Abstract
Plasmacytoid dendritic cells (PDCs) are a unique leukocyte population capable of secreting high levels of type I interferon (IFN) in response to viruses and bacterial stimuli. In vitro experiments have shown that upon maturation, human and murine PDCs develop into potent immunostimulatory cells; however, their ability to prime an immune response in vivo remains to be addressed. We report that CpG-matured murine PDCs are capable of eliciting in naive mice antigen-specific CTLs against endogenous antigens as well as exogenous peptides, but not against an exogenous antigen. Type I IFN is not required for priming, as injection of CpG-matured PDCs into type I IFN receptor–deficient mice elicits functional CTL responses. Mature PDCs prime CTLs that secrete IFN-γ and protect mice from a tumor challenge. In contrast, immature PDCs are unable to prime antigen-specific CTLs. However, mice injected with immature PDCs are fully responsive to secondary antigenic challenges, suggesting that PDCs have not induced long-lasting tolerance via anergic or regulatory T cells. Our results underline the heterogeneity and plasticity of different antigen-presenting cells, and reveal an important role of mature PDCs in priming CD8 responses to endogenous antigens, in addition to their previously reported ability to modulate antiviral responses via type I IFN.
Keywords: PDC, T cell priming, type I IFN, H-Y, tetramers
Introduction
DCs play a pivotal role in the control of innate and adaptive immune responses (1). They consist of a heterogeneous cell population, classified into distinct subsets according to surface phenotype, functional properties, and localization (2). In humans, an immature DC subset with plasmacytoid morphology (plasmacytoid DC [PDC]) represents a unique leukocyte population capable of secreting high levels of type I IFN in response to viruses and bacterial stimuli (3, 4). It has been shown recently that human PDCs behave as bona fide DCs, as they efficiently prime naive antigen-specific CD8 T cells (5), and are capable of restimulating CD4 and CD8 responses upon influenza virus infection (6). In both experimental systems, CD4 and CD8 T cells expanded by PDCs were capable of IFN-γ secretion. However, other investigators have shown that PDCs can differentiate allogeneic CD8 regulatory cells and Th2 responses (4, 7), suggesting that PDCs may have a certain degree of plasticity in their ability to prime T cell responses.
Murine PDCs have been identified recently on the basis of high type I IFN secretion and their unique surface phenotype (CD11cdull, B220+, CD11b−, and Gr-1+; references 8–10). To date, all functional studies on murine PDCs have been performed in vitro using PDCs either isolated from spleen or differentiated from bone marrow precursors. It has been shown that freshly isolated murine PDCs express lower levels of MHC and costimulatory molecules than the myeloid CD11chigh CD11b+ subset (myeloid DC [MDC]), possibly accounting for their reported poor stimulatory capacity for allogeneic and naive T cells (8–11). In contrast, PDCs matured with viral or CpG stimulation are potent APCs, capable of stimulating proliferation of allogeneic T cells and naive transgenic CD4 and CD8 T cells (8, 10, 12–14). Depending on antigen dose and Toll-like receptor engagement, murine PDCs show flexibility in their T cell polarizing capacity, generally eliciting Th1 responses at high and Th2 responses at low antigen doses (15). In addition, immature PDCs have been shown to differentiate T regulatory cells, capable of suppressing antigen-specific T cell proliferation (13, 16, 17).
Although in vitro experiments indicate that mature PDCs are potent immunostimulatory cells, it remains unclear whether they can prime antigen-specific immune responses in vivo in naive nontransgenic animals. To address this question, we set up an in vivo priming model in which we monitored ex vivo by tetramer analysis the proliferation of antigen-specific T cells after injection of PDCs either freshly isolated from the spleen or from FLT3 ligand (FLT3-L)–supplemented murine bone marrow cultures (18). We report that CpG-matured PDCs prime CTLs specific for endogenous but not exogenous antigens. CTLs primed by PDCs acquire potent in vivo cytolytic activity, are capable of IFN-γ secretion upon peptide stimulation, and protect mice from a subsequent tumor challenge. Priming is dependent on direct presentation of the antigen by the injected DCs and does not require responsiveness to type I IFN. Conversely, immature PDCs do not induce proliferation of antigen-specific CTLs in vivo. However, in contrast to what was observed in vitro, administration of immature PDCs does not prevent responses to subsequent challenges with viruses or DCs expressing the relevant antigen.
Materials and Methods
Mice.
C57BL/6, TAP-1−/− (on C57BL/6 background), 129A (lacking type I IFN receptor; reference 19), and 129 S1/SvEv mice were maintained at the John Radcliffe Hospital Biomedical Services and used at 7–12 wk of age according to institutional guidelines.
Peptides and Tetramers.
UTY246–254 (WMHHNMDLI), SMCY738–746 (KCSRNRQYL), LCMV-gp34–41 (AVYNFATC), OVA323–339 (ISQAVHAAHAEINEAGR), and OVA257–264 (SIINFEKL) peptides were purchased from Sigma-Aldrich and were HPLC purified. UTY246–254-H-2-Db, SMCY738–746-H-2-Db, OVA257–264-H-2-Kb, and LCMV gp34–41-H-2-Kb fluorescent tetrameric complexes (tetramers) were synthesized as described previously (20). The LCMV gp34–41-H-2-Kb tetramer was used instead of the LCMV gp33–41-H-2-Db tetramer (21) because, upon priming by LCMV gp33–41-peptide-pulsed DCs, responses to the octamer were dominant over those to the nonamer (unpublished data). Tetramers were validated by staining mice primed by vaccinia viruses encoding the relevant protein. Background levels of staining (<0.02% of total CTLs) were determined in naive mice.
Generation of Bone Marrow–derived DCs.
Culture medium was RPMI 1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 50 μg/ml kanamycin, 0.05 mM 2-mercaptoethanol (GIBCO BRL), and 10% FCS (Hyclone). Bone marrow cells were isolated by flushing femurs and tibia with complete medium. RBCs were lysed with RBC lysis solution (Puregene; Gentra Systems). The recovered cells were plated in culture medium containing 100 ng/ml FLT3-L (R&D Systems) at 106 cells/ml in six-well plates in a volume of 5 ml as described previously (18). Every 3–4 d, 2.5 ml of medium was replaced with fresh medium and FLT3-L. At day 10, half of the cultures were matured with 5 μg/ml phosphorothioate CpG DNA 1826 (Coley Pharmaceutical). In some experiments, bone marrow cultures were incubated with 500 μg/ml of soluble ovalbumin (fraction VII; Sigma-Aldrich) for 20 h before sorting (CpG was added to part of the cultures 4 h after ovalbumin). At day 11, the cultures were phenotyped by FACS® analysis with the following mAbs: CD11b-FITC, B220-PE, and CD19-APC (all obtained from BD Biosciences). PDCs (CD11b−B220+CD19−) and MDCs (CD11b+B220− CD19−) were isolated by a combination of magnetic (MACS; Miltenyi Biotec) and cell sorting (Moflo; DakoCytomation). The purity was always >97%, and CD11b+ cells were not detected upon reanalysis of PDC preparations.
Isolation of Splenic DCs.
C57BL/6 males were injected i.v. with 200 μg CpG DNA 1826. 16 h later, splenic DCs were enriched by magnetic sorting using CD11c beads (MACS; Miltenyi Biotec) after collagenase treatment of disrupted spleens. MDCs (CD11c+B220−Ly-6G/C−) and PDCs (CD11c+B220+Ly-6G/C+) were subsequently purified by cell sorting (Moflo; DakoCytomation) as described previously (11, 15).
Immunization Protocols.
Sorted cells were left unpulsed or pulsed with 1 μg/ml of peptide in serum-free medium for 2 h at 37°C, extensively washed, and diluted in PBS. 200 μl of cells was injected into the lateral tail vein. Animals were boosted by i.v. injection of 3 × 105 BM-DCs or 106 PFUs of UV-inactivated recombinant vaccinia encoding the UTY246–254 or SMCY738–746 minigenes or the full length ovalbumin protein (22).
Generation of Recombinant Vaccinia Viruses.
Recombinant vaccinia viruses (WR strains) encoding the UTY246–254 and SMCY738–746 minigenes were made by cloning each insert into the thymidine kinase gene using the vector pSC11 as described previously (23).
Isolation of PBLs and Tetramer Staining.
