Abstract
Epstein-Barr virus (EBV) nuclear antigen 1 (EBNA1)—the one EBV antigen that is expressed in all EBV-associated malignancies—has long been thought to go undetected by the cell-mediated immune system. However, recent studies show that EBNA1 can be presented to both CD4+ and CD8+ T cells, making it a potential new target for immunotherapy of EBV-related cancers.
EBV, which infects >90% of the human adult population, is considered to be the classic example for immune surveillance of persistent viral infections in humans (1). The virus establishes life-long latency in memory B cells (2). Different EBV latency programs are associated with many important tumors, like Hodgkin's disease and nasopharyngeal carcinoma, and also the EBV-transformed B cell lines (lymphoblastoid cell lines [LCLs]) that are so often used in human immunological research (3). Despite its significant transforming capacity, EBV causes tumors only in a small subset of infected individuals and is under effective immune control in most EBV carriers (4). In this respect, EBV is quite the opposite of HIV, which after establishing life-long latency in memory T cells is controlled only in a small subset of infected individuals, the so-called long-term nonprogressors, and causes AIDS in most infected individuals. Understanding the mechanisms of EBV-specific immune control might lead to the identification of crucial principles of immune surveillance failing in other persistent viral infections.
EBNA1 Maintains EBV Latency in Proliferating Cells.
EBV DNA only infrequently integrates into the host cell genome and is usually carried as circular DNA or episomes in latently infected cells (5). Therefore, EBV requires a mechanism for replicating viral DNA before mitosis and distributing episomes into progeny cells during cell division. EBNA1 fulfills these tasks by initiating replication through binding to the episome with its COOH-terminal domain and then cross-linking the episome to mitotic chromosomes as a protein anchor (3). Therefore, EBNA1 is expressed in all EBV-associated tumors (4) and EBV-positive proliferating cells in healthy EBV carriers (6). Expression of all other EBV-latent antigens can be absent in EBV+ tumors, like Burkitt's lymphoma, and in EBV+-replicating memory B cells. These features would make EBNA1 a potential tumor rejection antigen for EBV-associated malignancies and a target for EBV immune control in asymptomatic carriers. However, since Burkitt's lymphoma cells, which express EBNA1 as their only EBV latency antigen, are poorly immunogenic (7) and only EBNA1 and LMP2A mRNAs are detected in peripheral blood B cells (8), it has been assumed that EBV persists in this EBNA1-only latency program without detection by the immune system (8).
Immune Escape by EBNA1.
The idea that EBNA1 is immunologically invisible was enforced by several studies, which indicated that EBNA1 escapes recognition by cytotoxic T cells. EBNA1 contains a Gly-Ala repeat domain which was found to prevent antigen processing for cytolytic CD8+ T cell recognition (9). Presence of this Gly-Ala repeat domain in EBNA1 prevents processing by the proteasome, the main catalytic machinery for the generation of MHC class I ligands and therefore CD8+ T cell epitopes (10). More recently, the very same domain was found to prevent EBNA1 mRNA translation, and this effect alone diminishes CD8+ T cell recognition of EBNA1 by ∼30% (11).
EBV Persistence without EBNA1.
The view that the EBNA1-only latency program ensures EBV persistence in healthy EBV carriers was first challenged by D. Thorley-Lawson and colleagues who found that EBV persists mainly in peripheral memory B cells without expression of any EBV-latent antigen (2). These long-lived memory B cells need to express EBNA1 only occasionally during homeostatic proliferation with <1% of EBV-positive memory B cells containing EBNA1 at any given time point (6). These studies suggested that EBV does not rely on EBNA1 immune escape for its persistence but uses long-lived memory B cells without detectable viral antigen expression to persist life-long in healthy EBV carriers. Therefore, EBV persistence and immune recognition of EBNA1 do not exclude each other.
CD4+ T Cell Recognition of EBNA1.
The idea that EBNA1 is immunologically invisible was first challenged when EBV-specific CD4+ T cell responses were examined. Although initially only one EBNA1-specific CD4+ T cell clone was characterized (12), in more recent studies nearly all healthy EBV carriers were found to mount a CD4+ T cell response to this antigen (13, 14). Moreover, EBNA1 was recognized in more EBV-infected individuals than any other EBV-latent antigen (13). EBNA1-specific CD4+ T cell responses were found to be mainly T helper type 1 in nature (15) and could directly recognize LCLs (13, 15), Burkitt's lymphoma cells (16, 17), and freshly EBV-transformed B cells (18). Therefore, EBNA1-specific CD4+ T cells might orchestrate and maintain EBV-specific immune control and serve as effectors against EBV-associated malignancies.
