Abstract
Neutrophil apoptosis occurs both in the bloodstream and in the tissue and is considered essential for the resolution of an inflammatory process. Here, we show that p38–mitogen-activated protein kinase (MAPK) associates to caspase-8 and caspase-3 during neutrophil apoptosis and that p38-MAPK activity, previously shown to be a survival signal in these primary cells, correlates with the levels of caspase-8 and caspase-3 phosphorylation. In in vitro experiments, immunoprecipitated active p38-MAPK phosphorylated and inhibited the activity of the active p20 subunits of caspase-8 and caspase-3. Phosphopeptide mapping revealed that these phosphorylations occurred on serine-364 and serine-150, respectively. Introduction of mutated (S150A), but not wild-type, TAT-tagged caspase-3 into primary neutrophils made the Fas-induced apoptotic response insensitive to p38-MAPK inhibition. Consequently, p38-MAPK can directly phosphorylate and inhibit the activities of caspase-8 and caspase-3 and thereby hinder neutrophil apoptosis, and, in so doing, regulate the inflammatory response.
Keywords: apoptosis, Fas, inflammation, phosphopeptide mapping, TAT-tagged caspases
Introduction
Mature neutrophils are released from the bone marrow to the bloodstream (1), and from there they are recruited to inflamed foci by cytokines and chemoattractants. In the bloodstream, and even more extensively in the tissues, aging neutrophils are effectively eliminated by apoptosis (2, 3). Therefore, the induction and control of such cell death are considered to be essential factors in the resolution or progression of an inflammatory response. Knowledge of the signaling mechanisms that regulate neutrophil apoptosis is insufficient, partly due to methodological limitations; the short life span of neutrophils rules out transfection, and microinjection of neutrophils is not feasible (4).
Caspases are major executors of the apoptotic program in most cell types, including human neutrophils. The initiation of the caspase cascade is often triggered by activation of a death receptor, such as trimerization of the Fas receptor. An active Fas receptor recruits the proteins necessary to start the caspase cascade by catalyzing the processing of procaspase-8 to its active form, the caspase-8 heterodimer, composed of a p20 and a p12 subunit (5). Similar downstream enzymatic activation events have been described for all procaspases. Regarding regulation of active caspases, direct interactions with different cellular lipids and proteins have been implicated (6, 7). In the present search for a mechanism whereby p38–mitogen-activated protein kinase (MAPK) can signal survival in neutrophils, it is particularly interesting that a few works have indicated that biochemical modifications can impair caspase activities. First, it has been shown that procaspase-3 and caspase-3 can be nitrosylated and that this results in impaired processing and inactivation in human cell lines (8). Second, active Akt was demonstrated to phosphorylate and impair the processing of human procaspase-9 and also to phosphorylate (Ser-196) and inactivate human caspase-9 in different transfected cell lines (9). However, this amino acid residue is not found in mice procaspase-9 (10). More importantly, during Fas-induced apoptosis of neutrophils, no activation of Akt can be detected (11). Recently, p42/p44-MAPK has also been shown to phosphorylate a conserved residue (Thr-125) in caspase-9 and to decrease its activity in mammalian cell extracts (12). However, inhibition of p42/p44-MAPK has no effect on spontaneous and Fas-induced neutrophil apoptosis (11, 13). Despite these findings and the fact that p38-MAPK appears to play a crucial role in regulating survival in many cell types (14–17), no information on its mechanisms of action is available.
The constitutive p38-MAPK activity, found by us and others in freshly isolated neutrophils (11, 18), is most probably caused by factors present in the blood, and consequently the subsequent decrease in this MAPK activity (11) can be explained by such factors being washed away during the isolation of these cells. Inactivation of constitutive p38-MAPK activity and Fas-induced activation of caspase-3 occur concurrently, suggesting that neutrophil apoptosis is initiated by, and dependent on, such inactivation of p38-MAPK (11). The fact that the later reappearance of p38-MAPK activity in isolated neutrophils is preceded by a rise in caspase-3 activity (11) suggests that it could constitute an apoptosis-induced negative feedback mechanism of this process in a manner similar to that noted for Akt (19).
Materials and Methods
Cells and Evaluations.
Human neutrophils were isolated and maintained as described previously (11). Fas receptors were engaged by incubating the cells with an anti-Fas monoclonal Ab (CH-11, 150 ng/ml; Immunotech). All data are expressed as mean ± SD (n < 6) or SEM (n ≥ 6), and statistical significance of the differences was analyzed by paired Student's t test: *, P < 0.05, **, P < 0.01.
Immunoprecipitations and Western Blot Analysis.
Neutrophils were lysed, and cell debris was removed by centrifugation, as described previously (11). The remaining supernatant was precleared with protein-G plus agarose (Oncogene Research Products). Thereafter, 40 μl agarose conjugated with an anti-phospho–p38-MAPK (Thr180/Tyr182) IgG1 mAb (New England BioLabs, Inc.), an anti–p38-MAPK IgG mAb (Santa Cruz Biotechnology, Inc.), or, as controls, with anti–c-Myc IgG1 mAb (ClONTECH Laboratories, Inc.), anti-Fyn IgG1 Ab, or nonimmune anti–rabbit IgG (Santa Cruz Biotechnology, Inc.) were added to the samples. The samples were incubated under rotation at 4°C overnight. Alternatively, 20 μl agarose, conjugated with an anti–caspase-3 polyclonal IgG Ab (Santa Cruz Biotechnology, Inc.) was added to the samples, which were incubated as aforementioned for 2 h. The immunoprecipitates were washed four times with lysis buffer and these, or lysates of intact cells, were boiled in sample buffer (11), after which the proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. It is worth mentioning that in the anti–caspase-3 immunoprecipitates, we observed a low recovery of the active p20 kD of the caspases (Fig. 2). A possible explanation for this finding is a much larger abundance of procaspases resulting in a preferential immunoprecipitation of these proforms. The membranes were analyzed with an anti–caspase-8 polyclonal Ab (Chemicon or Santa Cruz Biotechnology, Inc.), an anti–caspase-3 polyclonal IgG Ab (BD Biosciences or Santa Cruz Biotechnology, Inc.), an anti-p38 MAPKα IgG Ab, a MAPKδ IgG mAb, or an HA IgG2a mAb (F-7) (Santa Cruz Biotechnology, Inc.), an anti–caspase-9 polyclonal IgG Ab (BD Biosciences), an anti–phospho-p38 MAPK (Thr180/Tyr182) IgG1 mAb (New England BioLabs, Inc.), or the anti–phospho-serine IgMκ mAb 16B4 (BIOMOL Research Laboratories, Inc.). Before immunoblotting, the membranes that contained radioactive labels were analyzed using a PhosphorImager. As indicated in the figure legends, certain blots were stripped and reprobed according to the instructions of the manufacturers.
