Abstract
Aided by the Plasmodium falciparum genome project, recent discoveries regarding the molecular basis of malaria pathogenesis have led to a better understanding of the interactions between host and parasite. Although vaccines that prevent infection by malaria parasites remain only hopes for the future, there are now more immediate prospects for vaccines that protect against specific disease syndromes. Here, we discuss the latest advances in the development of a vaccine that specifically targets pregnancy-associated malaria (PAM).
Pregnancy-associated Malaria.
It has long been observed that there is an increase in the severity of malaria during pregnancy, resulting in such negative outcomes as maternal anemia and reduction in birth weight, which in turn lead to increases in maternal and infant mortality (1). It has been estimated that as many as 300,000 fetal and infant deaths and 2,500 maternal deaths may be attributable to increased malaria susceptibility during pregnancy (2). The increased vulnerability of both mother and developing child to malaria results from the specific adherence and accumulation of parasite-infected erythrocytes within the placenta and a potentially important localized immune response (3, 4). Unlike other sequestration sites, inflammatory cells are a prominent component of infected placentas (1). In particular, monocytes are heavily recruited to the infected placenta by unknown processes and may have both immunoprotective and immunopathological activities. In their extreme, monocytic infiltrates have been associated with a “massive chronic intervillositis” or placental inflammation (1). More generally, malaria pigment-containing placental monocytes have been linked to maternal anemia and low birth weight, suggesting these may contribute to their development (1, 5).
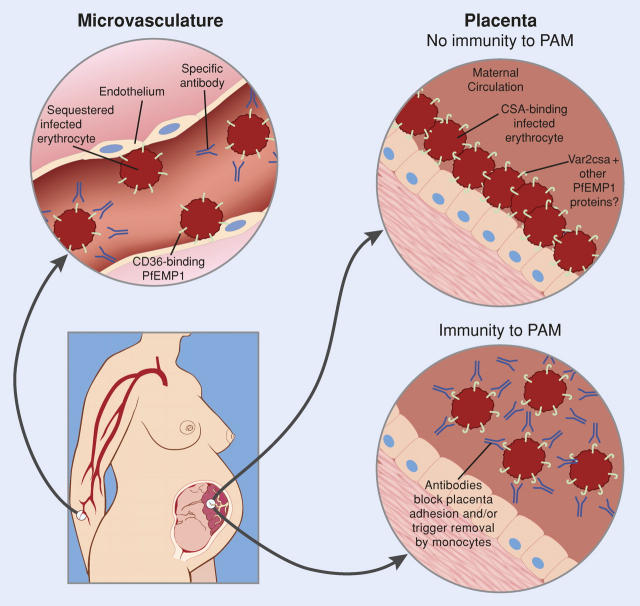
Over the last decade, a new model has emerged to explain PAM based on the distinctive adhesion properties of infected placental erythrocytes (Fig. 1), providing the first hint that a precise vaccine targeting this syndrome might be possible. Specifically, it was shown that placental chondroitin sulfate A (CSA) serves as a receptor for infected erythrocyte binding (6, 7). Infected RBCs isolated from placenta bind CSA, hyaluronic acid, and possibly other receptors, but not the primary receptor that mediates infected RBC binding to the microvasculature, CD36. In infected pregnant women, this results in the selection of a subpopulation of parasites expressing molecules that mediate adhesion of infected RBCs to CSA. From the perspective of vaccine development, there were two encouraging observations concerning this subpopulation of parasite-infected cells. First, there is a reduction in susceptibility to PAM in multigravid women, indicating that women can develop immunity to PAM after infection (1, 3, 4). Second, women develop broadly reactive antibodies to infected cells isolated from placentas (8), suggesting that it may be possible to stimulate a protective immune response that prevents PAM.
Figure 1.
Binding of infected erythrocytes to microvasculature endothelium versus placenta. Pregnant women, especially first-time pregnant mothers, are susceptible to massive sequestration of infected erythrocytes in the placenta. Placental isolates are antigenically and functionally distinct from those found systemically and do not bind the primary microvasculature receptor CD36, but instead bind to CSA. Because CSA-binding parasite variants are rare in infections before pregnancy, the parasite is able to exploit a gap in malaria immunity to establish high density infections in the placenta. During pregnancy, women develop antibodies to the infected erythrocyte surface that may either block infected erythrocyte binding or coat them for destruction by monocytes, thereby reducing disease severity in subsequent pregnancies. Modified with permission from the National Academy of Sciences (Gamain B., S. Gratepanche, L.H. Miller, and D.I. Baruch. Proc. Natl. Acad. Sci. USA. 2002. 99:10020–10024).