CD8+ T Cell Recognition of EBNA1.
The volte-face that places EBNA1 as potentially relevant antigen in EBV-specific immune control is now completed with two articles in this issue (20–21) and a study that appeared earlier this year in the February 2nd issue of the JEM (19). These studies document the recognition of EBV-transformed B cells by EBNA1-specific CD8+ T cells. All three studies utilize IFN-γ secretion as the assay for T cell recognition of EBNA1, and two reports demonstrate moderate cytotoxicity against LCLs (19, 21). Importantly, these EBNA1-specific CD8+ T cells are able to suppress LCL outgrowth in vitro (20). The data suggest that IFN-γ release, and probably to a lesser extent cytotoxicity, inhibit growth of EBV-transformed B cells. This is consistent with an earlier report in which CD8+ T cells could control the outgrowth of EBV-transformed B cells solely by IFN-γ secretion (22).
How do these new findings fit with the earlier descriptions of Gly-Ala repeat domain–mediated inhibition of EBNA1 recognition by CD8+ T cells? Lee et al. report that the Gly-Ala repeat domain reduces CD8+ T cell recognition of EBNA1 by a factor of four. This implies that recognition of full-length EBNA1 in LCLs is barely above the detection limit in classical cytotoxicity assays that have been used in previous studies (20). However, with more sensitive IFN-γ detection assays LCL recognition by EBNA1–specific CD8+ T cells can be documented, and this seems sufficient to control EBV-transformed B cells with the exception of Burkitt's lymphoma, which has a more general defect in MHC class I antigen processing (7).
Processing of EBNA1 for Presentation on MHC Class I.
The three JEM papers on EBNA1-specific CD8+ T cells (19–21) also contain interesting information on the antigen processing and degradation of EBNA1. It was noted earlier that full-length EBNA1 is very stable with a half-life >20 h in B cell lines (10), but deletion of the Gly-Ala repeat domain (ΔGA) lowers EBNA1's half-life substantially (23). Degradation of the short-lived EBNA1ΔGA variant is proteasome dependent, whereas the degradation of long-lived full-length EBNA1 is proteasome independent (10). In the study by Tellam et al., the authors demonstrate that the half-life of full-length EBNA1 depends on the cellular background: it is reduced to 4 h in primary epithelial cell lines, but EBNA1 degradation remains insensitive to proteasome inhibition as in EBV-transformed B cells (21). By contrast, in all three studies CD8+ T cell recognition is inhibited by various proteasome inhibitors (19–21). These data suggest that EBNA1-derived CD8+ T cell epitopes are not derived from full-length EBNA1 but from proteins that are prematurely truncated during translation or mal-folded after translation (known as defective ribosomal products or DRiPs). These DRiPs are preferentially degraded by proteasomes for MHC class I presentation (Fig. 1) (24). Indeed, Voo et al. showed that CD8+ T cell recognition of EBNA1 can be rapidly blocked by ementine, an irreversible inhibitor of protein biosynthesis (19). Along the same lines, Tellam et al. report that they can block EBNA1-specific CD8+ T cell recognition of LCLs by another protein biosynthesis inhibitor, cyclohexamide (21). Since, most EBNA1-derived CD8+ T cell epitopes are COOH-terminal of the Gly-Ala repeat domain, the question remains why this domain cannot prevent DRiP degradation by the proteasome. The study by Voo et al. offers a possible answer to this dilemma. They report that EBNA1-derived CD8+ T cell epitope presentation is not only dependent on proteasome-mediated proteolysis but also on serine protease degradation (19). Preprocessing of DRiPs by a serine protease could separate the CD8+ T cell epitopes from the Gly-Ala repeat domain and make them accessible for proteasome degradation.
