Abstract
In experiments to study the impact of deficiency in CD4+ T cell help on the magnitude of CD8+ cytotoxic T cell response to pathogens, it was noted that in CD4 gene knockout mice, the CD8 population made significant responses to several nominally major histocompatibility complex (MHC) class II–restricted epitopes in addition to the expected responses to MHC class I–restricted epitopes. A similar response by CD8+ T cells to class II–restricted epitopes was not observed in wild-type mice, or in mice that had been acutely depleted of CD4+ T cells just before the immunization. Coincident with this unexpected response to class II–restricted epitopes, it was also observed that the CD8+ response to the class I–restricted epitopes was consistently lower in CD4−/− mice than in wild-type mice. Further experiments suggested that these two observations are linked and that the CD8 population in CD4−/− mice may contain a majority of T cells that were actually selected by recognition of MHC class II molecules in the thymus. These results have implications for understanding CD4 versus CD8 lineage commitment in the thymus, and for the practical use of CD4−/− mice as models of helper deficiency.
Keywords: Listeria monocytogenes, thymic selection, T cell receptor, LCMV, lineage commitment
Introduction
Mice in which the CD4 gene has been disrupted provide a model system to analyze the contribution of the CD4 coreceptor to T cell differentiation in the thymus and the impact of helper T cell deficiency on the humoral and CD8 T cell response (1, 2). With regard to the former, it has been shown that in CD4−/− mice, several class II–restricted T cells emerge from the thymus as CD4 lineage cells that express neither CD8 nor CD4, so-called “CD4 wannabe” T cells (1, 2). These CD4−CD8− double-negative CD4 lineage T cells constitute 10–20% of the peripheral T cell pool (1–3). It has been supposed that these double-negative CD4 lineage T cells express TCRs with sufficiently high affinity for positively selecting ligands in the thymus, such that they do not require cooperation with the CD4 coreceptor to drive maturation to the CD4 lineage. In addition to these double-negative CD4 lineage T cells, in some class II–restricted TCR transgenic mice on certain MHC backgrounds, immature thymocytes that normally differentiate into the CD4 lineage are mis-selected into the CD8 lineage in mice lacking CD4 (4). Both of these outcomes can be explained on the basis of the strength of the signal model of CD4/CD8 lineage choice, which proposes that quantitative differences in signal strength direct differentiation to the CD4 versus CD8 lineage. According to this model, stronger signals during positive selection direct cells to the CD4 lineage, whereas weaker signals promote CD8 lineage choice (5–8). Thus, it was reasoned that some class II–restricted TCRs, which direct either deletion or CD4 lineage choice in mice that express CD4, provide a weaker signal in the absence of CD4, resulting in differentiation to the double-negative CD4 lineage or the CD8 lineage, respectively.
Many groups have used MHC class II–deficient and CD4-deficient mice as models to investigate the helper T cell contribution to the CD8 cytotoxic T cell response to various forms of antigenic challenge (1, 9–14). In our experience, the primary response of wild-type and MHC class II−/− mice to pathogens such as Listeria monocytogenes or lymphocytic choriomeningitis virus (LCMV) is indistinguishable when assayed for numbers of responding CD8 T cells by tetramer staining or intracellular cytokine staining (14). This is not the case in CD4−/− mice (15). We consistently found that only half or less than the number of effector CD8 cells specific for MHC class I–restricted peptides were generated in CD4-deficient animals compared with wild-type or class II–deficient mice. It did not seem likely that this reflected a dependency on CD4 T cell help for a full-blown CD8 response because wild-type levels of effector CD8 cells were generated in MHC class II knockout mice that have fewer helper T cells (16, 17). The experiments we present here have led to a different explanation for the weaker CD8 response to class I–restricted peptides in CD4−/− mice. We now suspect that the CD8 T cell pool in these animals is heavily contaminated with MHC class II–restricted cells, perhaps ≥50%. Together, our studies have implications for the strength of signal model of lineage commitment and reveal that CD4−/− mice are a poor model to study the requirement for T cell help in mounting CD8 T cell responses.
Materials and Methods
Mice.
Age-matched C57BL/6 (B6), B6.PL-Thy1 a/Cy (Thy 1.1+), B6.SJL-Ptprc aPep3b/BoyJ (Ly5.1+), and CD4−/− C57BL/6-CD4tm1Mak mice were purchased from the Jackson Laboratory. MHC class II−/− B6.129-H2-Ab1 tm1GlmN12 (ABBN12-M were backcrossed 12 times to C57BL/6) and β2-microglobulin (β2m)− / − B6.129-β2mtm1N12 (B2MN12-M backcrossed 12 times to C57BL/6) mice were purchased from Taconic. Experiments were initiated when mice were 6–12 wk of age and were performed according to Institutional Animal Care and Use Committee guidelines.
Bacterial and Viral Infections.
