Abstract
Reduced levels of wild-type mannose-binding lectin (MBL) may increase susceptibility for infection, other common diseases, and death. We investigated associations between MBL deficiency and risk of infection, other common diseases, and death during 24, 24, and 8 yr of follow-up, respectively. We genotyped 9,245 individuals from the adult Danish population for three MBL deficiency alleles, B, C, and D, as opposed to the normal noncarrier A allele. Hospitalization incidence per 10,000 person · yr was 644 in noncarriers compared with 631 in heterozygotes (log-rank: P = 0.39) and 658 in deficiency homozygotes (P = 0.53). Death incidence per 10,000 person · yr was 235 in noncarriers compared with 244 in heterozygotes (P = 0.44) and 274 in deficiency homozygotes (P = 0.12). After stratification by specific cause of hospitalization or death, only hospitalization from cardiovascular disorders was increased in deficiency homozygotes versus noncarriers (P = 0.02). When retested in two case control studies, this association could not be confirmed. Incidence of hospitalization or death from infections or other serious common disorders did not differ between deficiency homozygotes and noncarriers. In conclusion, in this large study in an ethnically homogenous Caucasian population, there was no evidence for significant differences in infectious disease or mortality in MBL-deficient individuals versus controls. Our results suggest that MBL deficiency is not a major risk factor for morbidity or death in the adult Caucasian population.
Keywords: complement, mannan, innate immunity, infection, genetics
Introduction
Infectious diseases are important causes of morbidity and mortality worldwide (1, 2). Our first set of immune responses during infection constitutes the innate immune system. A central player in this system is mannose-binding lectin (MBL), an acute phase protein with the capacity to bind to a broad range of bacteria and vira and subsequently kill them by activating the complement system (3–5). About 5% of Caucasians have an inherited deficiency of MBL leading to reduced plasma levels of wild-type MBL protein (5–7). This may impair normal innate immune function and increase susceptibility for infection (8). Because MBL may also play an important role in other serious common diseases like atherosclerosis (9, 10), chronic pulmonary disease (11, 12), and cancer (13, 14), MBL deficiency could also affect risk of these diseases and mortality.
MBL deficiency is associated with homozygosity for either of the three deficiency alleles of the MBL gene, the B, C, and D alleles (5–7). These mutant alleles are also designated O alleles as opposed to the normal noncarrier A allele. In previous studies, MBL deficiency has been associated with increased susceptibility for bacterial and viral infections (8), as well as with many other diseases like atherosclerosis (9, 10), leukemia (14), and miscarriage (15). Therefore, and because of the high population frequency of MBL deficiency, this condition could play an important role in public health. However, the significance of MBL deficiency in the adult general population remains relatively unknown because previous studies were mainly based on hospital cases or children (5, 9–25).
We tested the hypothesis that MBL deficiency homozygotes and heterozygotes compared with noncarriers are at increased risk of morbidity and mortality from infectious and other common diseases in the adult general population. For this purpose, we screened 9,245 individuals from the adult Danish population for the B, C, and D MBL deficiency alleles, and associated MBL genotype with risk of infection, other common diseases, and death during median follow-up periods of 24, 24, and 8 yr, respectively.
Materials and Methods
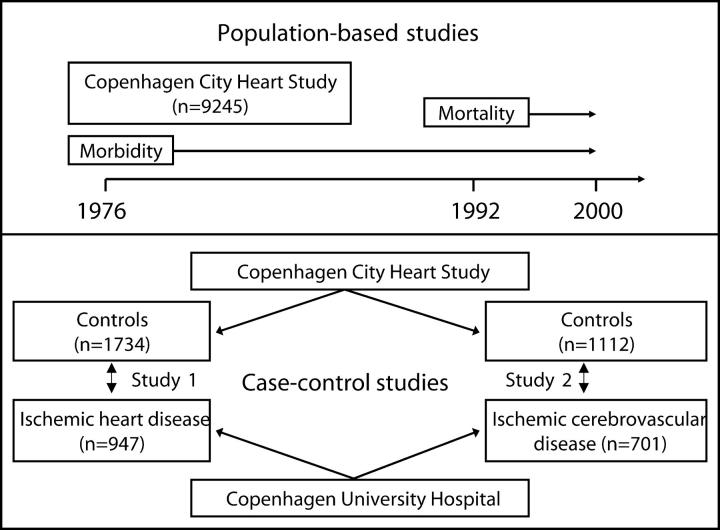
All subjects who participated in the 1991–1994 examination of the Copenhagen City Heart Study were included in this population-based study (Fig. 1, top; reference 26). The participants aged 20 yr and above were selected randomly after age stratification into 5-yr age groups from among residents of Copenhagen. Of the 17,180 individuals invited, 10,135 participated, 9,259 gave blood, and 9,245 were genotyped for the B, C, and D deficiency alleles of the MBL gene. Details of study procedures (26–29) and some characteristics of nonresponders (30, 31) are described elsewhere. More than 99% were Caucasians of Danish descent. All participants gave written informed consent, and the ethics committee for Copenhagen and Frederiksberg approved the study (no. 100.2039/91).
Figure 1.
Study design.
Participants at the Copenhagen City Heart Study examination filled out a self-administered questionnaire, which was validated by the participant and an investigator on the day of attendance. Participants reported on smoking habits (current smoker, ex-smoker, or never smoker), weekly physical activity (<2 h light activity, 2–4 h light activity, >4 h light activity, or 2–4 h heavy activity or >4 h heavy activity), and on weekly alcohol consumption. Recent respiratory infection was self-reported (cold, bronchitis, or lung infection) 4 wk before the Copenhagen City Heart Study examination. Blood pressure was measured by trained technicians using a London School of Hygiene Sphygmomanometer on the left arm after 5 min rest with the subject in the sitting position (32). Cholesterol was determined enzymatically by conventional methods (CHOD-PAP; Boehringer). Body mass index was weight divided by height squared. Spirometry-defined chronic obstructive pulmonary disease was FEV1 < 80% of predicted and FEV1/FVC < 0.7, excluding self-reported asthma.
Based on the World Health Organization International Classification of Diseases 8th and 10th Editions, additional information on morbidity was drawn from the Danish National Hospital Discharge Register (data from May 1, 1976 to December 31, 2000 was available), whereas data on mortality was drawn from the Danish National Register of Causes of Death (data from April 1, 1992 to December 31, 1999 was available for cause-specific deaths, whereas data for death only was available until December 31, 2001). Of the 1,726 deaths, which occurred in the population sample from 1991 through 2001, 1,065 (62%) had not yet been assigned with a certain death cause in the Danish National Register of Causes of Death. We grouped all diagnoses based on the Global Burden of Disease classification system (1, 2, 33). This paper focuses on groups of disease with high morbidity and mortality in developed countries. Subclassification of the disease groups was performed for endpoints particularly relevant for MBL deficiency.
