Abstract
It is well established that T lymphocytes undergo homeostatic proliferation in lymphopenic environment. The homeostatic proliferation requires recognition of the major histocompatibility complex on the host. Recent studies have demonstrated that costimulation-mediated CD28, 4-1BB, and CD40 is not required for T cell homeostatic proliferation. It has been suggested that homeostatic proliferation is costimulation independent. Here, we report that T cells from mice with a targeted mutation of CD24 have a remarkably reduced rate of proliferation when adoptively transferred into syngeneic lymphopenic hosts. The reduced proliferation cannot be attributed to abnormal survival and homing properties of the CD24-deficient T cells. T cell proliferation in allogeneic hosts is less affected by this mutation. These results demonstrate a novel function of CD24 expressed on T cells. Thus, although distinct costimulatory molecules are involved in antigen-driven proliferation and homeostatic proliferation, both processes can be modulated by costimulatory molecules.
Keywords: costimulation, homeostasis, autoimmune diseases, immunological memory, T cell traffic
Introduction
The immune system maintains a relatively constant number of lymphocytes by two distinct mechanisms. Activation-induced cell death (1, 2) results in the removal of lymphocytes after massive antigen-induced clonal expansions. When the lymphocytes are depleted, naive T cells undergo rapid expansion to replenish the T lymphocyte pools (3, 4). This phenomenon is termed homeostatic proliferation (5, 6). Homeostatic proliferation appears to be driven by self antigens (5, 7). In addition, activated T cells acquire features of memory T cells (8, 9). However, homeostatic T cell proliferation is distinct from antigen-driven proliferation in its requirements for costimulatory molecules. Although CD28–B7 interaction is required for optimal antigen-driven proliferation (10), several groups have reported that the lack of CD28 on T cells did not have an appreciable impact in homeostatic T cell proliferation (5, 8, 11). Moreover, interaction between 4-1BB and 4-1BBL, which is required for allogeneic T cell response in vivo (12), is unnecessary for homeostatic proliferation (11). Likewise, CD40–CD40L interaction, which is known to be indirectly involved in antigen-specific T cell response, is also not required for homeostatic proliferation (11). These observations lead to the hypothesis that homeostatic proliferation is driven by TCR signal only and does not require costimulation (11).
CD24 (heat-stable antigen) is a cell surface glycosyl-phosphatidylinositol–anchored protein (13) with a broad expression on a variety of cell types, including developing T (14) and most B lymphocytes (15) and a variety of APCs (16, 17). Although CD24 is down-regulated when T cells reach maturity (14), it is rapidly induced after the engagement of the TCR–CD3 complex (18, 19). Although we and others have demonstrated that CD24 on APCs mediates a CD28-independent costimulatory pathway for both CD4 and CD8 T cell responses (17, 20–24), the role of CD24 on T cells is largely unknown. Here, we report that CD24 expression on T cells is required for optimal homeostatic proliferation of both CD4 and CD8 T cells. Our results reveal an important function of CD24 expressed on T cells and suggest that homeostatic proliferation of T cells is regulated by T cell costimulation.
Materials and Methods
Mice and Antibodies.
C57BL6/J and BALB/c mice were purchased from Charles River Laboratories through a contract with the National Cancer Institute. B6.Thy1.1 mice were purchased from The Jackson Laboratory. Mice with the targeted mutation of CD24 were produced using embryonic stem cells from C57BL/6 mice as described previously (25) and were maintained under specific pathogen-free conditions in the animal facility at the Ohio State University. All studies involving animals have been approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
All conjugated antibodies used here were purchased from either eBioscience (Thy1.2, CD44, and CD62L) or BD Biosciences (CD4, CD8, H-2Kb, CD25 and IFN-γ, and isotype control).
Analysis of T Cell Division In Vivo.
