Abstract
The human gastric pathogen Helicobacter pylori spontaneously switches lipopolysaccharide (LPS) Lewis (Le) antigens on and off (phase-variable expression), but the biological significance of this is unclear. Here, we report that Le+ H. pylori variants are able to bind to the C-type lectin DC-SIGN and present on gastric dendritic cells (DCs), and demonstrate that this interaction blocks T helper cell (Th)1 development. In contrast, Le− variants escape binding to DCs and induce a strong Th1 cell response. In addition, in gastric biopsies challenged ex vivo with Le+ variants that bind DC-SIGN, interleukin 6 production is decreased, indicative of increased immune suppression. Our data indicate a role for LPS phase variation and Le antigen expression by H. pylori in suppressing immune responses through DC-SIGN.
Keywords: Helicobacter pylori, phase variation, DC-SIGN, dendritic cells, Th1/Th2 cell response
Introduction
The human Gram-negative bacterium Helicobacter pylori is able to persist for a lifetime on the gastric mucosa of 50% of the world population. In most individuals, colonization results in uncomplicated chronic gastritis, but in some patients H. pylori infection leads to gastric and duodenal ulcers, mucosa-associated lymphoid tissue lymphoma, and adenocarcinoma (1, 2). To date, the mechanism(s) that enables H. pylori to adapt to a large variety of genetically diverse hosts is unknown.
Most H. pylori express O-antigen Lewis (Le) blood group antigens in their LPS (Lex, Ley, H type 1, Lea, Leb, i-antigen, and sialyl Lex; for carbohydrate structure see Fig. 2 A; references 3–8). This restricted diversity in O-antigenic structures suggests a role for Le antigens in H. pylori infection. Lex/y expression is not a stable trait, but is subjected to phase variation (i.e., the occurrence of spontaneous, high frequency [up to 0.5%], reversible on and off switching of LPS epitopes), resulting in Lex+/Ley+ and Lex−/Ley− bacteria within a single strain (9). Le phase variation in H. pylori is caused by translational frame shifts in glycosyltransferase genes that occur during replication (10). Similar mechanisms of phase variation in Neisseria ssp. (11) and Haemophilus influenzae (12) have been described. These mechanisms have resulted in microorganisms that either adhere better to host cells or are more resistant to killing by complement (13). The role of Le antigens in adhesion to the gastric epithelium has been explored and the most recent data indicate that expression of Lex only plays a minor role in adherence of H. pylori to the gastric mucosa (14-16). Expression of Le antigens by H. pylori has been implicated in evasion of the host immune system by mimicking blood group antigens expressed on the gastric mucosa (17), but here controversial findings have also been reported (18). Furthermore, a correlation between Le expression and the degree of leukocyte infiltration (18) and that of symptomatic infection (19) has been described. However, expression of Le antigens is phase variable, resulting in several Le+ and Le− populations of H. pylori within a single strain. Therefore, the above mentioned studies may reflect the host response to heterogeneous populations of H. pylori Le+ and Le− phase variants, leaving the interpretation of these findings rather difficult. So far, the biological roles of Le antigen expression and phase variation remain unclear.
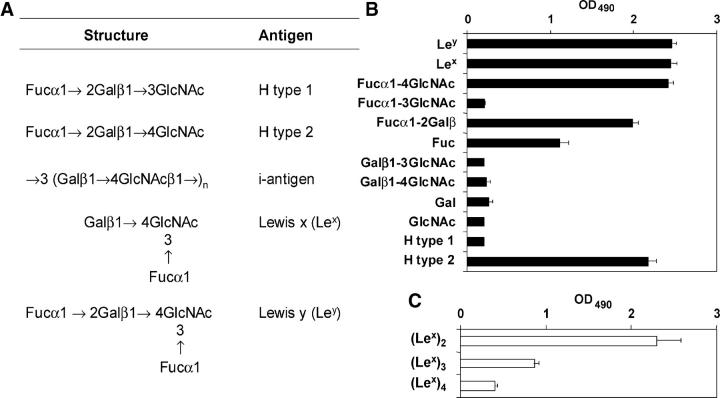
Figure 2.
Le blood group and related antigens and some of their substructures bind to DC-SIGN. (A) Le antigens expressed by H. pylori and discussed in this paper. (B and C) Carbohydrates, representing blood group antigens or their substructures, conjugated to polyacrylamide (B) or ceramide (C), were coated and binding of recombinant DC-SIGN-Fc was measured after incubation with peroxidase-labeled goat anti–human Fc. The mean (+ SD) of three independent experiments is shown.
H. pylori infection is marked by rapid recruitment of neutrophils followed by T and B lymphocytes, plasma cells, and macrophages (20). T lymphocyte responses in acute H. pylori infection are predominantly of the CD4+ Th1 cell phenotype (21), but data regarding accessory cells involved in presentation of H. pylori antigens to T cells are scarce. Despite their evident importance as link between innate and adaptive immunity, little attention has been paid to the role of DCs in H. pylori infection. Immature DCs are seeded throughout peripheral tissues and along mucosal surfaces to act as sentinels, and upon pathogen capture DCs are activated. Activated DCs process pathogens into antigenic peptides for presentation and migrate to secondary lymphoid organs where they activate naive T cells and steer adaptive T cell responses (22).
DCs express the C-type lectin DC-SIGN, which is involved in cell adhesion as well as antigen presentation to T cells (23). Accumulating evidence indicate DC-SIGN as pathogen receptor for viruses, parasites, fungi, and bacteria (24–27), including H. pylori (28). Binding to the carbohydrate recognition domain of DC-SIGN is dependent on high mannoses or Le sugars. The described phase variation of LPS Le antigens prompted us to analyze binding of H. pylori variants to DCs and the functional consequence of binding and the role of DC-SIGN herein. Here, we demonstrate that H. pylori LPS phase variation occurs in vivo and that clinically isolated Le+, but not Le−, phase variants can bind to DC-SIGN, which is present on DCs in gastric mucosa. This interaction has direct consequences on Th1/Th2 cell polarization.
Materials and Methods
Bacterial Strains.
H. pylori clinical isolates of different geographic origins (Netherlands, Canada, Poland, Italy, and China) have been described (5), as well as H. pylori strain NCTC 11637 and phase variants 1c, K4.1, K5.1, 3a, and 1b (9). H. pylori strain 4187E and generation of futA (gene HP0379) and futB (gene HP0651) knockout mutants have been described (10). LPS was purified by the hot-phenol-water procedure followed by gel-permeation chromatography (6). Clinical isolate H. pylori J223 was as described previously (6). Lex/y+ and Lex/y− variants of J223 were isolated as described previously (9). In brief, bacteria from the biopsy were inoculated on Dent plates (Columbia agar, 5% horse blood, plus Dent supplement), followed by one single passage in fluid phase (Brucella broth plus 3% FCS) and distribution over solid media, after which detection of LPS phase variants was performed by colony blotting (9). Genotyping by amplified fragment length polymorphism (AFLP) analysis was performed as described (29) with the following modifications. In a final volume of 20 μl, 20 ng of purified chromosomal DNA was digested with 1 U EcoRI (BD Biosciences) and 1 U MseI (New England Biolabs, Inc.). The final volume then was increased to 30 μl by the addition of 50 pmol each of the EcoRI adaptor and MseI adaptor, 1.2 U of T4 DNA ligase (BD Biosciences), 1 mM ATP, and ligase buffer. The adaptors were allowed to ligate to the restriction fragments for 16 h at 167°C, after which the sample was diluted with distilled water in a final volume of 50 μl. Texas red fluorescent-labeled EcoO primer (5′-AGACTGCGTACCAATTC-3′; Isogen Bioscience) and unlabeled MseO primer (5′-GACGATGAGTCCTGAG-3′) were used for DNA amplification in a thermal cycler (GeneAmp PCR system 9700; PerkinElmer) with 35 cycles as follows: denaturation (30 s at 94°C), annealing (30 s at 65–56°C), and DNA molecule extension (60 s at 72°C). In the first 12 cycles, the annealing temperature was lowered 0.7°C per cycle. After completion of the cycle program, each sample was analyzed according to the manufacturer's instructions in an automated DNA sequencer (Vistra 725; Amersham Biosciences). Fluorescent AFLP images were stored as TIFF files with TIFF software (Vistra 2; Amersham Biosciences). C-tract sequencing was performed as described previously (10).
mAbs and Glycoconjugates.
