Abstract
Interleukin (IL)-7 receptor (R) signaling is essential for T and B lymphopoiesis by promoting proliferation, differentiation, and survival of cells. Mice lacking either IL-7 or the IL-7Rα chain have abnormally low numbers of immature as well as mature T and B lymphocytes. Transgenic expression of the apoptosis inhibitor Bcl-2 rescues T cell development and function in IL-7Rα–deficient mice, indicating that activation of a proapoptotic Bcl-2 family member causes death of immature and mature T cells. BH3-only proteins such as Bim, which are distant proapoptotic members of the Bcl-2 family, are essential initiators of programmed cell death and stress-induced apoptosis. We generated Bim/IL-7Rα double deficient mice and found that loss of Bim significantly increased thymocyte numbers, restored near normal numbers of mature T cells in the blood and spleen, and enhanced cytotoxic T cell responses to virus infection in IL-7Rα−/− mice. These results indicate that Bim cooperates with other proapoptotic proteins in the death of IL-7–deprived T cell progenitors in vivo, but is the major inducer of this pathway to apoptosis in mature T cells. This indicates that pharmacological inhibition of Bim function might be useful for boosting immune responses in immunodeficient patients.
Keywords: apoptosis, Bim, Bcl-2, IL-7, T cells
Introduction
T and B lymphopoiesis depend on IL-7 and its cognate receptor, composed of the IL-7Rα chain and the γc chain, which also forms part of the receptors for IL-2, IL-4, IL-9, and IL-15 (1). Repeated injection of IL-7 into mice or IL-7 transgene expression promotes progressive lymphadenopathy (2, 3). Conversely, mice lacking IL-7 or IL-7Rα have abnormally reduced numbers of immature as well as mature T and B lymphocytes (4, 5). Mice lacking the γc chain have an even more severe lymphopoenia than IL-7 −/− or IL-7Rα−/− mice (6), most likely because cytokines in addition to IL-7, which require γc for signaling, also contribute to lymphopoiesis.
Cytokine signaling promotes cell proliferation, cell differentiation, and cell survival, but in many settings it is unclear which one of these processes is the most critical. In the case of IL-7R signaling, it appears that in T cell development, inhibiting apoptosis is the most critical function because expression of a Bcl-2 transgene restored normal numbers of thymocytes and peripheral T cells in IL-7Rα−/− mice (7, 8). In the B lymphoid lineage, Bcl-2 transgene expression caused an approximately threefold increase in peripheral B cells in IL-7Rα−/− mice, but B cell precursors in bone marrow remained abnormally low (9). This indicates that for B lymphopoiesis, IL-7R signaling is more important for cell proliferation and differentiation than for inhibiting apoptosis.
BH3-only proteins are a proapoptotic subgroup within the Bcl-2 protein family that share with their relatives only the short BH3 (Bcl-2 homology) domain, which is required for protein–protein interaction and proapoptotic activity. In mammals this group includes Bad, Bik/Blk/Nbk, Hrk/DP5, Bid, Bim/Bod, Noxa, Bmf, and Puma/Bbc3 (10). All of these proteins can bind to at least some of their antiapoptotic Bcl-2–like relatives (10). Biochemical and genetic experiments have demonstrated that BH3-only proteins initiate apoptosis signaling, whereas Bax/Bak-like proteins, the second proapoptotic subgroup within the Bcl-2 family, are required further downstream for cell death (10).
Experiments with knockout mice have identified the essential functions of BH3-only proteins as well as those of Bax/Bak. Bim is required for cytokine withdrawal–induced apoptosis of lymphoid and myeloid cells (11), for negative selection of autoreactive T and B cells (12–14), and for termination of T cell immune responses (15, 16). Although cells lacking single BH3-only proteins have specific resistance to certain apoptotic stimuli but not others, lymphocytes from Bax −/− Bak −/− mice are refractory to all Bcl-2–regulated apoptosis signaling (17), consistent with the model that BH3-only proteins function in distinct signaling pathways that converge upon Bax/Bak.
Because Bcl-2 overexpression rescues T cell development and function in IL-7Rα−/− mice (7, 8), it appears likely that activation of one or several BH3-only proteins is involved in the death of T lymphoid cells in IL-7Rα−/− mice. Bim is a candidate for this function because its loss inhibits IL-7 deprivation–induced apoptosis in resting (11) as well as activated T cells (16). Therefore, we generated Bim −/− IL-7Rα−/− mice and found that in the absence of IL-7Rα, loss of Bim could slightly augment T cell production in the thymus and restore near normal numbers of mature T cells in the spleen, which were capable of mounting a cytotoxic response to viral infection. These results indicate that Bim cooperates with other proapoptotic proteins in the death of IL-7–deprived T cell progenitors in vivo, but is the major inducer of this pathway to apoptosis in mature T cells.
Materials and Methods
Mice and In Vivo Challenge with HSV.
The generation of Bim −/− (11) and IL-7Rα−/− mice (The Jackson Laboratory; reference 4) has been described. Protocols for genotyping of the animals by PCR will be provided upon request. Experiments in which mice were infected with HSV-1 to measure CTL responses in vivo were performed as described previously (16), according to the guidelines of the Melbourne Directorate Animal Ethics Committee.
