Abstract
Linker for activation of B cells (LAB, also called NTAL; a product of wbscr5 gene) is a newly identified transmembrane adaptor protein that is expressed in B cells, NK cells, and mast cells. Upon BCR activation, LAB is phosphorylated and interacts with Grb2. LAB is capable of rescuing thymocyte development in LAT-deficient mice. To study the in vivo function of LAB, LAB-deficient mice were generated. Although disruption of the Lab gene did not affect lymphocyte development, it caused mast cells to be hyperresponsive to stimulation via the FcɛRI, evidenced by enhanced Erk activation, calcium mobilization, degranulation, and cytokine production. These data suggested that LAB negatively regulates mast cell function. However, mast cells that lacked both linker for activation of T cells (LAT) and LAB proteins had a more severe block in FcɛRI-mediated signaling than LAT−/− mast cells, demonstrating that LAB also shares a redundant function with LAT to play a positive role in FcɛRI-mediated signaling.
Keywords: LAT, MAPK, calcium flux, degranulation, mast cells
Introduction
Engagement of the high affinity IgE receptor (FcɛRI) at the surface of mast cells initiates signaling cascades leading to Ras–mitogen-activated protein kinase (MAPK) activation, calcium mobilization, degranulation, and cytokine production (1–4). FcɛRI, a member of the immunoreceptor superfamily, is composed of α, β, and the homodimeric γ chains (5). The α chain binds IgE, and the β and γ chains contain a signal-transducing immunoreceptor tyrosine-based activation motif (ITAM) in their cytoplasmic domains. Upon activation of FcɛRI by multivalent antigens bound on IgE, the Src family tyrosine kinase (protein tyrosin kinase [PTK]), Lyn, is activated and phosphorylates the tyrosine residues within the ITAMs of the β and γ chains. Syk then binds to the phosphorylated ITAMs via its tandem Src homology 2 domains and is further activated by Lyn (6, 7). These activated tyrosine kinases further phosphorylate downstream signaling molecules. The importance of these tyrosine kinases in mast cell function has been demonstrated in cells deficient in these PTKs. Syk-deficient mast cells fail to degranulate and secrete cytokines when stimulated through the FcɛRI (8). Compared with Syk deficiency, Lyn deficiency has a less severe phenotype. Lyn−/− mast cells have impaired tyrosine phosphorylation of proteins and delayed Ca2+ mobilization, but degranulation, MAPK activation, and cytokine production occur normally (9). This partial signaling defect caused by Lyn deficiency is likely due to the presence of another Src family PTK, Fyn (10). Although Fyn−/− mast cells are relatively normal in FcɛRI-mediated tyrosine phosphorylation, MAPK activation, and Ca2+ flux, they are defective in IgE-dependent degranulation (11). Fyn functions to phosphorylate Gab2, an adaptor molecule that is important in FcɛRI-mediated phosphotidylinositide 3-kinase activation (11, 12). Therefore, both Lyn and Fyn are required in FcɛRI-mediated signaling pathway.
After activation of these PTKs, many signaling proteins are phosphorylated. One of the prominently phosphorylated proteins downstream of FcɛRI engagement is linker for activation of T cells (LAT). LAT function has been clearly demonstrated in T cells. Upon T cell activation, it is phosphorylated and interacts with Grb2, Gads, and PLC-γ1 (13–15). Through binding to these molecules, LAT couples TCR engagement to MAPK activation and calcium flux. In LAT-deficient Jurkat cells, TCR-mediated MAPK activation and calcium flux are completely blocked (16, 17). LAT is also required during T cell development. LAT-deficient mice lack mature T cells, and thymocyte development is blocked at the double negative stage (18). In LAT−/− mast cells, although tyrosine phosphorylation of FcɛRI, Syk, and Vav is intact, FcɛRI-mediated phosphorylation of SLP-76, PLC-γ1, and PLC-γ2, MAPK activation, and calcium mobilization are significantly reduced, leading to a reduction in mast cell degranulation and cytokine production (19). Mutational studies show that four membrane-distal tyrosines in LAT are required for LAT function in FcɛRI-mediated signaling (20). In comparison with the LAT-deficient Jurkat cells, FcɛRI-mediated signaling and effector function in LAT−/− mast cells are not completely blocked. LAT−/− mast cells still flux calcium and produce cytokines after engagement of FcɛRI, albeit at reduced levels. These data suggest that FcɛRI-mediated signaling is not totally dependent on LAT.
Recently, a novel transmembrane adaptor protein, linker for activation of B cells (LAB)/non–T cell activation linker (NTAL), was identified (21, 22). Similar to LAT, LAB has multiple tyrosine residues in its cytoplasmic tail, five of which are within a Grb2-binding motif. LAB also has a palmitoylation motif and is localized to lipid rafts. LAB is expressed in B cells, NK cells, mast cells, and other cell types. Upon BCR or Fc receptor engagement, LAB is phosphorylated and interacts with Grb2; however, it is incapable of binding PLC-γ1 or PLC-γ2 (21–23). Functionally, LAB is able to rescue thymocyte development in LAT−/− mice. However, transgenic mice expressing LAB in T cells develop a Th2 autoimmune disease similarly to the LAT Y136F knockin mice, suggesting that LAB resembles the LAT mutant without the PLC-γ1 site (24). These data suggest that, although LAT and LAB share a subset of functions in signaling, they function differently.
To study how LAB functions in vivo, we generated LAB-deficient mice. Although disruption of the Lab gene had no significant impact on T and B cell development, it caused mast cell hyperresponsiveness to stimulation via FcɛRI, suggesting that LAB negatively regulates mast cell function. Interestingly, mast cells deficient in both Lat and Lab genes had further reduction in FcɛRI-mediated signaling and mast cell function in comparison with LAT−/− mast cells. Thus, LAB also plays a positive role in FcɛRI-mediated signaling.
Material and Methods
Generation of LAB−/− Mice by Gene Targeting.
Lab genomic fragments were amplified from ES cells by PCR and cloned into the XpPNT targeting plasmid. Seven exons, which encode the LAB protein from residues 1 to 113, were deleted. After linearized with NotI, this targeting construct was used to transfect embryonic stem (ES) cells. ES transfectants were selected in the presence of G418 and gancyclovir. Out of 250 G418–resistant ES clones, seven positive clones underwent homologous recombination by PCR screening and Southern blot analysis. Four of these targeted ES clones were injected into blastocysts from C57Bl/6 mice to generate chimeric mice. These mice were then used to cross with C57Bl/6 mice to produce LAB+/− mice. LAB−/− mice were obtained by interbreeding LAB+/− mice. Southern blot analysis of the genomic DNA from ES cells and mouse littermates was performed using ExpressHyb hybridization solution (CLONTECH Laboratories, Inc.). PCR genotyping of littermates was performed using three primers (5′-GGAAGTAACCAGGAGCCTGATGCTGC-3′, 5′-CGGGCTAGAGATGTCAGGCTTTATGG-3′, and 5′-TCGCAGCGCATCGCCTTCTATCG-3′). 2KO mice (LAB−/− LAT−/−) were obtained by breeding LAB−/− and LAT−/− followed by interbreeding LAB+/−LAT+/−. Fyn−/− and Lyn−/− mice were purchased from the Jackson Laboratories. All mice were used in accordance with National Institutes of Health guidelines.