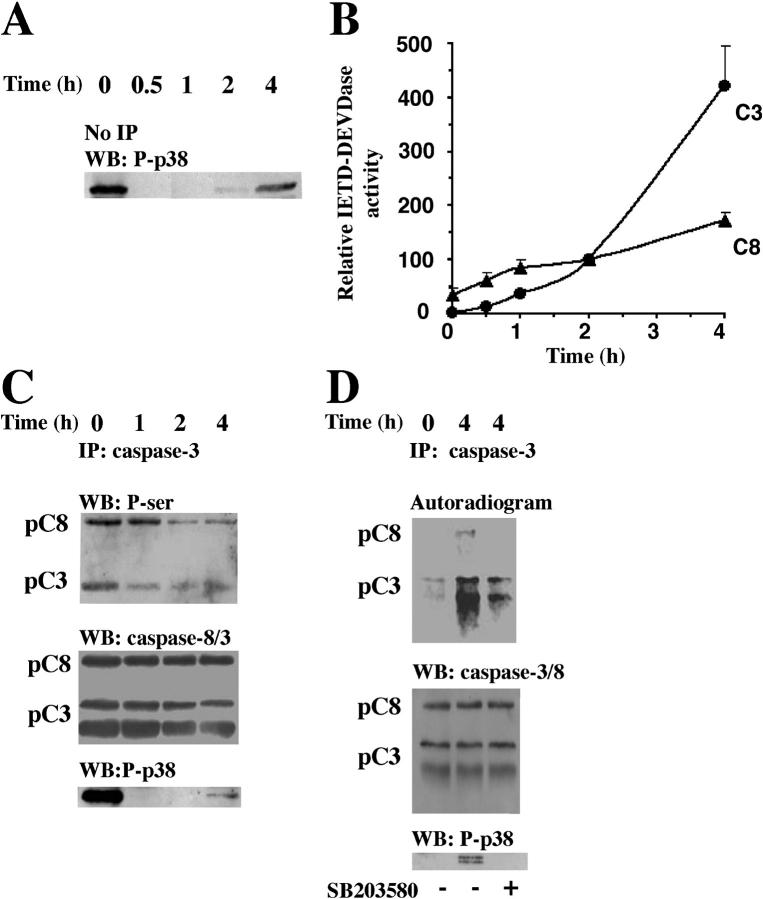
Figure 2.
p38-MAPK–dependent phosphorylations of procaspase-8 and procaspase-3 in intact cells. Neutrophils were incubated with anti-Fas Ab for the indicated times and lysed. (A) Samples were taken for Western blot analysis with an anti-phospho–p38-MAPK (P-p38) antibody. The blot shown is representative of at least eight separate experiments. (B) Alternatively, lysate samples were analyzed for IETDase (C8) and DEVDase (C3) activities (n = 6). To adjust for differences between blood batches, the caspase activities measured after 2 h were defined as 100%, and values at other time points were compared with that level. Caspase-3 immunoprecipitates were obtained from freshly isolated or 32P-labeled neutrophils after the indicated periods of exposure to anti-Fas Ab. (C) Unlabeled neutrophils were lysed, and the immunoprecipitates were immunoblotted with an anti–phospho-serine Ab, stripped, and reprobed with a mixture of anti–caspase-8 (detecting both the proform, pC8, and the active form) and anti–caspase-3 (detecting both the proform, pC3, and the active form) Abs and thereafter with an anti-phospho–p38-MAPK (P-p38) Ab. (D) The 32P-labeled neutrophils were lysed, and immunoprecipitates were analyzed by gel electrophoresis and blotted. The blots were developed with a PhosphorImager and subsequently analyzed with a mixture of the anti–caspase-8 and the anti–caspase-3 Abs, stripped, and reprobed with the anti-phospho–p38-MAPK Ab. The blots and the autoradiogram in C and D are representative of at least three separate experiments.
Expression of Human Caspases-8, Caspase-3, and Hamster Caspase-3.
The pET21b vectors containing COOH-terminally His6-tagged human procaspase-8 or procaspase-3 (20, 21) were provided by E.S. Alnemri (Kimmel Cancer Institute, Philadelphia, PA). The pET15b vector containing COOH-terminally His6-tagged hamster procaspase-3 (22) was provided by X. Wang (University of Texas Southwestern Medical Center, Dallas, TX). Exponentially growing Escherichia coli BL21(DE3)pLysS (Novagen) carrying the expression plasmids were induced at 30°C with 1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) and allowed to produce the procaspases for 1 h (23). Thereafter, the proteins were purified under native conditions using a Ni2+ affinity resin according to the instructions of the manufacturer (QIAGEN). Procaspase-8 and procaspase-3 were activated by preincubation for 15 min at 37°C.
Fluorometric Assays for Caspase Activities.
Ile-Glu-Thr-Asp (IETD)–aminomethylcoumarin (AMC; Upstate Biotechnology) and Asp-Glu-Val-Asp (DEVD)–AMC (Upstate Biotechnology), which are fluorogenic substrates for caspase-8 and caspase-3, respectively, were added to cell lysates, and the activities of the caspases (cleavage of AMC) were measured separately as described previously (11). The activities of recombinant human caspase-6 (Calbiochem), caspase-8, caspase-3, or Chinese hamster caspase-3 were assayed in caspase buffer (50 mM Tris, pH 8.0, 0.5 mM EDTA, 0.5 mM sucrose, 5% glycerol, and 10 mM DTT) supplemented with 20 μl of agarose-conjugated anti–active-phospho–p38-MAPK immunoprecipitate (from freshly isolated neutrophils) or, as controls, 20 μl of agarose-conjugated anti-cMyc or anti-Fyn (agarose conjugated) immunoprecipitate.
32P-Phosphorylation of the Caspases In Vivo.
5 × 107 neutrophils/ml were preincubated for 2 h in a calcium- and phosphate-free medium (136 mM NaCl, 5.9 mM KCl, 1.2 mM MgSO4, 5.0 mM NaHCO3, 5.5 mM glucose, and 20 mM HEPES, pH 7.4) supplemented with 2 mCi/ml [32P]orthophosphate; some cells were also exposed to 20 μM of SB203580 during the last 10 min of this incubation. Thereafter, the cells were washed and resuspended in RPMI 1640 supplemented with 5% FCS and stimulated with the anti-Fas monoclonal Ab for 4 h in the absence or presence of 20 μM of SB203580. Caspases were subsequently immunoprecipitated as aforementioned, using agarose-conjugated anti–caspase-3 polyclonal Ab.
Evaluation of Secondary Necrosis by Flow Cytometry.
The cells were incubated for 12 h in multi-well cell culture plates at 37°C in a humidified 5% CO2–95% air environment before studies of early and late apoptosis were performed by flow cytometry as described previously (11). In brief, to distinguish early from late apoptosis (secondary necrosis), the cells were stained simultaneously with FITC-conjugated annexin V and propidium iodide. The former binds to phosphatidylserine on the surface of both early and late apoptotic cells, whereas the latter stains cells that have lost their plasma membrane integrity, as is the case for late apoptotic cells.
p38-MAPK Phosphorylation Assay.
The p38-MAPK phosphorylation assay was initiated by adding 10 μCi γ-[32P]ATP (ICN Biomedicals) and 1 μg of substrate, that is, recombinant human caspase-8, caspase-3 or hamster caspase-3, BSA, or a purified extract from E. coli containing the empty vector in a reaction buffer (25 mM Tris, pH 7.5, 5 mM β-glycerophosphate, 0.1 mM Na3VO4 and 10 mM MgCl2) (11). The incubations with recombinant caspase-8 and caspase-3 were performed with or without 20 μM of SB203580 (Calbiochem). The effects of phosphorylation on caspase activities were assessed in the absence or presence of 10 mM cold ATP and substrate (i.e., active recombinant human caspase-8, caspase-3, caspase-6, hamster caspase-3, or BSA/extracts from bacteria containing an empty vector as controls). The assays were run for 60 min at 30°C. Thereafter, the degrees of phosphorylation were analyzed by autoradiography, and the activities of the two caspases were measured as aforementioned.