Immunofluorescent Staining and Cell Sorting.
Immunofluorescent staining, FACS analysis, and cell sorting were performed as described previously (18).
Tissue Culture and Mitogenic T Cell Activation In Vitro.
Activation of T cells in culture was performed as described previously (9).
Online Supplemental Material.
Fig. S1 shows FACS analysis of thymocytes from 6-wk-old WT, IL-7Rα−/−, Bim −/−, and Bim −/− IL-7Rα−/− mice stained with fluorochrome-conjugated antibodies to CD4 and CD8. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20041328/DC1.
Results and Discussion
To examine the role of Bim in the death of T and B cells in IL-7Rα−/− mice, we generated Bim −/− IL-7Rα−/− mice by intercrossing of the parental strains. To minimize the risk of having background genes affecting the phenotype, both parental strains were first backcrossed for >12 generations onto the C57BL/6 background. 6–10-wk-old WT, IL-7Rα−/−, Bim −/−, and Bim −/− IL-7Rα−/− mice were killed to measure their thymus and spleen cellularity, and subcellular composition was determined by immunofluorescent staining with surface marker–specific antibodies and flow cytometric analysis. All IL-7Rα−/− mice had an abnormally small thymus containing between 2 and 10% of the number of cells found in WT animals (Fig. 1). The Bim −/− IL-7Rα−/− mice had between three- to fivefold more thymocytes than the IL-7Rα−/− littermates (Fig. 1). All four major thymocyte subsets, CD4−8− pro-T, CD4+8+ immature thymocytes, and CD4+8− as well as CD4−8+ mature T cells, were present in IL-7Rα−/− mice, albeit all at abnormally low numbers (Fig. 1 and Fig. S1, which is available at http://www.jem.org/cgi/content/full/jem.20041328/DC1), consistent with the notion that IL-7R signaling is not essential for T cell differentiation, but required for normal magnitude of T cell production. Loss of Bim increased the numbers of all four thymocyte subsets in IL-7Rα−/− mice by ∼3- (for CD4+8+) to 10-fold (for mature CD4+8− and CD4−8+ thymocytes; Fig. 1). IL-7Rα−/− mice also have a severe deficit (5–10-fold fewer than WT animals) in mature CD4+8− and CD4−8+ T cells in the spleen and other peripheral lymphoid organs. Although loss of Bim increased thymic cellularity only by a relatively small extent, Bim −/− IL-7Rα−/− mice had normal or near normal numbers of mature CD4+8− and CD4−8+ T cells in their spleen (Fig. 1) and blood (not depicted). This indicates that Bim plays a role in the apoptosis of IL-7R signaling–deprived T cells during development in the thymus and in peripheral lymphoid organs. Because Bim −/− IL-7Rα−/− mice had almost normal numbers of splenic T cells, it appears that no other proapoptotic factor can compensate for Bim in their death. In contrast, Bim −/− IL-7Rα−/− mice had much fewer thymocytes than WT or Bcl-2 transgenic IL-7Rα−/− mice, indicating that IL-7R signaling blocks the proapoptotic function not only of Bim, but also of other BH3-only proteins. Puma and Bad are candidates for this function because Puma loss renders certain hemopoietic cells refractory to cytokine withdrawal (19, 20) and because Bad function has been reported to be regulated by growth factors (10). Most likely, all BH3-only protein–induced apoptosis elicited by loss of IL-7R signaling requires Bax and Bak because partial rescue of T cell production in IL-7Rα−/− mice was achieved by Bax deficiency (21). It is interesting to speculate why Bim −/− IL-7Rα−/− mice have almost normal numbers of splenic T cells despite having an abnormally small thymus. Because we have not observed any evidence for enhanced proliferation of mature T cells in Bim −/− IL-7Rα−/− mice, increased accumulation of mature T cells compared with IL-7Rα−/− mice is probably a consequence of enhanced thymic output plus increased survival in peripheral lymphoid organs.
Figure 1.