Antibodies and Flow Cytometry Analysis.
Antibodies used for immunoprecipitation were anti-LAT (13), LAB (22), Grb2, SLP-76, and PLC-γ2 (Santa Cruz Biotechnology), and PLC-γ1 (Upstate Biotechnology). Antibodies used for immunoblotting were anti-pTyr (4G10; Upstate Biotechnology, Inc.), SLP76 (Transduction laboratory), and Lyn (Santa Crutz Biotechnology, Inc.). Monoclonal anti-LAT (11B12) was generated by fusion of NSO cells with splenocytes from mice immunized with glutathione S-transferase–LAT (human) fusion protein. Antibodies for the MAPK assay were anti-phospho–Erk, –Jnk, and –p38 MAPK from Cell Signaling.
Antibodies used in FACS analysis of lymphocyte development were the following: APC-conjugated anti-CD8 and B220, FITC-conjugated anti-CD4 (eBioscience), FITC-conjugated anti-IgM and IgD, PE-conjugated anti-IgM and CD43 (BD Biosciences). For IgE receptor expression level, cells were stained with biotin-conjugated anti-FcɛRIα (eBioscience) followed by streptavidin-conjugated FITC. Flow cytometry was performed using the Becton Dickinson FACSCalibur and analyzed by the CELLQuest software.
BM-derived Mast Cells Culture, Activation, Immunoprecipitation, and Western Blot.
BM cells were taken from WT, LAB−/−, LAT−/−, and 2KO mouse femurs and were cultured in mast cell medium (Iscove's modified Dulbecoo's medium supplemented with 10% FBS, β-mercaptoethanol, penicillin, and streptomycin) in the presence of recombinant IL-3 (5 ng/ml) at 37°C for 3–6 wk before analysis.
BM-dervied mast cells (BMMCs) (2–5 × 106/ml) were preloaded with anti-DNP IgE (0.5 μg/ml, SPE-7 mAb; Sigma-Aldrich) in IMDM medium without IL-3 for 4 h. Cells were washed with IMDM and then stimulated with DNP-HSA (20 ng/ml) for the indicated time points. A total of 1×107 cells were lysed in 500 μl of ice cold Brij lysis buffer (1% Brij-97, 25 mM Tris-Cl, pH 7.6, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4) or RIPA lysis buffer (1% Triton, 0.5% sodium deoxycholic acid, 0.1% SDS, 25 mM Tris-Cl, pH 7.6, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4). Lysates were subjected to immunoprecipitation with protein A beads bound with various antibodies. Lipid raft and nonraft fractions were purified as described previously (25).
For Western blotting, samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with primary antibodies, nitrocellulose membranes were washed three times and probed with either anti–mouse or rabbit Ig conjugated to AlexaFluor 680 (Molecular Probes) or IRDye800 (Rockland). Membranes were then visualized with the LI-COR Bioscience Odyssey system (LI-COR).
Passive Systemic Anaphylaxis.
Mice were sensitized with 2 μg of anti–DNP-IgE by i.v. injection for 20–24 h. They were then injected i.v. with 200 μg of DNP-HSA for 1.5 min. Mice were killed with CO2, and blood was immediately collected by cardiac puncture. Histamine concentration in serum was determined using a competitive histamine ELISA assay kit (Immunotech).
Ca2+ Flux.
BMMCs (2–5 × 106/ml) were preloaded with anti-DNP IgE (0.5 μg/ml) in IMDM without IL-3 for 4 h. Cells were washed twice with IMDM and then loaded with 5 μM Fluo-4 AM (Molecular Probes) for 30 min. Cells were washed again and further incubated in IMDM for 30 min. DNP-HSA (30 ng/ml) and thapsigargin (1 μM) were used to induce calcium flux in these cells. Ca2+ flux was measured using a flow cytometer (FACSCalibur; BD Biosciences) to monitor the fluorescence emission.
Degranulation Assay.
Degranulation of BMMCs was determined by measuring the release of β-hexosaminidase. Briefly, BMMCs (2–5 × 106/ml) were preloaded with anti-DNP IgE (0.5 μg/ml) in medium without IL-3 for 4 h. Cells were then washed with IMDM. Sensitized cells (2 × 106 cells/ml) were stimulated with different concentrations of DNP-HSA (1, 10, 100, 1,000 ng/ml) and 1 μM thapsigargin for 10 min in Tyrode's buffer. The reactions were stopped by putting the tubes on ice followed by centrifugation. Supernatants were collected, and cell pellets were solubilized with 0.5% Triton X-100 in Tyrode's buffer. The enzymatic activity of β-hexosaminidase was measured with p-nitrophenyl N-acetyl-β-glucosaminide in 0.1 M sodium citrate (pH 4.5) for 60 min at 37°C. The reaction was stopped by addition of 0.2 M glycine (pH 10.7). The release of the product 4-p-nitrophenol was detected by measuring absorbance at 405 nm. The extent of degranulation was calculated by dividing the absorbance in the supernatant by the sum of absorbance in the supernatants and in the cell pellets solubilized in 0.5% Triton X-100.
Detection of Cytokine Production.
BMMCs were sensitized in 0.5 μg/ml for 4–16 h followed by stimulation with 20 ng/ml of DNP-HSA for 1 h. Total RNAs were isolated with the Trizol reagent (Invitrogen). First strand cDNAs were synthesized using the Superscript RT-PCR kit (Invitrogen). cDNAs were then used as templates for amplification of various cytokines. The primer pairs for amplification of different cytokines were the following: IL-3, 5′-GTGGCCGGGATACCCACCGTTTAAC-3′, 5′-TGGCAGGGCAGAGTCATTCGCAGA-3′; IL-4, 5′-GTCTCTCGTCACTGACGGCACAGAG-3′, 5′-GCATGGTGGCTCAGTACTACGAGTA-3′; IL-6, 5′-CGCTATGAAGTTCCTCTCTGCAAG-3′, 5′-GTAGCATCCATCATTTCTTTGTATC-3′; TNFα, 5′GCGACGTGGAACTGGCAG-AAGAGG-3′, 5′-GTACTTGGGCAGATTGACCTCAGC-3′; IFNγ, 5′-CTTCTTCAGCAACAGCAAGGCGAAA-3′, 5′-CCCCAGATACAACCCCGGAATCA-3′; actin, 5′-ACTCCTATGTGGGTGACGAG-3′, 5′-CAGGTCCAGACGCAGG-ATGGC-3′. The concentration of cytokines secreted by mast cells into the tissue culture supernatant was determined by mouse 18-Plex cytokine assay according to the manufacturer's protocol (Bio-Rad Laboratories, Inc.).
Results
Generation of LAB-deficient Mice.
To determine LAB function in vivo, we generated LAB-deficient mice by gene targeting. A 1.6-kb genomic fragment was cloned as the short arm, and a 6-kb genomic fragment was used as the long arm in the targeting construct to delete exons 2 through 8 (Fig. 1 A). These exons encode the NH2-terminal part of LAB protein from residues 1–113, which includes the transmembrane domain and palmitoylation site. This construct was used to transfect ES cells. Out of 250 transfected ES clones, seven clones underwent homologous recombination. Targeted ES cells were injected into blastocysts to produce chimeric mice. Crossing the chimeras with C57Bl/6 mice produced heterozygous mice (LAB+/−). These heterozygous mice (LAB+/−) were inbred to obtain homozygous mice for the disrupted Lab gene (LAB−/−).