Blood was taken from the tail vein, and PBLs were isolated after depletion of RBCs with RBC lysis solution (Puregene; Gentra Systems). Cells were resuspended in 25 μl of complete medium and incubated with 0.5 μg of tetramer for 25 min at 37°C. Cells were washed and incubated with rat anti–mouse CD8α (BD Biosciences) for 20 min at 4°C. Cells were washed twice and analyzed using a FACSCalibur™ with CELLQuest™ software.
In Vivo Killing Assay.
To assess cytotoxicity, immunized and control mice were injected with a mixture of four differentially labeled syngeneic splenocyte populations, loaded or not with 10 μg/ml UTY246–254 peptide; three populations were labeled with different concentrations of carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) and one with 10 μM of the dye 5-(and 6)-([{4-chloromethyl}benzoyl]amino) tetramethylrhodamine (Molecular Probes; references 24, 25). Labeled cells were pooled and injected at 107 cells/mouse into the tail vein. Cytotoxicity was assessed by FACS® analysis on blood taken from the lateral tail vein at different time points. The mean percentage lysis of peptide-loaded target cells was calculated relative to antigen-negative controls with the following formula: 100 − (100 × adjusted percent survival). Adjusted percent survival was calculated as follows: (percent survival Ag+/percent survival Ag−)/mean percent survival in control animals.
ELISPOT.
Blood was taken from the tail vein, and PBLs were isolated after depletion of RBCs with RBC lysis solution (Puregene; Gentra Systems). In some experiments, RBC-depleted splenocytes were used as responders. Analysis of IFN-γ production in response to stimulation with 10 μM peptide for 16 h was performed on MultiScreen-IP high protein–binding 96-well plates (Millipore) using MabTech mouse IFN-γ ELISPOT kit according to the manufacturer's instructions. In all experiments, stimulation with 1 μg/ml PHA served as positive control.
Tumor Immunity Assay.
10 d after priming, mice were challenged with subcutaneous injection of 106 B16-F10 tumor cells expressing the LCMV gp33–41 minigene (26). Mice were monitored for tumor growth every 3–4 d, and the tumor size for each group was calculated as the mean of the products of bisecting diameters (± SEM). Measurements were terminated for each group when the first animal developed ascitis, when the tumor became ulcerated, or when it grew in excess of 200 mm2.
Intracellular Cytokine Staining.
Spleens were harvested 8–9 d after priming, and lymphocytes were isolated after depletion of RBCs with RBC lysis solution (Puregene; Gentra Systems). Cells were plated in complete medium and stimulated with 10 μg/ml UTY246–254 peptide, 10−6 M PMA (Sigma-Aldrich), and 1 μg/ml ionomycin (Sigma-Aldrich) or left unstimulated. 5 μg/ml Brefeldin A (Sigma-Aldrich) was added after 1 h, and cells were collected after a total of 6 h. Cells were fixed in 2% paraformaldehyde, permeabilized in saponin buffer (27), and stained with antibodies to mouse IFN-γ–FITC, IL-2–PE, IL-10–PE, and IL-4–APC (BD Biosciences). Tetramer staining was performed on a sample of unstimulated splenocytes as described in previous paragraphs for blood PBLs.
Online Supplemental Material.
Fig. S1 shows the phenotype of immature and CpG-matured PDCs and MDCs isolated from FLT3-L–supplemented bone marrow cultures. Fig. S2 supplements Fig. 1 and shows priming of SMCY738–746-specific CTLs by CpG-matured PDCs. Fig. S3 shows the phenotype of immature and CpG-matured PDCs differentiated from type I IFN receptor–deficient mice (129A) and wild-type 129 S1 mice. Fig. S4 supplements Fig. 10 and shows priming of OVA257–264-specific CTLs by peptide-pulsed mature PDCs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20031059/DC1.
Figure 1.
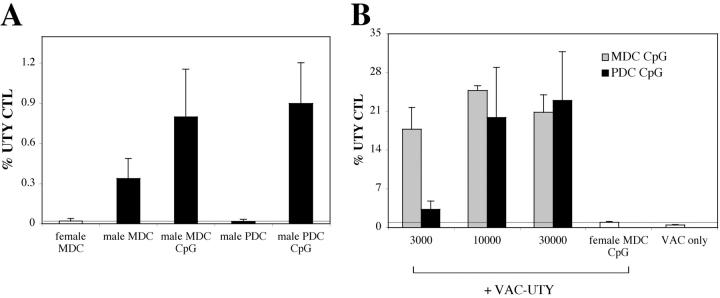
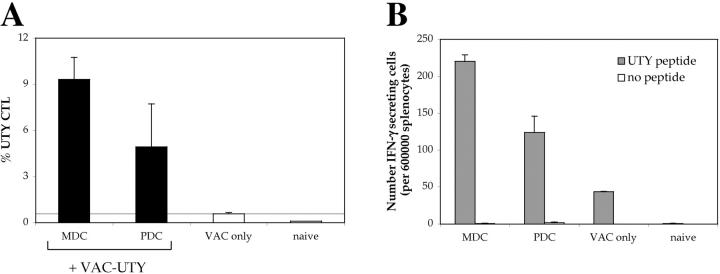
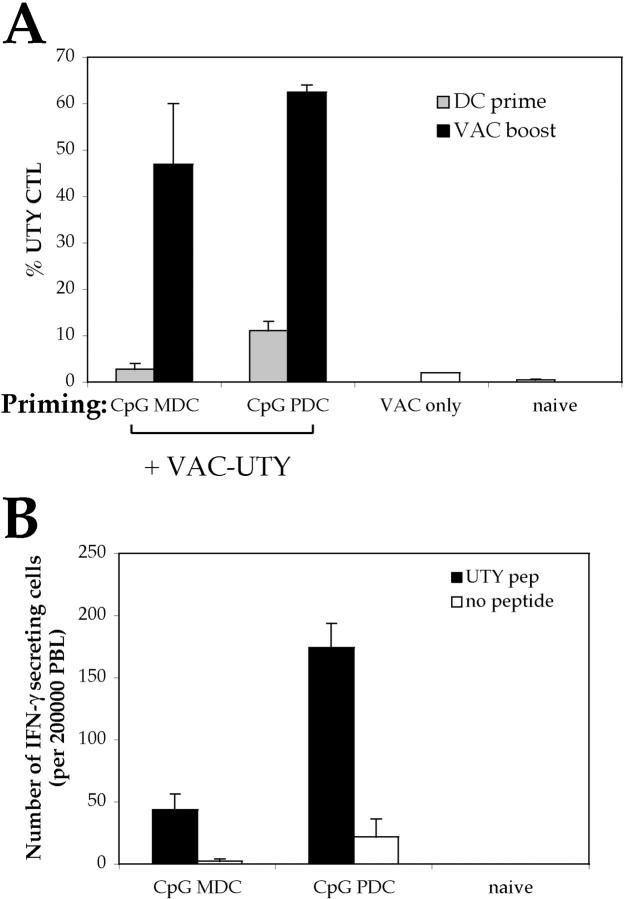
Intravenous injection of CpG-matured male PDCs induces CTL responses. (A) C57BL/6 mice (n = 5) were injected i.v. with 105 male MDC or PDCs, immature or CpG matured. Control animals were injected with female MDC. CTL responses were assessed in the blood by ex vivo FACS® analysis using UTY246–254-H-2-Db tetramers 7 d after priming. Mean proportions of tetramer+ cells as a percentage of CD8 cells (± SEM) for each group are shown. (B) C57BL/6 mice (n = 5) were injected with graded numbers of male CpG-matured MDC (gray bars) or PDCs (black bars) and boosted after 1 wk with UV-inactivated vaccinia-UTY246–254 minigene. CTL responses were assessed in the blood by ex vivo tetramer staining 8 d after boosting. Tetramer stainings of control mice, primed by female MDC or by vaccinia-UTY246–254 minigene alone, are shown (white bars).
Figure 10.