Immunoprecipitation of Active and Inactive Fas and Cleavage of Procaspase-8 In Vitro.
Immunoprecipitation of the active Fas receptor was performed as described previously (24). In brief, 5 × 107 neutrophils/ml were incubated with 2 μg/ml anti–APO-1 antibody (Bender MedSystems) for 5 min at 37°C, or the antibody was added after the cells were lysed (controls). Active receptor trimers or single inactive receptors (controls) were subsequently precipitated for 2 h with protein A–Sepharose (Amersham Biosciences), after which the beads were washed and resuspended in 25 μl of double-concentrated reaction buffer (25). These samples were added to 25 μl of the aforementioned p38-MAPK phosphorylation assay buffer containing 500 ng of procaspase-8 and the immunoprecipitates obtained using 20 μl of agarose-conjugated antibody against active p38-MAPK or an isotype-matched control antibody. These mixtures were incubated for 24 h at 4°C, and the samples were subsequently immunoblotted for caspase-8.
Assaying Procaspase-3 Cleavage In Vitro.
The assay was initiated by mixing 200 ng of active recombinant caspase-8 suspended in 25 μl of the previously described caspase buffer (double concentrated) with 25 μl of the aforementioned p38-MAPK phosphorylation assay buffer containing 300 ng of procaspase-3 and immunoprecipitates obtained using 20 μl of agarose-conjugated antibody against active p38-MAPK or an isotype-matched control antibody. The assay mixture was incubated for 35 min at 37°C, after which the samples were immunoblotted for caspase-3.
Tryptic Phosphopeptide Mapping and Phosphoamino Acid Analysis.
The radioactively labeled bands, corresponding to either caspase-8 or caspase-3, were localized by exposure on a PhosphorImager, excised from nitrocellulose filters and digested in situ with trypsin (modified sequencing grade; Promega) followed by two-dimensional phosphopeptide mapping, essentially as described by Blume-Jensen et al. (26). After exposure on a PhosphorImager, phosphopeptides were eluted from the plates in pH 1.9 buffer (formic acid/glacial acetic acid/double-distilled water; 44:156:1800, vol/vol/vol) and lyophilized. The fractions were subjected to two-dimensional phosphoamino acid analysis and automated Edman degradation in parallel. For Edman degradation, phosphopeptides were coupled to Sequelon-AA membranes (Millipore) according to the manufacturer's instructions and sequenced on a gas phase sequencer (model 470A; Applied Biosystems). The radioactivity in released phenylthiohydantoin derivatives from each cycle was quantitated by exposure on a PhosphorImager.
Plasmid Constructs and Site-directed Mutagenesis.
Human caspase-3 was amplified from the pET21b vector (provided by E.S. Alnemri) using the following primers: 5′-GCGGGTACCATGGAGAACACTGAAAACTCA-3′ and 5′-CGCGCATGCTTAGTGATAAAAATAGAGTTC-3′. The PCR fragment of caspase-3 was subcloned inframe into the KpnI/SphI sites of the bacterial expression vector pTAT-HA, provided by S. Dowdy (Howard Hughes Medical Institute, La Jolla, CA) (27, 28). To replace the single serine residue in caspase-3 with an alanine residue, we used the in vitro Quikchange™ site-directed mutagenesis kit (Stratagene) as indicated by the manufacturers and the oligonucleotide 5′-GAGGGGATCGTTGTAGAGCCCTAACTGGAAAACCC-3′ (the mutated bases Ser-150–Ala are underlined). The mutation was verified by sequence analysis.
Preparation and Use of TAT Fusion Proteins.
E. coli BL21-(DE3)pLysS (Novagen) carrying the expression vector pTAT-HA-procaspase-3 was incubated at 37°C with 0.2 mM IPTG overnight. Additional IPTG was added to the bacteria (final concentration = 0.450 mM) when the bacteria had reached OD600 of 0.6. The bacteria were allowed to further produce the vector protein for an additional 2 h period. Thereafter, the proteins were purified under either native (as aforementioned for in vitro assays) or denaturing conditions (urea, for introduction to intact cells) using a Ni2+ affinity resin according to the instructions of the manufacturer (QIAGEN). The preparation of procaspase-3 was desalted using chromatography columns (Micro Bio-Spin; Bio-Rad Laboratories). 2.5 × 105 cell were preincubated with 150 nM of either the wild-type TAT-HA-procaspase-3 or its mutant for 1 h (30 min on ice in the absence of and 30 min at 37°C in the presence of 20 μM of SB203580) before engagement of the Fas receptor. The uptake of TAT-tagged proteins was analyzed by Western blot. The cells were washed three times with PBS and lysed as described previously (11). To avoid preactivation of neutrophils by possible residual LPS in the recombinant protein preparations, the cells were incubated in the presence of 25 μg/ml polymixin B (a specific inhibitor of LPS bioactivity; reference 29) throughout the experiment.
Analysis of Nuclear Morphology.
Neutrophils were incubated for 4 h in multi-well cell culture plates at 37°C in a humidified 5% CO2–95% air environment before being stained with acridine orange and ethidium bromide as described previously (11) to assess nuclear morphology and cell viability.
Results
Regulatory Interactions between p38-MAPK and Caspases in Intact Cells.
Initially, we detected procaspase-8 and procaspase-3 (Fig. 1 A), but not procaspase-9 (not depicted), in immunoprecipitates of total p38α-MAPK, the most abundant p38-MAPK isoform in neutrophils (30). We also specifically immunoprecipitated active p38-MAPK from anti–Fas-treated cells, which showed a time-dependent increase in association of active p38-MAPK with the active p20 subunits of caspase-8 and caspase-3, levels of which were low or undetectable at time zero (Fig. 1 B). In immunoprecipitates obtained with an agarose-conjugated, isotype-matched control antibody, we could not detect any caspase-8 or caspase-3 (Fig. 1, A and B, top two blots). These findings imply intimate and regulatory interactions between these caspases and active p38-MAPK.
Figure 1.