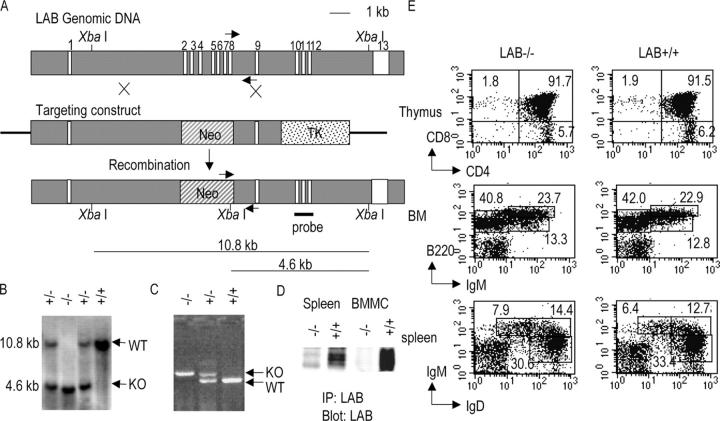
Figure 1.
Disruption of the Lab gene in mice. (A) The targeting construct was made to replace seven exons with the Neo cassette. (B) The genomic DNA from four littermates from LAB+/− interbreeding was digested with XbaI and analyzed by Southern blot using a probe as shown in A. The sizes of the DNA fragment detected by this probe were expected to be 10.8 kb for the WT allele and 4.6 kb for the targeted allele (KO). (C) PCR products were amplified from the genomic DNA of four littermates using three primers indicated by arrows in A. The size of the PCR products was expected to be 500 bp for WT allele and 600 bp for targeted allele (KO). (D) Absence of LAB protein in LAB−/− mice. Postnuclear lysates of splenocytes and BM-derived mast cells from WT and LAB−/− mice were immunoprecipitated with rabbit anti-LAB. Immunoprecipitates were then analyzed by Western blotting with monoclonal anti-LAB. (E). Normal T and B cell development in LAB−/− mice. Cells from thymus, spleen, and BM were stained with antibodies as indicated in each panel. Flow cytometry was performed and analyzed by the CELLQuest software.
Southern analysis using a probe in Fig. 1 A showed that the Lab gene was successfully targeted and the mutant allele was transmitted from the chimeric mice to their offspring. The sizes of DNA fragments of the targeted and WT alleles were the same as predicted (Fig. 1 B). Littermates were also genotyped by PCR using three primers (Fig. 1 C). Successful deletion of the Lab gene was further confirmed by immunoprecipitation of LAB from splenocyte and mast cell lysates followed by an anti-LAB blot. LAB protein was clearly absent in cells from LAB−/− mice (Fig. 1 D). LAB−/− mice were born at the expected Mendelian frequency and remained viable and apparently healthy in a pathogen-free environment. WT and LAB−/− mice were indistinguishable in terms of their appearance and size. To study whether LAT and LAB have any redundant roles in lymphocyte development and signaling, mice deficient in both LAT and LAB genes were generated by crossing LAB−/− with LAT−/− to produce LAT+/−LAB+/− mice. LAT+/−LAB+/− mice were inbred to produce double deficient mice (LAB−/−LAT−/−, simplified as 2KO mice throughout the text).
Normal T and B Cell Development in LAB−/− Mice.
The sizes of thymus, spleen, and LNs from LAB−/− mice were similar to those from WT mice. Analysis of cells from BM, thymus, spleen, and LN by FACS revealed normal development of T and B lymphocytes. The percentages of CD4+CD8+, CD4+, and CD8+ cells in the thymus of LAB−/− mice were similar to those in WT mice (Fig. 1 E). The percentages of CD4+ and CD8+ T cells were also normal in spleen and LN of LAB−/− mice (not depicted). This result was expected since LAB is not expressed in resting T cells. However, even though LAB is expressed in B cells disruption of the Lab gene had no effect on B cell development as the percentages of pro–B cells (IgM−B220+ CD43+), pre–B cells (IgM- B220+CD43-), and immature (B220loIgM+) and mature (B220hiIgM+) B cells in BM were similar to those in WT mice (Fig. 1 E and not depicted). No difference was observed in the percentages of splenic B cells expressing IgM, IgD, or both (Fig. 1 E). Dendritic and NK cell development was not affected in LAB−/− mice (not depicted). LAB function in B cells and immune responses is currently under investigation.
LAB Is Phosphorylated and Interacts with Grb2 upon FcɛRI Ligation.
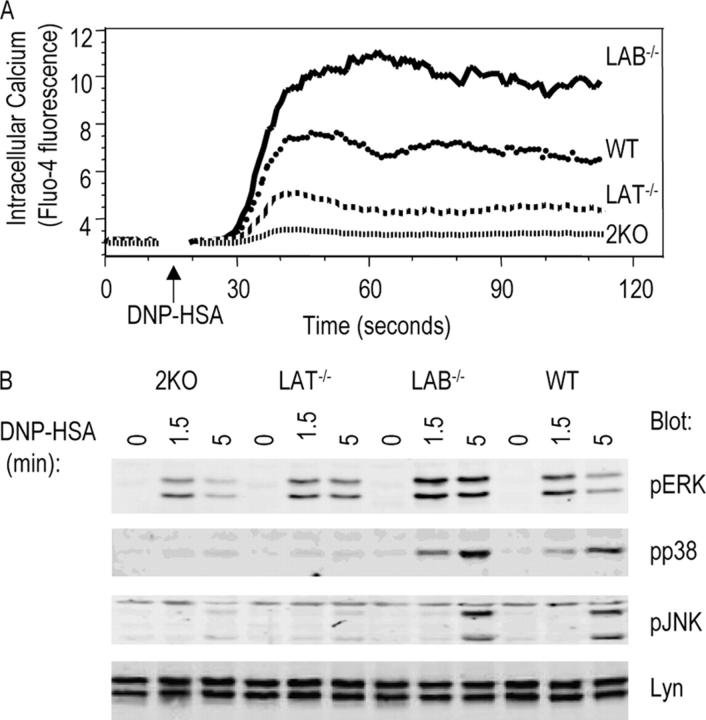
Lyn and Fyn tyrosine kinases play important roles in mast cell function. Upon FcɛRI engagement, these kinases are activated and subsequently phosphorylate downstream signaling proteins. Similar to LAT, LAB is also expressed in mast cells and is phosphorylated upon activation of mast cells via FcɛRI (21, 23). In fact, LAB protein was more abundant in mast cells than in splenocytes (Fig. 1 D). We first examined whether disruption of the Lyn or Fyn gene affects phosphorylation of LAB and LAT in mast cells. Long-term mast cell lines (BMMCs) were derived from BM cells of WT, Lyn−/−, and Fyn−/− mice. These cells were cultured in the medium with IL-3 for 3–6 wk and were used in subsequent experiments. After being sensitized with monoclonal anti-DNP IgE, mast cells were activated by cross-linking with DNP-HSA for 2 min before lysis. Postnuclear lysates were then subjected to immunoprecipitation with anti-LAB or LAT sera. Lysates and immunoprecipitates were resolved on SDS-PAGE and analyzed by Western blotting with anti-pTyr (phosphotyrosine), LAB, and LAT, respectively. As reported previously (9, 11), FcɛRI-mediated tyrosine phosphorylation of signaling proteins was dramatically reduced in Lyn−/− mast cells, whereas it was relatively normal in Fyn−/− cells (Fig. 2 A). Immunoprecipitation of LAT showed that LAT phosphorylation was significantly reduced in Lyn−/− cells compared with it in WT and Fyn−/− cells; however, it was still phosphorylated. Fyn deficiency had no effect on LAT phosphorylation. LAB was phosphorylated upon activation of WT mast cells (Fig. 2 A). Phosphorylated LAB appeared as a doublet on SDS-PAGE. Phosphorylation of LAB was almost completely abolished in Lyn−/−cells. In Fyn−/− cells, phosphorylation of LAB was slightly reduced compared with WT cells. These results indicated that Lyn, not Fyn, is required for tyrosine phosphorylation of the transmembrane adaptor proteins LAT and LAB.