A recombinant L. monocytogenes strain expressing a secreted form of chicken ovalbumin (rLmOva) and an erythromycin-resistant marker was provided by H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) (18). L. monocytogenes was grown to stationary phase, aliquoted, and frozen at −80°C. Before infecting mice, bacteria were thawed, grown to mid-log phase in brain–heart infusion broth, measured by optical density (A600), and diluted in PBS for injection. To generate an acute response, mice were infected with a primary dose equivalent to 2,000 CFU suspended in 200 μl PBS by tail vein injection and analyzed 7 d after infection. Bacteria numbers were accurately confirmed by spreading bacteria samples onto brain–heart infusion plates plus erythromycin. LCMV (Armstrong strain) stocks were plaque purified on Vero cells and grown in BHK-21 cells as described previously (19). For the generation of an acute virus-specific T cell response, mice were inoculated i.p. with 2 × 105 PFU of LCMV diluted in 500 μl PBS and analyzed 8 d after infection.
In Vivo Depletion of CD4 T Cells.
Mice were injected i.p. with 200 μg of purified anti–mouse CD4 mAb (clone GK1.5) at days −3, −1, and +3 after infection with rLmOva. Using a noncompeting FITC-conjugated antibody for CD4 (clone RM4.4), we observed that >99% of CD4+ T cells were depleted in the spleen and peripheral blood of mice on the day of infection.
Antibodies and Peptides.
The following purified, fluorescently conjugated antibodies were purchased from BD Biosciences: anti–IFN-γ–FITC (clone XMG1.2), anti–CD4-PerCP (clone L3T4), and anti–CD8-allophycocyanin (clone 53.6.7). Synthetic peptides representing the defined rLmOva epitopes (I-Ab–restricted listeriolysin O (LLO)190–201 and H-2Kb–restricted Ova257–264) or LCMV (I-Ab–restricted GP61–80 and H-2Db–restricted GP33–41) were obtained from Invitrogen.
Antigen-presenting Cell Cultures.
LPS blasts were generated from splenocytes cultured in complete RPMI 1640 (supplemented with 10% FCS, 2 mM l-glutamine, 10 mM Hepes, 0.5 μM 2-mercaptoethanol, 100 U/ml penicillin, and 100 mg/ml streptomycin) in the presence of 1 μg/ml LPS (Sigma-Aldrich) for 2 d at 37°C in 7% CO2.
Intracellular IFN-γ Staining.
rLmOva- or LCMV-specific CD8+ T cells were detected by measuring IFN-γ secretion in response to immunodominant MHC class I or II peptides using the Cytofix/Cytoperm Kit Plus (with GolgiPlug; BD Biosciences) according to manufacturer's instructions. For T cell stimulation, 3 × 106 splenocytes were incubated with or without synthetic peptides or 1 × 106 splenocytes were cocultured with 2 × 106 LPS blasts coated with synthetic peptide in a 96-well plate in a volume of 200 μl. Cells were stimulated with the MHC class I–restricted peptides (1 μM) Ova257–264 or GP33–41 and the I-Ab MHC class II–restricted peptides (10 μM) LLO190–201 or GP61–80 for 5 h in complete RPMI 1640 in the presence of 1 μg/ml of GolgiPlug (containing brefeldin A; BD Biosciences) at 37°C in 7% CO2. Cells were washed, stained with anti–CD8-allophycocyanin and anti-CD4-PerCp, washed, resuspended in permeabilization–fixation buffer, and stained with anti–IFN-γ–FITC for 30 min at 4°C. The cells were washed in PermWash solution, resuspended in PBS + 1% paraformaldehyde and analyzed on a FACSCalibur™ using CELLQuest™ software.
Assay for Cell-mediated Cytotoxicity.
Splenocytes from mice infected with bacteria or virus were assayed ex vivo for their ability to kill target cells pulsed with MHC class I or class II–restricted peptides. Splenocytes were seeded in threefold serial dilutions in triplicate in 96-well round-bottomed plates. LB27.4 target cells expressing MHC class I and class II were incubated with 51Cr and synthetic peptides for 1 h at 37°C. Target cells were washed three times to remove residual peptide and 51Cr and added to effector cells at 5,000 cells/well. After a 4-h incubation at 37°C, the plates were centrifuged at 1,500 revolutions/min for 5 min, and cell supernatants were collected. 100-μl supernatant samples were counted on an automatic counter (Wizard model 1470; PerkinElmer) to determine the amount of 51Cr release. Spontaneous and total release were measured by treating the targets cells with 10% RPMI 1640 or with 2% Triton X-100 detergent, respectively. The percent specific lysis was calculated as 100 × (experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm).
Adoptive Transfer of Purified CD8+ T Cells.
Lymph nodes and spleen were removed from naive B6.SJL and CD4−/− mice. Single cell suspensions were prepared, and red blood cells were lysed. The CD8+ T cell subsets were negatively purified by magnetic antibody cell sorting (MACS) with a CD8α+ T cell isolation kit following the manufacturer's instructions (Miltenyi Biotec). The purity of the flow-through fraction was >85% CD8+. 107 sorted CD8+ T cells from CD4-deficient mice (Thy 1.2+ and Ly 5.1−) and 107 sorted CD8+ T cells from B6.SJL (Thy 1.2+ and Ly 5.1+) were cotransferred into congenic B6.PL (Thy 1.2− and Ly 5.1−) host mice via tail vein injection. Mice were infected 24 h later with either rLmOva or LCMV. Intracellular IFN-γ staining was performed 7 and 8 d after infection, respectively.