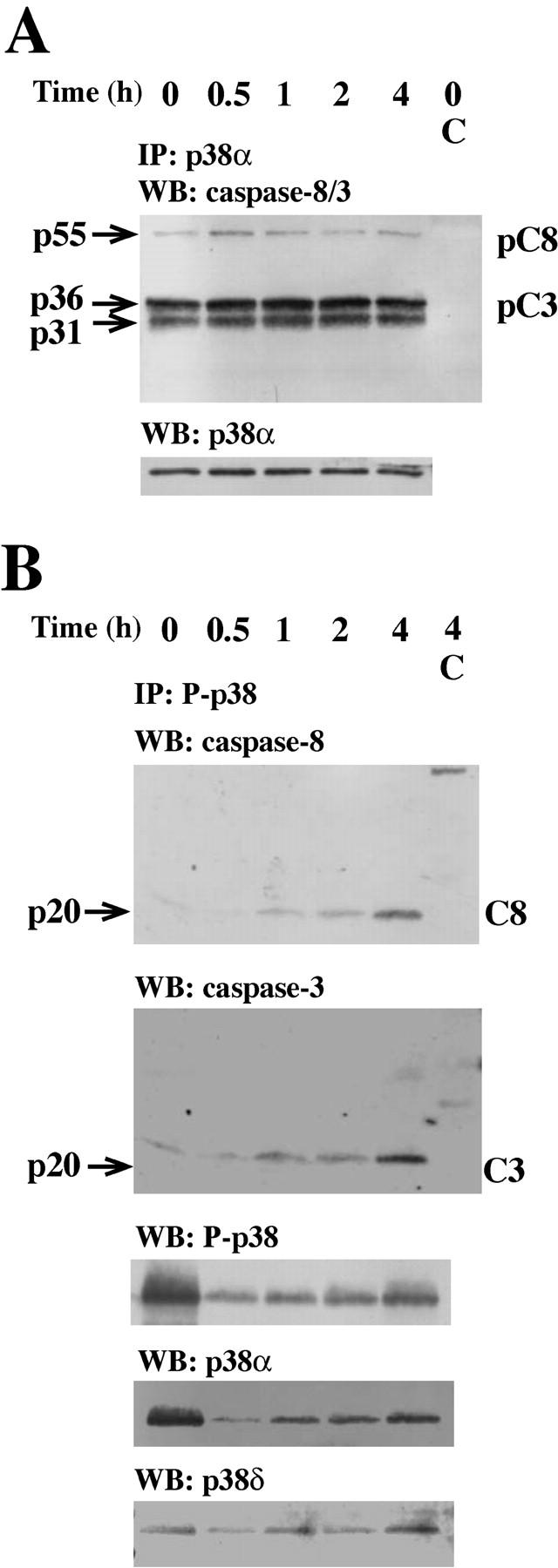
Caspase-8 and caspase-3 are coimmunoprecipitated with p38-MAPK. Neutrophils were incubated with anti-Fas Ab for the indicated times and lysed. (A) Samples were immunoprecipitated with an anti–p38-MAPKα or an isotype-matched control (C) antibody and subsequently assessed by Western blotting. The blots were either analyzed with a mixture of two antibodies respectively directed against caspase-8 (both the proform, pC8, and the active form, C8) and caspase-3 (both the proform, pC3, and the active form, C3), sequentially stripped, and reprobed with antibodies against p38-MAPKα (p38α). (B) Samples were immunoprecipitated with an anti-phospho–p38-MAPK or an isotype-matched control (C) antibody and subsequently assessed by Western blotting. The blots were sequentially analyzed with the antibodies against caspase-8, caspase-3, phospho–p38-MAPK (P-p38), p38-MAPKα (p38α), and p38-MAPKδ (p38δ). The blots in A and B are representative of at least seven separate experiments.
p38-MAPK activity was initially high and had almost disappeared after 30 min (Fig. 2 A), whereas caspase-3 activity was first detected after 30 min and reached a statistically significant increase after 1 h (Fig. 2 B), suggesting that the Fas-induced interactions between active p38-MAPK and caspases (Fig. 1 B) might require activation of these proteases. In support of that, the time-dependent increases in the activities of caspase-8 and caspase-3 (Fig. 2 B) paralleled the associations between the active p20 subunits of these caspases and p38-MAPK (Fig. 1 B). Assuming that recognition events between MAPKs and interacting substrates are necessary for the specificity of these kinases (31), our results suggest that active p38-MAPK might have a regulatory feedback effect on these caspases. We obtained support for this idea by the finding that inhibition of p38-MAPK significantly increased the percentage of cells exhibiting secondary necrosis (as revealed by positive propidium iodide staining) during spontaneous and Fas-induced apoptosis (spontaneous, 7.97 ± 4.6%; spontaneous + SB203580, 24.8 ± 13.6%; anti-Fas Ab, 10.6 ± 5.5%; and anti-Fas Ab + SB203580, 31.75 ± 10.4%; n = 4).
To evaluate possible p38-MAPK–induced phosphorylations of caspase-3, we immunoprecipitated this caspase with a precoupled antibody known to effectively bind this protein (Fig. 2, C and D). Unfortunately, we were unable to achieve equally efficient precipitation of caspase-8 with available anti–caspase-8 antibodies (unpublished data). However, this problem was partially overcome in our experiments because the anti–caspase-3 antibody also pulled down readily detectable amounts of caspase-8 (Fig. 2, C and D). First, we used an unspecific antiphosphoserine antibody to investigate possible phosphorylation events of caspase-8 and caspase-3 because MAPKs are known to phosphorylate their substrates on serine residues, and because this approach enabled analysis of possible caspase phosphorylations immediately after the cells were isolated. We observed distinct serine phosphorylations of both procaspase-8 and procaspase-3, which were most prominent when the p38-MAPK was active, and declined with different kinetics after the initial p38-MAPK activity had disappeared (Fig. 2 C). In addition, in parallel with the increase in p38-MAPK activity in whole cell lysates after 4 h (Fig. 2 A), the serine phosphorylations of the caspases also started to increase after 4 h (Fig. 2 C). Second, we labeled freshly isolated human neutrophils with 32P for 2 h and, thereafter, engaged their Fas receptors in the absence or presence of the p38-MAPK inhibitor SB203580. The time points indicated in Fig. 2 D cannot be compared with those in the other figures because the illustrated data represent cells that were preincubated with 32P for 2 h; instead, the caspase phosphorylations should be related to the evaluation of p38-MAPK activity in the same experiment (Fig. 2 D). The observed phosphorylations of the caspases were significantly (∼60%, as reveal by densitometric analysis), impaired by the p38-MAPK inhibitor SB203580 (Fig. 2 D). SB203580 is known to inhibit the α and β isoforms of p38-MAPK, but not the γ and δ isoforms (32). We detected the α and δ isoforms in the active p38-MAPK immunoprecipitates (Fig. 1 B), which could at least partly explain why SB203580 does not abolish p38-MAPK–induced phosphorylation of caspase-8 and caspase-3. However, at this stage, we cannot exclude that other alternative mechanisms are also responsible for the observed SB203580-insensitive phosphorylation of caspase-3. These experiments clearly demonstrate that engagement of Fas causes substantial 32P-phosphorylation of procaspase-8 and procaspase-3, and that SB203580 can reverse these biochemical modifications.
p38-MAPK-induced Phosphorylation of Caspase-8 (Ser-364) and Caspase-3 (Ser-150).
We used an in vitro assay to confirm that caspase-8 and caspase-3 are direct substrates of p38-MAPK. Active p38-MAPK immunoprecipitated from freshly isolated neutrophils was incubated with recombinant hamster caspase-3 (not depicted) as well as human caspase-8 or caspase-3 (Fig. 3, A and B) in the presence of γ-[32P]ATP. Both the procaspases and the 20-kD monomers of the active heterodimers of all three caspases were phosphorylated by the active p38-MAPK in the absence but not in the presence of SB203580.