H. pylori strains and phase variants were serotyped as described previously (5) with the following mAbs: Hp151, specific for Ley; 6H3, specific for monomeric Lex; 4D2, specific for H type 1 (all three provided by R. Negrini, General Hospital, Brescia, Italy); 54.1F6A, specific for polymeric Lex (provided by G. van Dam, Leiden University, Leiden, Netherlands); and NAM61-1A2, specific for i antigen (provided by D. Blanchard, Regional Blood Transfusion Service, Nantes, France; reference 10). mAbs AZN-D1 and AZN-D2 (24) were used to block DC-SIGN and mAb Clone 17 (provided by S. Gordon, University of Oxford, Oxford, UK) was used to block the mannose receptor (MR). Synthetic glycoconjugates (Syntesome) comprised monosaccharides and oligosaccharides that were multivalently linked to a polyacrylamide carrier. Ceramide-linked dimeric, trimeric, and tetrameric Lex antigens were provided by R.R. Schmidt (University of Konstanz, Konstanz, Germany) and synthesized as described previously (30).
Cells.
Immature DCs were generated by culturing monocytes in RPMI 1640/10% FCS in the presence of 500 U/ml IL-4 and 800 U/ml GM-CSF (both from Schering-Plough) for 5–8 d (31). K-562 cells, K-562-DC-SIGN cells, and RAW 264.7 macrophages were cultured as described previously (31).
Bacterial Binding.
H. pylori cells were labeled with FITC. 50,000 cells in Tris-saline-magnesium buffer with 0.5% BSA were preincubated with 20 μg/ml mAb, 200 μg/ml mannan, or 10 mM EDTA for 10 min at room temperature. FITC-labeled bacteria (10 bacteria/cell) were added and incubated for 45 min at 37°C. Samples were analyzed by flow cytometry.
Soluble DC-SIGN-Fc Adhesion Assay.
The soluble DC-SIGN adhesion assay was performed by ELISA (32). Antigens (either 3.75 × 106 bacterial cells/well or 5 μg/ml in the case of LPS or glycoconjugates) were coated in ELISA plates. Soluble DC-SIGN-Fc (1 μg/ml in Tris-saline-magnesium buffer) binding was determined by an anti–human Ig-Fc ELISA. When indicated, DC-SIGN-Fc was preincubated with 20 μg/ml mAb, 200 μg/ml mannan, or 5 mM EDTA for 10 min at room temperature.
Immunohistochemistry.
After informed consent by the patients, two antral biopsies were collected during gastroscopy. Tissue cryosections were fixed in acetone and incubated with AZN-D1 followed by anti–mouse horseradish peroxidase. Staining was performed with the ABC-AP Vectastain kit and AEC (Vector Laboratories), and sections were counterstained with hematoxylin. In parallel, the other biopsy was analyzed for H. pylori infection by incubation in urease medium for 12 h. No differences in morphology and staining were observed between noninfected and infected individuals.
DC Activation and Th1/Th2 Cell Differentiation.
To analyze maturation, 180,000 DCs were incubated with H. pylori for 1 h at multiplicities of infection (MOIs) of 1, 5, 10, and 20, and washed and cultured for 20 h. Cells were analyzed for maturation markers (CD80, CD86, CD83, and HLA-DR) by flow cytometry, and supernatant was collected for cytokine ELISA (27).
For T cell differentiation, DCs were cocultured with H. pylori (at an MOI of 10) for 2 d, washed, and incubated with CD45RA+ CD4+ T cells (5,000 T cells/20,000 DCs). In parallel, DCs were analyzed for the maturation markers described above and cytokine production after stimulation with J558 transfected with CD40L (provided by P. Lane, University of Birmingham, Birmingham, UK) in the presence or absence of 1,000 U/ml IFN-γ.
Quiescent T cells were restimulated with 10 ng/ml PMA and 1 μg/ml ionomycin for 6 h. During the last 5 h of that time, they were in the presence of 10 μg/ml brefeldin A (all from Sigma-Aldrich). Single cell production of IL-4 and IFN-γ was determined by intracellular flow cytometry (33).
Patients and Ex Vivo Challenge of Biopsies with H. pylori.
11 patients attending the Endoscopy Unit of the Outpatient Clinic of Gastroenterology of the Vrije Universiteit Medical Center, who were referred for diagnostic gastroscopy, were invited to participate in this investigation. Each patient provided informed consent in agreement with the Declaration of Helsinki to obtain two biopsy specimens additional to the clinically indicated biopsy specimens. The biopsy specimens used in these experiments were collected during two mornings in which the endoscopies were performed. Biopsies were cultured with 10 × 106 H. pylori for 48 h. Supernatant was collected and the biopsies were homogenized using a polytron. Homogenates and supernatants from nine patients who were shown to be negative for H. pylori by histology/immunohistochemistry, as scored by an experienced pathologist, were tested for the presence of IL-4, IL-6, IL-10, IL-12p40, IL-12p70, and IFN-γ by ELISA.
Results
DC-SIGN Is a Receptor for H. pylori and Binding of H. pylori to DC-SIGN Occurs via LPS.
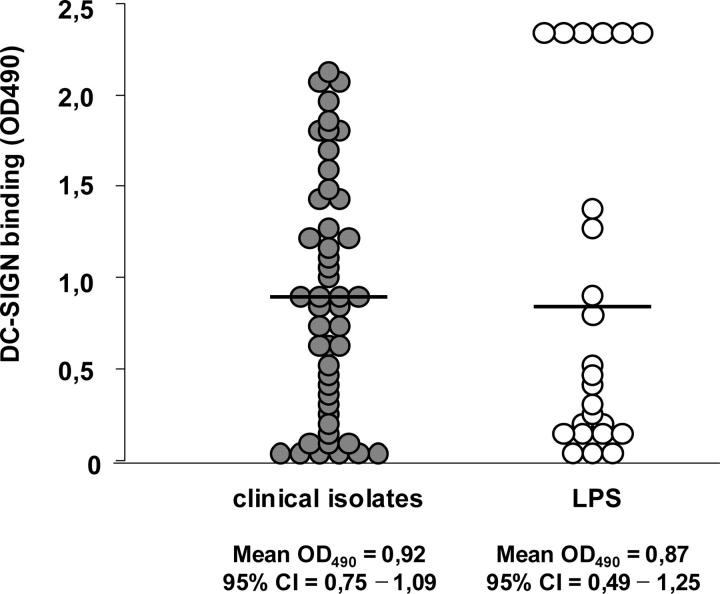
DC-SIGN binds Lex and Ley (28), so we investigated whether DC-SIGN functions as a receptor for H. pylori. Several clinical isolates of H. pylori from different continents (5) did bind to DC-SIGN fusion protein, which suggests that the H. pylori cell wall contains a common and universal ligand for DC-SIGN (Fig. 1). H. pylori binds DC-SIGN through its LPS, as was indicated by the binding of several batches of purified H. pylori LPS to DC-SIGN (Fig. 1). For 11 H. pylori strains, both whole bacterial cells and purified LPS were available, and the binding to DC-SIGN of whole cells and purified LPS correlates well (r = 0.8045; P < 0.0028; 95% confidence interval of 0.39–0.95; not depicted). The binding of H. pylori whole cells or purified LPS to DC-SIGN-Fc could be blocked by anti–DC-SIGN antibodies with mannan, or by chelating free Ca2+ ions with EGTA (not depicted), indicating that binding was specific.
Figure 1.
Clinically isolated H. pylori and purified LPS bind to DC-SIGN. H. pylori strains (•) isolated from patients from different continents (reference 5) and purified LPS (○) were coated on ELISA plates, and binding of recombinant DC-SIGN-Fc was assessed with peroxidase-labeled goat anti–human Fc.