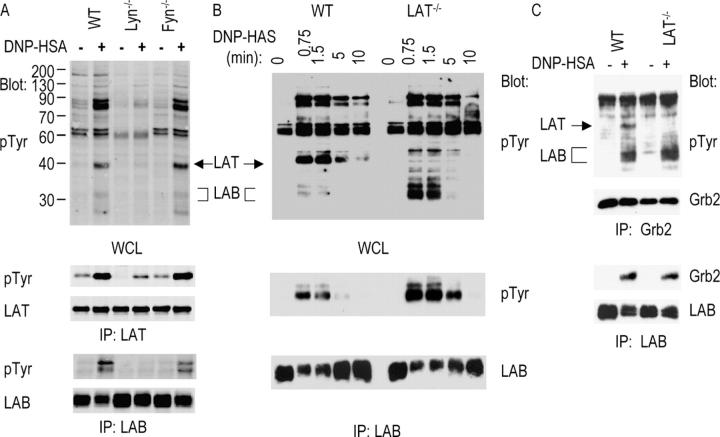
Figure 2.
FcɛRI-mediated LAB tyrosine phosphorylation and association with Grb2. BMMCs were sensitized with anti-DNP IgE and stimulated with DNP-HSA for 1.5 min or the indicated time. Postnuclear lysates were immunoprecipitated and blotted with indicated antibodies. (A) Phosphorylation of LAB and LAT in BMMCs from WT, Lyn−/−, and Fyn−/− mice. (B). Phosphorylation of LAB in BMMCs from WT and LAT−/− mice. (C) LAB association with Grb2 in WT and LAT−/− BMMCs.
Increased Tyrosine Phosphorylation of LAB in LAT−/− Mast Cells.
Previous studies showed that LAT is important in FcɛRI-mediated MAPK activation, Ca2+ flux, and degranulation; however, disruption of the Lat gene in mast cells fails to completely abolish FcɛRI-mediated signaling as LAT−/− mast cells still flux calcium and produce some cytokines upon activation (19). Because LAB and LAT contain similar tyrosine motifs and both are localized to lipid rafts, we speculated that LAB might compensate for the loss of LAT in LAT−/− mast cells. We examined whether phosphorylation of LAB is affected in LAT−/− mast cells. FcɛRI-mediated tyrosine phosphorylation of proteins was examined similarly as in Fig. 2 A. As shown in Fig. 2 B, although overall tyrosine phosphorylation of proteins in LAT−/− mast cells was similar to that in WT cells, there was an increase in phosphorylation of proteins with the molecular weight of ∼30 kD. Since LAB migrates on SDS-PAGE as a 30–32-kD protein, it is possible that this protein is LAB. Immunoprecipitation with anti-LAB sera revealed that LAB phosphorylation was indeed increased in LAT−/− mast cells (Fig. 2 B). Although it appeared that there was less LAB protein precipitated from activated mast cells at 0.75 and 1.5 min as shown in an anti-LAB blot, this was likely because our antibody did not recognize phosphorylated LAB well. We also determined whether LAB associates with Grb2 upon FcɛRI engagement. LAB and Grb2 were immunoprecipitated from lysates of resting and stimulated mast cells, followed by Western blotting with anti-pTyr and anti-Grb2. As shown in Fig. 2 C, Grb2 immunoprecipitated both phosphorylated LAT and LAB in WT, and LAB only in LAT−/− mast cells. Grb2 was also detected in anti-LAB immunoprecipitates from both mast cell lines. These data indicated that LAB is phosphorylated and interacts with Grb2 upon engagement of FcɛRI. In the absence of LAT, LAB phosphorylation was increased.
IgE-dependent Anaphylaxis in LAB−/− Mice.
Since LAB was phosphorylated and interacted with Grb2 upon FcɛRI engagement, we next examined whether disruption of LAB affects mast cell function in vivo. Passive systemic IgE-dependent anaphylaxis in WT and LAB-deficient mice was performed. This assay is dependent on in vivo mast cell degranulation and histamine release after engagement of FcɛRI. Mice were first injected via a tail vein with monoclonal anti-DNP IgE. At 20–24 h after injection, mice were challenged with DNP-HSA. At 1.5 min after challenge, mice were killed and blood was collected for measuring histamine concentration using competitive ELISA. As shown in Fig. 3 A, LAB−/− mice were capable of releasing histamine upon challenging with DNP-HSA similar to WT mice, if not slightly higher. These data indicate that LAB deficiency did not lead to a block in mast cell function in vivo.
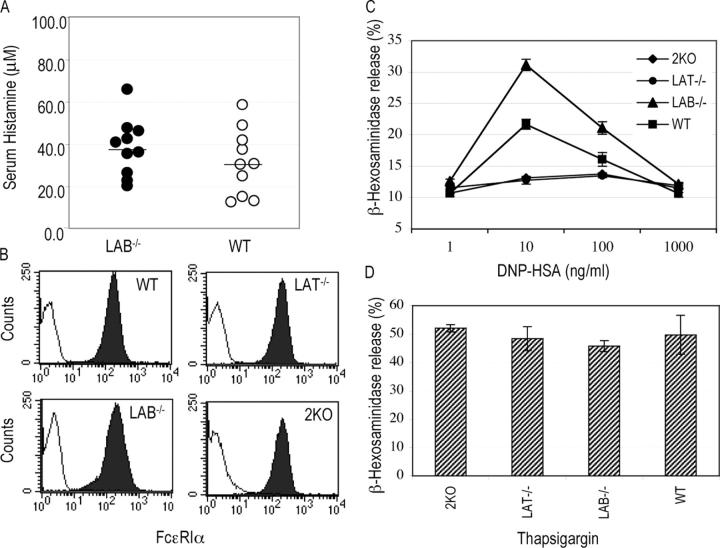
Figure 3.
The effect of LAB deficiency on IgE-mediated systemic anaphylaxis and degranulation. (A) IgE-mediated systemic anaphylaxis. Mice (n = 10) were sensitized with anti-DNP IgE and challenged with DNP-HSA. Histamine concentration in blood was measured by ELISA. (B) Surface IgE receptor expression on BMMCs from WT, LAT−/−, LAB−/−, and 2KO mice. BMMCs were stained with biotin–anti-FcɛRIα (filled curves) or biotin-IgG1 as an isotype control (opened curves) followed by streptavidin-FITC. (C and D) Degranulation was determined by measuring the release of granular β-hexosaminidase after stimulation with DNP-HSA (C) or thapsigargin (D).