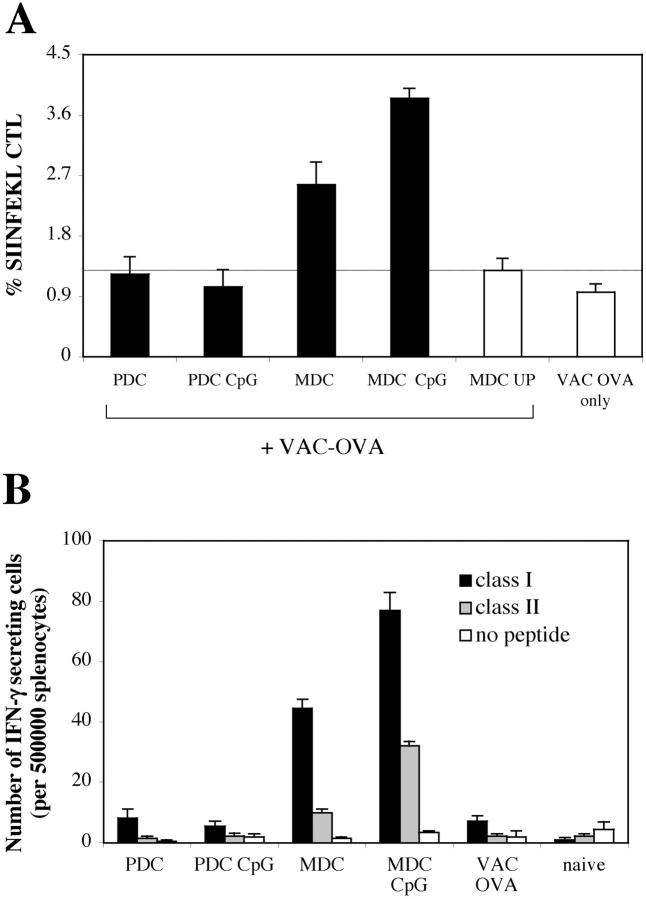
Lack of presentation of soluble ovalbumin by bone marrow–derived PDCs. BM cultures were pulsed on day 10 with 500 μg /ml of soluble ovalbumin in the presence or absence of CpG. MDC and PDCs were sorted on day 11. C57BL/6 mice (n = 3) were primed by intravenous injection of 105 cells and boosted after 10 d with vaccinia virus encoding full-length ovalbumin. (A) CTL responses were assessed in the blood by FACS® analysis using SIINFEKL-H-2-Kb tetramers 7 d after boosting. Mean proportions of tetramer+ cells as a percentage of CD8 cells (± SEM) for each group are shown. (B) IFN-γ ELISPOT was performed on splenocytes to assess responsiveness to ovalbumin MHC class I (SIINFEKL) and class II–restricted peptides (each at 10 μg/ml) 10 d after boosting. All animals showed comparable responses to PHA stimulation (not depicted).
Results
Intravenous Injection of CpG-matured Male PDCs Induces Functional CTL Responses.
Thus far, the stimulatory capacity of murine PDCs has been investigated only in vitro against transgenic T cells (8, 10, 12–14). Therefore, we developed an in vivo model to study the ability of PDCs to prime and polarize antigen-specific T cells from naive nontransgenic precursors. We differentiated both PDCs and control MDCs from murine bone marrow cultures supplemented with FLT3-L (18) and isolated the two DC populations by a combination of magnetic and cell sorting. In preliminary experiments, we observed that MDCs isolated from FLT3-L or GM-CSF + IL-4–supplemented cultures elicited qualitatively and quantitatively comparable CTL responses (unpublished data) and, therefore, restricted our subsequent analysis to FLT3-L–differentiated MDCs. The phenotype of bone marrow–derived PDCs and MDCs is shown in Fig. S1 (available at http://www.jem.org/cgi/content/full/jem.20031059/DC1).
In a first set of experiments, we investigated the response to an endogenous antigen, the male-specific transplantation antigen H-Y. When injected with male cells, female mice of the H-2b haplotype develop an immunodominant H-2-Db–restricted response against the UTY246–254 peptide (WMHHNMDLI) that can be monitored by tetramer analysis (28, 29).
UTY246–254-specific CTL responses were monitored ex vivo in the blood of female C57BL/6 mice injected with male PDCs or MDCs, either immature or CpG matured. Mature PDCs were as efficient as mature MDCs in eliciting UTY246–254-specific CTLs, as detected by tetramers (Fig. 1 A). In contrast, although immature MDCs always elicited a distinct CTL population (although lower in numbers as compared with mature MDCs), immature PDCs never induced proliferation of UTY246–254-specific CTLs above the detection limit of the tetramer staining (Fig. 1 A). Similar results were obtained when responses to the subdominant H-2-Db–restricted SMCY738–746 epitope (28) were monitored (unpublished data; Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20031059/DC1).
To better evaluate quantitatively the CTL responses, we titrated the numbers of DCs. At low numbers (3,000 cells/mouse), MDCs were far more efficient than PDCs in eliciting UTY246–254-specific CTLs (Fig. 1 B), whereas at 10,000 cells/mouse, PDCs and MDCs elicited a similar UTY246–254-specific CTL response. However, responses were weaker than in mice immunized with 105 cells (Fig. 1 A) and were detected only upon in vivo restimulation with vaccinia virus encoding the UTY246–254 minigene.
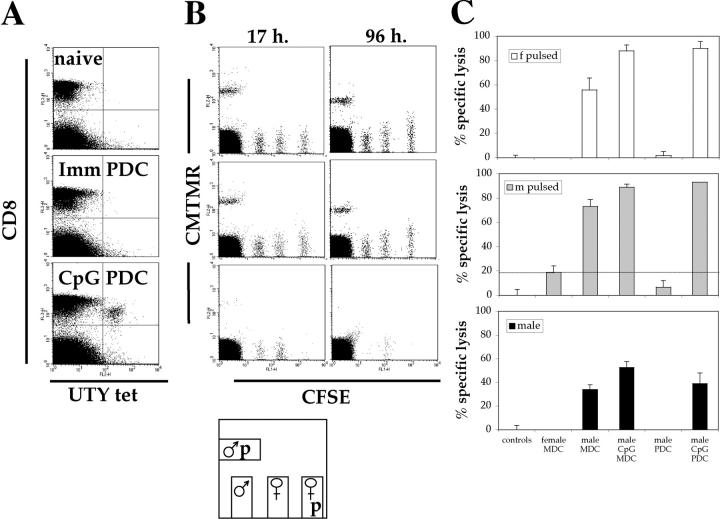
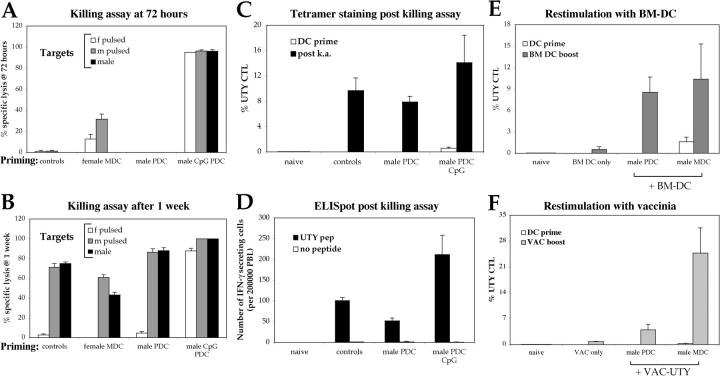
The functional state of the induced UTY246–254-specific CTLs was investigated by assessing their cytotoxic capacity and cytokine secretion upon antigen exposure. Cytotoxicity was assayed in vivo against syngeneic splenocyte targets, either male or peptide-pulsed female that had been labeled with a fluorescent dye and injected into the lateral vein 10 d after priming. Specific lysis of the antigen-expressing cells was determined against control female splenocytes not pulsed with the UTY246–254 peptide. UTY246–254-specific CTLs primed by mature PDCs efficiently lysed pulsed with 10 μg/ml of peptide female cells, as well as unpulsed male splenocytes (Fig. 2, B and C). Lysis of unpulsed male splenocytes, expressing a much lower density of UTY246–254–Db complexes than UTY246–254 peptide-pulsed cells, is indicative of expansion of high affinity CTLs. The majority of peptide-pulsed female targets were lysed within the first 17 h, whereas lysis of unpulsed male cells continued over the next 96 h, consistent with UTY246–254–Db complexes being presented at lower density but continuously over time (Fig. 2 B; not depicted). The cytolytic activity detected in mice primed by immature and mature MDCs correlated with the extent of CTL priming. No specific lysis above background was observed in mice primed by immature PDCs, mirroring the lack of detectable CTLs in the blood.
Figure 2.