During 1991 through 1993, we also recruited 947 patients with ischemic heart disease (IHD) referred for coronary angiography at Copenhagen University Hospital. Experienced cardiologists diagnosed IHD on the basis of characteristic symptoms of stable angina pectoris plus at least one of the following: severe stenosis on coronary angiography, myocardial infarction, or a positive result on exercise electrocardiography. We further identified 701 patients with ischemic cerebrovascular disease (ICVD) referred to Copenhagen University Hospital for outpatient ultrasonography of the carotid artery during 1994 through 2001. ICVD was diagnosed on the basis of ischemic stroke, transient ischemic attack, or amaourosis fugax, together with carotid artery stenosis ≥50%. All patients had computed tomography scans performed, and those with cerebral hemorrhage were excluded. All 947 and 701 patients with IHD and ICVD were genotyped. For each of these prevalent cases with ICVD and IHD, two control subjects of the same gender, age (within 1 yr), and smoking status (current smoker, ex-smoker, or never smoker) were randomly selected among Copenhagen City Heart Study participants without ICVD and IHD using a computer matching program for SPSS. For IHD and ICVD patients, 1,734 and 1,112 controls fulfilled all three matching criteria, respectively (Fig. 1, bottom).
We identified the B (Gly54Asp), C (Gly57Glu), and D (Arg52Cys) deficiency alleles of the MBL gene by RFLP-PCR using the primers 5′-CATCAACGGCTTCCCAGGCAAAGACGCG-3′ (sense) and 5′-AGGATCCAGGCAGTTTCCTCTGGAAGG-3′ (antisense; mismatches are underlined; reference 34). The presence of the B and D polymorphisms destroy a BanI and HhaI site, respectively, whereas the presence of the C polymorphism creates an MboII site. After restriction enzyme digestion with BanI, HhaI, and MboII, fragments of 90 plus 35 (normal allele) or 125 bp (B allele), and fragments of 97 plus 28 bp (normal allele), 125 bp (D allele), or 69 plus 28 plus 28 bp (C allele), were separated on two 4% agarose gels. Homozygotes and compound heterozygotes were reanalyzed to confirm the diagnosis. Less severe promoter polymorphisms (6, 7) were not detected using this assay.
Statistical analysis was performed with SPSS. P < 0.05 on a two-sided test was considered significant. Pearson's χ2-test, Kruskal Wallis test, or ANOVA were used for univariate analyses. Because deficiency B, C, and D alleles reduce plasma wild-type MBL at about similar magnitudes in caucasian Danish adults (34), our initial analyses were performed for the three alleles combined as O alleles versus the A allele. However, we also looked at heterozygotes, compound heterozygotes, and homozygotes for each of the B, C, and D alleles separately versus the A allele. As in previous studies, because genotype was already present at birth and preceded the outcomes studied, we evaluated morbidity and mortality incidences (Fig. 1, top) between genotypes using the log-rank test (27–29). Cox regression analysis with forced entry examined time to disease or death by using hazard ratios (relative risks) and 95% confidence intervals (CIs). The model adjusted for gender, age (deciles), smoking habits (current smoker, ex-smoker, and never smoker), systolic blood pressure (deciles), alcohol consumption (nonusers and deciles), plasma cholesterol (deciles), body mass index (deciles), and physical activity (<2 h light activity weekly, 2–4 h light activity, >4 h light activity, or 2–4 h heavy activity or >4 h heavy activity weekly). Unadjusted conditional logistic regression estimated odds ratios in matched case control studies 1 and 2 (Fig. 1, bottom).
Role of the Funding Source.
The sponsors of the study are public or nonprofit organizations and support science in general. They had no role in gathering, analyzing, or interpreting the data and could neither approve nor disapprove the submitted manuscript.
Results
MBL genotype frequencies in this sample from the Danish general population were 58% for A/A noncarriers, 37% for A/O heterozygotes, and 5% for O/O deficiency homozygotes. Frequencies for the B, C, and D alleles were 0.145, 0.017, and 0.076, respectively. Genotype frequencies did not differ from those predicted by the Hardy-Weinberg equilibrium (χ2-test for B allele: P = 0.75; C allele: P = 0.29; D allele: P = 0.87). Distribution of baseline characteristics in this population did not differ between individuals with the three different genotypes (Table I).
Table I.
Baseline Characteristics of Subjects Sampled from the General Population by MBL Deficiency Genotype
| Noncarriers | Heterozygotes | Homozygotes | P-valuea | |
|---|---|---|---|---|
| Women/Men | 2,962/2,398 | 1,880/1,502 | 275/228 | 0.91 |
| Age, yr | 57 ± 0.2 | 58 ± 0.3 | 58 ± 0.7 | 0.41 |
| Current smokers (%) | 2,621 (49) | 1,624 (48) | 246 (49) | 0.82 |
| Systolic blood pressure, mmHg | 138 ± 0.3 | 139 ± 0.4 | 140 ± 1.0 | 0.21 |
| Alcohol consumption, g/wk | 114 ± 2.1 | 113 ± 2.6 | 109 ± 6.7 | 0.61 |
| Plasma cholesterol, mmol/L | 6.1 ± 0.02 | 6.2 ± 0.02 | 6.2 ± 0.06 | 0.13 |
| Body mass index, kg/m2 | 26 ± 0.2 | 26 ± 0.1 | 26 ± 0.1 | 0.59 |
| Physical inactivityb(%) | 664 (13) | 390 (12) | 60 (12) | 0.36 |
Values are number of individuals (%) or mean ± SEM.
P-values by Pearson's χ2-test, Kruskal Wallis test, or analysis of variance. To approach normal distribution, body mass index was logarithmically transformed before statistical analysis.
Physical inactivity was <2 h of light physical activity per week.
Morbidity.
Incidence of any hospitalization per 10,000 person · yr was 644 in noncarriers compared with 631 in heterozygotes (log-rank: P = 0.39) and 658 in deficiency homozygotes (P = 0.53; Table II). After adjustment for gender, age, smoking habits, systolic blood pressure, alcohol consumption, cholesterol level, body mass index, and physical activity, the relative risks for any hospitalization were not significantly different from 1.0 in heterozygotes or deficiency homozygotes compared with noncarriers.
