/c mice (15 d after pump implant) were immunized into each footpad with 1 μg of highly immunogenic HA protein in IFA. 13 d after immunization, draining LN cells as well as mesenteric LN cells were collected and restimulated in vitro with either the entire HA protein or the HA peptide. Draining LN cells of immunized control mice proliferated vigorously to both protein and peptide stimuli (Fig. 5), whereas mesenteric LN cells from immunized mice and LN cells of nonimmunized BALB/c mice did not (not depicted). HA peptide infusion of animals before immunization resulted in the complete suppression of the in vitro recall response of draining LN cells to both in vitro stimuli (Fig. 5). Thus, the described peptide infusion is suited to induce tolerance to the entire protein from which the peptide is derived in normal mice.
Figure 5.
Ag-specific tolerance in peptide-infused nontransgenic BALB/c mice. Control BALB/c mice and HA peptide-infused BALB/c mice were immunized into the footpad with HA protein in IFA. Draining LN cells of the latter mice as well as popliteal LN cells of unmanipulated BALB/c mice were stimulated in vitro with either the whole HA protein or the infused HA peptide. Data are mean ± SD from triplicate wells and are representative of at least two independent experiments.
Discussion
The described experiments indicate that a relatively simple procedure of delivering peptide for a prolonged time period in subimmunogenic forms is suited to induce de novo CD4+25+ suppressor T cells from naive T cells in peripheral lymphoid organs in the absence of a functioning thymus as well as in the absence of a developing immune response. By all investigated criteria (i.e., surface phenotype, long-term stability in the absence of the inducing TCR ligand, Foxp3 expression, Ag-induced expansion in vivo, and suppressive activity in vitro and in vivo as well as cytokine production), the induced suppressor T cells are indistinguishable from intrathymically generated CD4+25+ T cells that were shown to have an essential role in preventing autoimmunity under physiological conditions.
The described experiments are akin to studies conducted by i.v. injection of soluble proteins that were described decades ago and that became known as “low zone tolerance” experiments (29, 30). It is well possible that, also under those condition, tolerance was due to the induction of suppressor cells as might also be the case in desensitization attempts that have the goal to reduce allergic reactions by applying relevant protein derivatives.
In vivo and ex vivo inductions of suppressor T cells by chronic antigenic stimulation was achieved by several groups (7, 24, 31–35), but gave rise to regulatory T cell subsets that appear to be distinct from natural CD25+ suppressor cells. Some in vivo protocols made use of chemicals that affect the entire immune system and, thus, may not be ideally suited to induce Ag-specific unresponsiveness (36). Ex vivo generation of Ag-specific CD25+ suppressor T cells with IL-2 and TGF-β that resemble naturally occurring suppressor T cells has been reported previously (36, 37), but little is known about the long-term survival of these cells in vivo in the absence of Ag. In one particular setting of suppressor T cell induction in vivo, it was found that CD4+25+ anergic cells could accumulate after i.v. or oral administration of Ag, but Foxp3 expression was not analyzed (25). Whether this resulted from de novo generation of suppressor cells or their selection was not entirely clear. Also, this particular protocol appeared to induce a transient immune response that was not observed after peptide infusion.
The mechanism by which the peripheral differentiation of naive T cells into CD25+ Ag-specific suppressor cells that resemble by all criteria natural suppressor cells as reported in the present work remains to be defined. It is possible that this process could be initiated by interactions with HA peptide–presenting nonactivated DCs such as epidermal Langerhans cells, which are at the site of osmotic pump implant, can endocytose efficiently, and are endowed with trafficking capacity to secondary lymphoid tissues where they can induce tolerance in the steady state (38, 39). Thus far, it has been demonstrated that Ag presentation by nonactivated DCs can result in recessive tolerance (40, 41), but it is possible that low doses of peptide presented over relatively long time periods (10 d) on nonactivated DCs represents a natural way of inducing active immunosuppression by which the immune system prevents immune responses to self components that do not induce tolerance in the thymus. Such a scenario may also lead to the suppression of antitumor responses when tumor-specific peptides are presented in subimmunogenic form. At present, it is not entirely clear how the immune system can evade this suppression, but strong activation of APCs may be one mechanism (42).