Figure 3.
p38-MAPK–induced phosphorylations of caspase-8 and caspase-3 in vitro. (A and B) Active phosphorylated p38-MAPK immunoprecipitates from freshly isolated neutrophils were incubated with [γ-32P]ATP and recombinant caspase-8 or -3 in the absence or presence of the p38-MAPK inhibitor SB203580. As controls, the same reaction was performed in the absence of caspases but in the presence of either purified proteins from E. coli transformed with an empty vector (C) or BSA. All blots were first developed with a PhosphorImager, cut, and analyzed with (A) the anti–caspase-8 (pC8 and C8) Ab or (B) the anti–caspase-3 (pC3 and C3) Ab, and, lastly, stripped and reprobed with the anti-phospho–p38-MAPK (P-p38) Ab. The illustrated autoradiograms and blots are representative of at least seven separate experiments. (C) Active phosphorylated p38-MAPK (P-p38) was immunoprecipitated and incubated with recombinant caspase-8 and caspase-3 as substrates, in the presence or absence of ATP and under the same conditions as aforementioned. Thereafter, the activities of caspase-8 and caspase-3 were measured separately. The results are presented as percentage of the activities found in samples incubated in the same way but with an immunoprecipitate obtained using an isotype-matched control antibody. The data are expressed as mean ± SEM of seven separate experiments. The substrates, recombinant procaspase-8 (pC8) in D and recombinant procaspase-3 (pC3) in E, were incubated in the presence of ATP and either the immunoprecipitated active phosphorylated p38-MAPK (P-p38) or an immunoprecipitate obtained using an isotype-matched control antibody (Control). Thereafter, the in vitro amounts of the procaspases (pC8 and pC3) and caspases (C8 and C3), after incubations in the presence of (D) immunoprecipitated active Fas (FasR; n = 3) or (E) active caspase-8 (n = 5), were analyzed by Western blotting.
Fig. 3 C (based on data obtained using the experimental conditions indicated in Fig. 3, A and B) shows that the p38-MAPK–induced phosphorylation of caspase-8 and caspase-3 (ATP present) reduced the enzymatic activities compared with the unphosphorylated controls (ATP absent). These results are presented as percentage of the activities found in control samples incubated with immunoprecipitates obtained using an isotype-matched control antibody; no phosphorylation of the caspases was detected under those conditions (unpublished data). Eliminating ATP in this assay restored the activities of the two caspases, even in the presence of active p38-MAPK (Fig. 3 C). The same effect was observed when human caspase-6 (67.1 ± 15.2% of control; n = 5) or hamster caspase-3 (77.4 ± 10.2% of control; n = 5) were incubated in the presence of active p38-MAPK (these values are expressed as percentage of the activities found in samples where ATP was absent). Thereafter, we investigated whether the p38-MAPK–induced phosphorylation of procaspase-8 and procaspase-3 affected the handling of these precursor molecules. In in vivo experiments, procaspase-8 is processed upon recruitment to the active Fas receptor (5); whereas procaspase-3 is cleaved by active caspase-8. Therefore, we incubated procaspase-8 or procaspase-3 in the absence or presence of active p38-MAPK, with the active Fas receptor (Fig. 3 D) or caspase-8 (Fig. 3 E). This revealed a p38-MAPK–dependent reduction in the levels of caspase-8 (Fig. 3 D) and caspase-3 (Fig. 3 E), and indicated that p38-MAPK–induced phosphorylation impaired the processing of procaspase-8, whereas such phosphorylation rendered both caspase-8 and caspase-3 less stable.
We further examined the phosphorylation of recombinant caspase-8 and caspase-3 by active p38-MAPK immunoprecipitates from freshly isolated neutrophils in the presence of γ-[32P]ATP. Phosphorylated procaspase-8 and procaspase-3 were separated by gel electrophoresis and transferred to nitrocellulose membranes before tryptic digestion in situ and two-dimensional phosphopeptide mapping. As shown in Fig. 4 A, p38-MAPK phosphorylates caspase-8 on a single peptide fragment, whereas it phosphorylates caspase-3 on two peptide fragments. These different phosphopeptides were scraped off the TLC plates and subjected to two-dimensional phosphoamino acid analysis. All three phosphopeptides were phosphorylated on serine residues (Fig. 4 B). The phosphorylated serine residue in the phosphopeptide that originated from caspase-8 (Fig. 4 A) was located at position 3, as shown by an automated Edman degradation assay (Fig. 4 C). The phosphopeptides originating from caspase-3 (Fig. 4 A) had their phosphoserine residues located either at position 1 (weakest spot) or 3 (strongest spot) (Fig. 4 C). After compiling all the theoretical tryptic peptides derived from a complete digestion of procaspase-8 and procaspase-3 and comparing the possible tryptic peptides with homologous amino acid sequences in caspase-6, we concluded that the peptide from procaspase-8 containing Ser-364 and from procaspase-3 containing Ser-150 were likely to be the phosphorylated peptides. The peptide derived from procaspase-3, which contains Ser-150, is proceed by two arginine residues. A partial tryptic digestion of the arginines will generate two different phosphopeptides, as we detected in the TLC plates. Ser-364 and Ser-150 are present in regions that lie 11 amino acids upstream of the active sites of caspase-8 and caspase-3, respectively (Fig. 5 A). This region, containing either a Ser or a Thr residue, is conserved among different human caspases (1, 2, 4, 5, 7, and 9 but not 10 or 14). In addition, this region is also well conserved among other vertebrates (for example, caspase-6, -7, -8, -9, and -10 from Xenopus laevis) and invertebrates (for example, caspase-1 from Drosophila melanogaster). Interestingly, the three-dimensional structure of this region is also preserved among caspases (Fig. 5 B), implying a common regulatory region for caspases that is conserved among species.
Figure 4.
Identification of phosphorylation sites on caspase-8 and caspase-3. (A) Active phosphorylated p38-MAPK immunoprecipitates from freshly isolated neutrophils were incubated with [γ-32P]ATP and recombinant procaspase-8 or procaspase-3. The proteins were separated by SDS–gel electrophoresis, and the separated proteins were digested in situ with trypsin. The obtained phosphopeptides were separated on cellulose TLC glass plates (elect.), followed by ascending chromatography (chrom.). The indicated electrophoresis direction is from the anode to the cathode. The plates were analyzed in a PhosphorImager as well as exposed to an X-ray film. (B) The phosphopeptides from caspase-8 or caspase-3 were eluted from the TLC plates and subjected to two-dimensional phosphoamino acid analysis. The locations of the phosphoamino acids (top) were compared with that of phosphoamino acid markers (bottom) as follows: serine (S), threonine (T), and tyrosine (Y). The phosphopeptides obtained from A were subjected to amino acid sequencing (C), and the radioactivity released in each cycle was measured by spotting onto TLC plates and exposure on a Fuji image analyzer. The phosphorylated serine residues, 364 for caspase-8 (C) and 150 for caspase-3 (C), are indicated in the sequence of the putative fragment from caspase-8 and caspase-3, respectively. The illustrated phosphomapping is representative of three experiments.
Figure 5.
Ser-364 and Ser-150 are conserved residues in caspases. (A) The homologous serine/threonine residue (first box) is found 11 amino acids upstream of the active site (second box) within the outlined caspases. These residues are found in the respective, large, p20 subunits of the caspases. (B) The known three dimensional structures of the p20 monomers of caspase-8 (34), caspase-3 (35), caspase-9 (36), and caspase-7 (37) are revealed with the three-dimensional structure viewer Cn3D. In these structures, the p38-MAPK putative phosphorylation site is depicted (yellow), below which is the active site of each caspase.