These observations stimulated a search for the molecules on placental adherent-infected RBCs that are recognized by antibodies—the putative adhesion receptors. A leading candidate for the placental adhesion receptor is the parasite variant surface antigen responsible for cytoadhesive activity and encoded by var genes (9). The multicopy var gene family was shown previously to encode multiple forms of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) (10). Individual parasites express a single var gene, exporting the encoded PfEMP1 molecule to the surface of the infected RBC where it acts as the primary cytoadherence determinant. This adhesive property leads to sequestration of parasitized RBCs within the deep vascular beds of many tissues. Here, the infected cells bind to molecules on the endothelial surface of blood vessels, resulting in obstruction of blood flow and tissue damage through poorly understood processes. In nonpregnant individuals for instance, cytoadherence of infected cells within the brain can lead to the potentially lethal syndrome of cerebral malaria (10). Exposure of PfEMP1 on the infected cell surface stimulates an antibody response by the host; however, by switching expression between different var genes, parasite populations can undergo antigenic variation and thus maintain a persistent infection (10). Different parasite strains contain distinct var gene repertoires allowing repeated infections with P. falciparum. These discoveries effectively linked both cytoadherence and antigenic variation to the var gene family and led to the hypothesis that expression of different var genes results in different cytoadherence phenotypes, in turn leading to sequestration in alternative tissues and ultimately resulting in variable disease outcomes. This raised the possibility that expression of a specific var gene or subset of var genes may be responsible for CSA binding, cytoadherence in the placenta, and PAM.
Indirect observations suggest the placenta does affect PfEMP1 protein expression. Since most PfEMP1 proteins bind the primary microvasculature receptor CD36 (11), this suggests that the placenta selects for parasites expressing a non-CD36 binding PfEMP1 protein that ensures localization in placenta instead of within the microvasculature. Which PfEMP1 proteins are expressed by placental isolates has been the subject of intense study and current debate. A variety of approaches, including gene expression, proteomics, CSA binding experiments, and serological investigation, have provided conflicting evidence regarding whether a var gene called var1csa is involved in placental binding (12–14). Despite questions about its natural role in PAM, antibodies to var1csa recombinant proteins do react with CSA binding lines, suggesting it has the potential to contribute to a PAM vaccine (15). More recently, a genome-wide transcriptional screen identified a distinct var gene, var2csa, which was transcriptionally up-regulated in a CSA-binding parasite line (16). In this issue, Salanti et al. (17) provide compelling new evidence that the protein encoded by var2csa is actually expressed at the surface of CSA-adherent lines and is an important target of protective antibodies from pregnant women. Infected pregnant mothers with higher levels of anti-var2csa antibodies have a fourfold lower risk of giving birth to a seriously underweight infant (17), providing the first direct evidence that protective antibodies recognize a PfEMP1 molecule. Unlike the majority of the var gene family, which tends to be extremely polymorphic and diverse between parasite strains, both var1csa and var2csa have diverged less extensively and are found within the genomes of most parasite isolates (14). The prospect that PAM may be mediated by expression of only one or two relatively conserved var genes is indeed encouraging for vaccine development. As these two antigens are moved forward as vaccine candidates, we are now confronted with the task of verifying that they do in fact represent the key molecules responsible for PAM and determining how best to test and ultimately implement a PAM-specific vaccine.
Vaccination Based on Variant Antigens.
The concept of a vaccine based on a variable antigen like PfEMP1 may strike some as counterintuitive because of the potential for escape mutants that would evade immunity. For example, if PAM is determined by a few members of a hyper-variable gene family like var, why do parasites not simply mutate these genes and evade immunity in multigravid women? This raises the key question of whether we have identified the optimal PAM vaccine targets in var1csa and var2csa, or whether placental isolates express other non-PfEMP1 proteins that are more invariant and are the main targets of protective immunity. This important question is further complicated by sero-epidemiological investigations that suggest that PAM immunity is acquired only gradually and may require chronic or multiple placental infections over more than one pregnancy (18, 19). An additional puzzling finding is that pregnant women have antibodies that react with CSA-binding parasites as early as the second trimester (18, 19), yet are still infected at term. Both observations could be explained if placental isolates do have some antigenic diversity within surface antigens but are not sufficiently divergent to prevent the eventual development of a broadly protective antibody response (8). These and other unresolved issues regarding PAM justify a more detailed look at the current most promising anti-PAM vaccine candidates, the proteins encoded by var1csa and var2csa.