Results
CD4−/− Mice Generate a Class II–restricted CD8+ T Cell Response after L. monocytogenes Infection.
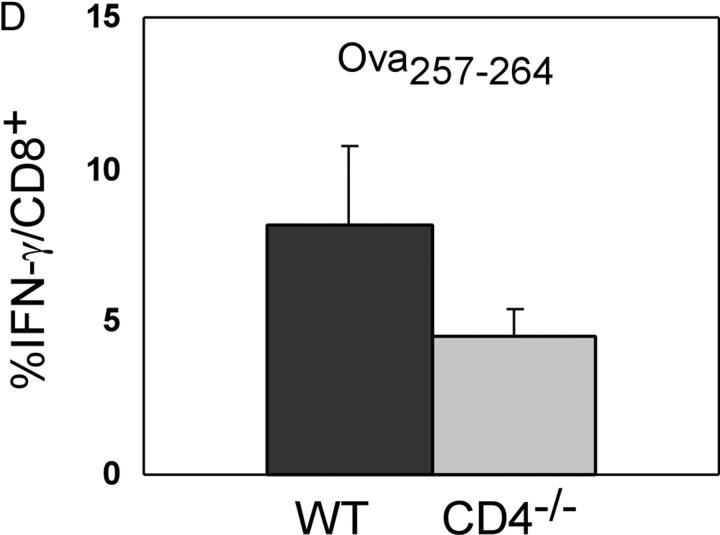
To investigate the role of CD4 T cells in protection against an intracellular bacterium, we analyzed T cell responses to L. monocytogenes in wild-type and CD4−/− mice. Because naturally derived H2b class I–restricted peptides from L. monocytogenes have not been identified, we took advantage of a recombinant form, rLmOva, that expresses the H-2Kb–restricted epitope, Ova257–264, as well as the endogenous I-Ab–restricted CD4 epitope, LLO190–201. Wild-type and CD4−/− mice were infected with a sublethal dose of rLmOva and T cell responses were assessed by intracellular IFN-γ staining and 51Cr release assays 7 d after infection. Wild-type mice responded as expected with strong CD4+ T cell responses to LLO190–201 and strong CD8+ T cell responses to Ova257–264 (Fig. 1, A and B). Surprisingly, the CD8+ T cell population in the CD4−/− mice showed good IFN-γ responses to both the class II–restricted LLO190–201 and class I–restricted Ova257–264 peptides. This pattern of response was also observed in a 4-h 51Cr release assay. In addition to secreting IFN-γ, the LLO190–201-specific T cells from both types of mice were able to kill targets in a short-term assay (Fig. 1 C). Of note was the fact that the magnitude of the CD8 response to Ova257–264 in CD4−/− mice was approximately half of that in wild type (Fig. 1 B). This was a consistent finding, and a summary of all the comparisons of the CD8 response of wild-type and CD4−/− mice to the Ova257–264 peptide is presented in Fig. 1 D.
Figure 1.
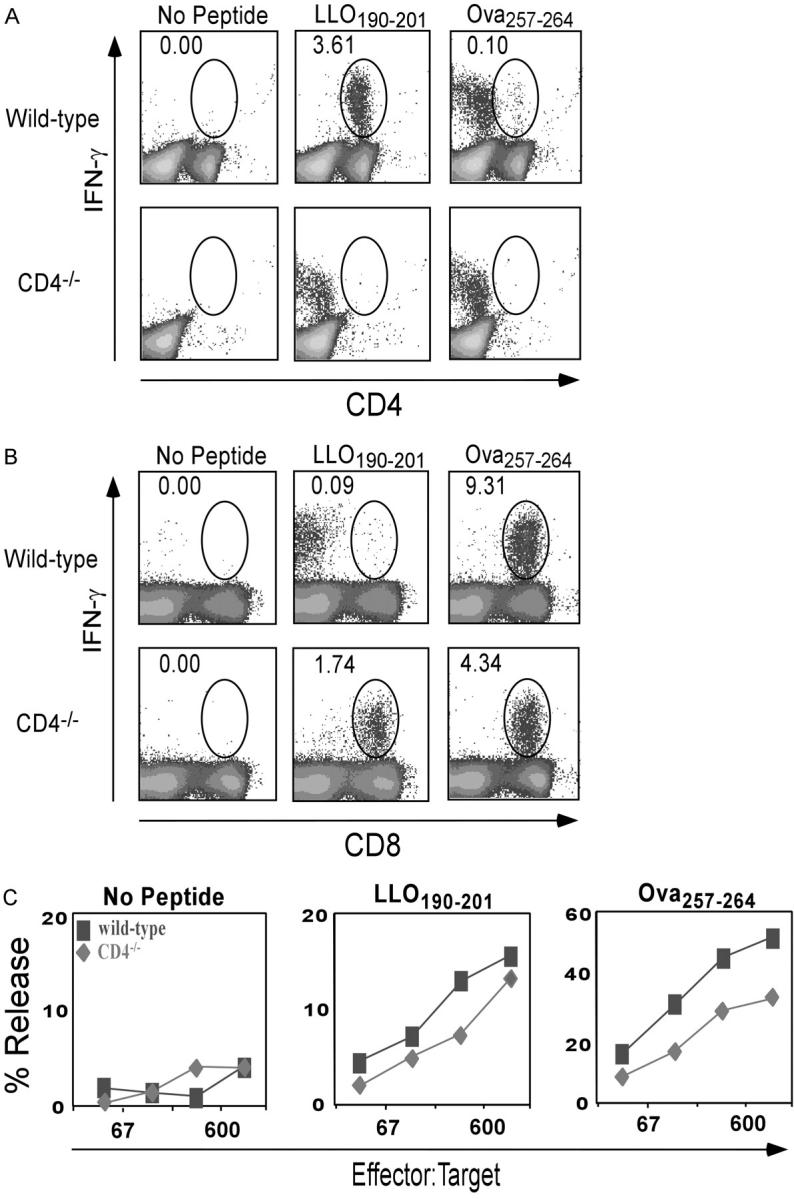