Table II.
Morbidity Leading to Hospitalization According to MBL Deficiency Genotype during 24 yr Follow-up
| Noncarriers
|
Heterozygotes
|
Homozygotes
|
||||||
|---|---|---|---|---|---|---|---|---|
| IncidenceN/10,000person · yr | RRa | IncidenceN/10,000person · yr | P-valueb | RRa(95% CI) | IncidenceN/10,000person · yr | P-valueb | RRa(95% CI) | |
| Any hospitalization | 644 | 1.0 | 631 | 0.39 | 0.98 (0.93–1.0) | 658 | 0.53 | 1.1 (0.96–1.2) |
| Infectious and parasitic diseases | 55 | 1.0 | 57 | 0.58 | 1.1 (0.93–1.2) | 50 | 0.48 | 0.91 (0.69–1.2) |
| Respiratory infections | 42 | 1.0 | 37 | 0.09 | 0.88 (0.76–1.0) | 40 | 0.75 | 0.96 (0.71–1.3) |
| Respiratory disorders | 51 | 1.0 | 49 | 0.53 | 0.99 (0.87–1.1) | 61 | 0.13 | 1.2 (0.97–1.6) |
| Cardiovascular disorders | 136 | 1.0 | 132 | 0.38 | 0.95 (0.87–1.0) | 162 | 0.02 | 1.2 (1.0–1.4) |
| Malignant neoplasms | 61 | 1.0 | 62 | 0.77 | 1.0 (0.90–1.1) | 65 | 0.64 | 1.0 (0.81–1.3) |
| Digestive disorders | 118 | 1.0 | 112 | 0.20 | 0.95 (0.87–1.0) | 135 | 0.10 | 1.2 (0.98–1.4) |
| Musculoskeletal disorders | 140 | 1.0 | 135 | 0.28 | 0.96 (0.89–1.0) | 140 | 0.96 | 1.0 (0.88–1.2) |
| Neuropsychiatric disorders | 57 | 1.0 | 57 | 0.85 | 0.97 (0.89–1.1) | 53 | 0.61 | 0.93 (0.71–1.2) |
Relative risks (RR) were adjusted for gender, age, smoking habits, systolic blood pressure, alcohol, cholesterol, body mass index, and physical inactivity.
P-values by log-rank test. Infectious and parasitic diseases: 001–136, 320, 323, 612–614, 620, 622, A00–B99, G00, G03–G04, N70–N73; respiratory infections: 460–486, 381–382, J00–J06, J10–J18, J20–J22, H65–H66; respiratory disorders: 490–519, J30–J98; cardiovascular disorders: 390–458, I00–I99; malignant neoplasms: 140–209, C00–C97; digestive disorders: 530–577, K20–K92; musculoskeletal disorders: 710–738, M00–M99; neuropsychiatric disorders: 290–315, 321–322, 324–358, F01–F99, G06–G98.
After stratification by major causes of morbidity (1), incidence of hospitalization from cardiovascular disorders per 10,000 person · yr was 136 in noncarriers compared with 132 in heterozygotes (P = 0.38) and 162 in deficiency homozygotes (P = 0.02; Table II). Relative risks for cardiovascular disorders after adjustment for potential confounders in heterozygotes and homozygotes compared with noncarriers were 0.95 (95% CI: 0.87–1.0) and 1.2 (1.0–1.4), respectively. This finding was retested in case control studies matched for gender, age, and smoking habits. Odds ratios for ICVD, IHD, or either of these diseases in deficiency homozygotes versus noncarriers were 0.82 (0.53–1.3), 1.1 (0.76–1.5), and 1.1 (0.81–1.4), respectively (not depicted). Incidence of hospitalization from infection or other common diseases did not differ between MBL genotypes (Table II).
After further stratification by specific hospitalization cause, there were trends toward decreased incidence of cystitis and increased incidence of chronic obstructive pulmonary disease, IHD, ICVD, and miscarriage in MBL deficiency homozygotes versus noncarriers (Table III). However, there were no significant changes in risk.
Table III.
Morbidity Leading to Hospitalization According to MBL Deficiency Genotype during 24 yr Follow-up
| Noncarriers
|
Heterozygotes
|
Homozygotes
|
||||||
|---|---|---|---|---|---|---|---|---|
| IncidenceN/10,000person · yr | RRa | IncidenceN/10,000person · yr | P-valueb | RRa(95% CI) | IncidenceN/10,000person · yr | P-valueb | RRa(95% CI) | |
| Infectious diseases | ||||||||
| Meningitis | 0.91 | 1.0 | 1.1 | 0.69 | 0.99 (0.39–2.5) | 0 | — | — |
| HIV/AIDS | 0.68 | 1.0 | 0.60 | 0.82 | 0.75 (0.23–2.5) | 0 | — | — |
| Tuberculosis | 16 | 1.0 | 18 | 0.45 | 1.1 (0.88–1.4) | 20 | 0.37 | 1.2 (0.80–1.9) |
| Pneumonia | 29 | 1.0 | 25 | 0.18 | 0.89 (0.75–1.1) | 32 | 0.47 | 1.1 (0.82–1.6) |
| Skin infection | 9.9 | 1.0 | 9.7 | 0.90 | 1.0 (0.77–1.4) | 12 | 0.41 | 1.2 (0.78–2.3) |
| Hepatitis | 1.7 | 1.0 | 1.4 | 0.68 | 0.86 (0.42–1.7) | 0.81 | 0.47 | 0.49 (0.07–3.7) |
| Diarrhoeal diseases | 7.9 | 1.0 | 8.3 | 0.74 | 1.1 (0.81–1.5) | 9.8 | 0.49 | 1.2 (0.68–2.3) |
| Cystitis | 16 | 1.0 | 13 | 0.14 | 0.85 (0.67–1.1) | 9.8 | 0.10 | 0.57 (0.32–1.0) |
| Pyelonephritis | 3.6 | 1.0 | 3.9 | 0.74 | 1.1 (0.67–1.7) | 5.7 | 0.24 | 1.7 (0.78–3.9) |
| Respiratory disorders | ||||||||
| COPD | 21 | 1.0 | 22 | 0.85 | 1.1 (0.87–1.3) | 29 | 0.10 | 1.4 (0.97–2.0) |
| Asthma | 8.9 | 1.0 | 8.2 | 0.59 | 0.89 (0.75–1.1) | 8.1 | 0.77 | 1.1 (0.82–1.6) |
| Cardiovascular disorders | ||||||||
| IHD | 43 | 1.0 | 46 | 0.30 | 1.1 (0.94–1.2) | 53 | 0.09 | 1.2 (0.92–1.6) |
| ICVD | 21 | 1.0 | 20 | 0.65 | 0.97 (0.80–1.2) | 28 | 0.10 | 1.2 (0.84–1.7) |
| Maternal conditions | ||||||||
| Miscarriage | 7.2 | 1.0 | 5.7 | 0.19 | 0.89 (0.62–1.3) | 11 | 0.10 | 1.7 (0.96–3.1) |
Relative risks (RR) were adjusted for age, gender, smoking habits, systolic blood pressure, alcohol, cholesterol, body mass index, and physical inactivity.