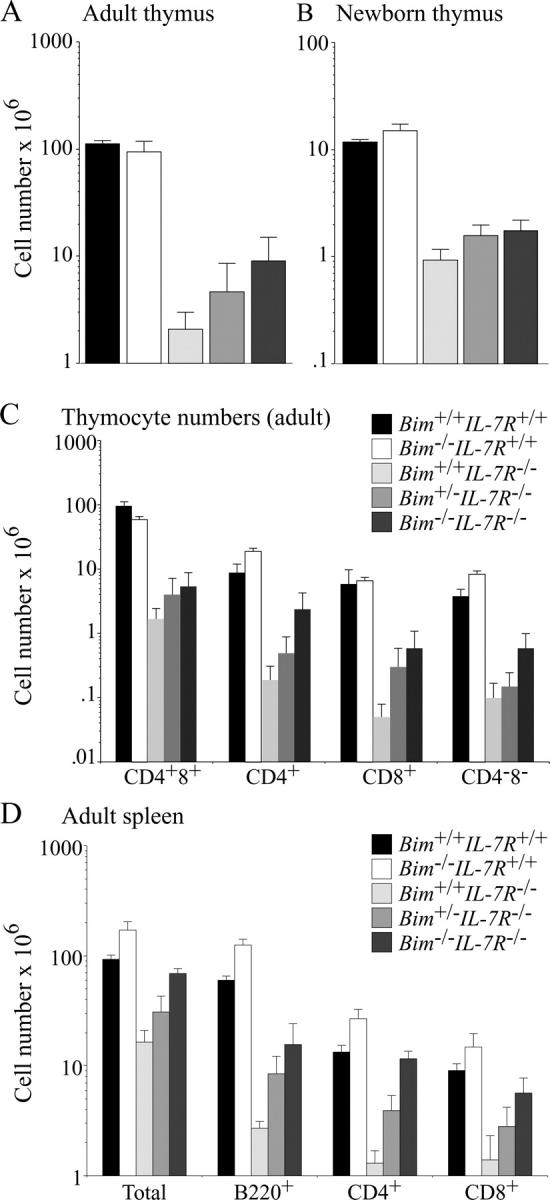
Loss of Bim increases thymus cellularity and numbers of splenic T and B cells in IL-7Rα−/− mice. 6–10-wk-old (A, C, and D) or newborn (B) WT, Bim −/−, IL-7Rα−/−, Bim +/− IL-7Rα−/−, and Bim −/− IL-7Rα−/− mice were killed to determine total leukocyte numbers in the thymus (A, B, and C) and spleen (D). Cell subset composition was determined by immunofluorescent staining with surface marker–specific antibodies (CD4 and CD8 for T cells and B220, CD19, and sIg for B cells) followed by flow cytometric analysis. Data are presented as means ± SD of at least five mice of each genotype.
IL-7R signaling plays a critical role at the pro–T2 and pro–T3 stages of T cell differentiation (4, 5), and the defect caused by IL-7Rα loss can be rescued by Bcl-2 overexpression (8). We investigated the impact of Bim deficiency on early T lymphopoiesis in IL-7Rα−/− mice by immunofluorescent staining of thymocytes with antibodies to CD25 and CD44 to identify pro–T1 (CD25−44+), pro–T2 (CD25+44+), pro–T3 (CD25+44−), and pro–T4 (CD25−44−) subsets. As reported previously (4), adult and neonatal IL-7Rα−/− mice had a striking deficit (∼40–80-fold reduction compared with WT mice) in pro–T2 and pro–T3 cells (Fig. 2, A and B). Loss of Bim increased the numbers of pro–T2 and pro–T3 cells in newborn IL-7Rα−/− mice by approximately fivefold (Fig. 2 B), providing a likely explanation for the increase in total thymocyte numbers found in Bim −/− IL-7Rα−/− mice compared with IL-7Rα−/− mice (Fig. 1). Adult Bim −/− IL-7Rα−/− mice had no significant increase in pro–T2 and pro–T3 cells compared with IL-7Rα−/− littermates (Fig. 2 A). Remarkably, adult Bim −/− mice had approximately fourfold fewer pro–T2 and pro–T3 cells compared with WT animals (Fig. 2 A). For the following reasons we speculate that accumulation of mature T cells suppresses T lymphocyte production in the thymus through an unknown negative feedback mechanism. We have previously found that not only pro–T cell numbers, but also the numbers of immature CD4+8+ thymocytes were abnormally low in Bim −/− mice (11) and similar effects were seen by others in Bax −/− Bak −/− double knockout mice (17). In both knockout strains, reduced thymic T cell production was only seen in adult, but not newborn animals, indicating that this negative feedback is activated by the build-up of excess mature T cells. Because we observed low numbers of pro–T2 and pro–T3 cells in adult Bim −/− IL-7Rα−/− mice, which have more mature T cells than IL-7Rα−/− mice, but not more than WT mice, it might be the ratio of mature T cells to pro–T cells and not the absolute numbers of mature T cells that elicits this proposed negative feedback.
Figure 2.