Primary response of wild-type and CD4−/− mice to MHC class I and class II–restricted peptides after rLmOva infection. 7 d after infection, splenocytes were stimulated in vitro with LLO190–201, Ova257–264, or no peptide and stained for levels of intracellular IFN-γ. (A) Response of CD4+ T cells to peptides. (B) Response of CD8+ T cells to peptides. Numbers indicate the percentage of IFN-γ–positive cells in the CD4+ or CD8+ population. There were 2.3 × 106 Ova257–264-specific CD8+ T cells in wild-type spleen and 106 in CD4−/− spleen. (C) Lysis of 51Cr-labeled control, Ova257–264, or LLO190–201-coated LB27.4 targets by spleen cells from wild-type (squares) and CD4−/− mice (diamonds) assayed directly ex vivo. These data are representative of four independent experiments of two mice per group. (D) Response of CD8+ T cells to Ova257–264 in wild-type and CD4−/− mice. Data represent the average of eight mice per group.
CD4−/− Mice Generate a Class II–restricted CD8+ T Cell Response after LCMV Infection.
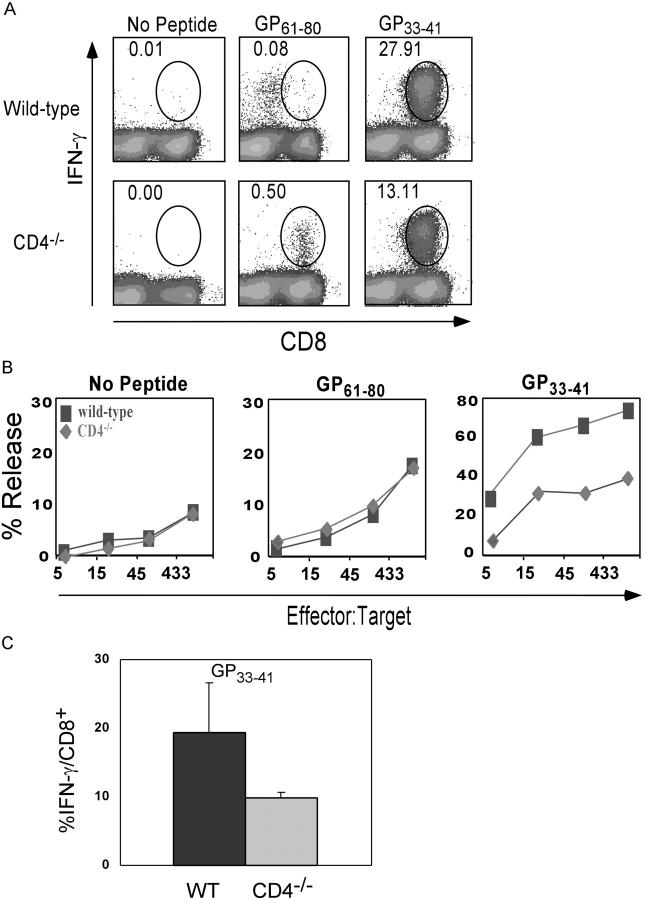
To address the possibility that generation of a CD8+ cell response to a class II–restricted peptide could be unique to this L. monocytogenes peptide, or the consequence of bacterial infection itself, we immunized wild-type and CD4−/− mice with LCMV. Naturally occurring H-2Db– and I-Ab–restricted antigenic peptides from LCMV infection have been identified, allowing for the study of both CD4 and CD8 T cell responses. Wild-type and CD4−/− mice were infected with a sublethal dose of LCMV Armstrong strain and T cell responses to class I–restricted GP33–41 and class II–restricted GP61–80 epitopes were assessed at day 8 after infection. Similar to the bacterial infection, viral infection of CD4−/− mice resulted in CD8+ T cells that responded to presentation of the class II–restricted peptide GP61–80, as determined by IFN-γ production and cytotoxic killing of peptide-coated target cells (Fig. 2, A and B). Similar to the Ova257–264 response, it was also consistently observed that the CD8 response to the class I–restricted GP33–41 peptide was reduced ∼50% in CD4−/− mice compared with wild type when expressed as the fraction of total CD8+ T cells (Fig. 2 C).