H. pylori LPS Binds DC-SIGN through Le Antigens.
Because DC-SIGN binds carbohydrates, LPS Le antigens might be involved in the binding of H. pylori to DC-SIGN. The binding of synthetic blood group antigens to soluble DC-SIGN was studied. Lex and Ley bound to DC-SIGN (Fig. 2 B), confirming previous results (28, 34), as did Lea and Leb (not depicted). Surprisingly, H type 2, but not H type 1, bound to DC-SIGN (Fig. 2 B), even though these carbohydrate structures comprise identical monosaccharides, as they are linked at a different position (Fig. 2 A). In H. pylori LPS, Lex can be present in a polymeric form, i.e., repetitive copies of single (monomeric) Lex (up to n = 8–9) in a long chain. Polymeric Ley does not exist. Although monomeric Lex strongly bound to DC-SIGN, binding surprisingly decreased with an increasing number of copies of Lex (Fig. 2 C). Next, substructures of Le carbohydrates were tested for binding to DC-SIGN. First, single sugar residues were tested. l-fucose, but not d-galactose or N-acetyl-d-glucosamine, bound to DC-SIGN (Fig. 2 B). Moreover, fucose-containing disaccharides were analyzed to determine the substructure to which DC-SIGN binds (Fig. 2 B). Of the disaccharides studied, Fucα1-4GlcNAc (present on Ley) and, strikingly, Fucα1-2Galβ (shared by Ley, Leb, and H type 1; Fig. 2 A) also bound to DC-SIGN. Thus, although complete Le H type 1 did not bind to DC-SIGN, its substructure-containing fucose did bind. Hence, H. pylori binds DC-SIGN through Le antigens, but the steric organization of the sugar residues involved in the interaction with DC-SIGN is also important.
Binding of H. pylori to DC-SIGN Requires Functional Expression of Bacterial α3-Fucosyltransferase Genes.
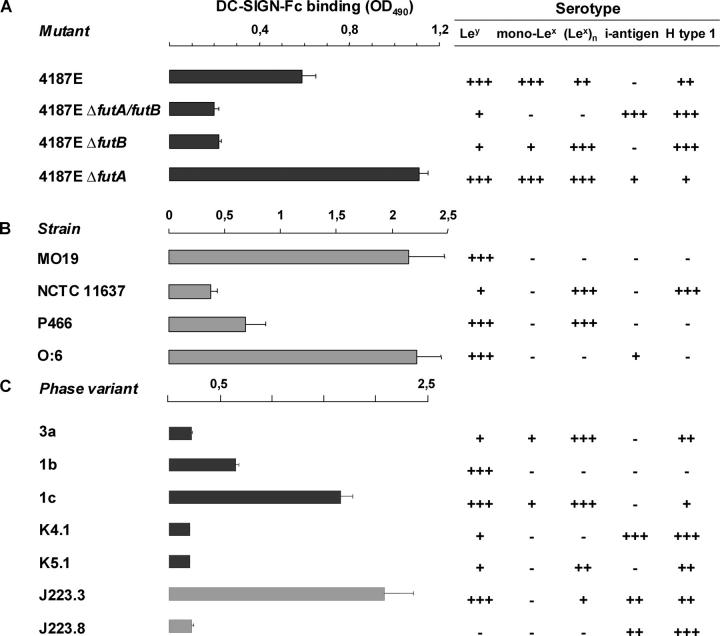
Biosynthesis of Lex/y antigens is dependent on the function of α3-fucosyltransferases FutA and FutB (10). We used H. pylori 4187E, its subsequent futA and futB mutants, and the futA/futB double knockout (10) to test our hypothesis that DC-SIGN binding to H. pylori cells is Lex/y dependent. Interruption of futA does not affect expression of mono Lex/y, whereas interruption of futB results in predominant expression of polymeric Lex only (Fig. 3 A). Disruption of both futA and futB yields mutants that almost completely lack Le expression (Fig. 3 A). Indeed, we found that although 4187E (that expresses Lex/y) did bind to DC-SIGN, futB and futA/B mutants that were disabled for expression of Ley and monomeric Lex did not bind to DC-SIGN (Fig. 3 A). Next, natural H. pylori strains with Lex/y serotypes corresponding to that of the 4187E futA/futB mutant strains were tested for binding to DC-SIGN. Strain NCTC 11637 (4) resembles the 4187E futB mutant by the predominant expression of polymeric Lex and as expected, LPS of NCTC 11637 did not bind to DC-SIGN (Fig. 3 B). H. pylori strains MO19, O:6, and P466 (3, 35), which represent the 4187E futA mutant phenotype and strongly express Ley, were all able to bind to DC-SIGN. We concluded that the binding of H. pylori to DC-SIGN depends on the expression of monomeric Lex and Ley, but not polymeric Lex.
Figure 3.
Binding of H. pylori is dependent on Le antigen expression. (A–C) H. pylori α3-fucosyltransferase mutants (A), strains (B), and phase variants (C) were coated, and binding of recombinant DC-SIGN-Fc was measured after incubation with peroxidase-labeled goat anti–human Fc. H. pylori were coated and incubated with mAbs specific for Le antigens indicated, and their serotype was determined after incubation with peroxidase-labeled anti–mouse Igs. The mean (+ SD) of three independent experiments is shown.
LPS Le Antigenic Phase Variation In Vitro within a Single Strain of H. pylori Affects Binding to DC-SIGN.
In a single H. pylori strain, reversible on-off switching of fucosyltransferases regulates the expression of Le antigens. This so-called phase variation results in Lex/y+ and Lex/y− bacteria within a single strain (9). Several LPS phase variants of NCTC 11637, which can be distinguished serologically, were tested for their binding to DC-SIGN (Fig. 3 C). As described above, LPS from NCTC 11637 expresses polymeric Lex only and did not bind to DC-SIGN (Fig. 2 C). LPS phase variants 1b and 1c, which during in vitro culture have turned on Ley expression, did bind to DC-SIGN (Fig. 3 C). In contrast, variants K4.1 and K5.1, which represent a switch back to the parental serotype and thus lack expression of Ley and monomeric Lex, did not bind to DC-SIGN (Fig. 3 C). Thus, the ability of NCTC 11637 to bind to DC-SIGN is regulated by phase variation during in vitro culture, i.e., on-off switching of Le antigen expression.
H. pylori LPS Phase Variation Drives Strain Diversification In Vivo, and Only Lex/y+ Variants of a Clinical Isolate Bind to DC-SIGN.
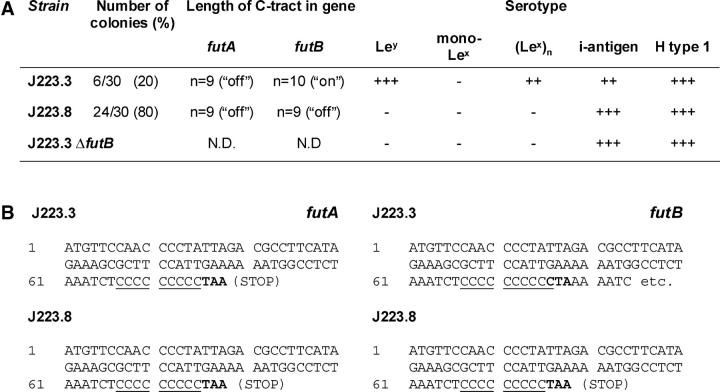
Phase variation in the host might determine the binding of H. pylori to DC-SIGN on DCs, but to date there is no evidence that phase variation occurs in vivo. We used a clinical isolate, H. pylori J223 (6), to demonstrate phase variation in the host. Chemical analysis previously revealed that J223 lacks expression of Lex/y (6), predicting that these bacteria do not bind to DC-SIGN, which indeed was the case (not depicted). However, in primary cultures from the biopsy, two variants of J223 could be detected by colony blotting in a ratio that could not result from in vitro phase variation during primary culture, as was concluded from the calculated frequencies of phase variation (<0.35%) and the time span of the experiment. This indicates that the two variants are derived from in vivo phase variation, i.e., phase variation has occurred in the stomach of the host. The predominant variant, J223.8, resembled the parent strain J223 and lacked expression of monomeric Lex and Ley, whereas the second variant, J223.3, did express Ley (Fig. 4 A). Sequencing of the α3-fucT gene C-tracts showed that both genes futA and futB are switched off in J223.8, but that futB was functionally expressed in variant J223.3, which is in agreement with the Le expression of these variants (Fig. 4 A). Interruption of futB in variant J223.3 resulted in a phenotype identical to that of variant J223.8 lacking Le antigens (Fig. 4 A), and switches from J223.3 to J223.8 and vice versa were observed on plate in vitro (not depicted). In addition, AFLP analysis revealed 96% homology between J223.3 and J223.8 that were cultured directly from the gastric biopsy, confirming that these variants are isogenic and not derived from two different strains (not depicted). Next, DC-SIGN binding of these two variants was analyzed (Fig. 3 C). Variant J223.3 was able to bind DC-SIGN, whereas J223.8 was not. Hence, we demonstrated that LPS phase variation in H. pylori occurs in vivo and yields populations that either bind DC-SIGN via Ley or escape binding to DC-SIGN by switching off Le expression.