Intravenous injection of CpG-matured male PDCs induces functional CTL responses. C57BL/6 mice (n = 5) were primed as described in Fig. 1 A. (A) CTL responses were assessed in the blood by ex vivo FACS® analysis using UTY246–254-H-2-Db tetramers 7 d after priming. One representative animal per group is shown. (B) 10 d after priming, cytolytic activity of the UTY246–254-specific cells was assessed in vivo against female syngeneic splenocytes unpulsed or peptide pulsed (CFSE labeled), male splenocytes unpulsed (CFSE labeled), or peptide pulsed (5-(and 6)-([{4-chloromethyl}benzoyl]amino) tetramethylrhodamine labeled) as summarized in the cartoon. Correlation between tetramer staining (A) and lysis of CFSE-labeled target cells at 17 and 96 h (B) is shown. The mouse primed by CpG-PDCs has a total of 2% UTY246–254-CTL (as a percentage of CD8 cells). (C) Analysis of mean antigen-specific lysis 17 h after target cell injection, calculated as described in Materials and Methods. Cells used for priming are shown on the x axis. Each panel depicts specific lysis of the labeled targets (top left).
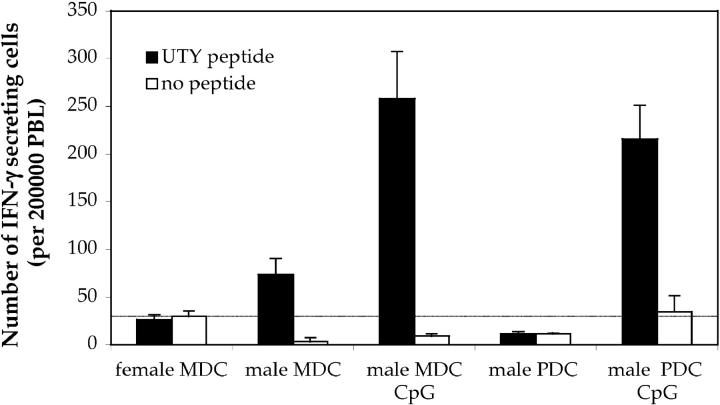
ELISPOT assays were performed to assess the capacity of UTY246–254-specific CTLs to secrete IFN-γ in response to peptide stimulation. As shown in Fig. 3, and in agreement with the tetramer data, mice primed by either mature PDCs or immature and mature MDCs were capable of recognizing the UTY246–254 peptide in an ex vivo assay, without further in vitro restimulation. No specific IFN-γ secretion was observed in mice primed by immature PDCs.
Figure 3.
Intravenous injection of CpG-matured male PDCs induces IFN-γ–secreting CTL. C57BL/6 mice (n = 5) were primed as described in Fig. 1. ELISPOT assay was performed on blood PBLs to assess IFN-γ secretion by antigen-specific cells in response to 10 μg/ml UTY246–254 peptide 7 d after priming. All animals showed comparable responses to PHA stimulation (not depicted).
Intravenous Injection of CpG-matured Male PDCs Induces Type I Polarization of Splenic UTY246–254-specific CTLs.
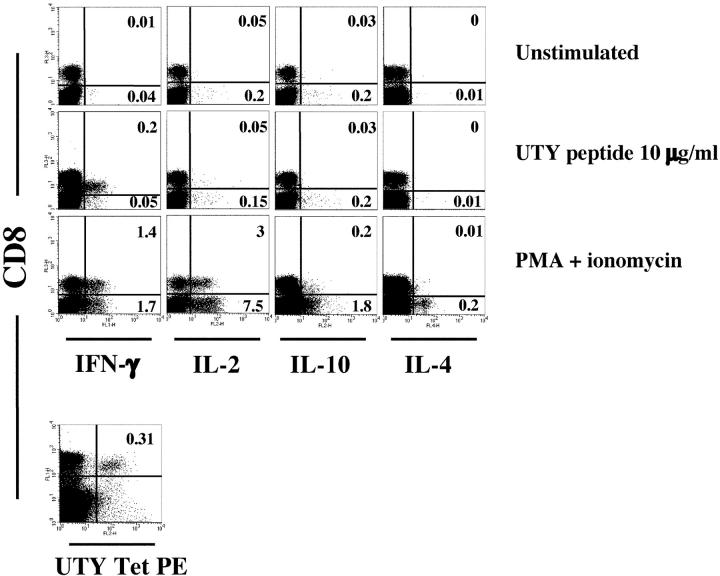
To further characterize the type of polarization induced by priming with mature PDCs, freshly isolated splenocytes were stimulated in vitro with the UTY246–254 peptide or PMA + ionomycin 8 d after priming. Analysis of intracellular cytokines revealed that CD8 T cells secreted the type 1 cytokine IFN-γ in response to both the specific peptide and PMA + ionomycin (Fig. 4). IL-2 was also detected in CD8 T cells stimulated with PMA + ionomycin. In contrast, CD8 cells did not secrete the type 2 cytokines IL-4 and IL-10 in response to either stimulation protocol. IL-4 and IL-10 were secreted only by the CD8 negative cells in response to PMA + ionomycin. A similar pattern of polarization was observed in mice primed by mature MDCs (unpublished data). Although the frequencies of tetramer+ cells in the spleen and in the blood were comparable, a higher proportion of splenic UTY246–254-specific CTLs secreted IFN-γ in response to the specific peptide (Fig. 4; not depicted), which may reflect homing of effector T cells to the spleen. We conclude that mature PDCs are able to efficiently prime antigen-specific CTLs, capable of cytolytic activity and IFN-γ secretion.
Figure 4.
Intravenous injection of CpG-matured male PDCs induces type I polarization of spleen CTL. C57BL/6 mice (n = 3) were injected i.v. with 105 male CpG-matured MDC or PDCs. Splenocytes from a mouse primed by CpG-matured PDCs were in vitro restimulated with UTY246–254 peptide or PMA and ionomycin, and intracellular cytokine accumulation was assessed as described in Materials and Methods 8 d after priming. The tetramer staining for the same sample is also shown. One experiment representative of three is shown. Similar profiles were obtained in mice primed by CpG-matured male MDC, whereas naive female C57BL/6 controls did not respond to the peptide.
Priming of UTY246–254-specific CTLs by Freshly Isolated CpG-matured Splenic PDCs.
It has been shown previously that freshly isolated splenic PDCs are less mature than their bone marrow–derived counterparts, hence they are also less immunostimulatory (15). Preliminary experiments showed that injection of immature splenic MDCs, in contrast to bone marrow–derived MDCs, did not elicit UTY246–254-specific CTLs detectable by ex vivo tetramer staining (unpublished data). Therefore, we isolated splenic PDCs and MDCs from male mice previously injected with CpG to induce in vivo DC maturation. Both MDCs and PDCs elicited UTY246–254-specific CTLs, although responses were much weaker than those elicited by equal numbers of bone marrow–derived DCs and were detectable only upon in vivo restimulation with vaccinia virus encoding the UTY246–254 minigene (Fig. 5 A). UTY246–254-specific CTLs primed by mature splenic PDCs and MDCs were functional, as shown by IFN-γ secretion in response to the cognate peptide in an ex vivo ELISPOT assay (Fig. 5 B).
Figure 5.
Intravenous injection of CpG-matured male splenic PDCs induces CTL responses. C57BL/6 mice (n = 3) were injected i.v. with 0.5 × 105 male splenic MDC or PDCs (isolated from CpG-treated animals) and boosted after 1 wk with UV-inactivated vaccinia-UTY246–254 minigene. (A) CTL responses were assessed in the blood by ex vivo FACS® analysis using UTY246–254-H-2-Db tetramers 8 d after boosting. Mean proportions of tetramer+ cells as a percentage of CD8 cells (± SEM) for each group are shown. (B) IFN-γ ELISPOT was performed on splenocytes to assess responsiveness to 10 μg/ml UTY246–254 peptide 9 d after boosting. All animals showed comparable responses to PHA stimulation (not depicted).
Priming of UTY246–254-specific CTLs Relies upon Direct Presentation of the Male Antigen by the Injected DCs.