P-values by log-rank test. Meningitis: 320, G00-G03; HIV/AIDS: 137, B20–B24; tuberculosis: 010–019, A15–A19, B90; pneumonia: 480–486, J12–J18; skin infection: 680–686, L00–L08; hepatitis: 070, B15–B19; diarrhoeal diseases: 000–001,004–006, 008–009, A00–A01, A03–A04, A06–A09; cystitis: 595, N30; pyelonephritis: 590, N10–N12; chronic obstructive pulmonary disease (COPD): 490–492, J41–J44; asthma: 493, J45–J46; IHD: 410–414, I20–I25; ICVD: 430–438, I60–I69; miscarriage: 643, O03.
Cross-sectionally, prevalence of recent respiratory infection was 26% in noncarriers compared with 25% in heterozygotes (χ2: P = 0.72) and 28% in deficiency homozygotes (P = 0.21; not depicted). Prevalence of spirometry-defined chronic obstructive pulmonary disease was 8.5% in noncarriers compared with 9.4% in heterozygotes (P = 0.19) and 11% in homozygotes (P = 0.14; not depicted).
When B, C, and D deficiency alleles were looked at separately versus noncarrier A alleles, none of the heterozygotes, compound heterozygotes, or homozygotes were associated with incidence of any hospitalization (Table IV). Compared with A/A noncarriers, A/D heterozygotes were associated with reduced risk of respiratory infection, A/C heterozygotes with reduced risk of cardiovascular disorders, B/D compound heterozygotes with increased risk of respiratory and digestive disorders, whereas D/D homozygotes were associated with increased risk of digestive disorders. If correction for multiple comparison is performed, all of these five significant associations become nonsignificant. Therefore, and because none of the significant findings are biologically plausible, these findings are likely due to chance alone rather than representing real phenomena. The remaining 65 calculated relative risks were not significant.
Table IV.
Morbidity Leading to Hospitalization According to MBL Deficiency Genotype during 24 yr Follow-up
| Heterozygotes
|
Compound heterozygotesor homozygotes
|
|||||||
|---|---|---|---|---|---|---|---|---|
| A/B | A/D | A/C | B/B | B/D | D/D | B/C | D/C a | |
| Any hospitalization | 1.0 (0.95–1.1) | 0.93 (0.86–1.0) | 0.98 (0.85–1.1) | 1.0 (0.88–1.2) | 1.1 (0.97–1.3) | 1.1 (0.81–1.5) | 1.0 (0.73–1.4) | 0.81 (0.52–1.3) |
| Infectious and parasitic diseases | 1.1 (0.94–1.3) | 0.98 (0.81–1.2) | 1.1 (0.81–1.6) | 0.74 (0.46–1.2) | 1.1 (0.72–1.6) | 1.2 (0.60–2.4) | 0.90 (0.37–2.2) | 0.28 (0.04–2.0) |
| Respiratory infections | 0.93 (0.78–1.1) | 0.74 (0.58–0.94)b | 1.0 (0.67–1.5) | 0.94 (0.59–1.5) | 1.2 (0.80–1.9) | 0.43 (0.11–1.7) | 0.67 (0.21–2.1) | 0.80 (0.20–3.2) |
| Respiratory disorders | 1.1 (0.93–1.2) | 0.89 (0.73–1.1) | 0.77 (0.51–1.2) | 1.2 (0.82–1.8) | 1.7 (1.2–2.4)b | 0.67 (0.25–1.8) | 0.96 (0.40–2.3) | — |
| Cardiovascular disorders | 0.96 (0.87–1.1) | 0.98 (0.87–1.1) | 0.77 (0.60–0.99)b | 1.3 (0.97–1.6) | 1.2 (0.93–1.5) | 1.4 (0.91–2.3) | 1.3 (0.78–2.1) | 0.44 (0.16–1.2) |
| Malignant neoplasms | 1.0 (0.90–1.2) | 0.94 (0.78–1.1) | 1.2 (0.88–1.6) | 1.1 (0.78–1.6) | 0.93 (0.63–1.4) | 0.82 (0.37–1.8) | 1.4 (0.73–2.7) | 0.79 (0.25–2.5) |
| Digestive disorders | 0.96 (0.87–1.1) | 0.95 (0.83–1.1) | 0.84 (0.64–1.1) | 1.1 (0.80–1.4) | 1.4 (1.1–1.8)b | 1.6 (1.0–2.5)b | 0.98 (0.54–1.8) | 0.26 (0.07–1.1) |
| Musculoskeletal disorders | 0.95 (0.86–1.0) | 0.96 (0.85–1.1) | 1.0 (0.83–1.3) | 0.96 (0.73–1.3) | 1.1 (0.88–1.5) | 0.95 (0.57–1.6) | 1.0 (0.59–1.8) | 1.1 (0.58–2.2) |
| Neuropsychiatric disorders | 0.96 (0.83–1.1) | 0.99 (0.82–1.2) | 0.93 (0.65–1.3) | 0.94 (0.61–1.4) | 1.2 (0.84–1.7) | 0.43 (0.14–1.3) | 0.86 (0.35–2.1) | — |
Values are relative risks (95% CI). Relative risks were adjusted for gender, age, smoking habits, systolic blood pressure, alcohol, cholesterol, body mass index, and physical inactivity. The A/A genotype was used as reference group.
The D/C genotype also included each of the following: C/C, B/B/D, B/B/C, and B/D/D.
P < 0.05 by Wald statistic. Infectious and parasitic diseases: 001–136, 320, 323, 612–614, 620, 622, A00–B99, G00, G03–G04, N70–N73; respiratory infections: 460–486, 381–382, J00–J06, J10–J18, J20–J22, H65–H66; respiratory disorders: 490–519, J30–J98; cardiovascular disorders: 390–458, I00–I99; malignant neoplasms: 140–209, C00–C97; digestive disorders: 530–577, K20–K92; musculoskeletal disorders: 710–738, M00–M99; neuropsychiatric disorders: 290–315, 321–322, 324–358, F01–F99, G06–G98.