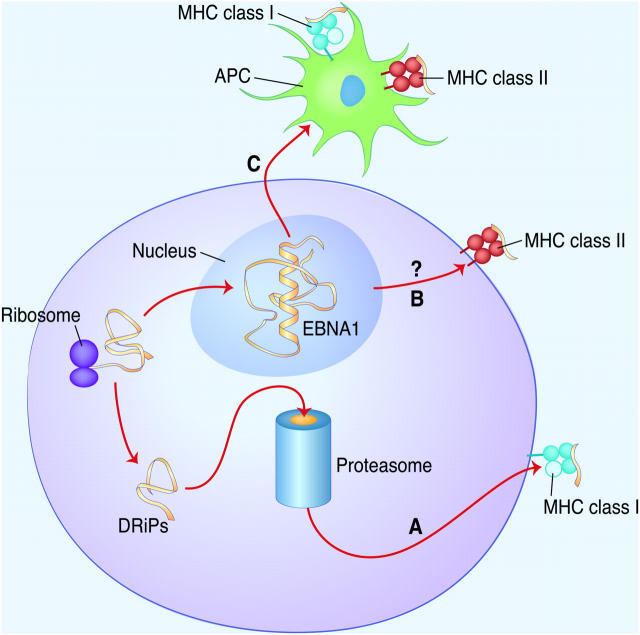
Figure 1.
Suggested antigen processing pathways of EBNA1. (A) Defective ribosomal products (DRiPs) of EBNA1 are degraded by the proteasome for MHC class I presentation (endogenous MHC class I antigen processing). (B) Full-length EBNA1 is not degraded by the proteasome and might be the source of MHC class II ligands (endogenous MHC class II antigen processing). (C) EBNA1 released by dying EBV-transformed B cells is taken up by APCs and presented for CD4+ and CD8+ T cell priming (exogenous MHC class I and II antigen processing).
Processing of EBNA1 for Presentation on MHC Class II.
Although full-length EBNA1 is not processed for MHC class I presentation, it might be vital for MHC class II presentation (Fig. 1). Two main processing pathways can be envisioned for EBNA1 delivery for MHC class II presentation. Full-length EBNA1 could gain access to the MHC class II loading compartment from within the cell, e.g., by autophagy, and sensitize EBV-associated tumor cells for CD4+ T cell recognition. Alternatively, EBNA1 from dying cells could be taken up by bystander cells and processed for presentation on MHC class II. This latter cross-presentation pathway seems crucial for the priming of EBV-specific immune control by DCs in vitro (25), but this pathway remains to be demonstrated in EBV+ lymphoma cells. Extracellular EBNA1 is also necessary for EBNA1-specific antibody responses, which can be seen in all healthy EBV carriers (26), and these antibodies could also facilitate EBNA1 cross-presentation (27). The fate of full-length EBNA1 and its connection to MHC class II presentation remains a challenge for future studies.
The Relevance of EBNA1 for EBV Immune Surveillance.
The observations on the recognition of EBNA1 have moved this latency antigen back into the focus of research on EBV immune surveillance. EBNA1, the one EBV latency antigen that is expressed in all EBV-associated malignancies and maintains EBV DNA in proliferating cells, might prove to be the Achilles heel of these tumors. It is now evident that EBNA1 can elicit Th1-type CD4+ and effector CD8+ T cells in healthy EBV carriers. In future studies, these responses need to be compared with EBNA1-specific T cell responses in patients with EBV-associated malignancies. The response to EBNA1 and other EBV antigens needs to be better charted in blood and lymphoid tissues of both healthy carriers and patients with tumors. Along these lines, the frequency of EBNA1-specific CD8+ T cell responses and if EBNA1-specific CD4+ and CD8+ T cell responses are altered in situations of EBV-associated malignancies remains to be determined.
The Potential of EBNA1 for Immunotherapy of EBV-associated Malignancies.
A more demanding proof of principle would entail EBNA1-based immunizations of patients with EBV+ tumors to determine if EBNA1 is indeed a key antigen in EBV-specific immune control. Passive and active immunotherapies have indeed been successful against EBV-associated malignancies. Adoptive transfer of autologous EBV-specific T cell populations into bone marrow transplant patients curbed the uncontrolled outgrowth of EBV-transformed B cells, known as lymphoproliferative disease (28). Since antigenic specificities of the transferred T cell lines were not determined, EBNA1-specific CD4+ and CD8+ T cells might have contributed to the success of this immunotherapy. In addition, vaccination with EBV latent antigens has been attempted in nasopharyngeal carcinoma patients (29). For this purpose, DCs, loaded with CD8+ T cell epitopes of the latent membrane protein 2 (LMP2) of EBV, were injected into inguinal lymph nodes of nasopharyngeal carcinoma patients. LMP2 is one of the two other EBV proteins expressed in nasopharyngeal carcinoma apart from EBNA1 (4). However, only 9 of 16 patients showed transiently increased LMP2-specific CD8+ T cell responses and only 2 of these experienced partial tumor regression with stable disease in only 1 patient (29). The investigators concluded that future vaccination approaches should aim to elicit sustained T cell responses and should stimulate both EBV-specific CD4+ and CD8+ T cells. Therefore, the same group has developed a recombinant modified vaccinia virus Ankara encoding an EBNA1–LMP2 fusion protein and proposes to elicit EBNA1-specific CD4+ and LMP2-specific CD8+ T cell responses in nasopharyngeal carcinoma patients (30). Since EBNA1 has now been shown to mediate protection against EBV-transformed B cells in vitro, more immunotherapies with this promising CD4+ and CD8+ T cell antigen should be developed to target EBV-associated malignancies.