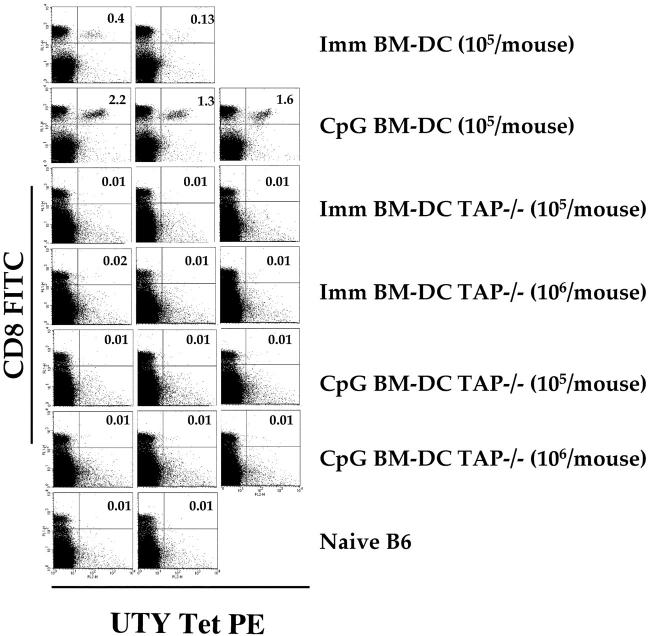
Priming of UTY246–254-specific CTLs by CpG-matured PDCs and MDCs could be due to direct presentation of the UTY246–254 peptide by the male APCs. Alternatively, proliferation of UTY246–254-specific CTLs could be due to uptake and presentation of male APC debris by resident female DCs, a mechanism known as cross-priming. Indeed, cross-priming has been shown to be effective for the generation of cytotoxic T cells, and it may be the dominant route for priming of some responses (30, 31). In vitro experiments showed that presentation of the UTY246–254 epitope is entirely TAP-dependent as UTY246–254-specific T cells did not recognize TAP-1–deficient male APCs (unpublished data). Therefore, we used TAP-1–deficient male BM-DCs as immunogens to distinguish between direct versus cross-presentation. Injection of as many as 106 male TAP-1−/− BM-DCs, either immature or CpG matured, failed to prime UTY246–254-specific CTLs in female C57BL/6 mice (Fig. 6). In contrast, control mice developed good responses after injection with 105 wild-type BM-DCs. After boosting with vaccinia-UTY246–254 minigene, only mice that had been primed with 106 male TAP-1−/− DC (i.e., 10 times more APCs than used in previous experiments) showed enhanced CTL responses (unpublished data). In agreement with the observation that UTY246–254-specific CTL responses cannot be efficiently generated upon priming by β-2m–deficient APCs (28), we conclude that the role of cross-priming in generating UTY246–254-specific CTLs in this system is marginal, and can only be appreciated when animals are injected with large numbers of APCs. Therefore, proliferation of UTY246–254-specific CTLs in our in vivo model can be accounted for by direct presentation of the endogenous antigen by the injected PDCs and MDCs.
Figure 6.
Direct presentation and not cross-priming accounts for expansion of UTY246–254-specific CTL. C57BL/6 mice were injected i.v. with the indicated numbers of male immature or CpG-matured BM-DCs (generated in FLT3-L). CTL responses were assessed in the blood by FACS® analysis using UTY246–254-H-2-Db tetramers 7 d after priming, and dot plot profiles for individual mice are shown. Only the mice injected with 106 BM-DCs showed secondary responses after boosting with vaccinia-UTY246–254 minigene (not depicted).
Lack of Priming by Intravenous Injection of Immature Male PDCs Does Not Prevent Subsequent Induction of Functional UTY246–254-specific Responses.
The inability of immature PDCs to prime antigen-specific CTLs could reflect the lack of expression of costimulatory or adhesion molecules essential to trigger naive T cell proliferation. Alternatively, immature PDCs could have induced the proliferation of anergic or regulatory cells nonreactive to further antigenic challenge or unable to bind UTY246–254-Db tetramers (32, 33). The possibility of anergy or negative regulation was ruled out by demonstrating that mice injected previously with immature PDCs developed cytolytic male-specific CD8 T cells upon boosting by splenocytes (Fig. 7, A and B). The acquisition of cytolytic activity correlated with the appearance of tetramer+ CTLs in the blood (Fig. 7 C), which also secreted IFN-γ in response to the cognate peptide in an ex vivo ELISPOT assay (Fig. 7 D).
Figure 7.
Intravenous injection of immature male PDCs does not prevent induction of subsequent UTY246–254 responses. C57BL/6 mice (n = 3) were primed as described in Fig. 1. 10 d after priming, cytolytic activity of the UTY246–254-specific cells was assessed in vivo against female or male syngeneic splenocytes unpulsed or peptide pulsed (as detailed in Fig. 1). The analysis of mean antigen-specific lysis 17 h (A) and 1 wk (B) after target cells injection is shown, calculated as described in Materials and Methods. The priming conditions are specified on the x axis. The first group of mice (controls) was injected with labeled splenocytes only and not with DC. The second group of mice was primed by female MDC, to control for responses to components of FCS used in the BM cultures. The high numbers of splenocytes used for the in vivo killing assay primes UTY246–254-specific CTL within 7 d (28), and this is reflected by the specific clearance of male splenocytes shown in B but absent at the earlier time point. The lack of clearance of peptide-pulsed female splenocytes reflects the short half life of the peptide on the surface of these cells, which, therefore, represent an important internal control for the experiment. (C) Mean proportions of tetramer+ cells as a percentage of CD8 cells (± SEM) for each group 7 d after PDC priming (white bar) and 1 wk after the in vivo killing assay (black bars). (D) IFN-γ ELISPOT performed on blood PBLs to assess responsiveness to 10 μg/ml UTY246–254 peptide 1 wk after the in vivo killing assay. All animals showed comparable responses to PHA stimulation (not depicted). The same groups of animals are shown in A–D. (E and F) Mean proportions of UTY246–254-H-2-Db tetramer+ cells as a percentage of CD8 cells (± SEM) after in vivo boosting with male immature DCs (E, staining performed at day 7) or UV-inactivated vaccinia-UTY246–254 minigene (F, staining performed at day 8).
As a further assessment of functional activity, we showed that previous injection of immature PDCs did not prevent expansion of UTY246–254-specific CTLs 1 wk after in vivo boosting with either male BM-DCs or vaccinia virus encoding the UTY246–254 minigene (Fig. 7, E and F).
We conclude that, despite the inability to detect UTY246–254-specific CTLs after one round of injection with immature PDCs (by either tetramer, ELISPOT, or in vivo killing assays), there was no evidence of tolerance to subsequent antigenic challenge.
Type I IFN Receptor–deficient CpG-matured Male PDCs Prime IFN-γ Secreting UTY246–254-specific CTLs.
PDCs are a unique leukocyte population capable of secreting high levels of type I IFN in response to viruses and bacterial stimuli (8–10). Because type I IFN is important for the survival and proliferation of memory CD8 T cells (34, 35), we investigated the priming capacity of CpG-matured PDCs in mice lacking type I IFN receptor (129A mice) (19).
As compared with 129 wild-type mice, 129A mice had normal numbers of PDCs ex vivo in the spleen and in vitro after culturing bone marrow cells in the presence of FLT3-L (unpublished data). In addition, 129A PDCs underwent maturation after CpG treatment, although to a lower extent than their wild-type counterpart (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20031059/DC1). Female 129A mice injected with male CpG-matured 129A PDCs developed antigen-specific CTLs, which could also be boosted by UV-inactivated vaccinia UTY246–254 minigene, as shown previously for C57BL/6 mice (Fig. 8 A). In addition, UTY246–254-specific CTLs primed by mature 129A PDCs secreted IFN-γ in response to the cognate peptide in an ex vivo ELISPOT assay (Fig. 8 B). These results suggest that in the absence of viral infection, type I IFN responsiveness is not essential for CTL priming by CpG-matured PDCs.
Figure 8.
Type I IFN receptor–deficient CpG-matured male PDCs prime IFN-γ–secreting CTL. Type I IFN receptor–deficient mice (129A; n = 2) were injected i.v. with 0.6 × 105 CpG-matured MDC or PDCs (also generated from 129A mice). 2 wk after priming, mice were boosted with UV-inactivated vaccinia-UTY246–254 minigene. (A) CTL responses were assessed in the blood by FACS® analysis using UTY246–254-H-2-Db tetramers 9 d after priming (gray bars) or 8 d after boosting (black bars). Tetramer stainings of control mice, naive or primed by vaccinia-UTY246–254 minigene alone, are shown as white bars (the priming conditions are specified on the x axis). Mean proportions of tetramer+ cells as a percentage of CD8 cells (± SEM) for each group are shown. (B) IFN-γ ELISPOT was performed on blood PBLs to assess responsiveness to 10 μg/ml UTY246–254 peptide 9 d after priming. All animals showed comparable responses to PHA stimulation (not depicted).