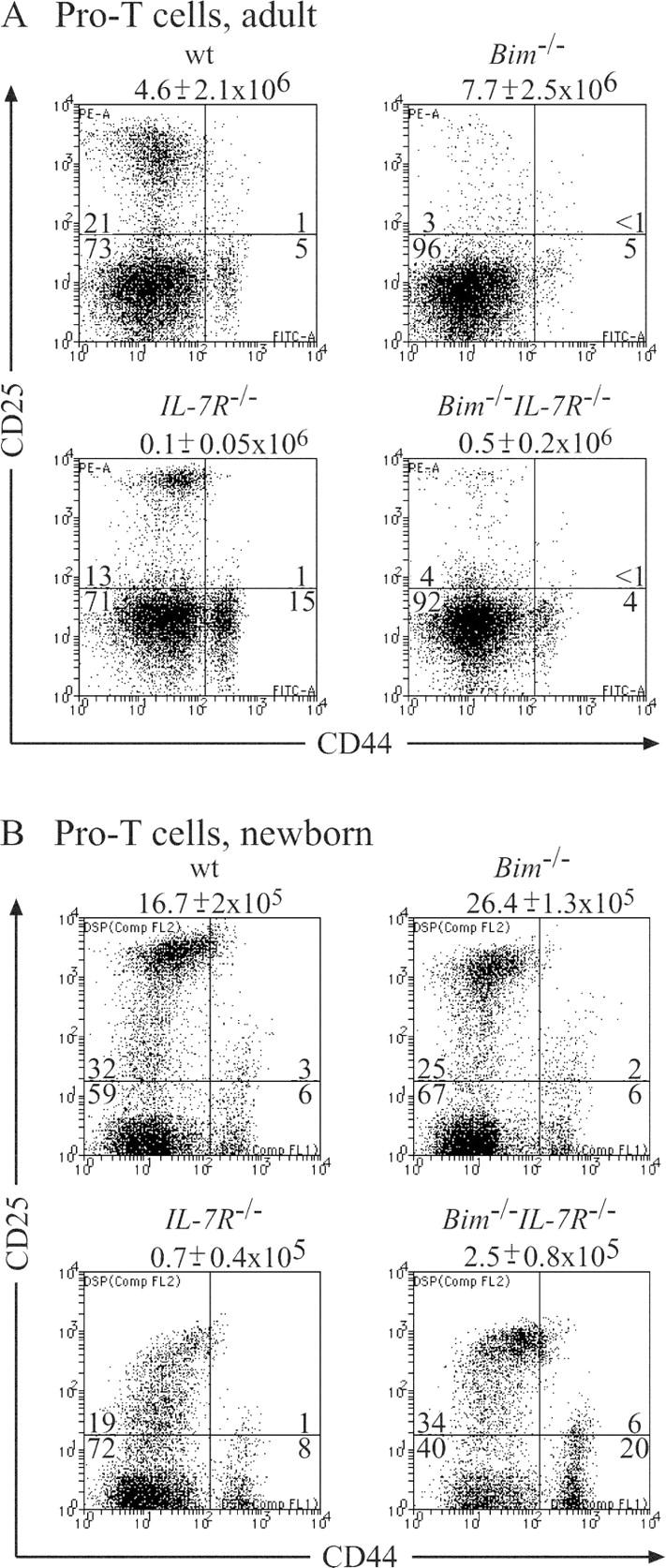
Effect of loss of Bim on the numbers of pro–T cells (CD3−4−8−) in the thymus of IL-7Rα−/− mice. 6–10-wk-old (A) or newborn (B) WT, IL-7Rα−/−, Bim −/−, and Bim −/− IL-7Rα−/− mice were killed and thymocytes were stained with Tricolor streptavidin and a cocktail of biotinylated antibodies to exclude immature CD4+8+ thymocytes, mature CD4+8− and CD4−8+ T cells, B cells, myeloid and erythroid cells, plus a Cy-5–labeled antibody to Thy1 to gate on T lymphoid cells. Pro–T cells were visualized by staining with a PE-labeled anti-CD25 antibody and a FITC-labeled anti-CD44 antibody. Total numbers of thymocytes are listed above the dot plots and the percentages of cells in each of the quadrant are indicated. Data shown are representative of at least five mice of each genotype.
IL-7R signaling is critical for the survival of resting as well as antigen-activated mature T lymphocytes (4, 5), and defects in mature T cell function caused by IL-7Rα deficiency can be rescued by Bcl-2 overexpression (7, 8). Because loss of Bim restored near normal numbers of mature CD4+8− and CD4−8+ T cells in IL-7Rα−/− mice, we investigated the ability of these T cells to function in an immune response. First, we stimulated purified T cells in culture with plate-bound mitogenic antibodies to CD3 alone or with anti-CD28 antibodies and measured proliferation by [3H]thymidine incorporation. As reported previously (4), IL-7Rα−/− CD4+8− and CD4−8+ T cells could proliferate in culture, but to a lesser extent than WT T cells, particularly when low concentrations of anti-CD3 antibodies were used. Loss of Bim enhanced proliferation of IL-7Rα−/− T cells significantly under all stimulatory conditions studied (not depicted).