T lymphocytes were purified from pooled spleen and lymph node cells by negative selection. In brief, pools of spleen and lymph node cells were incubated with a cocktail of antibodies specific for CD11b (Mac-1), Fc receptor (2.4G2), B220, and CD11c. The Dynal beads coated with goat anti–rat IgG were used to negatively select T cells. The purity of the cells was checked by flow cytometry to be >95%. The purified T cells (Thy1.2+, H-2b) were labeled with CFSE and injected intravenously into irradiated (600 R) recipient mice that were either congenic in Thy1 locus (B6. Thy1.1+) or allogeneic BALB/c (H-2d) mice, at a dose of 5 × 106/mouse. At given times after adoptive transfer, spleen cells were harvested and analyzed for the intensity of CFSE dye and other cell surface markers. In some experiments, mononuclear cells were harvested from the liver after perfusion with PBS as described previously (26).
Flow Cytometry.
The cell surface markers, including CD4, CD8, CD24, CD44, CD62L, Thy1.2, and H-2Kb, were analyzed by three- or four-color flow cytometry, using fluorochrome-conjugated monoclonal antibodies purchased from BD Biosciences. Apoptosis of T cells was analyzed by staining with PE–annexin V.
To assess intracellular cytokine production, spleen or lymph node cells were cultured for 4–6 h with 50 ng/ml PMA, 500 ng/ml ionomycin, and 2 μM GolgiStop (BD Biosciences). Cells were stained for cell surface markers CD4 and CD8 followed by intracellular staining for IFN-γ and/or isotype control using the CytoFix/CytoPerm kit (BD Biosciences).
Results and Discussion
Expression of CD24 on T Cells Is Not Required for Its Survival upon Adoptive Transfer.
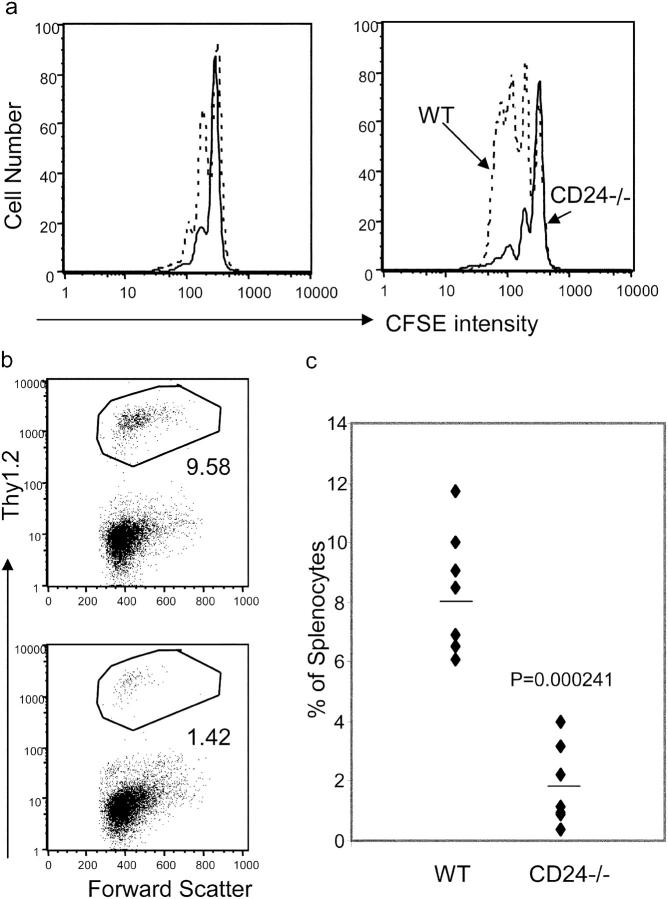
We analyzed CD24 expression among WT T cells before and after adoptive transfer. CD24−/− T cells were used as a control. As shown in Fig. 1 a, in comparison with CD24−/− T cells, WT T cells expressed significant levels of CD24 before adoptive transfer. The specificity of the antibody binding was confirmed both by the uses of isotype control and mice with targeted mutation of CD24. Upon adoptive transfer into lymphopenic hosts, the levels of CD24 were maintained from days 1 to 4 (Fig. 1 b).
Figure 1.