Figure 4.
LPS phase variation in H. pylori occurs in vivo. (A) After a short time of culturing directly from the biopsy, followed by one single passage in fluid phase and distribution over solid media, Lex/y+ phase variants of J223 were detected by colony blotting with mAbs specific for the indicated Le antigens. C-tract sequencing was performed to determine the on and off status of genes futA (HP0379) and futB (HP0651). The J223.3 futB mutant was generated by natural transformation with construct containing a chloramphenicol resistance marker cassette inserted in gene HP0651, and serotyped as indicated for J223. (B) Consequences of the C-tract length for functional expression of futA and futB.
DCs Are Present in the Lamina Propria of the Stomach and Express DC-SIGN.
The binding of clinically isolated strains of H. pylori to soluble DC-SIGN indicates a possible function for this DC-specific pathogen receptor in vivo. Here, we demonstrate that DC-SIGN–expressing cells with a DC-like morphology were detected in the lamina propria of the stomach (Fig. 5 A, arrows). The distribution or number of DC-SIGN–expressing cells in H. pylori–infected individuals was not different from that observed in noninfected persons (not depicted). Interestingly, several DC-SIGN+ cells were located directly beneath the stomach epithelial cell layer. Considering the ability of DCs to protrude into the intestinal lumen by penetrating the epithelial cell layer (36), our results implicate that DC-SIGN–expressing DCs are situated in close proximity to the stomach lumen and could be involved in H. pylori capture in vivo.
Figure 5.
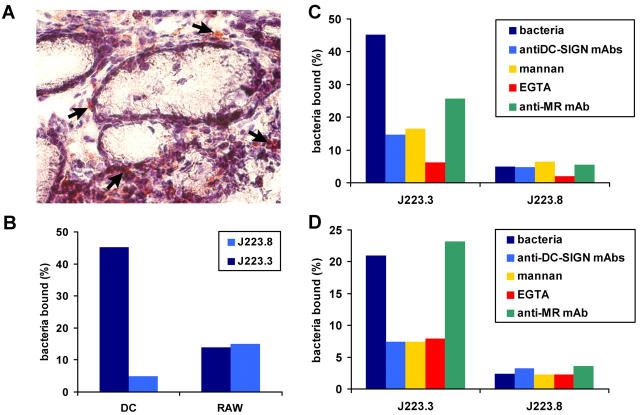
DC-SIGN is expressed on gastric DCs and is the major receptor for Le+ H. pylori. (A) A tissue section of stomach was fixed and stained with anti–DC-SIGN antibodies. A magnification of 20. Arrows indicate DC-SIGN+ DC-like cells in the lamina propria. (B–D) Monocyte-derived DCs and RAW 264 macrophages (B), monocyte-derived DCs (C), or K-562 cells transfected with DC-SIGN (D) were incubated with FITC-labeled H. pylori J223.3 or J223.8, and binding was analyzed using flow cytometry. In C and D, cells were preincubated with anti–DC-SIGN antibodies, mannan, EDTA, or anti-MR antibodies. One representative out of four experiments is shown.
DC-SIGN Is a Major Receptor on DCs for Lex/y+ H. pylori, whereas Lex/y− Isogenic Phase Variants Escape Binding.
To demonstrate that DC-SIGN–expressing cells could capture Lex/y+ H. pylori, we generated monocyte-derived DCs expressing DC-SIGN and studied their interaction with J223.3 and J223.8. Interestingly, the Ley+ variant J223.3 strongly bound to DCs, whereas J223.8 did not (Fig. 5 B). Preincubation of DCs with blocking antibodies against DC-SIGN or MR demonstrated that DC-SIGN was a major receptor for J223.3 H. pylori on DCs, whereas a part of J223.3 was bound by the C-type lectin MR (Fig. 5 C). These results are consistent with a recent study showing that Le clusters are specifically targeted to DC-SIGN, rather than MR (37). Macrophages lacking DC-SIGN bound J223.3 and J223.8 equally well, indicating that carbohydrate recognition of the Le antigens is the major determinant of the binding of H. pylori to DCs (Fig. 5 B). Specificity of DC-SIGN for Lex/y+ H. pylori was confirmed by the binding of J223.3 to DC-SIGN–transfected cells (K-SIGN; Fig. 5 D), but not to untransfected K562 cells (not depicted). Similarly to DCs, the binding of J223.3 to K-SIGN could be blocked using anti–DC-SIGN antibodies, mannan, or EGTA, showing that recognition is specific. As was shown for other ligands (23), J223.3 was internalized rapidly upon binding to DC-SIGN (not depicted). These results strongly suggest that DC-SIGN is an important pathogen receptor for Lex/y+ H. pylori on DCs and that bacteria can escape from recognition by DCs through phase variation, i.e., by changing their carbohydrate surface structures.
Binding to DC-SIGN Increases the H. pylori–mediated Production of IL-10.
Recognition of pathogens induces the up-regulation of costimulatory molecules on DCs and the production of cytokines to optimally induce protective T cell responses. DC-derived cytokines, especially IL-12, in combination with the interacting pathogen and cellular microenvironment, determine the differentiation of naive Th cells into Th1 or Th2 cells (38). To study the role of H. pylori–DC-SIGN interaction in the activation of DCs, these cells were cocultured with J223.3 or J223.8, and their phenotype and production of cytokines were analyzed. Both bacterial strains induced equal maturation, as assessed by expression of costimulatory molecules and maturation markers (not depicted). Moreover, blocking the binding of J223.3 to DC-SIGN did not change the maturation state of the DCs, indicating that DC-SIGN is not involved in maturation (not depicted). Other receptors, such as Toll-like receptor (TLR), which recognize distinct structures on pathogens and trigger DCs (39), might play a role in the observed DC maturation.