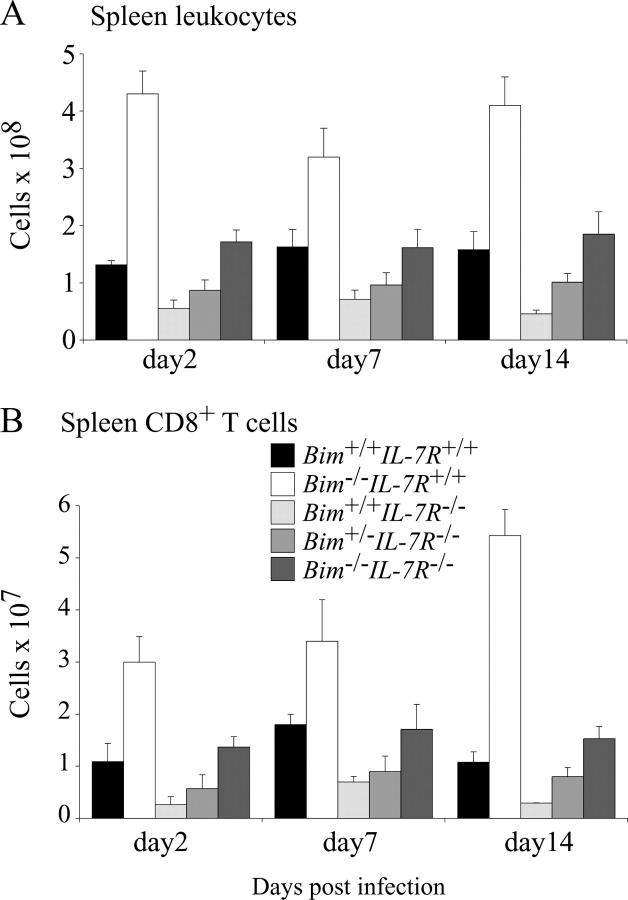
The impact of IL-7Rα deficiency on physiological T cell immune responses in vivo has not yet been studied in detail. Therefore, we infected WT, Bim −/−, IL-7Rα−/−, Bim +/− IL-7Rα−/−, and Bim −/− IL-7Rα−/− mice with HSV-1 by injection into both hind feet. CTL responses were measured after 2, 7, and 14 d by two methods that yielded comparable results. Spleen cells were stained with antibodies to CD8 plus PE-conjugated MHC class I tetrameric complexes incorporating the gB498–505 HSV glycoprotein peptide (SSIEFARL), the major epitope from HSV recognized by CD8+ T cells (16). Alternatively, splenic T cells were stimulated in culture with the gB498–505 peptide (or as a control with Kb-OVA257–264 peptide SIINFEKL) followed by intracellular staining for IFN-γ (16). Efficiency of viral clearance in these animals was tested by measuring HSV titers in their hind feet. Fig. 3 shows that at all time points after infection, total numbers of leukocytes, and CD4−8+ T cells were abnormally low in IL-7Rα−/− mice. In contrast, Bim −/− IL-7Rα−/− mice had normal numbers of these cells and, remarkably, even loss of a single Bim allele caused an increase of these populations (Figs. 3, A and B). As shown previously (16), infected WT mice produced substantial numbers of HSV-specific CTLs that were found first in the draining popliteal lymph nodes and later also in the spleen and other lymph nodes. The numbers of these CTLs peaked on day 7 after infection and by day 14, declined to ∼15% of peak levels (Figs. 4 and 5). Only a very small number (∼20-fold fewer compared with WT mice) of HSV-specific CTLs could be found on day 7 in IL-7Rα−/− mice and by day 14, no signal above background was detected (Figs. 4, A and B, and 5, A and B). In contrast, readily identifiable numbers of HSV-specific CTLs, reaching 50% of those seen in WT animals, were found on day 7 after infection in Bim −/− IL-7Rα−/− mice (Figs. 4, A and B, and 5, A and B). Unlike in WT mice, numbers of HSV-specific CTLs did not decline in the Bim −/− IL-7Rα−/− mice in which peak levels were still found on day 14 (Fig. 4 B). It is likely that this is a consequence of extended survival of antigen-activated CTLs because prolonged CTL survival was also seen in HSV-infected Bim −/− mice (16), but protracted antigenic stimulation cannot be ruled out as a cause (see below). Loss of even a single allele of Bim enhanced the CTL response to HSV in IL-7Rα−/− mice (Fig. 4 B), consistent with the increased basal numbers of T cells found in Bim +/− IL-7Rα−/− mice (Fig. 1). We have shown previously that loss of Bim can rescue T cell production and function in Bcl-2–deficient mice (22). Because of these observations and because IL-7R signaling increases Bcl-2 expression (23), we speculate that Bim loss promotes T cell accumulation and function in IL-7Rα−/− mice by allowing cells with abnormally low Bcl-2 levels, which would otherwise be killed by a Bim-dependent mechanism, to survive. When we analyzed HSV titers in the hind feet of infected mice, we found that only WT animals cleared the infection rapidly (by day 7), whereas animals of all other genotypes needed 14 d (Fig. 5 C). This raises two questions. How do IL-7Rα−/− mice clear HSV and why do Bim −/− IL-7Rα−/− mice take the same time for viral clearance as IL-7Rα−/− mice, even though they have much higher numbers of HSV-specific CTLs? First, IL-7Rα−/− T cells can proliferate in response to mitogenic stimulation, albeit less well than WT T cells (see above), and a small number of HSV-specific CTLs can be found in infected IL-7Rα−/− mice (Fig. 4, A and B). Therefore, we speculate that this diminished CTL response is still capable of eradicating HSV in this infection model. The alternative explanation, that cells other than lymphocytes surmount the infection, appears unlikely in view of the observation that B– and T cell–deficient RAG-1−/− mice die from this infection (Carbone, F., personal communication). Second, we believe that the severe reduction in lymph node cellularity and abnormal architecture not only found in IL-7Rα−/−, but also in Bim −/− IL-7Rα−/− mice (4 and not depicted), explains why HSV clearance is slow in the latter despite production of substantial numbers of specific CTLs in the spleen. It appears likely that for rapid HSV eradication in the feet, activation of a CTL response in the local lymph nodes is critical.