Expression of CD24 on T cells does not affect the distribution and survival of T cells upon adoptive transfer to lymphopenic host. (a) WT (top) and CD24−/− (bottom) spleen cells were stained with anti-CD3 in conjunction with either anti-CD24 or isotype control. (b) Levels of CD24 expression on T cells on days 1 (left) and 4 (right) after adoptive transfer into a lymphopenic environment. Purified T cells (Thy1.2+) were adoptively transferred into Thy1.1+ congenic mice that received 600 R of γ-irradiation before adoptive transfer. At different times after adoptive transfer, the spleen cells were analyzed. Data shown are FACS profiles of a representative mouse in each group and have been reproduced at least three times. (c) Percentage of donor T cells in the recipient at 6 and 18 h after adoptive transfer. Data shown are a summary of three to five independent experiments involving at least eight mice per group. The apparent reduction observed at 18 h is not statistically significant (P = 0.085). (d) Apoptosis of newly transferred T cells was not affected by CD24. Spleen cells were harvested at 18 h after adoptive transfer and stained with Thy1.2 (to marker donors), CD4, and annexin V. Data shown are profiles of gated Thy1.2 donor cells and have been reproduced three times.
To determine if CD24 was required for T cell survival and homing to the spleen, we compared the number of WT and CD24−/− T cells at 6 and 18 h after adoptive transfer. As shown in Fig. 1 c, the percentage of donor T cells in the spleen was essentially identical in the two groups at 6 h after adoptive transfer. Although the number of CD24−/− T cells was slightly lower at 18 h, the difference was not statistically significant (P = 0.085). Similar results were observed when donor T cells in the blood were analyzed (unpublished data). We also analyzed the rate of cell death by staining the spleen cells with annexin V. As shown in Fig. 1 d, low and comparable apoptotic cells were observed in both groups. Thus, the data presented in this section demonstrated that expression of CD24 does not affect distribution and survival of T cells upon adoptive transfer.
CD24 Expression on T Cells Is Necessary for Homeostatic Proliferation, but Less So for Response to Allogeneic Antigens.
To test whether CD24 expression on T cells plays a role in homeostatic proliferation, we isolated T cells from WT and CD24−/− C57BL/6 mice and injected them into irradiated Thy1.1 congenic mice. At 4 d after adoptive transfer, we isolated the spleen cells and compared them for the rate of division based on CFSE intensity. As shown in Fig. 2 a, a substantial proportion of WT CD4 T cells had undergone one or more divisions, whereas few CD24−/− CD4 T cells had divided. In comparison, CD8 T cells divided faster than CD4 T cells. The majority of WT CD8 T cells accumulated was the product of two to four divisions. In contrast, the majority of CD24−/− CD8 cells had not undergone any divisions. Correspondingly, the percentage of WT donor cells was fourfold greater than that of the CD24−/− donors (Fig. 2, b and c), although it is unclear whether the difference can be solely attributed to the rates of proliferation. Over a 6-d period, the difference in division further increased (Fig. 2 d). The differential division rate resulted in an almost 20-fold difference in the number of donor T cells in the recipients at 3 wk after adoptive transfer (Fig. 2 e). Consistent with previous papers (5, 11), targeted mutation of CD28 had no effect on T cell homeostatic proliferation in our model (unpublished data).
Figure 2.
A critical role for CD24 on T cells in homeostatic proliferation in a lymphopenic host. Purified T cells (Thy1.2+) were adoptively transferred into Thy1.1+ congenic mice that received 600 R of γ-irradiation before adoptive transfer. At different times after adoptive transfer, the spleen cells were analyzed for the rate of division (a and d) and accumulation of donor cells (b, c, and e). Donor cells used in a–d were labeled with CFSE before adoptive transfer, whereas those in e were not labeled. Data in a are representative profiles of groups of two mice and have been repeated four times. Data in b are representative profiles of gated Thy1.2+ T cells from five independent experiments, each involving three mice per group. Data in d are representative profiles of an experiment with three mice per group. Data in c are summary of three experiments in which the initial “take” of T cells was confirmed identical (Fig. 1). Similar data were confirmed with four other experiments in which the initial takes of T cells were not monitored. Data in e are a summary of an experiment involving four mice per group.