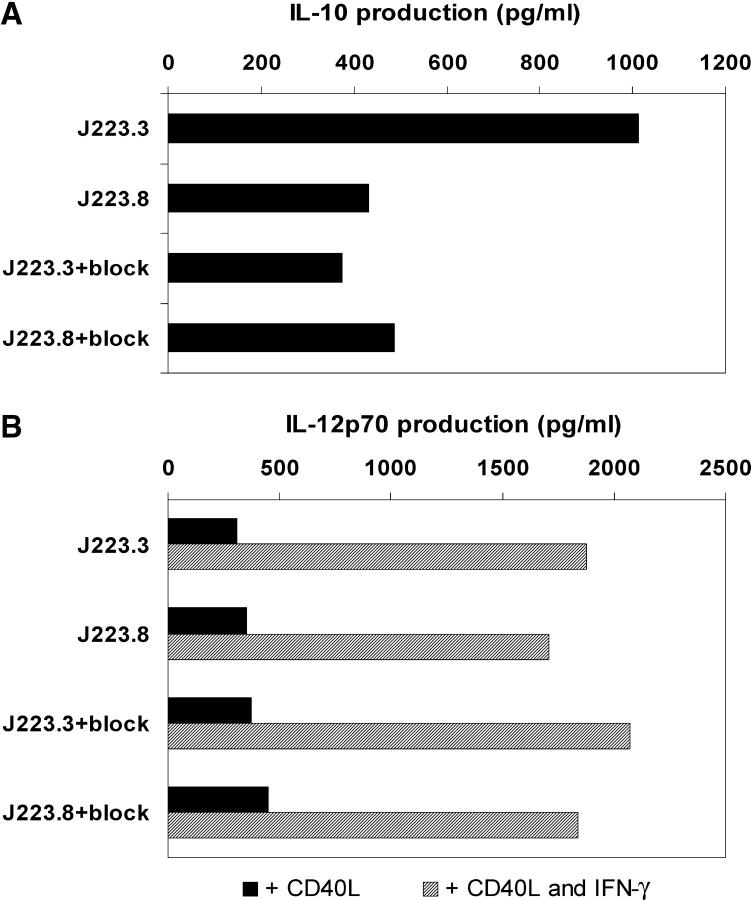
Analysis of the production of cytokines by DCs activated by the phase variants showed that the binding of H. pylori to DC-SIGN induces increased production of IL-10 at all MOIs tested. Strikingly, blocking the binding of J223.3 to DC-SIGN decreased the levels of IL-10 to approximately those induced by the non–DC-SIGN binder J223.8 (Fig. 6 A). Increased gastric mucosal IL-10 production has been reported in patients with chronic H. pylori infection, and is suggested to be protective, limiting tissue damage caused by inflammation, or to play a role in H. pylori persistence by damping the immune response against the bacterium (40). In vivo, other H. pylori proteins may influence DC production of IL-10 and IL-12 (41). Such proteins, however, are not likely to affect the cytokine production induced by the DC-SIGN binding and nonbinding bacteria, respectively, because we used isogenic phase variants of H. pylori that only differ in their Le expression. We observed no differences in IL-6 production that was produced in high amounts after challenge with DC-SIGN– and non–DC-SIGN–binding variants (not depicted). Although IL-12p40 was produced in our cultures (not depicted), no IL-12p70 could be detected. This could require additional activation of DCs by CD40 ligation, as occurs during interactions with T cells. Therefore, we mimicked the engagement by T cells by coculturing DCs with CD40L-transfected cells and measured IL-12p70 production. As shown in Fig. 6 B, no differences in secretion of bioactive IL-12 were observed upon DC activation by the two variants of H. pylori, not even in the presence of IFN-γ.
Figure 6.
Binding of H. pylori induces DC-SIGN–dependent increase of IL-10. (A) DCs were incubated with H. pylori J223.3 or J223.8 at an MOI of 20 in the presence or absence of anti–DC-SIGN antibodies for 1 h, washed, and cultured for 20 h. Supernatant was harvested and the amount of IL-10 was analyzed by ELISA. (B) Upon coculture of DCs with H. pylori J223.3 or J223.8, cells were incubated with CD40L-transfected J558 fibroblasts in the absence or presence of IFN-γ for 24 h. Supernatant was harvested and the amount of IL-12p70 was analyzed by ELISA. One representative experiment out of four is shown.
Binding of H. pylori to DC-SIGN Reduces Th1 Cell Induction of Naive T Cells by DCs.
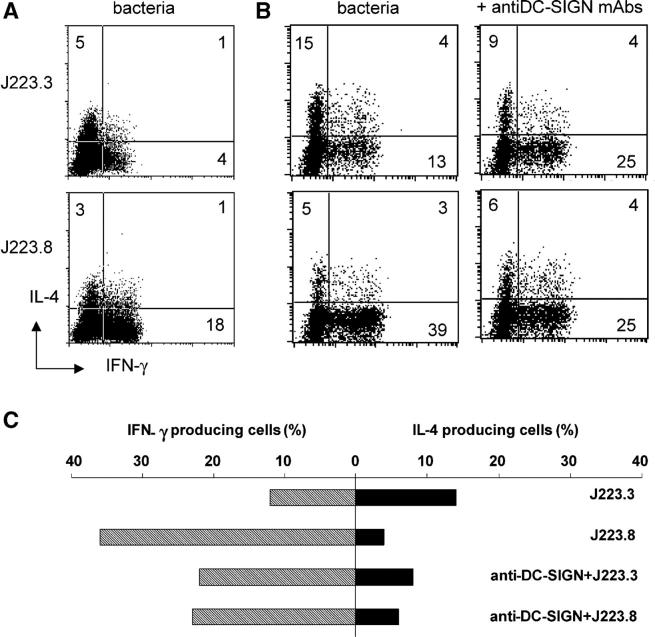
The cytokine profile of DCs that interacted with H. pylori through DC-SIGN did not demonstrate a specific polarization. In vivo, immature DCs encounter H. pylori at the mucosal surface and transport bacteria to lymph nodes for subsequent activation and differentiation of T cells. To mimic this, we primed DCs with either one of the H. pylori Le antigenic variants and subsequently cocultured washed DCs with naive T cells to analyze Th cell differentiation. The Th1/Th2 cell profiles of the resulting Th cells were assessed by measurement of intracellular IL-4 and IFN-γ production in individual T cells. Surprisingly, although J223.8-primed DCs gave rise to IFN-γ–producing Th cells, J223.3-primed DCs induced only low numbers of IL-4– and IFN-γ–producing Th cells (Fig. 7 A). This block of Th1 cell induction by DC-SIGN binding H. pylori was observed in most donors tested and was sometimes even accompanied by Th2 cell priming (Fig. 7 B, top). Strikingly, blocking the binding of J223.3 to DC-SIGN using specific antibodies resulted in a decreased percentage of IL-4–producing cells and an increased number of IFN-γ–producing Th cells (Fig. 7 B). Thus, prevention of J223.3 binding to DC-SIGN resulted in a shift from a mixed Th1/Th2 cell development to polarization of naive T cells toward a Th1 cell phenotype. As a control, we have added anti–DC-SIGN antibodies to other DC stimuli such as Escherichia coli LPS, showing that anti–DC-SIGN antibodies did not have an effect on skewing (not depicted). Our results indicate that recognition of Le antigens on H. pylori by DC-SIGN blocks the induction of a Th1 cell response and that phase variation modifies the Th cell polarization by DCs.
Figure 7.
Binding of H. pylori to DC-SIGN blocks skewing of naive T cells to Th1 cells. After preincubation with anti–DC-SIGN antibodies, DCs were incubated with H. pylori J223.3 or J223.8 at an MOI of 10 for 48 h, washed, and then cocultured with highly purified CD45RA+ CD4+ T cells. Quiescent T cells were restimulated with PMA and ionomycin, and IL-4 and IFN-γ was analyzed on a single cell basis by intracellular flow cytometry. Data from two representative donors (A and B/C) are shown from nine independent experiments.
Ex Vivo Challenge with DC-SIGN–binding H. pylori Reduces IL-6 Production in Gastric Biopsies.