Mutated Caspase-3 (S150A) Is Not Regulated by p38-MAPK.
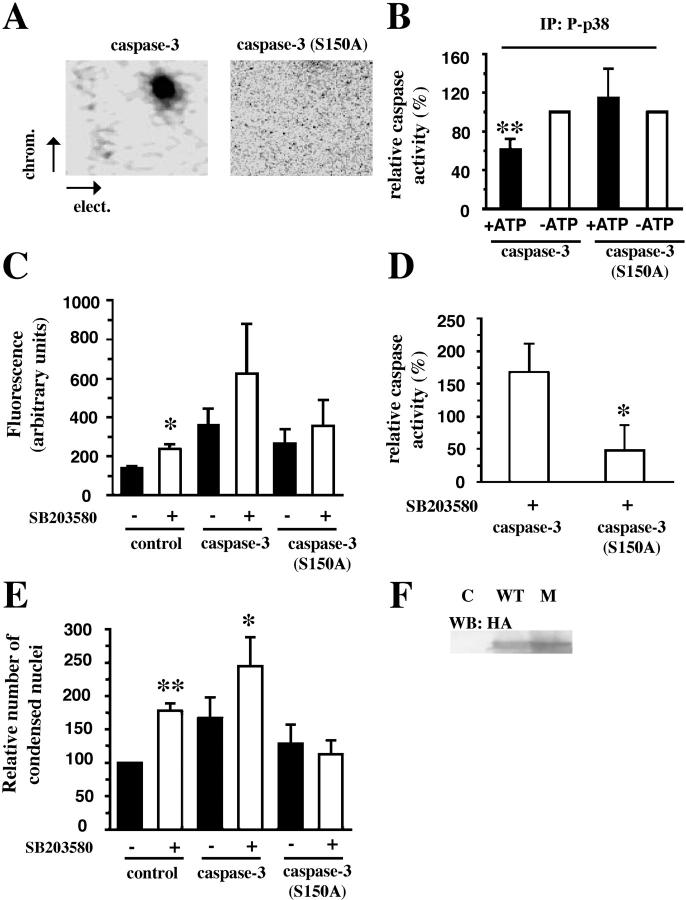
To directly test the role of caspase phosphorylation in the regulation of apoptosis in intact human neutrophils, we subcloned human procaspase-8 and procaspase-3 into TAT-HA vectors. TAT-HA–tagged caspase-8 exhibited no in vitro activity, probably due to a conformational change, whereas TAT-HA–tagged caspase-3 did. Consequently, we also prepared a TAT-HA–tagged mutated caspase-3 in which serine-150 was replaced with an alanine residue, and this protein was used together with the wild-type TAT-HA–tagged caspase-3. Despite overexposure, we could not detect any phosphorylation of the TAT-tagged mutated caspase-3 (Fig. 6 A). Furthermore, protease activity measurements of wild-type TAT-tagged caspase-3 and mutated TAT-tagged caspase-3 (S150A) revealed that, in contrast to wild type, the activity of the mutated caspase-3 is not modified by the presence of immunoprecipitated active p38-MAPK (Fig. 6 B). In vitro experiments revealed that caspase-3 (S150A) has a lower protease activity than wild-type caspase-3 (unpublished data). For this reason, and also to avoid any misinterpretations due to a difference in the loading of the two TAT-tagged proteins, we studied the effects of p38-MAPK inhibition (SB203580) on apoptosis in primary neutrophils preincubated with either wild-type or mutated caspase-3. The results show that caspase-3 activity is significantly increased by p38-MAPK inhibition in nontreated control cells and cells loaded with wild-type caspase-3, but not mutated caspase-3 (Fig. 6 C). The difference in protease activity between cells loaded with wild-type or mutated caspase-3 and incubated with the p38-MAPK inhibitor SB203580 is even clearer if we subtract the endogenous caspase-3 activity and express the activities as percentage of untreated cells (Fig. 6 D). These data nicely correlate with our findings that p38-MAPK inhibition (SB203580) increased the percentage of apoptotic cells (as revealed by acridine orange and ethidium bromide costaining) if they were preincubated with wild-type caspase-3, but not with the mutated caspase-3 (Fig. 6 E). These observations strongly support the idea that p38-MAPK counteracts neutrophil apoptosis by phosphorylation and inactivation of caspases.
Figure 6.
Mutation of Ser-150 abolishes the effect of the inhibition of p38-MAPK in vivo. (A) Active phosphorylated p38-MAPK immunoprecipitates from freshly isolated neutrophils were incubated with [γ-32P]ATP and recombinant wild-type TAT–caspase-3 or mutated TAT–caspase-3 (S150A). The phosphopeptide mapping was performed as in Fig. 4. The plates were exposed on a PhosphorImager as well as to film. The indicated electrophoresis direction is from the anode to the cathode. (B) Active phosphorylated p38-MAPK (P-p38) was immunoprecipitated and incubated with recombinant wild-type TAT–caspase-3 or mutated TAT-caspase-3 (S150A) as substrates in the presence (shaded bars) or absence (unshaded bars) of ATP. Thereafter, the activities of TAT–caspase-3 or TAT–caspase-3 (S150A) were measured separately. The results are presented as percentage of the activities found in samples depleted of ATP. The data are expressed as mean ± SD of five separate experiments. (C) Fas-induced caspase-3 activity in control cells or cells loaded with TAT–caspase-3 or TAT–caspase-3 (S150A) after 3 h after activation of the Fas-receptor. Unshaded bars represent cells treated with 20 μM SB203580 (n = 4–6). (D) The effect of SB203580 in cells loaded with TAT-fusion proteins (n = 4). (E) The Fas-treated cells were incubated for 4 h in the presence or absence of TAT-fusion protein and SB203580 and subsequently stained with acridine orange and ethidium bromide to assess their nuclear morphology. The effects are presented as percentage of untreated control cells (n = 4–6). (F) Neutrophils incubated in absence, as control (C) or presence of wild-type (WT) TAT-caspase-3 or (S150A) mutated (M) TAT–caspase-3. Samples were taken for Western blot analysis with an anti–HA-Ab (HA).
Discussion
We have shown previously (11), as have others (14, 15), that p38-MAPK constitutes a survival signal in isolated human and mice granulocytes, and that inactivation of this protein is a permissive event in spontaneous and Fas-induced neutrophil apoptosis (11). Furthermore, these previous findings suggested that active p38-MAPK could signal survival in primary neutrophils by keeping the activity of the caspases at low levels. Our initial finding, in the present work, of a constant association between procaspase-8 and procaspase-3 and p38-MAPK suggests a mechanism that could allow p38-MAPK to signal survival because such interaction might enable p38-MAPK–mediated biochemical modification and regulation of caspase-8 and caspase-3, and thereby also control of neutrophil apoptosis.