Figure 2.
Primary response of wild-type and CD4−/− mice to MHC class I and class II–restricted peptides after LCMV infection. CD8+ T cell responses were measured 8 d after infection by intracellular IFN-γ staining and 51Cr release cytotoxicity assays. (A) Intracellular IFN-γ staining in the CD8+ T cell population after in vitro peptide stimulation. Numbers indicate percentage of IFN-γ–positive cells in the CD8+ population. There were 17.7 × 106 GP33–41-specific CD8+ T cells in wild-type spleen and 6.9 × 106 in CD4−/− spleen. (B) 51Cr release assay of control, GP33–41, or GP61–80 peptide–coated LB27.4 cells by wild-type (squares) and CD4−/− (diamonds) splenocytes taken directly ex vivo. These data are representative of three independent experiments of two mice per group. (C) Response of CD8+ T cells to GP33–41 in wild-type and CD4−/− mice. Data represent the average of six mice per group.
Recognition of LLO190–201 by CD8 T Cells in CD4−/− Mice Is MHC Class II–restricted.
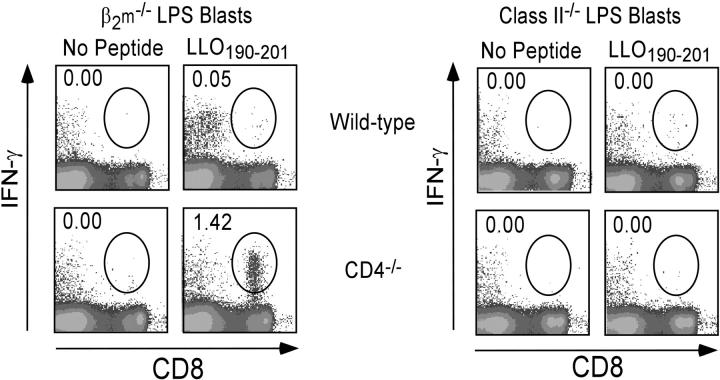
It is possible that the LLO190–201 peptide binds MHC class I as well as class II. To investigate whether the CD8+ T cell response to this peptide is indeed class II–restricted, synthetic peptides were loaded onto antigen-presenting cells that express either MHC class I or class II molecules. LPS blasts were generated from splenocytes isolated from β2m−/− and MHC class II−/− mice, pulsed with or without peptides, washed, and incubated with the indicated effectors. Using LLO190–201-coated LPS blasts from β2m−/− mice, we observed that CD8+ T cells from CD4−/− mice responded as measured by IFN-γ production, whereas cells from wild-type mice did not (Fig. 3, left). As expected, there were no observable CD8+ cell responses to LLO190–201-coated LPS blasts from class II−/− mice, indicating that the CD8+ population in CD4−/− mice was responding to the LLO190–201 peptide complexed with I-Ab (Fig. 3, right).
Figure 3.
The CD8+ response to LLO190–201 in CD4−/− mice requires peptide presentation on MHC class II molecules. 7 d after infection with rLmOva, splenocytes from wild-type or CD4−/− mice were stimulated with LLO190–201-coated β2m−/−or MHC class II−/− LPS blasts. Intracellular IFN-γ staining was performed to determine the percentage of LLO190–201-specific cells in the CD8+ T cell population. These data are representative of three independent experiments of two mice per group.
Class II–restricted CD8+ T Cells Are Undetectable in Acutely CD4-depleted Mice.
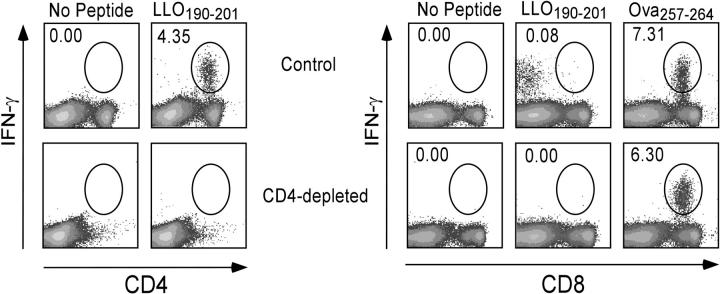
We addressed the possibility that class II–specific CD8+ T cells may be present in a normal mouse but are few or undetectable during acute infections due to peripheral competition with CD4+ T cells specific for the same epitope. To address this possibility, wild-type mice were depleted of CD4+ cells in vivo using anti-CD4 mAb just before immunization. As expected, few CD4+ cells were detected in the spleen 7 d after infection with rLmOva in the CD4-depleted mice (Fig. 4, left). The first difference observed in these CD4-depleted mice compared with CD4−/− mice was that the CD8+ T cell response to the H-2Kb–restricted Ova257–264 peptide was similar in both mice (Fig. 4, right). The second observation was that, unlike the CD8 response to LLO190–201 in CD4−/− mice, CD4-depleted mice did not respond to the class II–restricted peptide (Fig. 4, right). It is also noteworthy that the hint of a CD8 response to the class II–restricted peptide observed in normal B6 mice in Figs. 1–4 is not observed in CD4-depleted mice. These findings indicate that the mismatch in coreceptor and MHC recognition is due to abnormal development of the T cells in the CD4−/− mice and not due to peripheral competition for MHC–peptide complexes.