Mortality.
Incidence of death from any cause per 10,000 person · yr was 235 in noncarriers compared with 244 in heterozygotes (log-rank: P = 0.44) and 274 in deficiency homozygotes (P = 0.12; Table V). After adjustment for gender, age, smoking habits, systolic blood pressure, alcohol consumption, cholesterol level, body mass index, and physical activity, relative risks for death were not significantly different from 1.0 in heterozygotes or homozygotes compared with noncarriers.
Table V.
Mortality According to MBL Deficiency Genotype during 8 yr Follow-up
| Noncarriers
|
Heterozygotes
|
Homozygotes
|
||||||
|---|---|---|---|---|---|---|---|---|
| IncidenceN/10,000person · yr | RRa | IncidenceN/10,000person · yr | P-valueb | RRa (95% CI) | IncidenceN/10,000 person · yr |
P-valueb | RRa(95% CI) | |
| Death from any cause | 235 | 1.0 | 244 | 0.44 | 1.0 (0.95–1.2) | 274 | 0.12 | 1.1 (0.87–1.3) |
| Infectious and parasitic diseases | 1.9 | 1.0 | 2.7 | 0.53 | 1.5 (0.52–4.2) | 2.6 | 0.78 | 1.4 (0.17–13) |
| Respiratory infections | 4.3 | 1.0 | 2.7 | 0.40 | 0.66 (0.28–1.6) | 2.6 | 0.61 | 0.44 (0.05–3.6) |
| Respiratory disorders | 14 | 1.0 | 10 | 0.14 | 0.73 (0.45–1.2) | 18 | 0.56 | 1.2 (0.54–2.7) |
| Cardiovascular disorders | 41 | 1.0 | 31 | 0.04 | 0.73 (0.55–0.95) | 49 | 0.44 | 1.1 (0.70–1.8) |
| Malignant neoplasms | 58 | 1.0 | 63 | 0.45 | 1.1 (0.90–1.3) | 78 | 0.13 | 1.3 (0.87–1.9) |
| Digestive disorders | 7.0 | 1.0 | 5.3 | 0.41 | 0.82 (0.42–1.6) | 5.2 | 0.69 | 0.66 (0.15–2.8) |
| Musculoskeletal disorders | 1.4 | 1.0 | 0 | — | — | 0 | — | — |
| Neuropsychiatric disorders | 3.4 | 1.0 | 3.4 | 0.96 | 1.4 (0.56–3.3) | 0 | — | — |
Relative risks (RR) were adjusted for age, gender, smoking habits, systolic blood pressure, alcohol, cholesterol, body mass index, and physical inactivity.
P-values by log-rank test. Infectious and parasitic diseases: 001–136, 320, 323, 612–614, 620, 622, A00–B99, G00, G03–G04, N70–N73; respiratory infections: 460–486, 381–382, J00–J22, H65–H66; respiratory disorders: 490–519, J30–J99; cardiovascular disorders: 390–458, I00–I99; malignant neoplasms: 140–209, C00–C97; digestive disorders: 530–577, K20–K99; musculoskeletal disorders: 710–738, M00–M99; neuropsychiatric disorders: 290–315, 321–322, 324–358, F01–F99, G06–G98.
After stratification by major causes of death (2), mortality from cardiovascular disorders per 10,000 person · yr was 41 in noncarriers compared with 31 in heterozygotes (P = 0.04) and 49 in deficiency homozygotes (P = 0.44; Table V). After adjustment for potential confounders, relative risks for death from cardiovascular disorders in heterozygotes and homozygotes compared with noncarriers were 0.73 (95% CI: 0.55–0.95) and 1.1 (0.70–1.8), respectively. Incidence of death from infection or other common diseases did not differ between MBL genotypes.
When B, C, and D deficiency alleles were looked at separately versus noncarrier A alleles, B/B homozygotes were associated with increased incidence of death from any cause (Table VI). None of the heterozygotes, compound heterozygotes, or other homozygotes were associated with incidence of death from any cause. Compared with A/A noncarriers, A/B heterozygotes were associated with reduced risk of death from cardiovascular disorders. If correction for multiple comparison is performed, both significant associations become nonsignificant. Therefore, these findings are likely due to chance alone rather than representing real phenomena. The remaining 41 calculated relative risks were not significant.
Table VI.
Mortality According to MBL Deficiency Genotype during 8 yr Follow-up
| Heterozygotes
|
Compound heterozygotesor homozygotes
|
|||||||
|---|---|---|---|---|---|---|---|---|
| A/B | A/D | A/C | B/B | B/D | D/D | B/C | D/C a | |
| Death from any cause | 1.1 (0.93–1.2) | 1.0 (0.88–1.2) | 1.1 (0.83–1.5) | 1.4 (1.0–1.9)b | 0.96 (0.69–1.3) | 0.61 (0.27–1.4) | 0.98 (0.51–1.9) | 0.72 (0.29–1.7) |
| Infectious and parasitic diseases | 2.4 (0.85–7.0) | — | — | 3.2 (0.34–31) | — | — | — | — |
| Respiratory infections | 0.56 (0.19–1.7) | 0.73 (0.20–2.7) | 1.1 (0.14–8.8) | — | 0.93 (0.09–9.2) | — | — | — |
| Respiratory disorders | 0.67 (0.38–1.2) | 0.74 (0.33–1.6) | 1.2 (0.36–3.8) | 2.2 (0.77–6.3) | 1.0 (0.24–4.3) | 2.3 (0.30–17) | — | — |
| Cardiovascular disorders | 0.72 (0.52–0.99)b | 0.76 (0.50–1.2) | 0.65 (0.27–1.6) | 1.7 (0.89–3.2) | 0.77 (0.31–1.9) | 1.2 (0.28–4.7) | 0.60 (0.08–4.3) | 0.95 (0.13–7.0) |
| Malignant neoplasms | 1.1 (0.87–1.4) | 1.2 (0.85–1.6) | 0.90 (0.49–1.7) | 1.4 (0.76–2.4) | 1.2 (0.65–2.2) | 0.89 (0.22–3.6) | 1.9 (0.69–5.1) | 0.84 (0.12–6.0) |
| Digestive disorders | 1.0 (0.38–2.7) | 0.95 (0.83–1.1) | — | 1.3 (0.17–9.5) | 0.69 (0.09–6.5) | — | — | — |
| Musculoskeletal disorders | — | — | — | — | — | — | — | — |
| Neuropsychiatric disorders | 1.8 (0.69–4.8) | 0.42 (0.05–3.3) | 2.8 (0.34–23) | — | — | — | — | — |
Values are relative risks (95% CI). Relative risks were adjusted for gender, age, smoking habits, systolic blood pressure, alcohol, cholesterol, body mass index, and physical inactivity. The A/A genotype was used as a reference group.