Enhanced FcɛRI Signaling in LAB−/− Mast Cells.
LAB function in mast cells was further analyzed using mast cells derived from BM cells of WT and LAB−/− mice. We also derived mast cells from LAT−/− and 2KO mice to compare FcɛRI-mediated signaling in these cells with it in WT and LAB−/−cells. Mast cells developed normally from BM cells of WT, LAB−/−, LAT−/−, and 2KO mice in the presence of IL-3. These mast cell lines expressed similar levels of IgE receptor (Fig. 3 B) and c-kit (not depicted). We first determined whether the effector function of LAB−/− mast cells is normal by examining antigen-induced degranulation. Mast cells were sensitized with anti–DNP-IgE and cross-linked with different amounts of DNP-HSA. Release of β-hexosaminidase from granules was then assayed. As indicated in Fig. 3 C, antigen-induced degranulation peaked at a concentration of 10 ng/ml for mast cells from WT and LAB−/− mice; however, compared with the granule release in WT cells, FcɛRI-mediated granule release was significantly enhanced in LAB−/− cells. Similar to LAT−/− mast cells, 2KO mast cells failed to degranulate upon stimulation with DNP-HSA. These cell lines released similar amounts of β-hexosaminidase in response to stimulation by thapsigargin, which bypasses receptor engagement (Fig. 3 D).
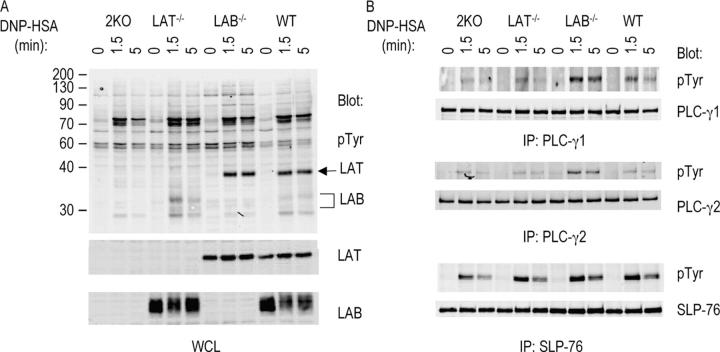
Next, we examined FcɛRI-mediated signaling in WT, LAT−/−, LAB−/−, and 2KO cells. Mast cells were sensitized with anti-DNP IgE followed by cross-linking with DNP-HSA for 0, 1.5, and 5 min before lysis. Lysates were analyzed by anti-pTyr blotting. As shown in Fig. 4 A, overall tyrosine phosphorylation of proteins, with exception of LAT and LAB, was similar in mast cells derived from WT, LAB−/−, LAT−/−, and 2KO mice. However, there was increased phosphorylation of proteins over 90 kD in LAB−/− cells. Immunoprecipitation of PLC-γ1 and PLC-γ2 showed that phosphorylation of these two proteins was increased in LAB−/− mast cells compared with that in WT, LAT−/−, and 2KO cells. Phosphorylation of SLP-76 was similar in WT and LAB−/− cell lines (Fig. 4 B).
Figure 4.
Phosphorylation of signaling proteins downstream of FcɛRI engagement. BMMCs were sensitized with anti-DNP IgE and then stimulated with DNP-HSA for the indicated time. (A) Protein phosphorylation in whole cell lysates was detected by antiphosphotyrosine (pTyr). LAT and LAB proteins were detected by anti-LAT and LAB, respectively. (B) Phosphorylation of PLC-γ1, PLC-γ2, and SLP-76 in WT, LAT−/−, LAB−/−, and 2KO BMMCs. Lysates were immunoprecipitated with anti–PLC-γ1, PLC-γ2, and SLP-76 antibodies. Phosphorylation of PLC-γ1, PLC-γ2, and SLP-76 was detected by Western blotting with anti-pTyr. Similar amounts of proteins were immunoprecipitated as indicated by blotting with anti–PLC-γ1, PLC-γ2, or SLP-76 antibodies.
LAT plays an essential role in TCR-mediated calcium mobilization by recruiting PLC-γ1 to the membrane for activation. In mast cells, LAT deficiency only leads to a modest reduction in FcɛRI-mediated calcium flux (19). To examine activation-induced intracellular calcium elevation, mast cells were sensitized with anti-DNP IgE, loaded with calcium-sensitive dye Fluo-4, and activated with DNP-HSA. LAT−/− mast cells showed a reduction in calcium flux compared with WT cells (Fig. 5 A). In contrast, LAB−/− mast cells showed an increased elevation of intracellular calcium after stimulation compared with WT cells, suggesting that LAB might negatively regulate calcium mobilization. Interestingly, 2KO mast cells, in which both genes were deleted, mobilized very minimal calcium upon stimulation.
Figure 5.
MAPK activation and Ca2+ flux in BMMCs from WT, LAT−/−, LAB−/−, and 2KO after FcɛRI engagement. (A) Ca2+ mobilization. BMMCs were sensitized with anti-DNP IgE and then loaded with Fluo-4 AM. DNP-HSA was used to induce Ca2+ mobilization. (B) Activation of Erk, p38, and Jnk in BMMCs. Whole cell lysates were blotted with antibodies against the activated forms of Erk, p38, and Jnk. Similar amounts of lysates were loaded on SDS-PAGE as indicated by an anti-Lyn blot.
Activation via the FcɛRI also leads to activation of MAPKs. To examine MAPK activation in WT, LAT−/−, LAB−/−, and 2KO mast cells, samples from Fig. 4 A were also analyzed by blotting with antibodies against active Erk, Jnk, and p38. Equal amounts of proteins loaded on each lane were indicated by an anti-Lyn blot. Disruption of the Lab gene in mast cells had no obvious effect on activation of p38 and Jnk; however, Erk activation in LAB−/− cells was significantly increased in comparison with Erk activation in WT, LAT−/−, and 2KO mast cells (Fig. 5 B). In contrast, LAT−/− mast cells failed to activate JNK and p38; however, Erk activation appeared to be normal in LAT−/− mast cells. Similar to LAT−/− mast cells, 2KO mast cells had no p38 and Jnk activation, but Erk activation in 2KO cells was reduced compared with activation in LAT−/− cells.
FcɛRI-mediated Cytokine Production.