Figure 4.
C57BL/6 mice acutely depleted of CD4+ cells do not mount a CD8+ T cell response to MHC class II–restricted peptides. C57BL/6 mice were depleted of CD4+ T cells by three injections of anti-CD4 mAb and infected with rLmOva. LLO190–201 and Ova257–264-specific CD4+ or CD8+ T cells were quantified by intracellular IFN-γ staining day 7 after infection and after in vitro stimulation with or without peptide. Numbers represent the percentage of Ag-specific cells in the CD4+ or CD8+ T cell population. This experiment is representative of two mice per group from two independent experiments.
Comparison of Wild Type and Knockout Response in the Same Environment.
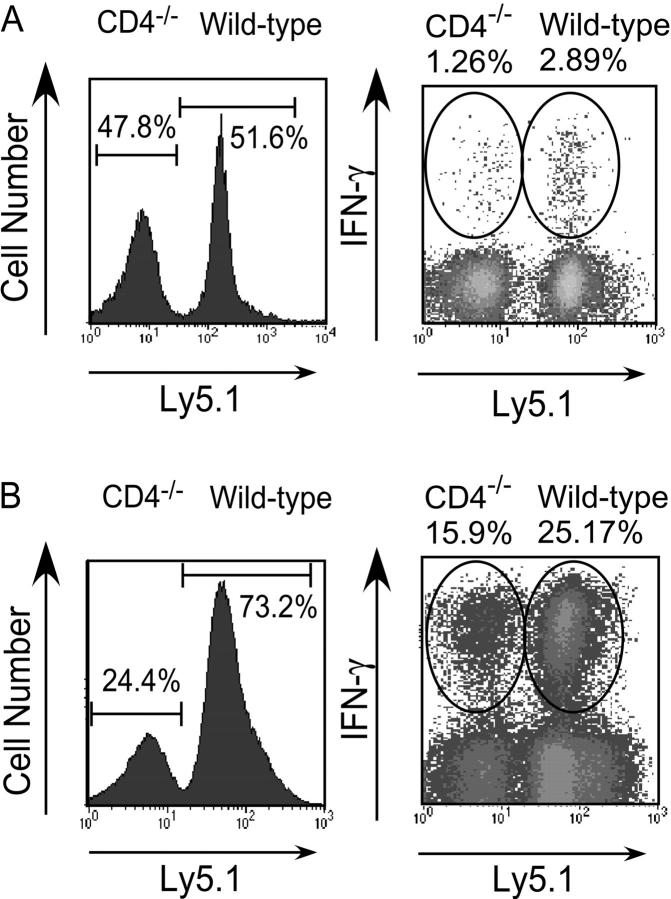
As aforementioned, we observed that the overall magnitude of the CD8+ T cell response to class I–restricted peptides assessed by intracellular cytokine staining or cytotoxicity was consistently lower in CD4−/− than in wild-type mice (Figs. 1 and 2). To determine whether this was due to helper deficiency in the CD4−/− mice, or is an intrinsic property of the CD8 population, purified CD8+ T cells from wild-type and CD4−/− mice were mixed in a 1:1 ratio, transferred into adoptive hosts and immunized with pathogen. After immunization with rLmOva, the IFN-γ response of transferred wild-type CD8+ T cells to the Ova peptide was roughly twofold higher than the response of the transferred CD4−/− cells in the same infected host (Fig. 5 A). After correcting for the slight skewing of total donor CD8 T cell numbers after L. monocytogenes infection, there were 2.5-fold more wild-type than CD4−/− effectors.
Figure 5.
Wild-type CD8+ T cells respond better than CD4−/− CD8+ T cells even when immunized in the same host. MACS-sorted CD8+ cells from Ly5.1 B6 and Ly5.2 CD4−/− mice were mixed in a 1:1 ratio and injected into Thy 1.1 hosts, which were immunized 1 d later with rLmOva or LCMV. For the analysis, donor T cells were gated as CD8+Thy1.1− and assessed for CD4−/− (Ly5.1−) or wild-type (Ly5.1+) origin. (A) 7 d after rLmOva immunization, the fraction of donor CD8+ T cells making IFN-γ in response to Ova peptide was quantitated. Numbers show the average of five adoptive hosts. (B) 8 d after LCMV immunization, IFN-γ synthesis by donor CD8 cells after in vitro stimulation with H2-Db-restricted GP33–41 peptide was assessed. Data are representative of two independent experiments.