The D/C genotype also included each of the following: C/C, B/B/D, B/B/C, B/D/D.
P < 0.05 by Wald statistics. Infectious and parasitic diseases: 001–136, 320, 323, 612–614, 620, 622, A00–B99, G00, G03–G04, N70–N73; respiratory infections: 460–486, 381–382, J00–J06, J10–J18, J20–J22, H65–H66; respiratory disorders: 490–519, J30–J99; cardiovascular disorders: 390–458, I00–I99; malignant neoplasms: 140–209, C00–C97; digestive disorders: 530–577, K20–K99; musculoskeletal disorders: 710–738, M00–M99; neuropsychiatric disorders: 290–315, 321–322, 324–358, F01–F99, G06–G98.
Discussion
In this large study in an ethnically homogenous Caucasian population, there was no evidence for differences in infectious disease or mortality in MBL-deficient individuals versus controls. Our results suggest that MBL deficiency is not a major risk factor for morbidity or death in the adult Caucasian population. These observations were independent of major risk factors for morbidity and mortality (35) such as gender, age, smoking, blood pressure, alcohol consumption, cholesterol level, body mass index, and physical inactivity.
Because ∼5% of Caucasians are MBL deficient, it is indeed important that these individuals are relatively unaffected by the deficiency. MBL replacement therapy is currently being evaluated (36–39). However, because MBL-deficient individuals do not differ in morbidity or mortality from noncarriers in this study, MBL reconstitution therapy does not appear to be of relevance for the average Caucasian MBL-deficient adult. Certainly, MBL replacement therapy might be warranted in the future, if randomized clinical trials can demonstrate improved clinical health status in certain patient subgroups with MBL deficiency. Today, however, diagnosing MBL deficiency among adults should not alter management because predicted long-term morbidity and mortality appear unaffected in these individuals.
Even though it is generally accepted that MBL is a central factor in the innate immune system (5), MBL deficiency did not increase risk for infection, other serious common diseases, or death in this study. This indicates that other immune systems, e.g., ficolins (5, 40), are able to take over MBL function in most adults with MBL deficiency. In concordance, MBL deficiency may only come into play when other parts of the immune system are compromised, e.g., by chemotherapy as shown in some (24, 41) but not all previous studies (42). MBL deficiency has been associated with increased risk for severe and recurrent infections, but almost solely in hospital-based studies (8). As opposed to this, our results suggest that most adults with MBL deficiency in the general population are asymptomatic. Thus, it appears that MBL deficiency may only increase risk of infection in certain contexts or subgroups of patients, or when additional impairments of the immune system are present (5).
Previous studies have shown an association between MBL deficiency and accelerated progression of atherosclerosis (9, 10, 43). This may lead to a higher incidence of cardiovascular disease as observed in this study. This finding, however, could not be confirmed in the matched case control studies in our study. Also, the number of deaths from cardiovascular disorders was not increased by MBL deficiency. Because of this, and because the risk estimate of 1.2 for cardiovascular disease was relatively small, we believe that MBL deficiency is of no greater clinical importance in cardiovascular disease. It cannot be ruled out, however, that MBL deficiency in certain contexts (44) or subgroups of patients may increase the risk of cardiovascular disease significantly.
A population-based study of 252 children found that MBL deficiency was associated with a twofold risk for acute respiratory tract infection (17). In our study during adulthood, MBL deficiency did not increase risk of respiratory infection outcomes on a population basis. Thus, it is possible that MBL deficiency could play a role in pulmonary host defense in children during the vulnerable period when the adaptive immune system is immature (17), while not having an effect in adults. It is also possible that risk of respiratory infection is increased by MBL deficiency in children of Inuit origin only (17), whereas risk is unaffected in children (45) and adults in Caucasian populations.
Normal levels of MBL or wild-type genotype have also been associated with higher incidence of disease by mycobacteria, indicating an advantage of the host in situations where opsonophagocytosis might be detrimental (46, 47). We were not able to support these previous observations. However, in contrast to this study, the former studies used different study designs or were performed in populations where incidence for mycobacteria-associated disease is high.
Participants were genotyped only if they attended the 1991–1994 examination of the Copenhagen City Heart Study. A selection bias may have occurred if death or morbidity prevented certain individuals from being genotyped. However, there are several arguments against such bias: (a) the observed MBL genotype distribution did not differ from that predicted by the Hardy-Weinberg equilibrium, (b) MBL genotype distribution observed in the Copenhagen City Heart Study was similar to that observed in other Caucasian populations (5), and (c) limiting the analyses to a follow-up period after the 1991–1994 examination yielded results similar to those presented. It should be noted that if one insists on only looking at results after correction for multiple comparisons, MBL genotype was not significantly associated with cardiovascular disease. As we studied a sample of the caucasian Danish general population, generalizability of our data to other races and to less affluent parts of the world may potentially be constrained. Bias caused by investigators' knowledge of disease or risk factor status seems unlikely because we selected from a general population and genotyped our sample without knowledge of disease status. Misclassification of genotypes seems unlikely because the diagnosis of the B and D alleles included a control site for restriction enzyme digestion, and because all subjects with the MBL deficiency genotype were reanalyzed to confirm the diagnosis.
In conclusion, we found no evidence for differences in infectious disease or mortality in MBL-deficient individuals versus controls. Our results suggest that MBL deficiency is not a major risk factor for infection, other serious common diseases, or death in the adult Caucasian population. These are important findings because MBL replacement therapy is currently under evaluation (36–39).
Acknowledgments
We thank Vibeke Wohlgehagen, Hanne Damm, and Birgit Hertz for expert technical assistance, and Henrik Scharling, The Copenhagen City Heart Study, for statistical support.
This study is supported by the Danish Heart Foundation and the Danish Lung Association.
Abbreviations used in this paper: CI, confidence interval; ICVD, ischemic cerebrovascular disease; IHD, ischemic heart disease; MBL, mannose-binding lectin.