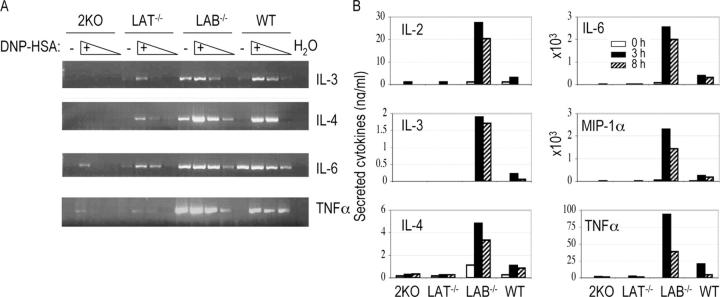
Engagement of the FcɛRI receptor also leads to production of multiple cytokines. To analyze the effect of LAB on cytokine production, mast cells were sensitized with anti-DNP IgE and cross-linked with DNP-HSA for 1 h. Total RNAs were then prepared from resting or stimulated WT, LAT−/−, LAB−/−, and 2KO cells. Cytokine RNA levels for IL-3, IL-4, IL-6, and TNFα were determined by RT-PCR. Amplification of the actin transcript indicated that similar amounts of cDNAs were used in each reaction (not depicted). As shown in Fig. 6 A, after being sensitized LAB−/− mast cells already produced significant amounts of RNAs for IL-3, IL-4, IL-6, and TNF before cross-linking. After cross-linking with DNP-HSA, LAB−/− cells produced more IL-4 and TNF-α than WT cells but similar amounts of IL-3 and IL-6 RNAs. Compared with WT cells, production of IL-3, IL-4, IL-6, and TNFα RNAs was dramatically reduced in LAT−/− mast cells as reported previously (19). Interestingly, production of these RNAs was further reduced in 2KO mast cells. We also quantitated the amounts of cytokines secreted into the tissue culture supernatants after stimulation using multiplex cytokine assay. As shown in Fig. 6 B, LAB−/− mast cells produced more IL-2, IL-3, Il-4, IL-6, MIP-1α, and TNFα after activation for 3 or 8 h than WT cells, whereas LAT−/− and 2KO cells produced very minimal amounts of cytokines. These results suggested that LAB negatively regulates FcɛRI-mediated cytokine production.
Figure 6.
Increased cytokine production in LAB−/− mast cells. (A) Semiquantitative RT-PCR for determination of cytokine RNAs. BMMCs were sensitized with anti-DNP IgE and stimulated with DNP-HSA for 1 h. RNAs were isolated from sensitized BMMCs before (−) and after cross-linking with DNP-HSA (+). Relative levels of cytokine RNAs were detected by RT-PCR using specific primer pairs for each cytokine. For stimulated samples, cDNAs after dilution at 1:5 and 1:25 were also used in PCR reactions. (B) Cytokine production by mast cells. Cytokines secreted into the tissue culture supernatant by mast cells before (0 h) and after cross-linking for 3 or 8 h were quantitated by a multiplex cytokine assay.
Competition of LAT and LAB for Raft Localization.
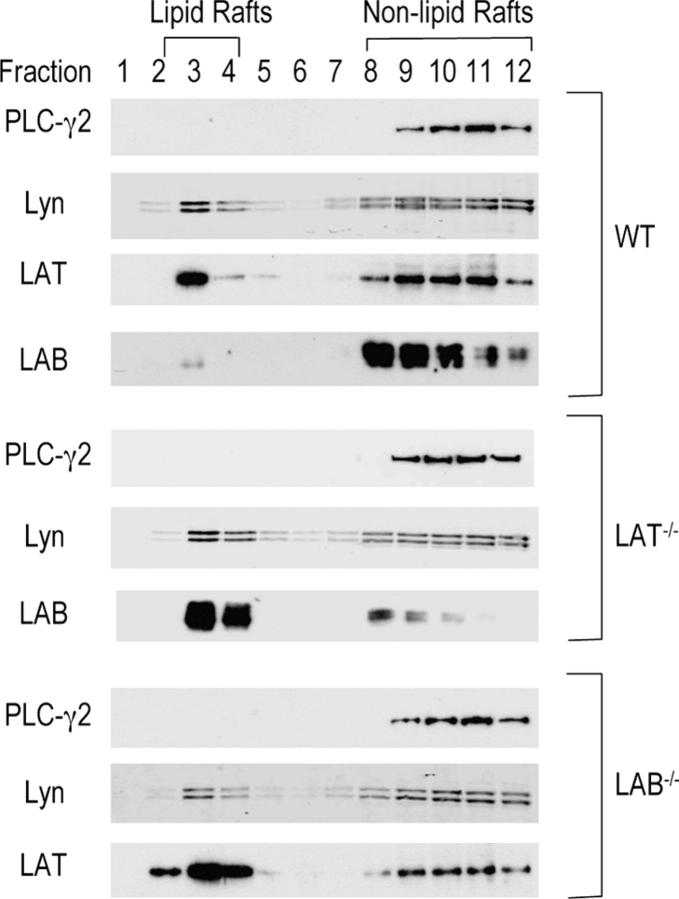
Our data showed that FcɛRI-mediated signaling was enhanced in LAB−/− mast cells. To find out how LAB deficiency affects FcɛRI-mediated signaling, we examined whether removal of either LAT or LAB by gene targeting affects raft localization of LAB or LAT, since both are localized in lipid rafts. We isolated lipid raft and nonraft fractions from WT, LAT−/−, and LAB−/− mast cells by ultracentrifugation on a sucrose gradient. Each fraction was analyzed by Western blotting with LAB, LAT, Lyn, and PLC-γ2 antibodies. In WT mast cells, LAT was present in both lipid raft (fractions 2–4) and nonraft fractions (fractions 8–12). Interestingly, unlike LAB in B cells, most of the LAB protein was present in nonraft fractions in WT mast cells (Fig. 7). In LAT−/− mast cells, most of the LAB protein was detected in raft fractions. Compared with WT mast cells, LAB−/− mast cells had more LAT in lipid raft fractions and less LAT in Triton-soluble fractions. The distribution of Lyn and PLC-γ2 was similar in WT, LAT−/−, and LAB−/− mast cells. These results suggested that LAT and LAB might compete for raft localization. In the absence of LAT, more LAB protein is targeted to lipid rafts, leading to increased tyrosine phosphorylation of LAB as indicated in Fig. 2 B. In the absence of LAB, more LAT moves into rafts, thus enhancing FcɛRI-mediated signaling.
Figure 7.
Localization of LAB and LAT in lipid rafts. BMMCs from WT, LAT−/−, and LAB−/− mice were lysed in 1% Triton before ultracentrifugation on a sucrose gradient. 12 fractions were collected starting from the top of the sucrose gradient. Each fraction was resolved on SDS-PAGE and blotted with anti-LAB, LAT, PLC-γ2, and Lyn, respectively.
Discussion
In our study, we used mast cells derived from LAB-deficient mice to study how this adaptor molecule functions in FcɛRI-mediated signaling and effector function. Our data suggested that LAB negatively regulates FcɛRI-mediated signaling. We also compared FcɛRI-mediated signaling in mast cells derived from the WT, LAT−/−, LAB−/−, and 2KO mice. Our data indicated that, although LAT plays a dominant role, LAB also has a positive role in FcɛRI-mediated signaling and effector function.