The fact that naive CD25− T cells can be instructed in vivo, as reported here, or in vitro (13, 26) to become Foxp3-expressing CD25+ suppressor cells raises the question of whether naturally occurring Foxp3-expressing CD25+ suppressor T cells that develop in the thymus constitute a lineage distinct from that of their peripherally induced counterparts, or whether such cells reflect the existence of a single Foxp3-expressing naturally occurring CD25+ suppressor lineage that can develop under noninflammatory steady state conditions in primary as well as secondary lymphoid tissues.
It needs to be tested whether the procedure described here will be suited to induce prospective tolerance to a large variety of Ags, including transplantation Ags. By means of single peptides, efficient suppression could be achieved because suppressor cells cannot only suppress responses of T cells with the same Ag specificity but also responses of neighboring T cells that happen to interact with other ligands in the vicinity of suppressor T cells (bystander suppression). Thus, it will be of interest to monitor whether, eventually, the in vivo induction of Ag-specific suppressor cells by the described procedure will make general immune suppression the therapy of the past.
Acknowledgments
The authors thank M. Handley for FACS® sorting and X. Li for technical assistance.
This work was supported by the Koerber Foundation (to H. von Boehmer) and National Institutes of Health grant AI53102.
Abbreviations used in this paper: BrdU, 5-bromodeoxyuridine; CFSE, 5,6-carboxyfluorescein diacetate-succinimidyl ester; INS-HA, rat insulin promoter; Tx, thymectomized.
References
- 1.Bach, J.F. 2003. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 3:189–198. [DOI] [PubMed] [Google Scholar]
- 2.Apostolou, I., A. Sarukhan, L. Klein, and H. Von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. [DOI] [PubMed] [Google Scholar]
- 3.Stephens, L.A., and D. Mason. 2000. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25−subpopulations. J. Immunol. 165:3105–3110. [DOI] [PubMed] [Google Scholar]
- 4.Olivares-Villagomez, D., A.K. Wensky, Y. Wang, and J.J. Lafaille. 2000. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J. Immunol. 164:5499–5507. [DOI] [PubMed] [Google Scholar]
- 5.Filaci, G., and N. Suciu-Foca. 2002. CD8+ T suppressor cells are back to the game: are they players in autoimmunity? Autoimmun. Rev. 1:279–283. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483–513. [DOI] [PubMed] [Google Scholar]
- 7.Barrat, F.J., D.J. Cua, A. Boonstra, D.F. Richards, C. Crain, H.F. Savelkoul, R. de Waal-Malefyt, R.L. Coffman, C.M. Hawrylowicz, and A. O'Garra. 2002. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levings, M.K., R. Sangregorio, C. Sartirana, A.L. Moschin, M. Battaglia, P.C. Orban, and M.G. Roncarolo. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 10.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 12.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 13.Walker, M.R., D.J. Kasprowicz, V.H. Gersuk, A. Benard, M. Van Landeghen, J.H. Buckner, and S.F. Ziegler. 2003. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J. Clin. Invest. 112:1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 15.Bennett, C.L., J. Christie, F. Ramsdell, M.E. Brunkow, P.J. Ferguson, L. Whitesell, T.E. Kelly, F.T. Saulsbury, P.F. Chance, and H.D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21. [DOI] [PubMed] [Google Scholar]
- 16.Wildin, R.S., F. Ramsdell, J. Peake, F. Faravelli, J.L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20. [DOI] [PubMed] [Google Scholar]
- 17.Chatila, T.A., F. Blaeser, N. Ho, H.M. Lederman, C. Voulgaropoulos, C. Helms, and A.M. Bowcock. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan, M.S., M.P. Riley, H. von Boehmer, and A.J. Caton. 2000. Anergy and suppression regulate CD4+ T cell responses to a self peptide. Eur. J. Immunol. 30:136–144. [DOI] [PubMed] [Google Scholar]