References
- 1.Murray, C.J.L., and A.D. Lopez. 1997. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 349:1436–1442. [DOI] [PubMed] [Google Scholar]
- 2.Murray, C.J.L., and A.D. Lopez. 1997. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 349:1269–1276. [DOI] [PubMed] [Google Scholar]
- 3.Super, M., S. Thiel, J. Lu, R.J. Levinsky, and M.W. Turner. 1989. Association of low levels of mannan-binding protein with a common defect of opsonisation. Lancet. 2:1236–1239. [DOI] [PubMed] [Google Scholar]
- 4.Neth, O., D.L. Jack, A.W. Dodds, H. Holzel, N.J. Klein, and M.W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmskov, U., S. Thiel, and J.C. Jensenius. 2002. Collectins and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547–578. [DOI] [PubMed] [Google Scholar]
- 6.Terai, I., K. Kobayashi, M. Matsushita, H. Miyakawa, N. Mafune, and H. Kikuta. 2003. Relationship between gene polymorphisms of mannose-binding lectin (MBL) and two molecular forms of MBL. Eur. J. Immunol. 33:2755–2763. [DOI] [PubMed] [Google Scholar]
- 7.Garred, P., F. Larsen, H.O. Madsen, and C. Koch. 2003. Mannose-binding lectin deficiency–revisited. Mol. Immunol. 40:73–84. [DOI] [PubMed] [Google Scholar]
- 8.Eisen, D.P., and R.M. Minchinton. 2003. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 37:1496–1505. [DOI] [PubMed] [Google Scholar]
- 9.Rugonfalvi-Kiss, S., V. Endrész, H.O. Madsen, K. Burián, J. Duba, Z. Prohászka, I. Karádi, L. Romics, É. Gönczöl, G. Füst, et al. 2002. Association of chlamydia pneumoniae with coronary artery disease and its progression is dependent on the modifying effect of mannose-binding lectin. Circulation. 106:1071–1076. [DOI] [PubMed] [Google Scholar]
- 10.Hegele, R.A., M.R. Ban, C.M. Anderson, and J.D. Spence. 2000. Infection susceptibility alleles of mannose-binding lectin are associated with increased carotid plaque area. J. Investig. Med. 48:198–202. [PubMed] [Google Scholar]
- 11.Yang, I.A., S.L. Seeney, J.M. Wolter, E.M. Anders, J.G. McCormack, A.M. Tunnicliffe, G.C. Rabnott, J.G. Shaw, A.G. Dent, S.T. Kim, et al. 2003. Mannose-binding lectin gene polymorphism predicts hospital admissions for COPD infections. Genes Immun. 4:269–274. [DOI] [PubMed] [Google Scholar]
- 12.Nagy, A., G.T. Kozma, M. Keszei, A. Treszl, A. Falus, and C. Szalai. 2003. The development of asthma in children infected with chlamydia pneumoniae is dependent on the modyfying effect of mannose-binding lectin. J. Allergy Clin. Immunol. 4:729–734. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa, T., B. Ma, K. Uemura, S. Oka, N. Kawasaki, and T. Kawasaki. 2003. Role of mannan-binding protein, MBL, in innate immunity. Anticancer Res. 23:4467–4471. [PubMed] [Google Scholar]
- 14.Schmiegelow, K., P. Garred, B. Lausen, B. Andreassen, B.L. Petersen, and H.O. Madsen. 2002. Increased frequency of mannose-binding lectin insufficiency among children with acute lymphoblastic leukemia. Blood. 100:3757–3760. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen, O.B., D.C. Kilpatrick, V. Souter, K. Varming, S. Thiel, and J.C. Jensenius. 1999. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand. J. Immunol. 49:193–196. [DOI] [PubMed] [Google Scholar]
- 16.Garred, P., J.J. Strøm, L. Quist, E. Taaning, and H.O. Madsen. 2003. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J. Infect. Dis. 188:1394–1403. [DOI] [PubMed] [Google Scholar]
- 17.Koch, A., M. Melbye, P. Sørensen, P. Homøe, H.O. Madsen, K. Mølbak, C.H. Hansen, L.H. Andersen, G.W. Hahn, and P. Garred. 2001. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 285:1316–1321. [DOI] [PubMed] [Google Scholar]
- 18.Garred, P., H.O. Madsen, B. Hofmann, and A. Svejgaard. 1995. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 346:941–943. [DOI] [PubMed] [Google Scholar]
- 19.Summerfield, J.A., M. Sumiya, M. Levin, and M.W. Turner. 1997. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. BMJ. 314:1229–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibberd, M.L., M. Sumiya, J.A. Summerfield, R. Booy, and M. Levin. 1999. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet. 353:1049–1053. [DOI] [PubMed] [Google Scholar]
- 21.Garred, P., H.O. Madsen, U. Balslev, B. Hofmann, C. Pedersen, J. Gerstoft, and A. Svejgaard. 1997. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 349:236–240. [DOI] [PubMed] [Google Scholar]
- 22.Garred, P., T. Pressler, H.O. Madsen, B. Frederiksen, A. Svejgaard, N. Høiby, M. Schwartz, and C. Koch. 1999. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J. Clin. Invest. 104:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy, S., K. Knox, S. Segal, D. Griffiths, C.E. Moore, K.I. Welsh, A. Smarason, N.P. Day, W.L. McPheat, D.W. Crook, et al. 2002. MBL genotype and risk of invasive pneumococcal disease: a case-control study. Lancet. 359:1569–1573. [DOI] [PubMed] [Google Scholar]
- 24.Neth, O., I. Hann, M.W. Turner, and N.J. Klein. 2001. Deficiency of mannose-binding lectin and burden of infection in children with malignancy: a prospective study. Lancet. 358:614–618. [DOI] [PubMed] [Google Scholar]
- 25.Yuen, M.-F., C.-S. Lau, Y.-L. Lau, W.-M. Wong, C.-C. Cheng, and C.-L. Lai. 1999. Mannose-binding lectin gene mutations are associated with progression of liver disease in chronic hepatitis B infection. Hepatology. 29:1248–1251. [DOI] [PubMed] [Google Scholar]