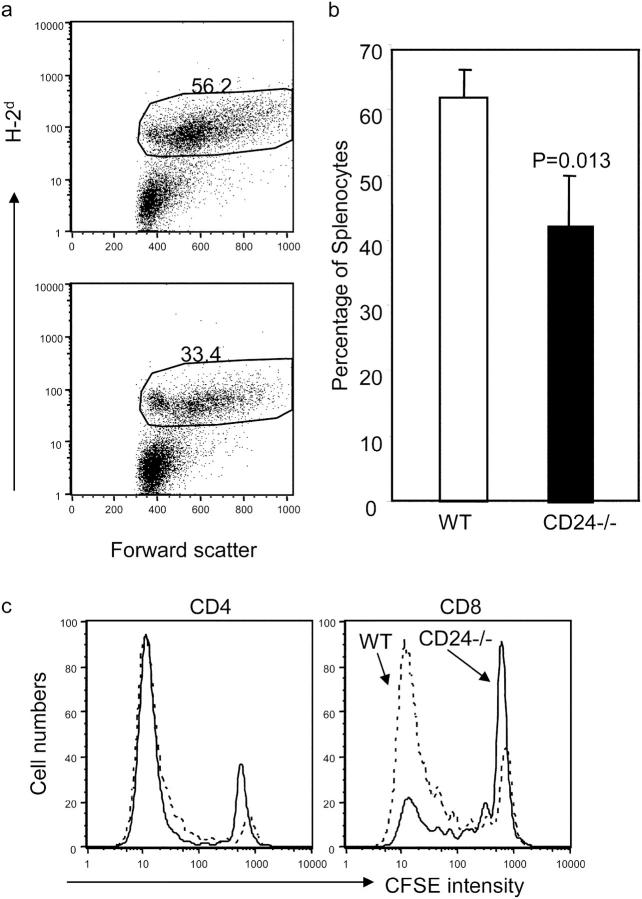
An interesting issue is whether CD24 is required for antigen-driven proliferation. To analyze this issue in a polyclonal T cell pool, we transferred CFSE-labeled WT and CD24−/− T cells into irradiated BALB/c mice. Because of a strong allogeneic-reactive T cell response, a robust proliferation of donor T cells was observed, regardless of whether WT or CD24−/− T cells were used as donors. As shown in Fig. 3 a, >30% of the spleen cells in the host spleen are of donor origin. In the allogeneic hosts, the donor cells were larger in size in comparison with the host cells. Although the amount of WT T cells was consistently higher than that of the CD24−/− T cells, the difference was usually not >40% (Fig. 3 b). When the number of T cell divisions was analyzed by CFSE intensity of the donor cells, it was clear that most of the WT and CD24−/− donor T cells had undergone more than seven divisions. Among CD4 T cells, the distribution of CFSE intensity was grossly similar between WT and CD24−/− donors (Fig. 3 c, left). However, among the CD8 compartment, more WT than CD24−/− T cells reached the maximal number of divisions that can be traced by CFSE (Fig. 3 c, right). Thus, CD24 is less crucial for the division of T cells in response to allogeneic antigens in vivo, especially for CD4 T cells. The difference of ∼40%, mostly among CD8 T cells, can be attributed to the role of CD24 in homeostatic proliferation and/or antigen-specific proliferation. Because the host is both allogeneic and lymphopenic, the contribution of each component cannot be easily determined at this point. The lesser contribution of CD24 to antigen-driven proliferation is consistent with our previous reports that the targeted mutation of CD24 alone does not have a significant impact on CD4 and CD8 T cell responses (20, 22).
Figure 3.