- 19.Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 20.Klein, L., K. Khazaie, and H. von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisson, S., G. Darrasse-Jeze, E. Litvinova, F. Septier, D. Klatzmann, R. Liblau, and B.L. Salomon. 2003. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J. Exp. Med. 198:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki, S., T. Iyoda, K. Tarbell, K. Olson, K. Velinzon, K. Inaba, and R.M. Steinman. 2003. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker, L.S., A. Chodos, M. Eggena, H. Dooms, and A.K. Abbas. 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagler-Anderson, C., A.K. Bhan, D.K. Podolsky, and C. Terhorst. 2004. Control freaks: immune regulatory cells. Nat. Immunol. 5:119–122. [DOI] [PubMed] [Google Scholar]
- 25.Thorstenson, K.M., and A. Khoruts. 2001. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188–195. [DOI] [PubMed] [Google Scholar]
- 26.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirberg, J., A. Baron, S. Jakob, A. Rolink, K. Karjalainen, and H. von Boehmer. 1994. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex–restricted receptor. J. Exp. Med. 180:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchison, N.A. 1964. Induction of immunological paralysis in two zones of dosage. Proc. Roy. Soc. 161:275–292. [DOI] [PubMed] [Google Scholar]
- 30.Chiller, J.M., G.S. Habicht, and W.O. Weigle. 1971. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 171:813–815. [DOI] [PubMed] [Google Scholar]
- 31.Chen, Y., V.K. Kuchroo, J. Inobe, D.A. Hafler, and H.L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 265:1237–1240. [DOI] [PubMed] [Google Scholar]
- 32.Weiner, H.L. 1997. Oral tolerance for the treatment of autoimmune diseases. Annu. Rev. Med. 48:341–351. [DOI] [PubMed] [Google Scholar]
- 33.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A.H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundstedt, A., E.J. O'Neill, K.S. Nicolson, and D.C. Wraith. 2003. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 170:1240–1248. [DOI] [PubMed] [Google Scholar]
- 35.Miller, A., O. Lider, A.B. Roberts, M.B. Sporn, and H.L. Weiner. 1992. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc. Natl. Acad. Sci. USA. 89:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwitz, D.A., S.G. Zheng, J.D. Gray, J.H. Wang, K. Ohtsuka, and S. Yamagiwa. 2004. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin. Immunol. 16:135–143. [DOI] [PubMed] [Google Scholar]
- 37.Zheng, S.G., J.H. Wang, M.N. Koss, F. Quismorio, Jr., J.D. Gray, and D.A. Horwitz. 2004. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-β suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 172:1531–1539. [DOI] [PubMed] [Google Scholar]
- 38.Hemmi, H., M. Yoshino, H. Yamazaki, M. Naito, T. Iyoda, Y. Omatsu, S. Shimoyama, J.J. Letterio, T. Nakabayashi, H. Tagaya, et al. 2001. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 13:695–704. [DOI] [PubMed] [Google Scholar]
- 39.Akbari, O., R.H. DeKruyff, and D.T. Umetsu. 2001. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2:725–731. [DOI] [PubMed] [Google Scholar]
- 40.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J.V. Ravetch, R.M. Steinman, and M.C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawahata, K., Y. Misaki, M. Yamauchi, S. Tsunekawa, K. Setoguchi, J. Miyazaki, and K. Yamamoto. 2002. Peripheral tolerance to a nuclear autoantigen: dendritic cells expressing a nuclear autoantigen lead to persistent anergic state of CD4+ autoreactive T cells after proliferation. J. Immunol. 168:1103–1112. [DOI] [PubMed] [Google Scholar]
- 42.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]