There are at least four MHC class I–restricted LCMV epitopes in C57BL/6 mice that all stimulate significant CD8 responses. The response to LCMV generates a massive, antigen-specific expansion of CD8+ T cells (20, 21) and this resulted in a dramatic skewing of wild-type/mutant CD8 T cell numbers. In adoptive hosts of an equal mixture of wild-type and mutant CD8+ T cells, at 8 d after LCMV infection, the total number of wild-type cells outnumbers the CD4−/− numbers by a 3:1 ratio (Fig. 5 B, left). Adoptive hosts that did not receive LCMV maintained a 1:1 ratio of wild-type and mutant CD8+ T cells (unpublished data). The weaker response to the single Ova epitope did not result in such a dramatic shift in the ratio of wild-type to mutant cells after rLmOva immunization (Fig. 5 A). The IFN-γ response to the H2-Db/GP33–41 epitope is also skewed in favor of the wild-type CD8+ T cells (Fig. 5 B). When one takes into account the differential expansion of the wild-type and mutant cells, there are ∼4.8 times as many GP33–41-specific effectors of wild-type origin compared with CD4−/− origin. We conclude that the environment of the CD4−/− mouse does not explain their reduced CD8+ T cell response to class I–restricted peptides from L. monocytogenes or LCMV. Rather, something intrinsic to the CD8 population itself is the cause.
Discussion
We provide evidence for the surprising conclusion that in CD4−/− mice, the CD8 population contains a large fraction of MHC class II–restricted cells in addition to the conventional class I–restricted cells. In both of the immunization systems we studied, these CD8+ class II–restricted T cells were readily apparent. It has also been reported that the response to murine hepatitis virus in CD4−/− mice shows a similar, readily detectable CD8 T cell response to class II–restricted antigen, though in this case the epitopes were not defined (22). The class II–restricted response of CD8 lineage cells, which is readily seen in CD4−/− mice, is not obvious in control animals, where it is difficult to detect any CD8 response to the same MHC class II–restricted peptides. We have shown that this difficulty in detecting class II–restricted CD8 cells in a wild-type mouse is not because conventional CD4 T cells suppress the CD8 response to the class II/peptide epitopes; even when the CD4 T cell population is depleted just before immunization, no class II–restricted CD8 T cell response is revealed (Fig. 4). On the contrary, we observed that the few events in the IFN-γ staining profiles suggesting that CD8+ T cells in normal mice can respond to class II–restricted peptides were decreased in acutely CD4-depleted mice. This suggests that these events were caused by bystander activation of CD8+ T cells driven by peptide activation of CD4 cells in the cultures. However, we do not rule out the possibility that there is a very weak response of CD8+ T cells to class II–restricted peptides in normal B6 mice.
Indeed, it is likely that the class II–restricted CD8 T cells in the CD4−/− animals arise as a result of misdirection during positive selection in the thymus (4). We assume that the cells are selected by recognition of class II in the thymus, where some immature thymocytes express a randomly generated TCR with sufficient affinity for class II molecules on the thymic cortical epithelial cells that they can be selected into the CD8 lineage when the CD4 coreceptor is absent.
We propose that the main reason why the CD8 response to class I–restricted peptides in CD4−/− mice appears to be weaker than the response in control mice is that their TCR repertoire is heavily contaminated by MHC class II–restricted cells. It is not explained by the deficiency in T cell help, as demonstrated best by their weaker response even when immunized alongside equal numbers of CD8 T cells from wild-type mice (Fig. 5). It is difficult to estimate precisely the extent of this contamination. When we compare the fraction of total CD8 cells making a peptide-specific response in intact wild-type or mutant mice, as we did in Figs. 1 and 2, we find that CD4−/− give roughly half the wild-type level. However, this does not take into account the relative starting numbers of CD8 cells or their degree of expansion after immunization. When equal starting numbers of CD8 T cells were mixed and immunized together, we found that for the Ova257–264 and GP33–41 epitopes, the wild-type CD8 T cells gave 2.5-fold and 4.8-fold more progeny than the mutant CD8 T cells, respectively. This comparison suggests that the CD8 repertoire in CD4−/− mice may actually contain more class II–restricted receptors than class I–restricted receptors!
One intriguing question that remains is which immature thymocytes bearing class II–restricted receptors in CD4−/− mice are selected into the CD8 lineage, and which ones are selected into the double-negative CD4 lineage. According to the strength of signal model for CD4/CD8 lineage choice, double-positive thymocytes with higher avidity for the selecting ligand would commit to the CD4 lineage, and lower avidity cells would preferentially commit to the CD8 lineage (5–8). Although one can readily observe the response of the CD8 lineage cells to class II–restricted peptides in CD4−/− mice, a response by the double-negative CD4 lineage population is not apparent in our studies. One caveat to this is that we have mainly used IFN-γ staining to detect these cells, and it is possible that the response of CD4 lineage cells would be detectable using tetramers for these specificities, or by staining for other cytokines. However, we have evidence that a similar conclusion is reached even when intracellular IL-2 staining is used for detection (unpublished data). In addition, previous work on the response of CD4 lineage cells in CD4−/− mice has suggested that, whereas they can efficiently mount a Th1 response typified by IFN-γ synthesis, they are much less able to mount a Th2 response (23, 24). Finally, it is clear that the response of CD4 T cells in wild-type animals is readily detected by intracellular staining for IFN-γ. Another factor that may determine our inability to detect the response to class II–restricted peptides in the double negative CD4 lineage cells in CD4−/− mice is simple numbers. They make up only 10–20% of the peripheral T cell pool, whereas we estimate ≥50% of CD8 lineage cells are class II–restricted. At present, it is not precisely known what factors determine the outcome of MHC class II recognition in the thymus of CD4−/− mice, whether it be deletion, selection into the double-negative “CD4 wannabe” lineage, selection into the CD8 lineage, or no selection. Similarly, what controls the response to antigen presented on MHC class II molecules of peripheral T cells that lack the CD4 coreceptor and are either double negative or CD8+ is unknown.