Acknowledgments
I thank Ralph Steinman, Casper Paludan, and Dorothee Schmid for critically reading the manuscript. I would like to apologize to those colleagues whose contributions to this research field could not be cited due to space limitations.
I thank the Leukemia and Lymphoma Society and the New York Academy of Medicine for supporting my research.
References
- 1.Klein, G. 1994. Epstein-Barr virus strategy in normal and neoplastic B cells. Cell. 77:791–793. [DOI] [PubMed] [Google Scholar]
- 2.Babcock, G.J., L.L. Decker, M. Volk, and D.A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity. 9:395–404. [DOI] [PubMed] [Google Scholar]
- 3.Kieff, E., and A. Rickinson. 2001. Epstein-Barr Virus and its replication. Fields Virology. D.M. Knipe and P.M. Howley, editors. Lippincott-Raven, Philadelphia, PA. 2511–2573.
- 4.Rickinson, A.B., and E. Kieff. 2001. Epstein-Barr Virus. Fields Virology. P.M. Knipe and P.M. Howley, editors. Lippincott-Raven, Philadelphia, PA. 2575–2627.
- 5.Humme, S., G. Reisbach, R. Feederle, H.J. Delecluse, K. Bousset, W. Hammerschmidt, and A. Schepers. 2003. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc. Natl. Acad. Sci. USA. 100:10989–10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochberg, D., J.M. Middeldorp, M. Catalina, J.L. Sullivan, K. Luzuriaga, and D.A. Thorley-Lawson. 2004. Demonstration of the Burkitt's lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc. Natl. Acad. Sci. USA. 101:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe, M., R. Khanna, C.A. Jacob, V. Argaet, A. Kelly, S. Powis, M. Belich, D. Croom-Carter, S. Lee, S.R. Burrows, et al. 1995. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur. J. Immunol. 25:1374–1384. [DOI] [PubMed] [Google Scholar]
- 8.Chen, F., J.Z. Zou, L. di Renzo, G. Winberg, L.F. Hu, E. Klein, G. Klein, and I. Ernberg. 1995. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP1. J. Virol. 69:3752–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake, N., S. Lee, I. Redchenko, W. Thomas, N. Steven, A. Leese, P. Steigerwald-Mullen, M.G. Kurilla, L. Frappier, and A. Rickinson. 1997. Human CD8+ T cell responses to EBV EBNA1: HLA class I presentation of the (Gly-Ala)-containing protein requires exogenous processing. Immunity. 7:791–802. [DOI] [PubMed] [Google Scholar]
- 10.Levitskaya, J., A. Sharipo, A. Leonchiks, A. Ciechanover, and M.G. Masucci. 1997. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc. Natl. Acad. Sci. USA. 94:12616–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin, Y., B. Manoury, and R. Fahraeus. 2003. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 301:1371–1374. [DOI] [PubMed] [Google Scholar]
- 12.Khanna, R., S.R. Burrows, P.M. Steigerwald-Mullen, D.J. Moss, M.G. Kurilla, and L. Cooper. 1997. Targeting Epstein-Barr virus nuclear antigen 1 (EBNA1) through the class II pathway restores immune recognition by EBNA1-specific cytotoxic T lymphocytes: evidence for HLA-DM-independent processing. Int. Immunol. 9:1537–1543. [DOI] [PubMed] [Google Scholar]
- 13.Münz, C., K.L. Bickham, M. Subklewe, M.L. Tsang, A. Chahroudi, M.G. Kurilla, D. Zhang, M. O'Donnell, and R.M. Steinman. 2000. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 191:1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leen, A., P. Meij, I. Redchenko, J. Middeldorp, E. Bloemena, A. Rickinson, and N. Blake. 2001. Differential immunogenicity of Epstein-Barr virus latent-cycle proteins for human CD4+ T-helper 1 responses. J. Virol. 75:8649–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bickham, K., C. Münz, M.L. Tsang, M. Larsson, J.F. Fonteneau, N. Bhardwaj, and R. Steinman. 2001. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J. Clin. Invest. 107:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paludan, C., K. Bickham, S. Nikiforow, M.L. Tsang, K. Goodman, W.A. Hanekom, J.F. Fonteneau, S. Stevanovic, and C. Münz. 2002. EBNA1 specific CD4+ Th1 cells kill Burkitt's lymphoma cells. J. Immunol. 169:1593–1603. [DOI] [PubMed] [Google Scholar]