Alloantigen-driven T cell proliferation is less dependent on CD24 expression on T cells. T cells from WT or CD24−/− C57BL/6 mice were labeled with CFSE and adoptively transferred into irradiated BALB/c mice. At 3 d after adoptive transfer, the spleen cells were analyzed for the accumulation (a and b) and proliferation of donor T cells. (a) Percentage of donor T cells in the host spleen cells at 3 d after adoptive transfer. (b) Summary of experiments involving three mice per group. (c) Division of CD4 (left) and CD8 (right) donor T cells in the host spleen at 3 d after adoptive transfer. Data shown are representative of three independent experiments, each involving three mice per group.
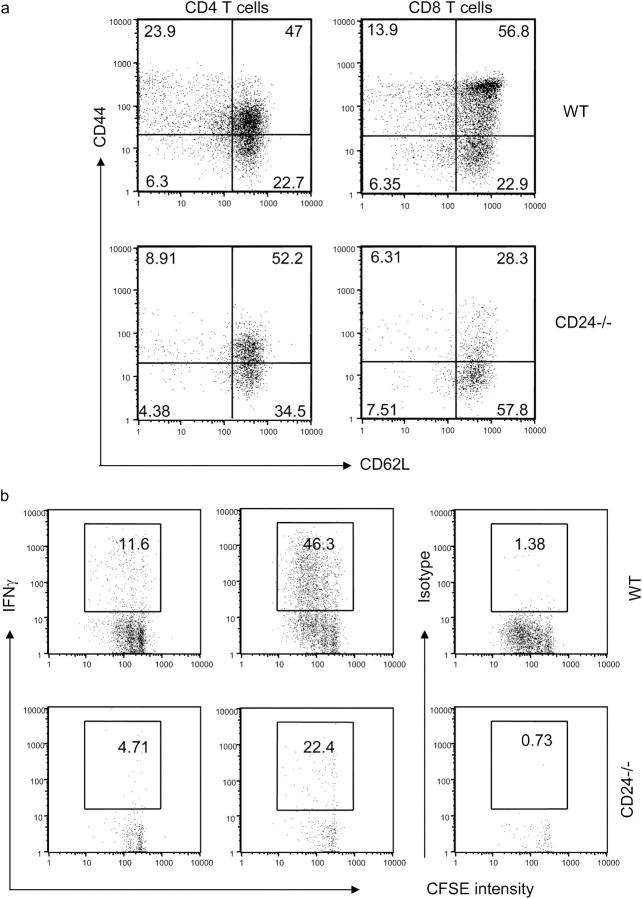
It has been reported that homeostatic proliferation of T cells is accompanied by the acquisition of memory markers (8, 9). To test whether CD24 plays a role, we analyzed the memory markers on WT and CD24−/− T cells, including CD44 and CD62L. The data are presented in Fig. 4 a. Consistent with previous reports, the majority of WT CD8 T cells expressed high levels of CD44 upon adoptive transfer. A significant proportion of donor cells also down-regulated CD62L. Compared with CD8 T cells, there was less up-regulation of CD44, but more down-regulation of CD62L among WT CD4 T cells. As expected, the CD24−/− cells were less activated. This was demonstrated by substantially fewer CD62LlowCD44high cells and more CD44lowCD62Lhigh cells.
Figure 4.
(a) Expression of CD24 on T cells promotes acquisition of memory cell markers. WT or CD24−/− T cells were adoptively transferred into the irradiated congeneic mice. At 4 d after adoptive transfer, the spleen cells were harvested and analyzed for acquisition of memory markers. The differential expression of activation markers has been reproduced in three independent experiments, each involving two to three mice per group. (b) Production of IFN-γ after short-term stimulation with PMA and ionomycin. Spleen cells from 4-d reconstituted mice were harvested and stimulated for 6 h. The intracellular IFN-γ was analyzed by flow cytometry. Pools of three spleens were used in each group. Data shown are representative of two independent experiments.