- 26.Schnohr, P., G. Jensen, P. Lange, H. Scharling, and M. Appleyard. 2001. The Copenhagen City Heart Study–Østerbroundersøgelsen. Tables with data from the third examination 1991–1994. Eur. Heart J. Suppl. 3:H1–H83. [Google Scholar]
- 27.Juul, K., A. Tybjærg-Hansen, R. Steffensen, S. Kofoed, G. Jensen, and B.G. Nordestgaard. 2002. Factor V leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood. 100:3–10. [DOI] [PubMed] [Google Scholar]
- 28.Bojesen, S.E., A. Tybjærg-Hansen, and B.G. Nordestgaard. 2003. Integrin β3 Leu33Pro homozygosity and risk of cancer. J. Natl. Cancer Inst. 95:1150–1157. [DOI] [PubMed] [Google Scholar]
- 29.Dahl, M., A. Tybjærg-Hansen, P. Lange, J. Vestbo, and B.G. Nordestgaard. 2002. Change in lung function and morbidity from chronic obstructive pulmonary disease in α1-antitrypsin MZ heterozygotes: a longitudinal study of the general population. Ann. Intern. Med. 136:270–279. [DOI] [PubMed] [Google Scholar]
- 30.Jensen, G. 1984. Epidemiology of chest pain and angina pectoris, with special reference to treatment needs. Acta Med. Scand. Suppl. 682:1–120. [PubMed] [Google Scholar]
- 31.Dahl, M., B.G. Nordestgaard, P. Lange, J. Vestbo, and A. Tybjærg-Hansen. 2001. Molecular diagnosis of intermediate and severe α1-antitrypsin deficiency: MZ individuals with chronic obstructive pulmonary disease may have lower lung function than MM individuals. Clin. Chem. 47:56–62. [PubMed] [Google Scholar]
- 32.Dahl, M., A. Tybjærg-Hansen, H. Sillesen, G. Jensen, R. Steffensen, and B.G. Nordestgaard. 2003. Blood pressure, risk of ischemic cerebrovascular and ischemic heart disease, and longevity in α1-antitrypsin deficiency. The Copenhagen City Heart Study. Circulation. 107:747–752. [DOI] [PubMed] [Google Scholar]
- 33.Mathers, C.D., C. Stein, D.M. Fat, C. Rao, M. Inoue, N. Tomijima, C. Bernard, A.D. Lopez, and C.J.L. Murray. 2002. Global burden of disease 2000. Version 2 methods and results. Global Programme on Evidence for Health Policy Discussion Paper no. 50 World Health Organisation, Geneva. pp.55–59.
- 34.Madsen, H.O., P. Garred, J.A.L. Kurtzhals, L.U. Lamm, L.P. Ryder, S. Thiel, and A. Svejgaard. 1994. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 40:37–44. [DOI] [PubMed] [Google Scholar]
- 35.Ezzati, M., A.D. Lopez, A. Rodgers, S.V. Hoorn, and C.J. Murray. 2002. Selected major risk factors and global and regional burden of disease. Lancet. 360:1347–1360. [DOI] [PubMed] [Google Scholar]
- 36.Valdimarsson, H., M. Stefansson, T. Vikingsdottir, G.J. Arason, C. Koch, S. Tiehl, and J.C. Jensenius. 1998. Reconstitution of opsonizing activity by infusion of mannan-binding lectin (MBL) to MBL-deficient humans. Scand. J. Immunol. 48:116–123. [DOI] [PubMed] [Google Scholar]
- 37.Valdimarsson, H., T. Vikingsdottir, P. Bang, S. Saevarsdottir, J.E. Gudjonsson, O. Oskarsson, M. Christiansen, L. Blou, I. Laursen, and C. Koch. 2004. Human plasma-derived mannose-binding lectin: a phase I safety and phamacokinetic study. Scand. J. Immunol. 59:97–102. [DOI] [PubMed] [Google Scholar]
- 38.Jensenius, J.C., P.H. Jensen, K. McGuire, J.L. Larsen, and S. Thiel. 2003. Recombinant mannan-binding lectin (MBL) for therapy. Biochem. Soc. Trans. 31:763–767. [DOI] [PubMed] [Google Scholar]
- 39.Garred, P., T. Pressler, S. Lanng, H.O. Madsen, C. Moser, I. Laursen, F. Balstrup, C. Koch, and C. Koch. 2002. Mannose-binding lectin (MBL) therapy in an MBL-deficient patient with severe cystic fibrosis lung disease. Pediatr. Pulmonol. 33:201–207. [DOI] [PubMed] [Google Scholar]
- 40.Matsushita, M., M. Kuraya, N. Hamasaki, M. Tsujimura, H. Shiraki, and T. Fujita. 2002. Activation of the lectin complement pathway by H-ficolin (Hakata Antigen). J. Immunol. 168:3502–3506. [DOI] [PubMed] [Google Scholar]
- 41.Peterslund, N.A., C. Koch, J.C. Jensenius, and S. Thiel. 2001. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 358:637–638. [DOI] [PubMed] [Google Scholar]
- 42.Kilpatrick, D.C., L.A. Mclintock, E.K. Allan, M. Copland, T. Fujita, N.E. Jordanides, C. Koch, M. Matsushita, H. Shiraki, K. Stewart, et al. 2003. No strong relationship between mannan binding lectin or plasma ficolins and chemotherapy-related infections. Clin. Exp. Immunol. 134:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madsen, H.O., V. Videm, A. Svejgaard, J.L. Svennevig, and P. Garred. 1998. Association of mannose-binding lectin deficiency with severe atherosclerosis. Lancet. 352:959–960. [DOI] [PubMed] [Google Scholar]
- 44.Best, L.G., M. Davidson, K.E. North, J.W. MacCluer, Y. Zhang, E.T. Lee, B.V. Howard, S. DeCroo, and R.E. Ferrel. 2004. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians. Circulation. 109:471–475. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen, H.E., V. Siersma, S. Andersen, B. Gahrn-Hansen, C.H. Mordhorst, B. Norgaard-Pedersen, B. Roder, T.L. Sorensen, R. Temme, and B.F. Vestergaard. 2003. Respiratory syncytial virus infection—risk factors for hospital admission: a case-control study. Acta Paediatr. 92:1314–1321. [PubMed] [Google Scholar]
- 46.Hoal-Van Helden, E.G., J. Epstein, T.C. Victor, D. Hon, L.A. Lewis, N. Beyers, D. Zurakowski, A.B. Ezekowitz, and P.D. Van Helden. 1999. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr. Res. 45:459–464. [DOI] [PubMed] [Google Scholar]
- 47.Søborg, C., H.O. Madsen, Å.B. Madsen, T. Lillebaek, A. Kok-Jensen, and P. Garred. 2003. Mannose-binding lectin polymorphisms in clinical tuberculosis. J. Infect. Dis. 188:777–782. [DOI] [PubMed] [Google Scholar]