It is known that the proapoptotic stimulus triggered by an anti-Fas Ab directly recruits procaspase-8 to the Fas receptor, which facilitates the processing and activation of this proenzyme and in that way initiates the caspase cascade (5). We noted in the present work that such engagement of Fas receptors on human neutrophils also causes active p38-MAPK to associate with the active p20 subunits of caspase-8 and caspase-3. In addition, our experiments comparing the kinetics of p38-MAPK and caspase-3 revealed an interesting relationship between the activities of these proteins. In freshly isolated neutrophils, we found that p38-MAPK was highly active, whereas caspase-3 displayed no activity at all and caspase-8 very little. Not until after 30 min of Fas engagement did caspase-8 and caspase-3 exhibit increased activity, and this pattern coincided with the absence of p38-MAPK activity. Furthermore, we have observed previously that this loss of p38-MAPK activity does not occur in the presence of the survival factor GM-CSF (11). These results suggest that the initial deactivation of p38-MAPK is essential for Fas-induced stimulation of the caspase cascade in neutrophils. Of interest in the present context, we noted that p38-MAPK regained its activity once the caspases showed significantly increased activities and that inhibition of this activity caused an increased secondary necrosis in neutrophils. Therefore, we believe that this regain in activity represents a regulatory feedback mechanism that protects the cells from overactivation of caspases, which would lead to premature secondary necrosis and unwanted tissue damage in vivo. Indeed, such apoptosis-induced feedback has already been demonstrated for the well-known survival factor Akt (19). A physical basis for the aforementioned p38-MAPK–mediated regulation of caspase activities is indicated by the fact that we detected active p20 subunits of caspase-8 and caspase-3 in immunoprecipitates of active p38-MAPK from Fas-engaged neutrophils. These associations agree with the notion that p38-MAPK can induce biochemical modifications of caspase-8 and caspase-3 because such docking of MAPKs has been suggested to play an important role in their substrate specificities (31).
To ascertain whether the serine/threonine p38-MAPK induces phosphorylation of caspase-8 and caspase-3, we immunoprecipitated these two enzymes and performed Western blotting to measure if they were phosphorylated on such residues. We observed distinct serine phosphorylations of both procaspase-8 and procaspase-3, which were most prominent in freshly isolated neutrophils (i.e., when p38-MAPK was active), but declined with different kinetics after the initial p38-MAPK activity had disappeared. Furthermore, the serine phosphorylations of the caspases started to increase after 4 h, in parallel with the regain of p38-MAPK activity. In addition, when we labeled freshly isolated human neutrophils with 32P for 2 h and engaged their Fas receptors in the absence or presence of the p38-MAPK inhibitor SB203580, we found that the caspases were clearly phosphorylated by a p38-MAPK–dependent mechanism. Accordingly, the SB203580-induced increase in caspase-8 and caspase-3 activities in neutrophils undergoing Fas-provoked apoptosis (11) may be due to the aforementioned observation that active p38-MAPK phosphorylates caspase-8 and caspase-3, as in the case of Akt and human caspase-9 in transfected cell lines (9), and that such phosphorylations lead to inactivation of caspase-8 and caspase-3. This postulation was confirmed in experiments in vitro, which clearly showed that caspase-8 and caspase-3 are directly phorphorylated by p38-MAPK on serine-364 and serine-150, respectively, and that these biochemical modifications significantly reduced their enzymatic activities. The most straightforward interpretation of our results is that the p38-MAPK–induced phosphorylations cause activity-impairing conformational changes in the caspases. It also appears as if the observed phosphorylations of procaspase-8 and procaspase-3 affect the processing to the active caspases, and that the p38-MAPK–provoked phosphorylations of caspase-8 and caspase-3 also result in more rapid degradation of these two enzymes.
To finally test the aforementioned survival function of p38-MAPK in primary human neutrophils, we introduced TAT-tagged wild-type and mutated caspase-3 (S150A). The results clearly show that the Fas-induced apoptotic response in neutrophils containing mutated caspase-3 was not potentiated upon inhibition of p38-MAPK. The present data and other results implicating p38-MAPK as both a positive and a negative regulator of cell survival, depending on the type of cell studied (33), suggest that the role of p38-MAPK in a particular cell type is decided by the downstream substrates that are phosphorylated by the kinase.
Our results provide a novel and logical explanation for the mechanism underlying survival signaling by p38-MAPK in primary neutrophils: the active form of p38-MAPK associates with caspase-8 and caspase-3, which constitutes the basis for p38-MAPK–induced phosphorylations on serine-362 and serine-150 of these caspases. These biochemical modifications impair the activities, and possibly also the stability, of these caspases and thereby weaken their capacity to induce apoptosis.
Acknowledgments
The authors are grateful to E.S Alnemri, X. Wang, and S. Dowdy for providing the pET21b, pET15b, and HA-TAT vectors, respectively, and to P. Ödman for linguistic revision of the manuscript.
This work was supported by the Swedish Cancer Association, the SSF Inflammation program, King Gustaf V Memorial Foundation, U-MAS Research Foundations, the Österlund Foundation, and the Royal Physiographic Society in Lund.
Abbreviations used in this paper: AMC, aminomethylcoumarin; IPTG, isopropyl-1-thio-β-d-galactopyranoside; MAPK, mitogen-activated protein kinase.
References
- 1.Cartwright, G.E., G.W. Athens, and M.M. Wintrobe. 1964. The kinetics of granulopoiesis in normal man. Blood. 24:780–803. [PubMed] [Google Scholar]
- 2.Savill, J.S., A.H. Wyllie, J.E. Henson, M.J. Walport, P.M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grigg, J.M., J.S. Savill, C. Sarraf, C. Haslett, and M. Silverman. 1991. Neutrophil apoptosis and clearance from neonatal lungs. Lancet. 338:720–722. [DOI] [PubMed] [Google Scholar]
- 4.Glogauer, M., J. Hartwig, and T. Stossel. 2000. Two pathways through Cdc42 couple the N-formyl receptor to actin nucleation in permeabilized human neutrophils. J. Cell Biol. 150:785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551–1570. [DOI] [PubMed] [Google Scholar]
- 6.Mejillano, M., M. Yamamoto, A.L. Rozelle, H. Sun, X. Wang, and H.L. Yin. 2001. Regulation of apoptosis by phosphatidylinositol 4,5-bisphosphate inhibition of caspases, and caspase inactivation of phosphatidylinositol phosphate 5-kinases. J. Biol. Chem. 276:1865–1872. [DOI] [PubMed] [Google Scholar]