Memory T cells differ from naive T cells in their ability to produce cytokine within hours of stimulation (27). We stimulated spleen cells from mice that received WT or CD24−/− T cells with PMA and ionomycin and analyzed the intracellular IFN-γ production. As shown in Fig. 4 b, ∼12% of the WT CD4 cells synthesized IFN-γ within 6 h of stimulation. In the same experiments, the percentage of IFN-γ–producing cells among the CD24−/− CD4 T cells was ∼2.5–3-fold less than their WT counterpart. Although more CD8 T cells produced IFN-γ, there was also a two- to threefold reduction among CD24−/− donor cells in the percentage of cytokine-producing cells.
Reduction of Dividing CD24−/− T Cells Was Not Due to Accelerated Apoptosis.
A potential explanation for the reduced number of divided CD24−/− T cells in the lymphopenic host is that CD24 may be required for the survival of dividing T cells. To test this possibility, we analyzed the apoptosis of the dividing T cells based on their binding to annexin V and intensity of CFSE. As shown in Fig. 5 a, very few, if any, annexin V+ donor T cells were found in the spleen, and the proportion of annexin V+ cells was similar in the two groups. Because the liver is the site for apoptosis of activated T cells, one may expect an increase in CD24−/− T cells in the liver if CD24 deficiency promoted T cell death. Therefore, we analyzed migration of donor T cells in the liver. As shown in Fig. 5 b, approximately three- to fivefold fewer CD24−/− than WT T cells were detected in the liver. Moreover, the majority of WT T cells in the liver were products of multiple rounds of divisions, whereas CD24−/− T cells barely divided. Thus, the reduction of the dividing CD24−/− T cells was neither due to accelerated apoptosis nor trafficking to liver. Nevertheless, it is unclear at this stage whether the difference in the accorded numbers of accumulated T cells in the spleen is solely due to differential proliferation of T cells.
Figure 5.
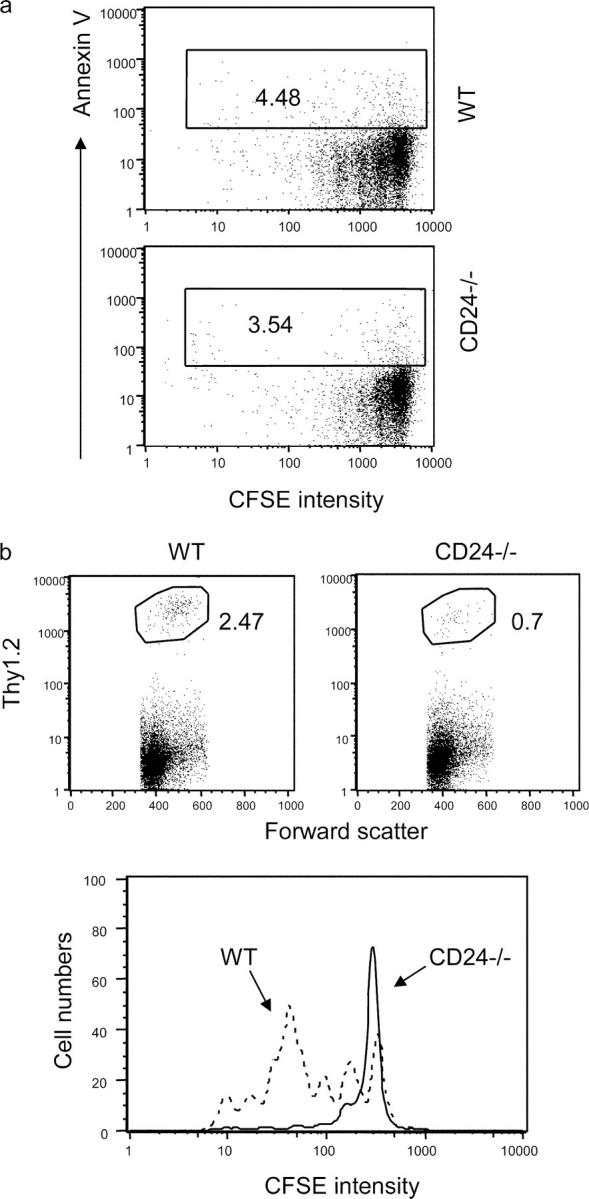
CD24 deficiency promotes neither apoptosis of dividing T cells nor their migration into the liver. WT or CD24−/− T cells were adoptively transferred into the irradiated congeneic mice. At 4 d after adoptive transfer, the spleen cells (a) or liver mononuclear cells (b) were harvested and analyzed for apoptosis (a), accumulation (b, top), or rate of division in the liver (b, bottom). Data shown in a and b (bottom) are gated donor cells, whereas those in b (top) are gated liver mononuclear cells. Data shown are representative of two to three independent experiments, each with two to three mice per group.