Priming by CpG-matured PDCs Induces Protective CTL Responses.
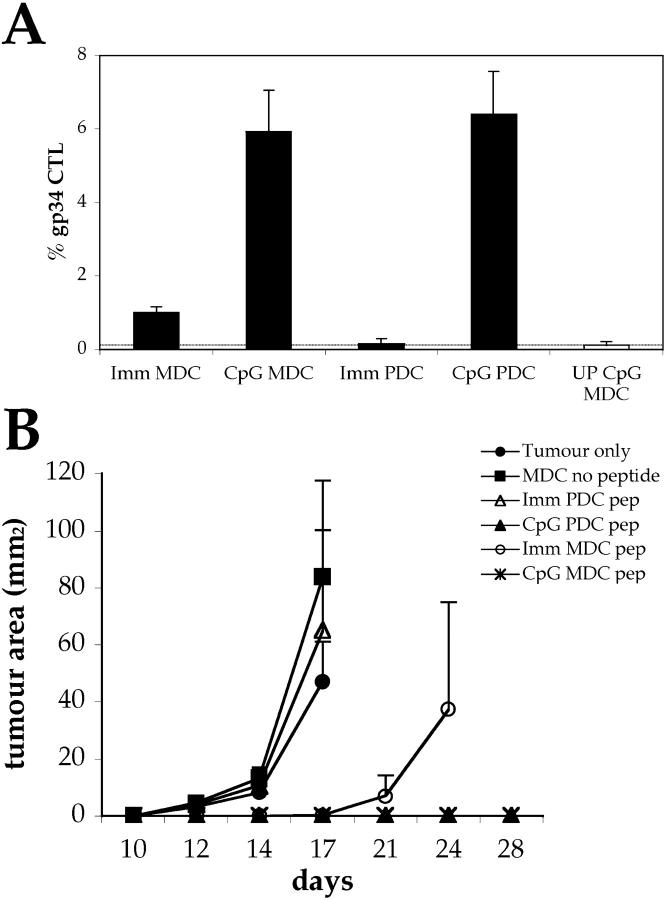
We have shown that PDCs can efficiently present an endogenous antigen and prime naive CTL precursor. We extended these results by analyzing the ability of peptide-pulsed PDCs to prime antigen-specific CTLs. We pulsed PDCs and MDCs with the LCMV gp34–41 peptide (Kb restricted) and monitored ex vivo LCMV gp34-specific CTLs by tetramer staining. In accordance with what was observed previously, CpG-matured PDCs and MDCs induced similar proliferations of LCMV gp34-CTL; immature MDCs were somewhat intermediate, and no priming was induced by immature PDCs (Fig. 9 A).
Figure 9.
Priming by CpG-matured PDCs induces protective CTL responses. C57BL/6 mice (n = 5) were primed by intravenous injection of 2 × 105 immature or CpG-matured MDC or PDCs pulsed with 1 μg/ml LCMV gp34–41 peptide. Control animals were injected with unpulsed CpG-DCs. (A) CTL responses were assessed in the blood by FACS® analysis using LCMV gp34–41-H-2-Kb tetramers 7 d after priming. Mean proportions of tetramer+ cells as a percentage of CD8 cells (± SEM) for each group are shown. (B) Progression of B16-F10-gp33 tumors implanted s.c. 10 d after priming. Mean tumor sizes per group ± SEM are shown. An additional control group (n = 5) that did not receive DCs was included.
These animals were challenged at day 10 with B16-F10-gp33, a derivative of the aggressive melanoma B16 that grows rapidly when injected in C57BL/6 mice (26). As shown in Fig. 9 B, mice that had been primed by CpG-matured PDCs or MDCs were completely protected from tumor growth and remained tumor free for up to 2 mo (not depicted). Mice primed by immature MDCs succumbed to the tumors, although they did so 2 wk later than mice injected with immature PDCs (which did not develop LCMV gp34-CTL) or unimmunized control groups.
Lack of Presentation of Exogenous Antigens by Bone Marrow–derived PDCs.
To further characterize the antigen-processing capacity of PDCs as compared with classical MDCs, we investigated the presentation of a model exogenous antigen, soluble ovalbumin. We sorted PDCs and MDCs from bone marrow cultures prepulsed with ovalbumin in the presence or absence of a maturation stimulus to induce cross-presentation (36). Ex vivo SIINFEKL-Kb tetramer staining detected primary CTL responses only upon injection of mature MDCs (unpublished data). After boosting with vaccinia virus encoding full-length ovalbumin, mice primed by ovalbumin-loaded PDCs did not expand SIINFEKL-specific CTLs above controls, regardless of the PDC maturation stimulus (Fig. 10 A). In contrast, CTL responses primed by MDCs were boosted two- to fourfold.
As a control, both CpG-matured PDCs and MDCs pulsed with the OVA257–264 peptide elicited functional SIINFEKL-specific CTLs detectable in the blood by tetramer staining (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20031059/DC1). Mice primed by MDC showed significant IFN-γ secretion in response to ovalbumin class I and class II peptides, whereas the response in mice primed by PDCs was comparable to that of control mice infected with vaccinia-OVA only (Fig. 10 B).
Discussion
In this paper, we have shown that CpG-matured murine PDCs efficiently prime antigen-specific CTLs in vivo from naive nontransgenic precursors. PDC-primed CTLs display cytolytic activity in vivo against target cells expressing the relevant antigen and are capable of protecting mice from a subsequent challenge with a tumor recombinant for the immunizing peptide. CTL priming occurs after injection of peptide-pulsed PDCs, but more importantly, we demonstrated that PDCs present endogenous antigens for which the density of MHC–peptide complexes at the cell surface is much lower than after peptide pulsing. However, PDCs are not able to present exogenous antigens, in contrast to MDCs, thus underlining the heterogeneity within the DC populations.
Previous works showed that, upon in vitro maturation, PDCs develop into potent immunostimulatory cells, although not to the same extent as control MDCs (8, 10, 12–14). In our in vivo experiments, mature PDCs and MDCs injected at high numbers elicited comparable responses, qualitatively and quantitatively, whether presenting the endogenous antigen or peptide pulsed. Only when limiting numbers of DCs were injected, MDCs were more stimulatory than PDCs. In line with our results, Dalod et al. (37) showed recently that PDCs purified after in vivo challenge with murine cytomegalovirus (MCMV) became as potent as other DC subsets for activation of naive CD8 T cells. Dalod et al. did not address whether PDCs could also present endogenous viral antigens because MCMV did not infect PDCs, but only induced their maturation. Our results showing priming of UTY246–254-specific CTL responses by male PDCs strongly suggest that PDCs, infected by viruses inducing their maturation, would be capable of priming virus-specific T cell responses.
In contrast with the majority of the analyses, Krug et al. have shown that splenic PDCs failed to stimulate a strong proliferation of naive CD4 and CD8 T cells, even after in vivo viral stimulation, questioning whether PDCs belong to the DC system (11). Conversely, we have shown that, although less efficient than bone marrow–derived PDCs, freshly isolated in vivo–matured splenic PDCs are able to prime UTY246–254-specific CTLs. It is possible that maturation induced by either CpG (our model) and MCMV (37) or VSV (11) may not generate functionally equivalent cells as a consequence of different Toll-like receptor engagement and, therefore, account for the observed discrepancies. With respect to the model antigen used, we analyzed ex vivo CTL responses specific for the immunodominant H-Y UTY246–254-H-2-Db epitope and the subdominant SMCY738–746-H-2-Db epitope in nontransgenic animals. In contrast, Krug et al. have looked at the endogenous presentation of the SMCY738–746-H-2-Db epitope using TCR transgenic mice (38). These mice fail to reject male skin grafts, and SMCY738–746 TCR transgenic CD8 T cells do not induce graft versus host disease when transferred in male nude mice (39, 40), suggesting that this receptor may have poor reactivity for its ligand. In addition, SMCY738–746 TCR transgenic CD8 T cells have a defect in cytotoxicity in response to male targets that can be overcome at elevated densities of the cognate peptide (41, 42), which may be present on the surface of MDCs but not PDCs (11).