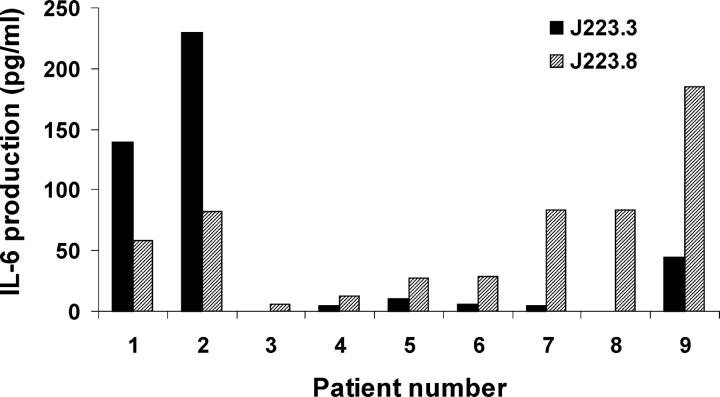
In our experiments so far, we used monocyte-derived DCs. These DCs might differ from DC-SIGN+ DCs in the stomach in their response to H. pylori. To mimic more closely the in vivo encounter with H. pylori, gastric biopsies were cocultured with DC-SIGN–binding or –nonbinding variants and supernatants, as well as homogenates that were analyzed for cytokine production. No cytokines were detected in the homogenates (not depicted). However, in most supernatants, low levels of IL-12p70 were detected, indicating that DCs were present in almost all biopsies (not depicted). In most patients, no significant differences were observed between the amounts of IL-12p70 produced upon challenge with either one of the variants of J223, consistent with our results with isolated DCs. No IL-10 production could be detected, nor secretion of IL-4 or IFN-γ, which is in agreement with previous reports (42). In contrast, when IL-6 was analyzed, in seven out of nine patients (patient numbers 3–9) higher levels of IL-6 were produced upon coculture with nonbinding H. pylori, as compared with DC-SIGN–binding bacteria (Fig. 8). The reason(s) for the higher IL-6 production of patients 1 and 2 in the presence of nonbinding H. pylori is unclear, but it may include host variability. Alternatively, in patient 1 a relapse of ulcerative colitis (3 mo before our study) might underlie the observed IL-6 response in this patient. In conclusion, DCs in gastric biopsies are activated by H. pylori ex vivo and DC-SIGN–binding variants induce reduced amounts of IL-6. Lower levels of IL-6 render T cells more sensitive to suppression (43), which implies that by targeting DC-SIGN, H. pylori can decrease T cell activation.
Figure 8.
Ex vivo challenge with DC-SIGN–binding H. pylori induces lower expression of IL-6 in stomach biopsies. Gastric biopsies were cocultured with 10 × 106 H. pylori J223.3 or J223.8 for 48 h and supernatants were harvested for analysis of cytokines, as indicated in Materials and Methods, by ELISA.
Discussion
The mechanisms by which H. pylori is able to persistently colonize half the population are unknown. Worldwide, H. pylori strains express LPS Le antigens and Le expression is subject to phase variation (44). Here, we demonstrate that H. pylori phase variation occurs in vivo and that H. pylori expressing monomeric forms of Le antigens specifically target the C-type lectin DC-SIGN on DCs. This interaction induces inhibition of Th1 cell differentiation, as compared with nonbinding phase variants. This is the first study that shows interactions between H. pylori and its natural host on the level of individual LPS phase variants derived from a single clinically isolated strain. Our data implicate for the first time a role for Le antigen expression and LPS phase variation in modulation of the host immune response that operates via the C-type lectin DC-SIGN.
H. pylori adds to the growing list of pathogens that are bound by DC-SIGN via common pathogen recognition patterns consisting of high mannose and/or Le carbohydrates (24–28, 34). Interestingly, several of these pathogens target DC-SIGN to modulate DC functions and escape immune activation (45). HIV uses DC-SIGN to target and internalize into DCs, but escapes antigen-processing routes to infect T cells (24). Recently, it has been demonstrated that the mycobacterial component ManLAM binds to DC-SIGN, subsequently suppressing TLR-4–mediated DC maturation and promoting IL-10 secretion by DCs (27). Here, we show that H. pylori targets to DC-SIGN by phase variation in its LPS. H. pylori–expressing Ley or short chains of Lex (monomeric Lex up to (Lex)4), but not polymeric Lex, are able to bind DC-SIGN. Therefore, it is the ability to bind DC-SIGN, rather than the expression of Le antigens, that enables H. pylori to modulate the immune response.
Distinct from ManLAM occupation of DC-SIGN, H. pylori did not change DC maturation, but production of IL-10 was increased (Fig. 6 A). Surprisingly, when analyzing naive T cell differentiation, the Le-containing H. pylori variants alter the T cell–differentiating capacity of DCs and block Th1 cell polarization, particularly by binding to DC-SIGN, resulting in a mixed Th1/Th2 cell response (Fig. 7). Interestingly, phase variants of H. pylori that have switched off fucosyltransferase FutB, resulting in loss of Le-containing determinants on their LPS, escape binding to DC-SIGN and induce a strong Th1 cell response. It is likely that in addition to H. pylori, other pathogens that target DC-SIGN also induce a shift in the Th1/Th2 cell balance. Interestingly, Schistosoma mansoni and Leishmania mexicana that also bind DC-SIGN both favor Th2, and not Th1, cell responses for chronic infections (34, 45).
To extend our in vitro data on monocyte-derived DCs and mimic the in vivo challenge of stomach DCs with H. pylori, stomach biopsies were used in which DC-SIGN was found to be expressed (Fig. 5 A). In addition, upon incubation of biopsies with bacteria, IL-12p70 was produced, indicating that DCs were indeed present. Clearly, stomach lamina propria DCs differ from monocyte-derived DCs in their cytokine profiles upon H. pylori encounter because no IL-10 could be detected. This finding seems to contradict a previously reported increase in gastric mucosal secretion of IL-10 in patients with H. pylori + chronic inflammation (40). However, the proposed sources of IL-10 in that study, i.e., mononuclear phagocytes or lymphocytes, are absent in H. pylori − gastric mucosa. Instead, in supernatants of ex vivo–challenged biopsies, IL-6 production was readily detected.
IL-6 is a proinflammatory cytokine that can be produced by DCs and many other cell types. In our ex vivo system, it cannot be determined which cells are responsible for IL-6 production, but interestingly, DC-SIGN binding by H. pylori reduced the amounts of IL-6 produced compared with the nonbinding variants. Although interaction between H. pylori LPS and selectins expressed on the vascular endothelium (46) may influence cytokine production, our results indicate that DCs, and more specifically DC-SIGN on DCs, are directly or indirectly involved in suppressing IL-6 secretion. Future studies challenging ex vivo biopsies in the presence of blocking anti–DC-SIGN antibodies combined with more sensitive cytokine detection methods, may reveal whether host receptors other than DC-SIGN are involved in immune modulation by H. pylori Le antigens.
Recently, IL-6 produced by TLR-activated antigen-presenting cells was shown to render T cells insensitive to suppression by regulatory T cells (T reg cells; 43). CD4+ CD25+ T reg cells have been shown to reduce immunopathology in H. pylori infection in mice by reducing the activation of Th1 cells (47). In our system, this would imply that upon binding to DC-SIGN in vivo, lower levels of IL-6 are produced, leading to increased T cell sensitivity to suppression. Although interesting, involvement of T reg cells in the clinical outcome of H. pylori infection in humans and the possible effect of bacterial Le phase variation on T reg cell function warrants further study. Further insight in the role of DC-SIGN in gastric pathology could be obtained in the future by comparing DC-SIGN binding capacity of the H. pylori strain(s) isolated, with disease severity by histological analysis (Sidney grading).
Pathogens are recognized by distinct classes of innate immune receptors and the combination of receptors triggered will determine the functional outcome on DCs. Several recent studies have demonstrated that cross-talk between C-type lectins and TLRs can occur (27, 48–50). Upon simultaneous recognition of zymosan by TLR-2 and the C-type lectin dectin-1, IL-12 and TNF-α production by DCs is induced by collaborative signaling of these two pathogen receptors (49). In contrast, upon binding of ManLAM, both DC-SIGN and MR deliver a negative signal that interferes with TLR-mediated DC maturation and IL-12 production (27, 48). Interestingly, a recent study has shown that binding of certain ligands to the MR can inhibit DC maturation and induce T reg cells, whereas other ligands failed to change DC functions (51). Thus, it would be of interest to study the role of the MR upon interaction with Le-expressing H. pylori; however, this falls outside the scope of this paper. Because intact H. pylori activates TLR-2 as well as TLR-4 (unpublished data), it is likely that these receptors mediate the observed DC maturation and cytokine induction. Our results indicate that H. pylori occupation of DC-SIGN modulates TLR-mediated activation of DCs, leading to a suppression of Th1 cell differentiation. Direct signal transduction upon ligand binding to DC-SIGN has not been proven yet, although the presence of an intracellular ITAM motif similarly to dectin-1 (52) implies that DC-SIGN is a signaling receptor. To date, DC-SIGN is the only C-type lectin expressed by DCs that recognizes Le antigens. The H. pylori phase variants used here that are completely identical except for Le carbohydrates would be instrumental to analyze interference of TLR signals by DC-SIGN in DCs.