Disruption of Lat, Lab, or both did not affect IL-3–dependent mast cell growth, suggesting that these two molecules are not required in IL-3 receptor–mediated signaling. In LAB−/− mast cells, FcɛRI-mediated tyrosine phosphorylation of cellular proteins was relatively normal compared with that in WT cells, indicating that proximal PTK activation was not affected by deficiency of LAB. However, phosphorylation of PLC-γ1 and PLC-γ2 was increased. Increased tyrosine phosphorylation of PLC-γ1 and PLC-γ2 normally correlates with an increase in its enzymatic activity, leading to increase in inositol 1,4,5-trisphosphate and diacylglycerol (DAG) production. Indeed, when we measured FcɛRI-mediated calcium flux we observed a significant increase in LAB−/− cells, likely caused by increased inositol 1,4,5-trisphosphate production. In addition, FcɛRI-mediated Erk activation was increased, a consequence of PKC or Ras-GRP activation by increased production of DAG. Enhanced calcium flux and Erk activation further led to an increase in mast cell effector function, such as degranulation and cytokine production. These data suggested that, different from LAT, LAB functions as a negative regulator in FcɛRI-mediated signaling. These results were apparently different from those obtained using siRNA to knock down LAB protein (23). Reduced LAB expression was found to abolish FcɛRI-mediated signaling and mast cell function. The reason for this discrepancy is not clear. One possibility is that LAB siRNA might also affect expression of other protein(s) involved in FcɛRI-mediated signaling.
Previous studies showed that FcɛRI-mediated signaling is not completely blocked in LAT−/− mast cells. LAT−/− cells still flux calcium and produce cytokines, although at reduced levels. Thus, the impact of LAT deficiency is less severe on FcɛRI-mediated signaling compared with its effect on TCR-mediated signaling. In LAT-deficient Jurkat cells, TCR-mediated calcium flux and MAPK activation are completely blocked. This suggests that coupling of calcium flux and MAPK activation to FcɛRI engagement is not solely dependent on LAT. Our data indicated that LAB might play a similar role as LAT. When both LAT and LAB were deficient in mast cells, FcɛRI-mediated calcium flux was completely absent and Erk activation was further reduced compared with Erk activation in LAT−/− mast cells. In the LAT−/− cells, FcɛRI-mediated IL-3, IL-6, and TNF production was reduced. Cytokine production in 2KO mast cells was further reduced as detected by RT-PCR. These data suggested that LAB also functions positively in FcɛRI-mediated signaling by coupling FcɛRI engagement to MAPK activation and calcium flux.
LAT and LAB share similar structural motifs. Although both adaptor proteins are capable of binding Grb2, LAB is unable to bind PLC-γ1. When the Lab gene was used to reconstitute LAT-deficient Jurkat cells, it could partially rescue Ca2+ flux (26) or MAPK activation (21). Transgenic mice that express LAB in T cells under the CD2 promoter have a similar autoimmune phenotype as the LAT Y136F knockin mice (24, 27, 28). These mice have splenomegaly and massive lymphocyte infiltration. T cells in these mice are hyperactivated and produce large amounts of Th2 cytokines. Therefore, LAB functions like the LAT mutant without the PLC-γ1 site. In LAT−/− mast cells, more LAB was phosphorylated. Although LAB does not bind PLC-γ1 or PLC-γ2 directly, perhaps LAB could recruit PLC-γ1 or PLC-γ2 to the membrane through binding of the Gads–SLP76–PLC-γ complex. Since recruitment through LAB might be not as efficient as through LAT, FcɛRI-mediated calcium flux is thus compromised in LAT−/− cells. When both molecules are absent as in 2KO cells, there is no recruitment PLC-γ1 or PLC-γ2 to the membrane, resulting in a complete block in FcɛRI-mediated calcium flux.
It is surprising that disruption of the Lab gene enhanced FcɛRI-mediated signal transduction. Since LAB and LAT have similar structures, we expected to see reduced FcɛRI-mediated signaling when the Lab gene was deleted. The reason for this enhanced mast cell function is not entirely clear. One possibility is that LAB and LAT might compete for binding of Grb2. Without LAB, LAT could bind more Grb2 and their associated proteins, thus enhancing FcɛRI-mediated MAPK activation and calcium flux. A more likely possibility, as suggested in Fig. 7, is that LAT and LAB compete for localization to lipid rafts. In WT mast cells, LAT is the dominant one. It keeps most of the LAB protein away from lipid rafts. In the absence of LAT, more LAB moves into lipid rafts, leading to more tyrosine phosphorylation of LAB and further compensation for loss of LAT. This is probably the reason why LAT deficiency only causes a partial block in FcɛRI-mediated signaling. In the absence of LAB, more LAT moves into lipid rafts and binds more signaling proteins, thus enhancing FcɛRI-mediated phosphorylation of PLC-γ1 or PLC-γ2, MAPK activation, calcium flux, degranulation, and cytokine production. Therefore, LAB might negatively regulate FcɛRI-mediated signaling by competing with LAT to localize to lipid rafts. However, whether lipid rafts exist and function still remains controversial (29). Our data in Fig. 7 might simply indicate changes of LAT or LAB in its physical properties, such as palmitoylation, or subcellular localization when the other one is absent. How and at what levels LAB competes with LAT to localize to rafts remain to be investigated in the future.
Although we observed enhanced FcɛRI-mediated signaling in mast cells derived from BM cells of LAB−/− mice, we did not see a significant enhancement of anaphylaxis in vivo. It is possible that there is a compensatory mechanism in vivo to control hyperactive mast cells. Interestingly, the basal IgE level in naïve LAB−/− mice is higher than the basal IgE level in WT mice (unpublished data). Mast cells in LAB−/− mice might bind less anti–DNP-IgE after sensitization than cells in WT mice, since exogenous IgE has to compete with endogenous IgE to bind FcɛRI. Even though FcɛRI-mediated signaling is enhanced in LAB−/− mast cells, engagement of the FcɛRI in LAB−/− mice might not be as efficient as it in WT mice. It is also possible that cross-linking with DNP-HSA, which induces anaphylaxis in sensitized mice, already causes a very strong stimulation in WT mice; therefore, it might be difficult to observe enhanced mast cell function using this assay. In addition, because there are some variations in the anaphylaxis assay even among different mice with the same genetic background, it is difficult to detect enhanced mast cell-mediated anaphylaxis even if it does occur in vivo.
Compared with the effect of LAT deficiency on T cell development, disruption of Lab did not have a significant impact on B cell development as normal populations of pro–, pre–, and mature B cells are present in LAB-deficient mice. Therefore, even though LAB can substitute LAT in T cell development and partially rescues calcium flux in LAT-deficient cells, LAB does not function like LAT in B cells. It is possible that in B cells BCR-mediated signaling does not use a LAT-like molecule to signal. In T cells, recruitment of SLP-76 and PLC-γ1 to the membrane and further activation of Ras-MAPK and calcium flux depend on LAT; however, in B cells SLP-65/BLNK can be recruited to the BCR by interacting directly with the Igα chain (30, 31). BLNK then recruits PLC-γ1/γ2 to the membrane by interacting with them directly. It is also possible that other membrane-associated molecules might exist to function like LAT in B cells. Although LAB deficiency had no effect on B cell development, we could not exclude the possibility that B cell function is affected by disruption of Lab. We are currently investigating whether BCR-mediated signaling, B cell function, and immune responses are normal in these mice.
Acknowledgments
The authors thank the Duke University Cancer Center Flow Cytometry, DNA Sequencing, Human Vaccine Institute, and Transgenic Mouse facilities for their excellent services. We thank Dr. Motonari Kondo for carefully reading our manuscript.
This work was supported by National Institutes of Heath grants AI048674 and AI056156.
The authors have no conflicting financial interests.