- 17.Voo, K.S., T. Fu, H.E. Heslop, M.K. Brenner, C.M. Rooney, and R.F. Wang. 2002. Identification of HLA-DP3-restricted peptides from EBNA1 recognized by CD4+ T cells. Cancer Res. 62:7195–7199. [PubMed] [Google Scholar]
- 18.Nikiforow, S., K. Bottomly, G. Miller, and C. Münz. 2003. Cytolytic CD4+-T-cell clones reactive to EBNA1 inhibit Epstein-Barr Virus-induced B-cell proliferation. J. Virol. 77:12088–12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voo, K.S., T. Fu, H.Y. Wang, J. Tellam, H.E. Heslop, M.K. Brenner, C.M. Rooney, and R.F. Wang. 2004. Evidence for the presentation of major histocompatibility complex class I–restricted Epstein-Barr Virus nuclear antigen 1 peptides to CD8+ T lymphocytes. J. Exp. Med. 199:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S.P., J.M. Brooks, H. Al-Jarrah, W.A. Thomas, T.A. Haigh, G.S. Taylor, S. Humme, A. Schepers, W. Hammerschmidt, J.L. Yates, et al. CD8 T cell recognition of endogenously expressed EBNA1: a reappraisal of protection mediated by the glycine-alanine repeat. J. Exp. Med. 199:1409–1420. [DOI] [PMC free article] [PubMed]
- 21.Tellam, J., G. Connolly, K.J. Green, J.J. Miles, D.J. Moss, S.R. Burrows, and R. Khanna. Endogenous presentation of CD8+ T cell epitopes from Epstein-Barr virus encoded nuclear antigen 1: functional evidence for DRiPs as a source of endogenously processed epitopes. J. Exp. Med. 199:1421–1431. [DOI] [PMC free article] [PubMed]
- 22.Shi, Y., and C.T. Lutz. 2002. Interferon-γ control of EBV-transformed B cells: a role for CD8+ T cells that poorly kill EBV-infected cells. Viral Immunol. 15:213–225. [DOI] [PubMed] [Google Scholar]
- 23.Tellam, J., M. Sherritt, S. Thomson, R. Tellam, D.J. Moss, S.R. Burrows, E. Wiertz, and R. Khanna. 2001. Targeting of EBNA1 for rapid intracellular degradation overrides the inhibitory effects of the Gly-Ala repeat domain and restores CD8+ T cell recognition. J. Biol. Chem. 276:33353–33360. [DOI] [PubMed] [Google Scholar]
- 24.Schubert, U., L.C. Anton, J. Gibbs, C.C. Norbury, J.W. Yewdell, and J.R. Bennink. 2000. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 404:770–774. [DOI] [PubMed] [Google Scholar]
- 25.Bickham, K., K. Goodman, C. Paludan, S. Nikiforow, M.L. Tsang, R.M. Steinman, and C. Münz. 2003. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J. Exp. Med. 198:1653–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henle, W., G. Henle, J. Andersson, I. Ernberg, G. Klein, C.A. Horwitz, G. Marklund, L. Rymo, C. Wellinder, and S.E. Straus. 1987. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc. Natl. Acad. Sci. USA. 84:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rooney, C.M., L.K. Aguilar, M.H. Huls, M.K. Brenner, and H.E. Heslop. 2001. Adoptive immunotherapy of EBV-associated malignancies with EBV-specific cytotoxic T-cell lines. Curr. Top. Microbiol. Immunol. 258:221–229. [DOI] [PubMed] [Google Scholar]
- 29.Lin, C.L., W.F. Lo, T.H. Lee, Y. Ren, S.L. Hwang, Y.F. Cheng, C.L. Chen, Y.S. Chang, S.P. Lee, A.B. Rickinson, and P.K. Tam. 2002. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 62:6952–6958. [PubMed] [Google Scholar]
- 30.Taylor, G.S., T.A. Haigh, N.H. Gudgeon, R.J. Phelps, S.P. Lee, N.M. Steven, and A.B. Rickinson. 2004. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J. Virol. 78:768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]