We have not studied the capacity of mature bone marrow–derived PDCs to present endogenous antigens to CD4 T cells. However, as the development of the UTY246–254-specific CTL response is strictly dependent on T cell help from H-Y-Ab CD4 cells (28), we speculate that mature PDCs might have primed CD4 as well as CD8 male-specific cells.
It has been shown that human immature MDCs induce differentiation of CD4 and CD8 regulatory T cells, both in vivo and in vitro (43, 44). In the mouse, targeting of antigen in vivo to immature DCs induces CD4+ CD25+ regulatory T cells and CD8 T cell tolerance (45, 46). Upon injection of immature MDCs, we elicited functional CTLs, although in lower numbers than with mature MDCs, and this can be explained by the fact that these cells may have undergone a limited degree of maturation during the culture/sorting period (unpublished data). Regulatory T cells have also been differentiated in vitro by CD40L-activated human PDCs (7) and murine immature PDCs (13, 16, 17), and it has been hypothesized that thymic PDCs might be involved in Treg differentiation (13). However, in our in vivo priming model, we did not elicit any antigen-specific CTLs when mice were injected with immature PDCs (male DCs or peptide pulsed), making it difficult to test for any regulatory activity. Nevertheless, exposure to immature PDCs did not prevent induction of immunity after subsequent challenge with DCs, recombinant virus, or splenocytes, ruling out establishment of long-term tolerance. Although these results might at first seem difficult to reconcile with published data, they may be explained by the different experimental systems used. Indeed, previous in vivo studies have been performed with adoptively transferred naive transgenic T cells, whereas our paper relies on endogenous CTL precursors present in much lower numbers. The development of antibodies to specifically target antigens to PDCs in vivo, as described for CD8α+ DCs via DEC-205 (45, 46), will help to address whether immature PDCs may also induce tolerance and T regulatory cells.
With respect to T cell polarization, we have shown that CTLs primed by CpG-matured PDCs secreted mainly IFN-γ in response to the cognate antigen both by ex vivo ELISPOT and intracellular cytokine assays. Other investigators have reported high IFN-γ by transgenic CTLs polarized in vitro by mature PDCs pulsed with OVA257–264 or LCMV gp33–41 peptides (12, 37). A flexibility of murine PDCs in directing Th1 and Th2 development of CD4 transgenic T cells has been described, depending on antigen dose and Toll-like receptor engagement (15). This is likely to reflect differences in polarization of CD4 and CD8 cells, consistent with the lack of secretion of IL-4 by PMA + ionomycin–stimulated CD8 splenocytes (Fig. 3). We have not been able to induce UTY246–254-specific CTL proliferation when mice were injected with immature PDCs (male PDCs or peptide pulsed); therefore, we cannot comment on the CTL polarizing capacity of PDCs at different stages of maturation.
We have shown that, in the absence of viral infection, type I IFN is not required for priming because injection of CpG-matured PDCs into type I IFN receptor–deficient mice (129A) elicits functional CTL responses. In contrast, during a viral infection, the importance of PDC-derived type I IFN in initiating innate immune responses and in the cross-talk with other DC subsets for induction of adaptive immunity has been very elegantly demonstrated (37, 47). However, we cannot exclude the possibility that other recently described members of the IFN family may play a key role in PDC stimulatory capacity (48, 49), and some redundancy in the IFN system may be envisaged. Such redundancy may be implied by the observation that 129A mice have PDCs (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20031059/DC1) in contrast to what was reported for IFN consensus sequence-binding protein (a transcription factor involved in the IFN signaling pathway)–deficient mice that lack PDCs and have a defect in the activation of CD8α+ DCs (50–52). In addition, 129A PDCs mature in response to CpG treatment, although to a lower extent than their wild-type counterpart, consistent which was with what was reported previously (53–55). This level of maturation is indeed sufficient for in vivo priming of functional CTL responses, although in other experimental models, amplification of DC maturation by IFN signaling may be required for optimal regulation of the immune response (37).
Although in vivo mature PDCs can induce proliferation of functional CTLs specific for an endogenous antigen as well as for exogenously loaded peptides, they are unable to present exogenous antigens, even in the presence of maturation signals known to activate cross-presentation in MDCs (36). This observation is consistent with a recent paper showing that PDCs were far less efficient compared with other DC subsets in presenting s.c. injected Leishmania major antigens (56). Lack of presentation of exogenous antigens could be due to poor endocytic activity compared with MDCs (unpublished data; references 57, 58), or to differences in the antigen-processing and -presenting machinery between MDCs and PDCs, consistent with the different expression pattern of cathepsins in human MDCs and PDCs (59). Our results underline the heterogeneity and plasticity within the APC family, highlighted previously between CD8α+ and CD8α− MDCs (60–63). We envision that in vivo PDCs may contribute to priming antigen-specific CD8 and CD4 T cell responses by efficiently presenting endogenous antigens, whereas their role in priming T cell responses to exogenous antigens would be negligible; in this respect, differing from both CD8α+ and CD8α− MDCs. In addition, PDCs could modulate the function of other DC subsets by inducing their functional maturation and promoting cross-presentation via type I IFN secretion (64). Finally, these data validate in vivo our previous results on the ability of human PDCs to prime antigen-specific naive T cells (5), and provide a rationale for the combined use of mature MDCs and PDCs in vaccine trials to optimize the induction of innate and adaptive immune responses.
Acknowledgments
We would like to thank Drs. A. O'Garra for helpful discussion and C. Hetherington for care of the animal colonies.
This work was funded by Cancer Research (project grant C399/A2291) and the Cancer Research Institute.
Abbreviations used in this paper: CFSE, carboxyfluorescein succinimidyl ester; FLT3-L, FLT3 ligand; MCMV, murine cytomegalovirus; MDC, myeloid DC; PDC, plasmacytoid DC.
The online version of this article includes supplemental material.
References
- 1.Banchereau, J., and R. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 2.Shortman, K., and Y.J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151–161. [DOI] [PubMed] [Google Scholar]
- 3.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. [DOI] [PubMed] [Google Scholar]
- 4.Siegal, F., N. Kadowaki, M. Shodell, P. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 5.Salio, M., M. Cella, W. Vermi, F. Facchetti, M.J. Palmowski, C.L. Smith, D. Shepherd, M. Colonna, and V. Cerundolo. 2003. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur. J. Immunol. 33:1052–1062. [DOI] [PubMed] [Google Scholar]
- 6.Fonteneau, J.F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y.J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 101:3520–3526. [DOI] [PubMed] [Google Scholar]
- 7.Gilliet, M., and Y.J. Liu. 2002. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144–1150. [DOI] [PubMed] [Google Scholar]
- 9.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 98:3520–3526. [DOI] [PubMed] [Google Scholar]
- 10.Nakano, H., M. Yanagita, and M.D. Gunn. 2001. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krug, A., R. Veeraswamy, A. Pekosz, O. Kanagawa, E.R. Unanue, M. Colonna, and M. Cella. 2003. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brawand, P., D.R. Fitzpatrick, B.W. Greenfield, K. Brasel, C.R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 169:6711–6719. [DOI] [PubMed] [Google Scholar]
- 13.Martin, P., G.M. Del Hoyo, F. Anjuere, C.F. Arias, H.H. Vargas, L.A. Fernandez, V. Parrillas, and C. Ardavin. 2002. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 100:383–390. [DOI] [PubMed] [Google Scholar]
- 14.O'Keeffe, M., H. Hochrein, D. Vremec, B. Scott, P. Hertzog, L. Tatarczuch, and K. Shortman. 2003. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 101:1453–1459. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y.J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilsborough, J., T.C. George, A. Norment, and J.L. Viney. 2003. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 108:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanagita, M., Y. Suzuki, H. Nakano, and M.D. Gunn. 2003. Splenic immature PDC induce the development of IL-10 producing CD4+ T regulatory cells. Kestone Conference on Dendritic Cells. 133a.