Together, the data reported here revealed a crucial role for CD24 in homeostatic proliferation of T cells in a lymphopenic environment. Although CD24 is widely used as a marker for T cell differentiation, its function has remained elusive. Hubbe et al. reported that anti-CD24 can synergize with anti-CD28 in inducing T cell proliferation in vitro (18), which suggests that the CD24 expressed on T cells is capable of transducing a costimulatory signal. In addition, we showed that although CD24 was not essential in the priming of autoreactive T cells in the mouse model of experimental autoimmune encephalomyelitis, CD24−/− T cells failed to induce experimental autoimmune encephalomyelitis when transferred in WT hosts (28). It would be of interest to determine whether its contribution to homeostatic proliferation explains its critical role in the pathogenesis of CD4 T cells in the experimental autoimmune encephalomyelitis model.
Much like antigen-driven proliferation, homeostatic proliferation requires the expression of MHC molecules and intact antigen-processing machinery (5, 7). Thus, both processes require engagement of the TCR. However, unlike antigen-specific T cell responses, homeostatic proliferation was not affected by blocking costimulation mediated by CD28–B7, CD40–CD40L, and 4-1BB–41BB-L interactions (5, 7, 11). The latter observation leads to the hypothesis that homeostatic proliferation is independent of costimulation (11). Our results demonstrated that CD24 on T cells plays a major role in the homeostatic proliferation of T cells in the lymphopenic environment. To our knowledge, this is the first demonstration of any costimulatory molecules playing a significant role in homeostatic proliferation.
Acknowledgments
We thank L. Yin for the maintenance of the CD24−/− mouse colony and L. Shaw for secretarial assistance.
This work is supported by grants from National Institutes of Health.
The authors have no conflicting financial interests.
References
- 1.Liu, Y., and C.A. Janeway Jr. 1990. Interferon γ plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J. Exp. Med. 172:1735–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohman, B.L., and R.M. Welsh. 1998. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J. Virol. 72:7815–7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldrath, A.W., and M.J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min, B., R. McHugh, G.D. Sempowski, C. Mackall, G. Foucras, and W.E. Paul. 2003. Neonates support lymphopenia-induced proliferation. Immunity. 18:131–140. [DOI] [PubMed] [Google Scholar]
- 5.Ernst, B., D.S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 6.Surh, C.D., and J. Sprent. 2000. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192:F9–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge, Q., V.P. Rao, B.K. Cho, H.N. Eisen, and J. Chen. 2001. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc. Natl. Acad. Sci. USA. 98:1728–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho, B.K., V.P. Rao, Q. Ge, H.N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldrath, A.W., L.Y. Bogatzki, and M.J. Bevan. 2000. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linsley, P.S., and J.A. Ledbetter. 1993. The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 11:191–212. [DOI] [PubMed] [Google Scholar]
- 11.Prlic, M., B.R. Blazar, A. Khoruts, T. Zell, and S.C. Jameson. 2001. Homeostatic expansion occurs independently of costimulatory signals. J. Immunol. 167:5664–5668. [DOI] [PubMed] [Google Scholar]
- 12.Blazar, B.R., B.S. Kwon, A. Panoskaltsis-Mortari, K.B. Kwak, J.J. Peschon, and P.A. Taylor. 2001. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J. Immunol. 166:3174–3183. [DOI] [PubMed] [Google Scholar]