- 7.Pandey, P., R. Farber, A. Nakazawa, S. Kumar, A. Bharti, C. Nalin, R. Weichselbaum, D. Kufe, and S. Kharbanda. 2000. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 19:1975–1981. [DOI] [PubMed] [Google Scholar]
- 8.Mannick, J.B., A. Hausladen, L. Liu, D.T. Hess, M. Zeng, Q.X. Miao, L.S. Kane, A.J. Gow, and J.S. Stamler. 1999. Fas-induced caspase denitrosylation. Science. 284:651–654. [DOI] [PubMed] [Google Scholar]
- 9.Cardone, M.H., N. Roy, H.R. Stennicke, G.S. Salvesen, T.F. Franke, E. Stanbridge, S. Frisch, and J.C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science. 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- 10.Fujita, E., A. Jinbo, H. Matuzaki, H. Konishi, U. Kikkawa, and T. Momoi. 1999. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem. Biophys. Res. Commun. 264:550–555. [DOI] [PubMed] [Google Scholar]
- 11.Alvarado-Kristensson, M., M.I. Porn-Ares, S. Grethe, D. Smith, L. Zheng, and T. Andersson. 2001. p38 Mitogen-activated protein kinase and phosphatidylinositol 3-kinase activities have opposite effects on human neutrophil apoptosis. FASEB J. 10.1096/fj.01-0817fje. [DOI] [PubMed]
- 12.Allan, L.A., N. Morrice, S. Brady, G. Magee, S. Pathak, and P.R. Clarke. 2003. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat. Cell Biol. 5:647–655. [DOI] [PubMed] [Google Scholar]
- 13.Frasch, S.C., J.A. Nick, V.A. Fadok, D.L. Bratton, G.S. Worthen, and P.M. Henson. 1998. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J. Biol. Chem. 273:8389–8397. [DOI] [PubMed] [Google Scholar]
- 14.Villunger, A., L.A. O'Reilly, N. Holler, J. Adams, and A. Strasser. 2000. Fas ligand, Bcl-2, granulocyte colony-stimulating factor, and p38 mitogen-activated protein kinase: regulators of distinct cell death and survival pathways in granulocytes. J. Exp. Med. 192:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kankaanranta, H., P.M. De Souza, P.J. Barnes, M. Salmon, M.A. Giembycz, and M.A. Lindsay. 1999. SB 203580, an inhibitor of p38 mitogen-activated protein kinase, enhances constitutive apoptosis of cytokine-deprived human eosinophils. J. Pharmacol. Exp. Ther. 290:621–628. [PubMed] [Google Scholar]
- 16.Nemoto, S., J. Xiang, S. Huang, and A. Lin. 1998. Induction of apoptosis by SB202190 through inhibition of p38beta mitogen-activated protein kinase. J. Biol. Chem. 273:16415–16420. [DOI] [PubMed] [Google Scholar]
- 17.Zechner, D., R. Craig, D.S. Hanford, P.M. McDonough, R.A. Sabbadini, and C.C. Glembotski. 1998. MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J. Biol. Chem. 273:8232–8239. [DOI] [PubMed] [Google Scholar]
- 18.Aoshiba, K., S. Yasui, M. Hayashi, J. Tamaoki, and A. Nagai. 1999. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J. Immunol. 162:1692–1700. [PubMed] [Google Scholar]
- 19.Tang, D., H. Okada, J. Ruland, L. Liu, V. Stambolic, T.W. Mak, and A.J. Ingram. 2001. Akt is activated in response to an apoptotic signal. J. Biol. Chem. 276:30461–30466. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes-Alnemri, T., R.C. Armstrong, J. Krebs, S.M. Srinivasula, L. Wang, F. Bullrich, L.C. Fritz, J.A. Trapani, K.J. Tomaselli, G. Litwack, and E.S. Alnemri. 1996. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc. Natl. Acad. Sci. USA. 93:7464–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasula, S.M., T. Fernandes-Alnemri, J. Zangrilli, N. Robertson, R.C. Armstrong, L. Wang, J.A. Trapani, K.J. Tomaselli, G. Litwack, and E.S. Alnemri. 1996. The Ced-3/interleukin 1beta converting enzyme-like homolog Mch6 and the lamin-cleaving enzyme Mch2alpha are substrates for the apoptotic mediator CPP32. J. Biol. Chem. 271:27099–27106. [DOI] [PubMed] [Google Scholar]
- 22.Wang, X., N.G. Zelenski, J. Yang, J. Sakai, M.S. Brown, and J.L. Goldstein. 1996. Cleavege of Sterol regulatory element binding proteins (SREBPs) by CPP32 during apoptosis. EMBO J. 15:1012–1020. [PMC free article] [PubMed] [Google Scholar]
- 23.Stennicke, H.R., and G.S. Salvesen. 1999. Caspases: preparation and characterization. Methods. 17:313–319. [DOI] [PubMed] [Google Scholar]
- 24.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K.J. Tomaselli, K. Debatin, P.H. Krammer, and M.E. Peter. 1988. Two CD95 (APO-1/Fas) signalling pathways. EMBO J. 17:1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmström, T.H., I. Schmitz, T.S. Soderstrom, M. Poukkula, V.L. Johnson, S.C. Chow, P.H. Krammer, and J.E. Eriksson. 2000. MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly. EMBO J. 19:5418–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blume-Jensen, P., C. Wernstedt, C.H. Heldin, and L. Rönnstrand. 1995. Identification of the major phosphorylation site for protein kinase C in kit/stem cell factor receptor in vitro and in intact cells. J. Biol. Chem. 270:14192–14200. [DOI] [PubMed] [Google Scholar]
- 27.Wadia, J.S., and S.F. Dowdy. 2002. Protein transduction technology. Curr. Opin. Biotechnol. 13:52–56. [DOI] [PubMed] [Google Scholar]
- 28.Becker-Hapak, M., S.S. McAllister, and S.F. Dowdy. 2001. TAT-mediated protein transduction into mammalian cells. Methods. 24:247–256. [DOI] [PubMed] [Google Scholar]
- 29.Lazou Ahrén, I., A. Bjartell, A. Egensten, and K. Riesbeck. 2001. Lipopolysaccharide-binding protein increases toll-like receptor 4-dependent activation by nontypeable Haemophilus influenzae. J. Infec. Dis. 184:926–930. [DOI] [PubMed] [Google Scholar]
- 30.Hale, K.K., D. Trollinger, M. Rihanek, and C.L. Manthey. 1999. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J. Immunol. 162:4246–4252. [PubMed] [Google Scholar]
- 31.Tanoue, T., R. Maeda, M. Adachi, and E. Nishida. 2001. Identification of a docking groove on ERK and p38 MAPK kinases that regulates the specificity of docking interactions. EMBO J. 20:466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar, S., P.C. McDonnell, R.J. Gum, A.T. Hand, J.C. Lee, and P.R. Young. 1997. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235:533–538. [DOI] [PubMed] [Google Scholar]
- 33.Nebreda, A.R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257–260. [DOI] [PubMed] [Google Scholar]
- 34.Watt, W., K.A. Koeplinger, A.M. Mildner, R.L. Heinrikson, A.G. Tormasselli, and K.D. Watenpaugh. 1999. The atomic-resolution structure of human caspase-8 a key activator of apoptosis. Struct. Fold. Des. 7:1135–1143. [DOI] [PubMed] [Google Scholar]
- 35.Erlanson, D.A., J. Lan, C. Wiesmann, T.N. Luong, R.L. Simmons, W.L. Delano, I.C. Choong, M.T. Burdett, W.M. Flanagan, D. Lee, et al. 2003. In situ assembly of enzyme inhibitors using extended tethering. Nat. Biotechnol. 21:308–314. [DOI] [PubMed] [Google Scholar]
- 36.Shiozaki, E.N., J. Chai, D.J. Rigotti, S.J. Riedl, P. Li, S.M. Srinivasula, E.S. Alnemri, R. Fairman, and Y. Shi. 2003. Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell. 11:519–527. [DOI] [PubMed] [Google Scholar]
- 37.Chai, J., Q. Wu, E. Shiozaki, S.M. Srinivasula, E.S. Alnemri, and Y. Shi. 2001. Crystal structure of a procaspas-7 zymogen: mechanisms of activation and substrate binding. Cell. 107:399–407. [DOI] [PubMed] [Google Scholar]