- 18.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X.L. Xu, G. Trinchieri, A. O'Garra, and Y.J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller, U., U. Steinhoff, L.F. Reis, S. Hemmi, J. Pavlovic, R.M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science. 264:1918–1921. [DOI] [PubMed] [Google Scholar]
- 20.Altman, J., P. Moss, P. Goulder, D. Barouch, M. McHeyzer-Williams, J. Bell, A. McMichael, and M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274:94–96. [DOI] [PubMed] [Google Scholar]
- 21.Hudrisier, D., M.B. Oldstone, and J.E. Gairin. 1997. The signal sequence of lymphocytic choriomeningitis virus contains an immunodominant cytotoxic T cell epitope that is restricted by both H-2D(b) and H-2K(b) molecules. Virology. 234:62–73. [DOI] [PubMed] [Google Scholar]
- 22.Restifo, N.P., I. Bacik, K.R. Irvine, J.W. Yewdell, B.J. McCabe, R.W. Anderson, L.C. Eisenlohr, S.A. Rosenberg, and J.R. Bennink. 1995. Antigen processing in vivo and the elicitation of primary CTL responses. J. Immunol. 154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 23.Gileadi, U., A. Gallimore, P. Van der Bruggen, and V. Cerundolo. 1999. Effect of epitope flanking residues on the presentation of N-terminal cytotoxic T lymphocyte epitopes. Eur. J. Immunol. 29:2213–2222. [DOI] [PubMed] [Google Scholar]
- 24.Palmowski, M.J., E.M. Choi, I.F. Hermans, S.C. Gilbert, J.L. Chen, U. Gileadi, M. Salio, A. Van Pel, S. Man, E. Bonin, et al. 2002. Competition between CTL narrows the immune response induced by prime-boost vaccination protocols. J. Immunol. 168:4391–4398. [DOI] [PubMed] [Google Scholar]
- 25.Hermans, I.F., J.D. Silk, J. Yang, M.J. Palmowski, U. Gileadi, C. McCarthy, M. Salio, F. Ronchese, and V. Cerundolo. 2003. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-medicated cytotoxicity against multiple targets in vitro and in vivo. 10.1016/j.jim.2003.10.017. [DOI] [PubMed]
- 26.Prevost-Blondel, A., C. Zimmermann, C. Stemmer, P. Kulmburg, F.M. Rosenthal, and H. Pircher. 1998. Tumor-infiltrating lymphocytes exhibiting high ex vivo cytolytic activity fail to prevent murine melanoma tumor growth in vivo. J. Immunol. 161:2187–2194. [PubMed] [Google Scholar]
- 27.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millrain, M., P. Chandler, F. Dazzi, D. Scott, E. Simpson, and P.J. Dyson. 2001. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J. Immunol. 167:3756–3764. [DOI] [PubMed] [Google Scholar]
- 29.Greenfield, A., D. Scott, D. Pennisi, I. Ehrmann, P. Ellis, L. Cooper, E. Simpson, and P. Koopman. 1996. An H-YDb epitope is encoded by a novel mouse Y chromosome gene. Nat. Genet. 14:474–478. [DOI] [PubMed] [Google Scholar]
- 30.Bevan, M.J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigal, L.J., S. Crotty, R. Andino, and K.L. Rock. 1999. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 398:77–80. [DOI] [PubMed] [Google Scholar]
- 32.Blohm, U., E. Roth, K. Brommer, T. Dumrese, F.M. Rosenthal, and H. Pircher. 2002. Lack of effector cell function and altered tetramer binding of tumor-infiltrating lymphocytes. J. Immunol. 169:5522–5530. [DOI] [PubMed] [Google Scholar]
- 33.Demotte, N., D. Colau, S. Ottaviani, D. Godelaine, A. Van Pel, T. Boon, and P. van der Bruggen. 2002. A reversible functional defect of CD8+ T lymphocytes involving loss of tetramer labeling. Eur. J. Immunol. 32:1688–1697. [DOI] [PubMed] [Google Scholar]
- 34.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tough, D., S. Sun, X. Zhang, and J. Sprent. 1999. Stimulation of naive and memory T cells by cytokines. Immunol. Rev. 170:39–47. [DOI] [PubMed] [Google Scholar]
- 36.Delamarre, L., H. Holcombe, and I. Mellman. 2003. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J. Exp. Med. 198:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalod, M., T. Hamilton, R. Salomon, T.P. Salazar-Mather, S.C. Henry, J.D. Hamilton, and C.A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kisielow, P., H. Bluthmann, U.D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 333:742–746. [DOI] [PubMed] [Google Scholar]
- 39.Bassiri, H., J.F. Markmann, N.M. Desai, J.I. Kim, H.S. Teh, and C.F. Barker. 1993. Allograft rejection by T cell receptor transgenic mice. J. Surg. Res. 54:437–444. [DOI] [PubMed] [Google Scholar]
- 40.Rocha, B., and H. von Boehmer. 1991. Peripheral selection of the T cell repertoire. Science. 251:1225–1228. [DOI] [PubMed] [Google Scholar]
- 41.Arsov, I., and S. Vukmanovic. 1997. Altered effector responses of H-Y transgenic CD8+ cells. Int. Immunol. 9:1423–1430. [DOI] [PubMed] [Google Scholar]
- 42.Opferman, J.T., B.T. Ober, and P.G. Ashton-Rickardt. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science. 283:1745–1748. [DOI] [PubMed] [Google Scholar]
- 43.Dhodapkar, M.V., R.M. Steinman, J. Krasovsky, C. Munz, and N. Bhardwaj. 2001. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A. Enk. 2000. Induction of interleukin 10–producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonifaz, L., D. Bonnyay, K. Mahnke, M. Rivera, M.C. Nussenzweig, and R.M. Steinman. 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196:1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahnke, K., Y. Qian, J. Knop, and A.H. Enk. 2003. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 101:4862–4869. [DOI] [PubMed] [Google Scholar]
- 47.Dalod, M., T.P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C.A. Biron. 2002. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotenko, S.V., G. Gallagher, V.V. Baurin, A. Lewis-Antes, M. Shen, N.K. Shah, J.A. Langer, F. Sheikh, H. Dickensheets, and R.P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69–77. [DOI] [PubMed] [Google Scholar]
- 49.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T.E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, et al. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63–68. [DOI] [PubMed] [Google Scholar]
- 50.Aliberti, J., O. Schulz, D.J. Pennington, H. Tsujimura, C. Reis e Sousa, K. Ozato, and A. Sher. 2003. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 101:305–310. [DOI] [PubMed] [Google Scholar]
- 51.Schiavoni, G., F. Mattei, P. Sestili, P. Borghi, M. Venditti, H.C. Morse III, F. Belardelli, and L. Gabriele. 2002. ICSBP is essential for the development of mouse type I interferon–producing cells and for the generation and activation of CD8α+ dendritic cells. J. Exp. Med. 196:1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsujimura, H., T. Tamura, and K. Ozato. 2003. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J. Immunol. 170:1131–1135. [DOI] [PubMed] [Google Scholar]
- 53.Hemmi, H., T. Kaisho, K. Takeda, and S. Akira. 2003. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J. Immunol. 170:3059–3064. [DOI] [PubMed] [Google Scholar]
- 54.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, et al. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA. 100:10872–10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshino, K., T. Kaisho, T. Iwabe, O. Takeuchi, and S. Akira. 2002. Differential involvement of IFN-beta in Toll-like receptor-stimulated dendritic cell activation. Int. Immunol. 14:1225–1231. [DOI] [PubMed] [Google Scholar]
- 56.Shah, J.A., P.A. Darrah, D.R. Ambrozak, T.N. Turon, S. Mendez, J. Kirman, C.Y. Wu, N. Glaichenhaus, and R.A. Seder. 2003. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J. Exp. Med. 198:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grouard, G., M. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorg, R., G. Kogler, and P. Wernet. 1999. Identification of cord blood dendritic cells as an immature CD11c-population. Blood. 93:2302–2307. [PubMed] [Google Scholar]
- 59.Fiebiger, E., P. Meraner, E. Weber, I.F. Fang, G. Stingl, H. Ploegh, and D. Maurer. 2001. Cytokines regulate proteolysis in major histocompatibility complex class II–dependent antigen presentation by dendritic cells. J. Exp. Med. 193:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.den Haan, J.M., S.M. Lehar, and M.J. Bevan. 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iyoda, T., S. Shimoyama, K. Liu, Y. Omatsu, Y. Akiyama, Y. Maeda, K. Takahara, R.M. Steinman, and K. Inaba. 2002. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 195:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pooley, J.L., W.R. Heath, and K. Shortman. 2001. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 166:5327–5330. [DOI] [PubMed] [Google Scholar]
- 63.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D.F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009–1015. [DOI] [PubMed] [Google Scholar]