Successful colonization by H. pylori requires a certain Th1/Th2 cell balance, which is determined by the genetic composition of the host (53). Panthel et al. (53) assessed the ability of H. pylori to colonize BALB/c (intrinsic Th2 cell responders) and C57BL/6J (an intrinsic Th1 cell responsive strain) wild-type mice, as compared with their IL-4 and IL-12 knockouts. Although disruption of IL-12 in BALB/c mice resulted in enhanced colonization, disruption of IL-4 in these mice has little effect. In contrast, colonization of C57BL/6J knockout mice deficient in either IL-4 or IL-12 was decreased. These findings support the idea that H. pylori would benefit from a mechanism to modulate the host Th1/Th2 cell balance during colonization, e.g., by phase-variable interaction with DC-SIGN, leading to suppression of Th1 cell responses, as we show here. The genetically very diverse human population might be considered one broad spectrum of potential hosts, in which individuals differ in their capacity to produce Th1 and Th2 cell cytokines, and the opposite ends of this spectrum are resembled by the murine C57BL/6J and the BALB/c genotypes, respectively. Our results suggest that H. pylori may suppress the local Th1 cell response through Le phase variation and thereby may create microenvironmental niches (53) with different Th1/Th2 cell balances within the stomach of a single host. In vivo, phase variation occurs within the human stomach (our results). By mechanisms that are still unknown, the host may positively select for the (mixture of) phase variants that meet the required Th1/Th2 cell balance for colonization of that particular host, resulting in selective outgrowth of one variant and persistent colonization.
Data on acute H. pylori infection are scarce, but in rhesus macaques acute H. pylori infection leads to a predominant Th1 cell response (21), which is concordant with the Th1 cell response found in association with gastric pathology in H. pylori–infected patients (54, 55). However, 80–90% of H. pylori–infected human individuals will never have symptoms (20). Although in humans peptic ulceration is associated with H. pylori–specific, local gastric predominant Th1 cell responses, the majority of gastric T cell clones reactive to H. pylori antigens in asymptomatic chronic gastritis are of the Th0 cell phenotype, secreting both Th1 and Th2 cell cytokines (56). What host genetic factors are involved in the shift from acute Th1 cell to a mixed Th1/Th2 cell response in chronic asymptomatic gastritis are currently unknown, leaving challenging topics for future research.
In conclusion, we demonstrated that H. pylori targets DC-SIGN to block a polarized Th1 cell response by phase-variable expression of Le antigens. Our results suggest a new and likely universal role for Le antigen expression and phase variation by H. pylori, and may help to explain the success of this pathogen to persistently colonize a large variety of genetically polymorphic hosts worldwide.
Acknowledgments
We are grateful to M.A. Monteiro for providing H. pylori LPS and R.R. Schmidt for making ceramide-linked (Lex)2-4 glycoconjugates. We thank M.M. Gerrits for AFLP analysis of the J223 phase variants.
A. Engering is supported by a grant from the Dutch Organization for Scientific Research (NWO grant no. 916.36.009).
The authors have no conflicting financial interests.
M.P. Bergman and A. Engering contributed equally to this work.
Abbreviations used in this paper: AFLP, amplified fragment length polymorphism; Le, Lewis; MOI, multiplicity of infection; MR, mannose receptor; TLR, Toll-like receptor; T reg cell, regulatory T cell.
References
- 1.Ernst, P.B., and B.D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615–640. [DOI] [PubMed] [Google Scholar]
- 2.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R.J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784–789. [DOI] [PubMed] [Google Scholar]
- 3.Aspinall, G.O., and M.A. Monteiro. 1996. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry. 35:2498–2504. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall, G.O., M.A. Monteiro, H. Pang, E.J. Walsh, and A.P. Moran. 1996. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen chain and core oligosaccharide regions. Biochemistry. 35:2489–2497. [DOI] [PubMed] [Google Scholar]
- 5.Simoons-Smit, I.M., B.J. Appelmelk, T. Verboom, R. Negrini, J.L. Penner, G.O. Aspinall, A.P. Moran, S.F. Fei, B.S. Shi, W. Rudnica, et al. 1996. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J. Clin. Microbiol. 34:2196–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro, M.A., K.H. Chan, D.A. Rasko, D.E. Taylor, P.Y. Zheng, B.J. Appelmelk, H.P. Wirth, M. Yang, M.J. Blaser, S.O. Hynes, et al. 1998. Simultaneous expression of type 1 and type 2 Lewis blood group antigens by Helicobacter pylori lipopolysaccharides. Molecular mimicry between H. pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. J. Biol. Chem. 273:11533–11543. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro, M.A., P. Zheng, B. Ho, S. Yokota, K. Amano, Z. Pan, D.E. Berg, K.H. Chan, L.L. MacLean, and M.B. Perry. 2000. Expression of histo-blood group antigens by lipopolysaccharides of Helicobacter pylori strains from Asian hosts: the propensity to express type 1 blood-group antigens. Glycobiology. 10:701–713. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro, M.A., B.J. Appelmelk, D.A. Rasko, A.P. Moran, S.O. Hynes, L.L. MacLean, K.H. Chan, F.S. Michael, S.M. Logan, J. O'Rourke, et al. 2000. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis X, and H. pylori UA915 expressing Lewis B classification of H. pylori lipopolysaccharides into glycotype families. Eur. J. Biochem. 267:305–320. [DOI] [PubMed] [Google Scholar]
- 9.Appelmelk, B.J., B. Shiberu, C. Trinks, N. Tapsi, P.Y. Zheng, T. Verboom, J. Maaskant, C.H. Hokke, W.E. Schiphorst, D. Blanchard, et al. 1998. Phase variation in Helicobacter pylori lipopolysaccharide. Infect. Immun. 66:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelmelk, B.J., S.L. Martin, M.A. Monteiro, C.A. Clayton, A.A. McColm, P. Zheng, T. Verboom, J.J. Maaskant, D.H. van den Eijnden, C.H. Hokke, et al. 1999. Phase variation in Helicobacter pylori lipopolysaccharide due to changes in the lengths of poly(C) tracts in alpha3-fucosyltransferase genes. Infect. Immun. 67:5361–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang, Q.L., and E.C. Gotschlich. 1996. Variation of gonococcal lipooligosaccharide structure is due to alterations in poly-G tracts in lgt genes encoding glycosyl transferases. J. Exp. Med. 183:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roche, R.J., and E.R. Moxon. 1995. Phenotypic variation of carbohydrate surface antigens and the pathogenesis of Haemophilus influenzae infections. Trends Microbiol. 3:304–309. [DOI] [PubMed] [Google Scholar]
- 13.van Putten, J.P., and B.D. Robertson. 1995. Molecular mechanisms and implications for infection of lipopolysaccharide variation in Neisseria. Mol. Microbiol. 16:847–853. [DOI] [PubMed] [Google Scholar]
- 14.Edwards, N.J., M.A. Monteiro, G. Faller, E.J. Walsh, A.P. Moran, I.S. Roberts, and N.J. High. 2000. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol. Microbiol. 35:1530–1539. [DOI] [PubMed] [Google Scholar]
- 15.Takata, T., E. el Omar, M. Camorlinga, S.A. Thompson, Y. Minohara, P.B. Ernst, and M.J. Blaser. 2002. Helicobacter pylori does not require Lewis X or Lewis Y expression to colonize C3H/HeJ mice. Infect. Immun. 70:3073–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahdavi, J., T. Boren, C. Vandenbroucke-Grauls, and B.J. Appelmelk. 2003. Limited role of lipopolysaccharide Lewis antigens in adherence of Helicobacter pylori to the human gastric epithelium. Infect. Immun. 71:2876–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth, H.P., M. Yang, R.M. Peek Jr., K.T. Tham, and M.J. Blaser. 1997. Helicobacter pylori Lewis expression is related to the host Lewis phenotype. Gastroenterology. 113:1091–1098. [DOI] [PubMed] [Google Scholar]