Figure 3.
Total numbers of leukocytes and CD4−8+ splenic T cells in HSV-infected WT, Bim −/−, IL-7Rα−/−, Bim +/− IL-7Rα−/−, and Bim −/− IL-7Rα−/− mice. Control WT, Bim −/−, IL-7Rα−/−, Bim +/− IL-7Rα−/−, and Bim −/− IL-7Rα−/− mice were infected with HSV by injection into both hind feet. On days 2, 7, and 14 after infection, total numbers of leukocytes (A) and CD4−8+ T cells (B) in the spleen were determined by cell counting combined with immunofluorescent staining with surface marker–specific antibodies and FACS analysis. Data shown represent means ± SD of at least three mice of each genotype.
Figure 4.
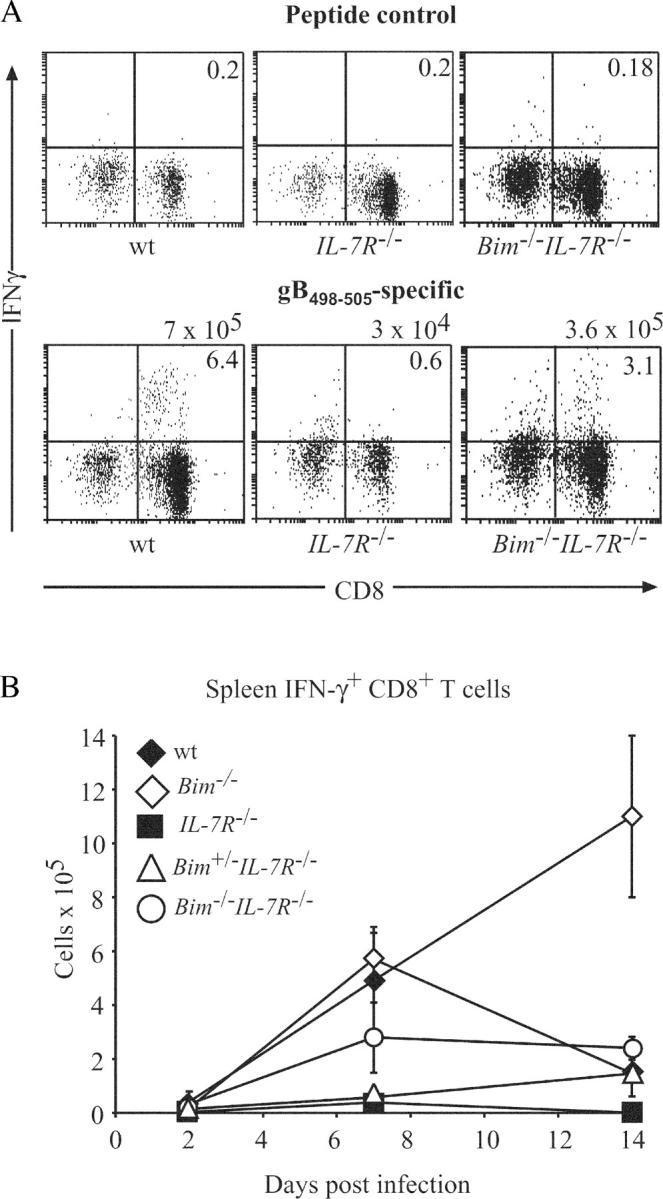
Loss of Bim enhances CTL immune responses to HSV in IL-7Rα−/− mice. Control WT, Bim −/−, IL-7Rα−/−, Bim +/− IL-7Rα−/−, and Bim −/− IL-7Rα−/− mice were infected with HSV by injection into both hind feet. (A) On days 2, 7, and 14 after infection, HSV-specific CTLs in the spleen were enumerated by stimulating cells in culture with the gB498–505 peptide (the major epitope from HSV recognized by CTLs in C57BL/6 mice) or as a control with Kb-OVA257–264 peptide SIINFEKL, followed by intracellular staining for IFN-γ. Total numbers of HSV-specific CTLs are listed above the dot plots and the percentages of CD8+ IFN-γ+ T cells are indicated in the top right quadrants. (B) The means ± SD of such analyses using at least three mice of each genotype at each time point are shown.
Figure 5.