Acknowledgments
We thank P.J. Fink, A. Gallegos, and M. Williams for comments on the manuscript.
This work was supported by National Institutes of Health grants AI19335 and CA09537 and the Howard Hughes Medical Institute.
Abbreviations used in this paper: β2m, β2-microglobulin; LCMV, lymphocytic choriomeningitis virus; LLO, listeriolysin O; rLmOva, recombinant Listeria monocytogenes strain expressing a secreted form of chicken ovalbumin.
References
- 1.Rahemtulla, A., W.P. Fung-Leung, M.W. Schilham, T.M. Kundig, S.R. Sambhara, A. Narendran, A. Arabian, A. Wakeham, C.J. Paige, R.M. Zinkernagel, et al. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 353:180–184. [DOI] [PubMed] [Google Scholar]
- 2.Killeen, N., S. Sawada, and D.R. Littman. 1993. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 12:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locksley, R.M., S.L. Reiner, F. Hatam, D.R. Littman, and N. Killeen. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 261:1448–1451. [DOI] [PubMed] [Google Scholar]
- 4.Matechak, E.O., N. Killeen, S.M. Hedrick, and B.J. Fowlkes. 1996. MHC class II-specific T cells can develop in the CD8 lineage when CD4 is absent. Immunity. 4:337–347. [DOI] [PubMed] [Google Scholar]
- 5.Itano, A., P. Salmon, D. Kioussis, M. Tolaini, P. Corbella, and E. Robey. 1996. The cytoplasmic domain of CD4 promotes the development of CD4 lineage T cells. J. Exp. Med. 183:731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogquist, K.A. 2001. Signal strength in thymic selection and lineage commitment. Curr. Opin. Immunol. 13:225–231. [DOI] [PubMed] [Google Scholar]
- 7.Germain, R.N. 2002. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2:309–322. [DOI] [PubMed] [Google Scholar]
- 8.Singer, A. 2002. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr. Opin. Immunol. 14:207–215. [DOI] [PubMed] [Google Scholar]
- 9.von Herrath, M.G., M. Yokoyama, J. Dockter, M.B. Oldstone, and J.L. Whitton. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett, S.R., F.R. Carbone, F. Karamalis, J.F. Miller, and W.R. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belz, G.T., D. Wodarz, G. Diaz, M.A. Nowak, and P.C. Doherty. 2002. Compromised influenza virus-specific CD8 (+)-T-cell memory in CD4 (+)-T-cell-deficient mice. J. Virol. 76:12388–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen, E.M., E.E. Lemmens, T. Wolfe, U. Christen, M.G. von Herrath, and S.P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 421:852–856. [DOI] [PubMed] [Google Scholar]
- 13.Shedlock, D.J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 300:337–339. [DOI] [PubMed] [Google Scholar]
- 14.Sun, J.C., and M.J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shedlock, D.J., J.K. Whitmire, J. Tan, A.S. MacDonald, R. Ahmed, and H. Shen. 2003. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J. Immunol. 170:2053–2063. [DOI] [PubMed] [Google Scholar]
- 16.Cosgrove, D., D. Gray, A. Dierich, J. Kaufman, M. Lemeur, C. Benoist, and D. Mathis. 1991. Mice lacking MHC class II molecules. Cell. 66:1051–1066. [DOI] [PubMed] [Google Scholar]
- 17.Grusby, M.J., R.S. Johnson, V.E. Papaioannou, and L.H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 253:1417–1420. [DOI] [PubMed] [Google Scholar]
- 18.Pope, C., S.K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed, R., J.A. Byrne, and M.B. Oldstone. 1984. Virus specificity of cytotoxic T lymphocytes generated during acute lymphocytic choriomeningitis virus infection: role of the H-2 region in determining cross-reactivity for different lymphocytic choriomeningitis virus strains. J. Virol. 51:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butz, E.A., and M.J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 8:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murali-Krishna, K., J.D. Altman, M. Suresh, D.J. Sourdive, A.J. Zajac, J.D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. [DOI] [PubMed] [Google Scholar]
- 22.Heemskerk, M.H., M.W. Schilham, H.M. Schoemaker, G. Spierenburg, W.J. Spaan, and C.J. Boog. 1995. Activation of virus-specific major histocompatibility complex class II-restricted CD8+ cytotoxic T cells in CD4-deficient mice. Eur. J. Immunol. 25:1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown, D.R., N.H. Moskowitz, N. Killeen, and S.L. Reiner. 1997. A role for CD4 in peripheral T cell differentiation. J. Exp. Med. 186:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowell, D.J., J. Magram, C.W. Turck, N. Killeen, and R.M. Locksley. 1997. Impaired Th2 subset development in the absence of CD4. Immunity. 6:559–569. [DOI] [PubMed] [Google Scholar]