- 13.Kay, R., P.M. Rosten, and R.K. Humphries. 1991. CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J. Immunol. 147:1412–1416. [PubMed] [Google Scholar]
- 14.Crispe, I.N., and M.J. Bevan. 1987. Expression and functional significance of the J11d marker on mouse thymocytes. J. Immunol. 138:2013–2018. [PubMed] [Google Scholar]
- 15.Hardy, R.R., K. Hayakawa, D.R. Parks, and L.A. Herzenberg. 1984. Murine B cell differentiation lineages. J. Exp. Med. 159:1169–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez del Hoyo, G., P. Martin, C.F. Arias, A.R. Marin, and C. Ardavin. 2002. CD8alpha+ dendritic cells originate from the CD8alpha− dendritic cell subset by a maturation process involving CD8alpha, DEC-205, and CD24 up-regulation. Blood. 99:999–1004. [DOI] [PubMed] [Google Scholar]
- 17.De Bruijn, M.L., P.A. Peterson, and M.R. Jackson. 1996. Induction of heat-stable antigen expression by phagocytosis is involved in in vitro activation of unprimed CTL by macrophages. J. Immunol. 156:2686–2692. [PubMed] [Google Scholar]
- 18.Hubbe, M., and P. Altevogt. 1994. Heat-stable antigen/CD24 on mouse T lymphocytes: evidence for a costimulatory function. Eur. J. Immunol. 24:731–737. [DOI] [PubMed] [Google Scholar]
- 19.Zhou, Q., Y. Wu, P.J. Nielsen, and Y. Liu. 1997. Homotypic interaction of the heat-stable antigen is not responsible for its co-stimulatory activity for T cell clonal expansion. Eur. J. Immunol. 27:2524–2528. [DOI] [PubMed] [Google Scholar]
- 20.Wu, Y., Q. Zhou, P. Zheng, and Y. Liu. 1998. CD28-independent induction of T helper cells and immunoglobulin class switches requires costimulation by the heat-stable antigen. J. Exp. Med. 187:1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, Y.-C., L. Zhu, R. McHugh, K.W. Sell, and P. Selvaraj. 1995. Expression of heat-stable antigen on tumor cells provides co-stimulation for tumor-specific T cell proliferation and cytotoxicity in mice. Eur. J. Immunol. 25:1163–1167. [DOI] [PubMed] [Google Scholar]
- 22.Liu, Y., R.H. Wenger, M. Zhao, and P.J. Nielsen. 1997. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J. Exp. Med. 185:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, Y., B. Jones, A. Aruffo, K.M. Sullivan, P.S. Linsley, and C.A. Janeway Jr. 1992. Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J. Exp. Med. 175:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enk, A.H., and S.I. Katz. 1994. Heat-stable antigen is an important costimulatory molecule on epidermal Langerhans' cells. J. Immunol. 152:3264–3270. [PubMed] [Google Scholar]
- 25.Nielsen, P.J., B. Lorenz, A.M. Muller, R.H. Wenger, F. Brombacher, M. Simon, T. von der Weid, W.J. Langhorne, H. Mossmann, and G. Kohler. 1997. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood. 89:1058–1067. [PubMed] [Google Scholar]
- 26.Gao, J.X., X. Liu, J. Wen, M.A. Caligiuri, I. Stroynowski, P. Zheng, and Y. Liu. 2003. Two-signal requirement for activation and effector function of natural killer cell response to allogeneic tumor cells. Blood. 102:4456–4463. [DOI] [PubMed] [Google Scholar]
- 27.Cho, B.K., C. Wang, S. Sugawa, H.N. Eisen, and J. Chen. 1999. Functional differences between memory and naive CD8 T cells. Proc. Natl. Acad. Sci. USA. 96:2976–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai, X.F., J.Q. Liu, X. Liu, Y. Guo, K. Cox, J. Wen, P. Zheng, and Y. Liu. 2000. The heat-stable antigen determines pathogenicity of self-reactive T cells in experimental autoimmune encephalomyelitis. J. Clin. Invest. 105:1227–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]