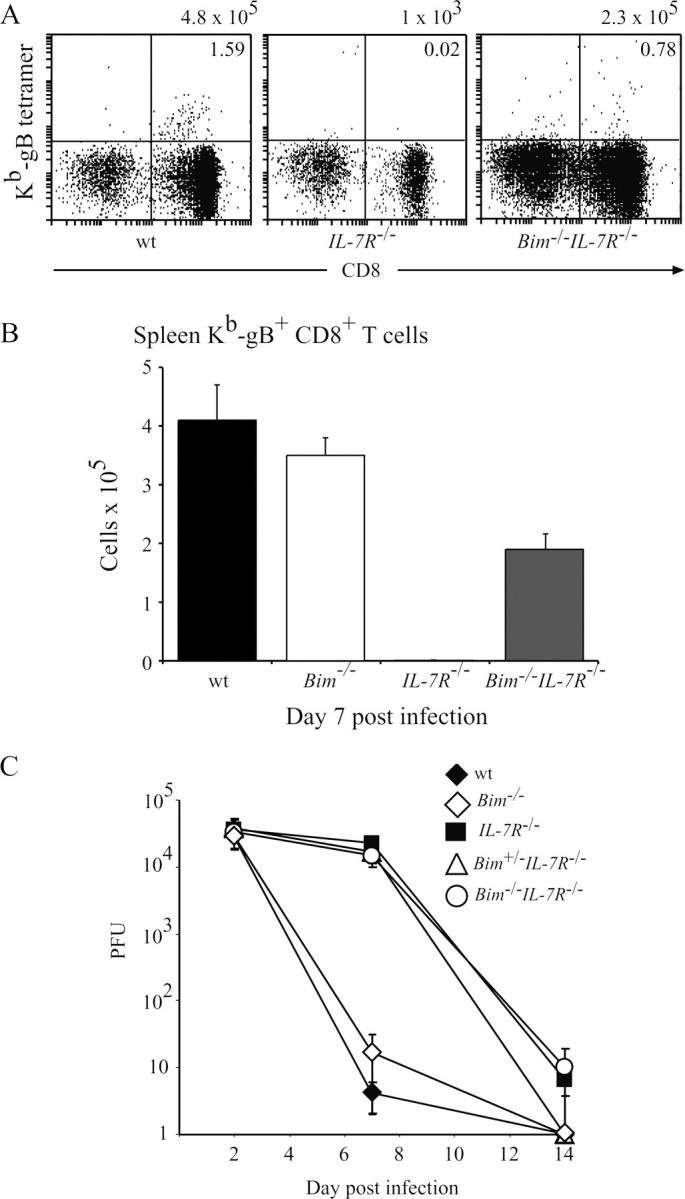
Loss of Bim enhances CTL immune responses to HSV in IL-7Rα−/− mice, but does not accelerate viral clearance. Control WT, Bim −/−, IL-7Rα−/−, Bim +/− IL-7Rα−/−, and Bim −/− IL-7Rα−/− mice were infected with HSV by injection into both hind feet. (A) On day 7 after infection, HSV-specific CTLs in the spleen were enumerated by staining with antibodies to CD8 plus PE-conjugated MHC class I tetrameric complexes incorporating the gB498–505 HSV glycoprotein peptide SSIEFARL, the major epitope from HSV recognized by CD8+ T cells. Total numbers of HSV-specific CTLs are listed above the dot plots and the percentages of CD8+ T cells binding to gB498–505 HSV presented by MHC class I are indicated in the top right quadrants. (B) The means ± SD of such analyses using at least three mice of each genotype are shown. (C) The kinetics of viral clearance in the infected mice as determined by viral plaque assays on extracts from feet are shown. Data shown represent means ± SD of viral PFUs for both feet from at least three mice of each genotype for each time point.
An approximately threefold increase in mature splenic B cells was observed in Bim −/− IL-7Rα−/− mice compared with IL-7Rα−/− mice (Fig. 1), but similar deficits in B cell precursors were found in the bone marrow of animals of both genotypes (not depicted). This phenotype is similar to that observed in the B lineage of Bcl-2 transgenic IL-7Rα−/− mice (9), indicating that the critical function of IL-7R signaling in B lymphopoiesis is to promote cell division and inhibiting apoptosis is subsidiary. The accumulation of mature B cells in Bim −/− IL-7Rα−/− mice is probably due to increased survival of cells at later stages of development.
In summary, our results show that Bim plays a role in the death of T lymphoid cells deprived of IL-7R signaling in vivo during development in the thymus, and even more prominently at the mature stage in peripheral lymphoid organs and during immune responses. This may indicate that the ability of IL-7 therapy to restore immune responses in certain experimental models of immunodeficiency (24) may, at least in part, be due to inhibition of Bim-mediated T cell apoptosis. Consequently, like the proposed IL-7 therapy (24), therapies designed to lower Bim expression levels, for example by using RNA interference based technology, might be a useful approach for treating immunodeficient patients.
Acknowledgments
We are grateful to Profs. J. Adams and S. Cory for the use of Bim-deficient mice. We thank J. Morrow and K. Pioch for animal care, Drs. F. Battye, V. Lapatis, C. Tarlinton, D. Kaminaris, and C. Clark for cell sorting, Profs. J. Adams and S. Cory, and Drs. A. Harris, D. Huang, D. Vaux, and L. O'Reilly for insightful discussions.
This work was supported by fellowships and grants from the National Health and Medical Research Council (Canberra), the Charles and Sylvia Viertel Charitable foundation, the Wellcome Trust (Senior Overseas Fellowship to G.T. Belz), the Virtual Research Institute of Ageing (Boehringer Japan), the Leukemia and Lymphoma Society of America, and the National Institutes of Health.
The authors have no conflicting financial interests.