Abbreviations used in this paper: BMMC, BM-derived mast cell; DAG, diacylglycerol; ES, embryonic stem; ITAM, immunoreceptor tyrosine-based activation motif; LAB, linker for activation of B cells; LAT, linker for activation of T cells; MAPK, mitogen-activated protein kinase; NTAL, non–T cell activation linker; PTK, protein tyrosine kinase.
References
- 1.Nadler, M.J., and J.P. Kinet. 2002. Uncovering new complexities in mast cell signaling. Nat. Immunol. 3:707–708. [DOI] [PubMed] [Google Scholar]
- 2.Ott, V.L., and J.C. Cambier. 2000. Activating and inhibitory signaling in mast cells: new opportunities for therapeutic intervention? J. Allergy Clin. Immunol. 106:429–440. [DOI] [PubMed] [Google Scholar]
- 3.Rivera, J. 2002. Molecular adapters in Fc(epsilon)RI signaling and the allergic response. Curr. Opin. Immunol. 14:688–693. [DOI] [PubMed] [Google Scholar]
- 4.Nadler, M.J., S.A. Matthews, H. Turner, and J.P. Kinet. 2000. Signal transduction by the high-affinity immunoglobulin E receptor Fc epsilon RI: coupling form to function. Adv. Immunol. 76:325–355. [DOI] [PubMed] [Google Scholar]
- 5.Ravetch, J.V. 1994. Fc receptors: rubor redux. Cell. 78:553–560. [DOI] [PubMed] [Google Scholar]
- 6.Minoguchi, K., M. Benhamou, W.D. Swaim, Y. Kawakami, T. Kawakami, and R.P. Siraganian. 1994. Activation of protein tyrosine kinase p72syk by Fc epsilon RI aggregation in rat basophilic leukemia cells. p72syk is a minor component but the major protein tyrosine kinase of pp72. J. Biol. Chem. 269:16902–16908. [PubMed] [Google Scholar]
- 7.El-Hillal, O., T. Kurosaki, H. Yamamura, J.P. Kinet, and A.M. Scharenberg. 1997. syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc. Natl. Acad. Sci. USA. 94:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costello, P.S., M. Turner, A.E. Walters, C.N. Cunningham, P.H. Bauer, J. Downward, and V.L. Tybulewicz. 1996. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 13:2595–2605. [PubMed] [Google Scholar]
- 9.Nishizumi, H., and T. Yamamoto. 1997. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J. Immunol. 158:2350–2355. [PubMed] [Google Scholar]
- 10.Odom, S., G. Gomez, M. Kovarova, Y. Furumoto, J.J. Ryan, H.V. Wright, C. Gonzalez-Espinosa, M.L. Hibbs, K.W. Harder, and J. Rivera. 2004. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J. Exp. Med. 199:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parravicini, V., M. Gadina, M. Kovarova, S. Odom, C. Gonzalez-Espinosa, Y. Furumoto, S. Saitoh, L.E. Samelson, J.J. O'Shea, and J. Rivera. 2002. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 3:741–748. [DOI] [PubMed] [Google Scholar]
- 12.Gu, H., K. Saito, L.D. Klaman, J. Shen, T. Fleming, Y. Wang, J.C. Pratt, G. Lin, B. Lim, J.P. Kinet, and B.G. Neel. 2001. Essential role for Gab2 in the allergic response. Nature. 412:186–190. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R.P. Trible, and L.E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 92:83–92. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, W., R.P. Trible, M. Zhu, S.K. Liu, C.J. McGlade, and L.E. Samelson. 2000. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J. Biol. Chem. 275:23355–23361. [DOI] [PubMed] [Google Scholar]
- 15.Liu, S.K., N. Fang, G.A. Koretzky, and C.J. McGlade. 1999. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9:67–75. [DOI] [PubMed] [Google Scholar]
- 16.Finco, T.S., T. Kadlecek, W. Zhang, L.E. Samelson, and A. Weiss. 1998. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 9:617–626. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, W., B.J. Irvin, R.P. Trible, R.T. Abraham, and L.E. Samelson. 1999. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int. Immunol. 11:943–950. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, W., C.L. Sommers, D.N. Burshtyn, C.C. Stebbins, J.B. DeJarnette, R.P. Trible, A. Grinberg, H.C. Tsay, H.M. Jacobs, C.M. Kessler, et al. 1999. Essential role of LAT in T cell development. Immunity. 10:323–332. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh, S., R. Arudchandran, T.S. Manetz, W. Zhang, C.L. Sommers, P.E. Love, J. Rivera, and L.E. Samelson. 2000. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 12:525–535. [DOI] [PubMed] [Google Scholar]
- 20.Saitoh, S., S. Odom, G. Gomez, C.L. Sommers, H.A. Young, J. Rivera, and L.E. Samelson. 2003. The four distal tyrosines are required for LAT-dependent signaling in FcepsilonRI-mediated mast cell activation. J. Exp. Med. 198:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brdicka, T., M. Imrich, P. Angelisova, N. Brdickova, O. Horvath, J. Spicka, I. Hilgert, P. Luskova, P. Draber, P. Novak, et al. 2002. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J. Exp. Med. 196:1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen, E., M. Zhu, W. Zhang, S. Koonpaew, and W. Zhang. 2003. LAB: A new membrane-associated adaptor molecule in B cell activation. Nat. Immunol. 4:117–123. [DOI] [PubMed] [Google Scholar]
- 23.Tkaczyk, C., V. Horejsi, S. Iwaki, P. Draber, L.E. Samelson, A.B. Satterthwaite, D.H. Nahm, D.D. Metcalfe, and A.M. Gilfillan. 2004. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and Fc epsilon RI aggregation. Blood. 104:207–214. [DOI] [PubMed] [Google Scholar]
- 24.Janssen, E., M. Zhu, B. Craven, and W. Zhang. 2004. Linker for activation of B cells: a functional equivalent of a mutant linker for activation of T cells deficient in phospholipase C-gamma1 binding. J. Immunol. 172:6810–6819. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, W., R.P. Trible, and L.E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 9:239–246. [DOI] [PubMed] [Google Scholar]
- 26.Koonpaew, S., E. Janssen, M. Zhu, and W. Zhang. 2004. The importance of three membrane-distal tyrosines in the adaptor protein NTAL/LAB. J. Biol. Chem. 279:11229–11235. [DOI] [PubMed] [Google Scholar]
- 27.Sommers, C.L., R.K. Menon, A. Grinberg, W. Zhang, L.E. Samelson, and P.E. Love. 2001. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. J. Exp. Med. 194:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguado, E., S. Richelme, S. Nunez-Cruz, A. Miazek, A.M. Mura, M. Richelme, X.J. Guo, D. Sainty, H.T. He, B. Malissen, and M. Malissen. 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 296:2036–2040. [DOI] [PubMed] [Google Scholar]
- 29.Munro, S. 2003. Lipid rafts: elusive or illusive? Cell. 115:377–388. [DOI] [PubMed] [Google Scholar]
- 30.Engels, N., B. Wollscheid, and J. Wienands. 2001. Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-alpha. Eur. J. Immunol. 31:2126–2134. [DOI] [PubMed] [Google Scholar]
- 31.Kabak, S., B.J. Skaggs, M.R. Gold, M. Affolter, K.L. West, M.S. Foster, K. Siemasko, A.C. Chan, R. Aebersold, and M.R. Clark. 2002. The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways. Mol. Cell. Biol. 22:2524–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]