- 18.Heneghan, M.A., C.F. McCarthy, and A.P. Moran. 2000. Relationship of blood group determinants on Helicobacter pylori lipopolysaccharide with host lewis phenotype and inflammatory response. Infect. Immun. 68:937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasko, D.A., M. Keelan, T.J. Wilson, and D.E. Taylor. 2001. Lewis antigen expression by Helicobacter pylori. J. Infect. Dis. 184:315–321. [DOI] [PubMed] [Google Scholar]
- 20.Suerbaum, S., and P. Michetti. 2002. Helicobacter pylori infection. N. Engl. J. Med. 347:1175–1186. [DOI] [PubMed] [Google Scholar]
- 21.Mattapallil, J.J., S. Dandekar, D.R. Canfield, and J.V. Solnick. 2000. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 118:307–315. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 23.Engering, A., T.B. Geijtenbeek, S.J. van Vliet, M. Wijers, E. van Liempt, N. Demaurex, A. Lanzavecchia, J. Fransen, C.G. Figdor, V. Piguet, and Y. van Kooyk. 2002. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168:2118–2126. [DOI] [PubMed] [Google Scholar]
- 24.Geijtenbeek, T.B., D.S. Kwon, R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. Kewal Ramani, D.R. Littman, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587–597. [DOI] [PubMed] [Google Scholar]
- 25.Colmenares, M., A. Puig-Kroger, O.M. Pello, A.L. Corbi, and L. Rivas. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J. Biol. Chem. 277:36766–36769. [DOI] [PubMed]
- 26.Cambi, A., K. Gijzen, J.M. de Vries, R. Torensma, B. Joosten, G.J. Adema, M.G. Netea, B.J. Kullberg, L. Romani, and C.G. Figdor. 2003. The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur. J. Immunol. 33:532–538. [DOI] [PubMed] [Google Scholar]
- 27.Geijtenbeek, T.B.H., S.J. van Vliet, E.A. Koppel, M. Sanchez-Hernandez, C.M.J.E. Vandenbroucke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appelmelk, B.J., D. Van, S.J. van Vliet, C.M. Vandenbroucke-Grauls, T.B. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635–1639. [DOI] [PubMed] [Google Scholar]
- 29.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as an new tool in bacterial taxonomy. Microbiol. 142:1881–1893. [DOI] [PubMed] [Google Scholar]
- 30.Toepfer, A., and R.R. Schmidt. 1992. An efficient synthesis of the Lewis x (Lex) antigen family. Tetrahedron Lett. 33:5161–5164. [Google Scholar]
- 31.Geijtenbeek, T.B., R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, G.J. Adema, Y. van Kooyk, and C.G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 100:575–585. [DOI] [PubMed] [Google Scholar]
- 32.Geijtenbeek, T.B., G.C. van Duijnhoven, S.J. van Vliet, E. Krieger, G. Vriend, C.G. Figdor, and Y. van Kooyk. 2002. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J. Biol. Chem. 277:11314–11320. [DOI] [PubMed] [Google Scholar]
- 33.Smits, H.H., E.C. de Jong, J.H. Schuitemaker, T.B. Geijtenbeek, Y. van Kooyk, M.L. Kapsenberg, and E.A. Wierenga. 2002. Intercellular adhesion molecule-1/LFA-1 ligation favors human Th1 development. J. Immunol. 168:1710–1716. [DOI] [PubMed] [Google Scholar]
- 34.Van Die, I., S.J. van Vliet, N.A. Kwame, R.D. Cummings, C.M. Bank, B. Appelmelk, T.B. Geijtenbeek, and Y. van Kooyk. 2003. The dendritic cell specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis-x. Glycobiology. 13:471–478. [DOI] [PubMed] [Google Scholar]
- 35.Aspinall, G.O., M.A. Monteiro, R.T. Shaver, L.A. Kurjanczyk, and J.L. Penner. 1997. Lipopolysaccharides of Helicobacter pylori serogroups O:3 and O:6–structures of a class of lipopolysaccharides with reference to the location of oligomeric units of D-glycero-alpha-D-manno-heptose residues. Eur. J. Biochem. 248:592–601. [DOI] [PubMed] [Google Scholar]
- 36.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J.P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361–367. [DOI] [PubMed] [Google Scholar]
- 37.Frison, N., M.E. Taylor, E. Soilleux, M.T. Bousser, R. Mayer, M. Monsigny, K. Drickamer, and A.C. Roche. 2003. Oligolysine-based oligosaccharide clusters: selective recognition and endocytosis by the mannose receptor and DC-SIGN. J. Biol. Chem. 728:32922–32929. [DOI] [PubMed] [Google Scholar]
- 38.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. [DOI] [PubMed] [Google Scholar]
- 39.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science. 293:253–256. [DOI] [PubMed] [Google Scholar]
- 40.Bodger, K., J.I. Wyatt, and R.V. Heatley. 1997. Gastric mucosal secretion of interleukin-10: relations to histopathology, Helicobacter pylori status, and tumour necrosis factor-alpha secretion. Gut. 40:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voland, P., N. Hafsi, M. Zeitner, S. Laforsch, H. Wagner, and C. Prinz. 2003. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect. Immun. 71:3837–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, and A.M. Svennerholm. 2001. Induction of chemokine and cytokine responses by Helicobacter pylori in human stomach explants. Scand. J. Gastroenterol. 36:1022–1029. [DOI] [PubMed] [Google Scholar]
- 43.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 44.Appelmelk, B.J., M.A. Monteiro, S.L. Martin, A.P. Moran, and C.M. Vandenbroucke-Grauls. 2000. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 8:565–570. [DOI] [PubMed] [Google Scholar]
- 45.Kooyk, Y., B. Appelmelk, and T.B. Geijtenbeek. 2003. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 9:153–159. [DOI] [PubMed] [Google Scholar]
- 46.Galustian, C., N. Elviss, H. Chart, R. Owen, and T. Feizi. 2002. Interactions of the gastrotropic bacterium Helicobacter pylori with the leukocyte-endothelium adhesion molecules, the selectins–a preliminary report. FEMS Immunol. Med. Microbiol. 36:127–134. [DOI] [PubMed] [Google Scholar]
- 47.Raghavan, S., M. Fredriksson, A.M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477–7485. [DOI] [PubMed] [Google Scholar]
- 49.Gantner, B.N., R.M. Simmons, S.J. Canavera, S. Akira, and D.M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown, G.D., J. Herre, D.L. Williams, J.A. Willment, A.S.J. Marshall, and S. Gordon. 2003. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chieppa, M., G. Bianchi, A. Doni, A. Del Prete, M. Sironi, G. Laskarin, P. Monti, L. Piemonti, A. Biondi, A. Mantovani, et al. 2003. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 171:4552–4560. [DOI] [PubMed] [Google Scholar]
- 52.Engering, A., T.B. Geijtenbeek, and Y. van Kooyk. 2002. Immune escape through C-type lectins on dendritic cells. Trends Immunol. 23:480–485. [DOI] [PubMed] [Google Scholar]
- 53.Panthel, K., G. Faller, and R. Haas. 2003. Colonization of C57BL/6J and BALB/c wild-type and knockout mice with Helicobacter pylori: effect of vaccination and implications for innate and acquired immunity. Infect. Immun. 71:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bamford, K.B., X. Fan, S.E. Crowe, J.F. Leary, W.K. Gourley, G.K. Luthra, E.G. Brooks, D.Y. Graham, V.E. Reyes, and P.B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 114:482–492. [DOI] [PubMed] [Google Scholar]
- 55.D'Elios, M.M., M. Manghetti, M. De Carli, F. Costa, C.T. Baldari, D. Burroni, J.L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962–967. [PubMed] [Google Scholar]
- 56.D'Elios, M.M., M. Manghetti, F. Almerigogna, A. Amedei, F. Costa, D. Burroni, C.T. Baldari, S. Romagnani, J.L. Telford, and G. Del Prete. 1997. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur. J. Immunol. 27:1751–1755. [DOI] [PubMed] [Google Scholar]