M. Pellegrini and P. Bouillet contributed equally to this work.
M. Pellegrini's present address is Dept. of Immunology, Faculty of Medicine, University of Toronto, 1 King's College Circle, Toronto, Ontario, M5S 1AB, Canada.
References
- 1.Noguchi, M., Y. Nakamura, S.M. Russell, S.F. Ziegler, M. Tsang, X. Cao, and W.J. Leonard. 1993. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science. 262:1877–1880. [DOI] [PubMed] [Google Scholar]
- 2.Morrissey, P.J., P. Conlon, K. Charrier, S. Braddy, A. Alpert, D. Williams, A.E. Namen, and D. Mochizuki. 1991. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J. Immunol. 147:561–568. [PubMed] [Google Scholar]
- 3.Fisher, A.G., C. Burdet, C. Bunce, M. Merkenschlager, and R. Ceredig. 1995. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int. Immunol. 7:415–423. [DOI] [PubMed] [Google Scholar]
- 4.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E.G. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, X., E.W. Shores, J. Hu-Li, M.R. Anver, B.L. Kelsall, S.M. Russell, J. Drago, M. Noguchi, A. Grinberg, E.T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 2:223–238. [DOI] [PubMed] [Google Scholar]
- 7.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. [DOI] [PubMed] [Google Scholar]
- 8.Maraskovsky, E., L.A. O'Reilly, M. Teepe, L.M. Corcoran, J.J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1 −/− mice. Cell. 89:1011–1019. [DOI] [PubMed] [Google Scholar]
- 9.Maraskovsky, E., J.J. Peschon, H. McKenna, M. Teepe, and A. Strasser. 1998. Overexpression of Bcl-2 does not rescue impaired B lymphopoiesis in interleukin-7 receptor-deficient mice but can enhance survival of mature B cells. Int. Immunol. 10:1367–1375. [DOI] [PubMed] [Google Scholar]
- 10.Huang, D.C., and A. Strasser. 2000. BH3-only proteins-essential initiators of apoptotic cell death. Cell. 103:839–842. [DOI] [PubMed] [Google Scholar]
- 11.Bouillet, P., D. Metcalf, D.C. Huang, D.M. Tarlinton, T.W. Kay, F. Kontgen, J.M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 12.Bouillet, P., J.F. Purton, D.I. Godfrey, L.C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J.M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926. [DOI] [PubMed] [Google Scholar]
- 13.Davey, G.M., C. Kurts, J.F. Miller, P. Bouillet, A. Strasser, A.G. Brooks, F.R. Carbone, and W.R. Heath. 2002. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2–inhibitable pathway mediated by Bim. J. Exp. Med. 196:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enders, A., P. Bouillet, H. Puthalakath, Y. Xu, D.M. Tarlinton, and A. Strasser. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation–induced apoptosis and deletion of autoreative B cells. J. Exp. Med. 198:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hildeman, D.A., Y. Zhu, T.C. Mitchell, P. Bouillet, A. Strasser, J. Kappler, and P. Marrack. 2002. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity. 16:759–767. [DOI] [PubMed] [Google Scholar]
- 16.Pellegrini, M., G. Belz, P. Bouillet, and A. Strasser. 2003. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl. Acad. Sci. USA. 100:14175–14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathmell, J.C., T. Lindsten, W.-X. Zong, R.M. Cinalli, and C.B. Thompson. 2002. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 3:932–939. [DOI] [PubMed] [Google Scholar]
- 18.Marsden, V., L. O'Connor, L.A. O'Reilly, J. Silke, D. Metcalf, P. Ekert, D.C.S. Huang, F. Cecconi, K. Kuida, K.J. Tomaselli, et al. 2002. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 419:634–637. [DOI] [PubMed] [Google Scholar]
- 19.Villunger, A., C. Scott, P. Bouillet, and A. Strasser. 2003. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 101:2393–2400. [DOI] [PubMed] [Google Scholar]
- 20.Jeffers, J.R., E. Parganas, Y. Lee, C. Yang, J. Wang, J. Brennan, K.H. MacLean, J. Han, T. Chittenden, J.N. Ihle, et al. 2003. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 4:321–328. [DOI] [PubMed] [Google Scholar]
- 21.Khaled, A.R., W.Q. Li, J. Huang, T.J. Fry, A.S. Khaled, C.L. Mackall, K. Muegge, H.A. Young, and S.K. Durum. 2002. Bax deficiency partially corrects interleukin-7 receptor α deficiency. Immunity. 17:561–573. [DOI] [PubMed] [Google Scholar]
- 22.Bouillet, P., S. Cory, L.C. Zhang, A. Strasser, and J.M. Adams. 2001. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev. Cell. 1:645–653. [DOI] [PubMed] [Google Scholar]
- 23.von Freeden-Jeffry, U., N. Solvason, M. Howard, and R. Murray. 1997. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 7:147–154. [DOI] [PubMed] [Google Scholar]
- 24.Fry, T.J., and C.L. Mackall. 2002. Interleukin-7: from bench to clinic. Blood. 99:3892–3904. [DOI] [PubMed] [Google Scholar]