As mentioned above, both var1csa and var2csa are unusually conserved compared with other members of the var gene family. Although it might be assumed that this conservation simply reflects the shared ability of these proteins to bind CSA, this does not explain why homologues are strikingly conserved over their entire extracellular region and not simply at specific binding domains (16, 20). This is unexpected because PfEMP1 proteins have multiple binding domains, whose adhesion properties appear to function independently (9). It is almost as though the entire extracellular binding region has been simultaneously selected, perhaps to bind multiple placental receptors (CSA, HA, and others) or for some other unidentified reason. Also, although the sequence conservation of var1csa and var2csa is indeed extreme, compared with most other parasite proteins they are still highly polymorphic. For example, the sequences of var2csa from different parasite isolates have ∼80% identity (20). It is possible that these allelic polymorphisms could explain why the acquisition of PAM immunity is not more immediate. To inform vaccine design, it will be imperative to develop a more comprehensive picture of var1csa and var2csa sequence conservation both between different parasite strains and from different geographical locations. In addition, as long as many details of PAM remain unresolved it is important to emphasize that additional work should be done to establish whether placental infection induces the expression of new parasite proteins at the erythrocyte surface other than those encoded by var1csa and var2csa.
Toward a PAM Vaccine.
With the identification of PAM vaccine candidates, strategies for testing and evaluating these antigens will need to be developed. If our intention is to stimulate the type of immunity naturally acquired by multigravid women, it is reasonable to question how well we actually understand PAM immunity. In pregnant women, antibodies develop that can block infected erythrocyte binding to CSA or simply react with the infected erythrocyte surface and presumably opsinize them for uptake by monocytes. Both antibody types increase with PAM exposure and have been correlated with improved pregnancy outcomes (21, 22), but it is not yet clear if either is more important for protection. The fact that HIV infection impairs the development of humoral immunity to placental isolates (23) could explain the increased susceptibility to malaria seen in pregnant women coinfected with HIV, providing further support for the protective role of antibodies in PAM. Vaccine adjuvants should presumably favor cytophilic antibody isotypes that will support antibody-dependent cellular mechanisms of protection, although in vitro assays to measure antibody-dependent cellular immunity to placental isolates have not been developed.
Options for testing PAM vaccines are currently limited. Although P. falciparum cytoadherence can be studied in the New World Monkeys species, Aotus and Saimiri (24), models for cerebral malaria and pregnancy malaria have not yet been developed. Work is proceeding, however, on a P. coatneyi–rhesus monkey model for pregnancy malaria (25) that may have some utility. One challenge is that different Plasmodium species evolved distinct variant antigen families (25, 26), and significantly only some Plasmodium species encode strong binding function into the variant genes and are sequestered (24). Further study of the simian Plasmodium species could increase our understanding of var/PfEMP1 protein family evolution and validate these species as models for human disease. As an example, P. coatneyi–infected erythrocytes produce knob-like protrusions at the erythrocyte surface that have some similarities to P. falciparum and are sequestered from blood circulation like P. falciparum (25). It will be important to determine whether the genes and mechanisms responsible for sequestration are conserved. Primate models, in addition to allowing vaccine therapeutics to be tested for efficacy, safety, and mechanisms of action, could provide new insights into malaria pathogenesis from placental or cerebral complications and the opportunity to study placental binding phenotypes and antibody development over the course of pregnancy. In the absence of animal models, clinical testing of var1csa and/or var2csa in women may be just around the corner.
Since the sequences of var1csa and var2csa vary between strains, to be efficacious a PAM vaccine will need to stimulate a broad antibody response. It is an encouraging sign that var2csa recombinant proteins were recognized by pregnant mother sera from both East and West Africa and that rabbit sera to these recombinant proteins were able to cross-react with a CSA-binding line from a different strain (17). How well these antisera recognize different placental isolates will be the next important test. Several forms of each antigen may need to be combined in vaccine mixes that may need to be tailored to particular geographic regions. This will add additional complexity and expense to the ultimate vaccine cost, since different antigen mixtures, protein purification processes, or nonprotein-based vaccine platforms may need to be tested and optimized. The possibility of a PAM vaccine also raises important questions for deployment. Most significant are the timing and mechanisms by which women could be immunized before their first pregnancies, especially in Africa where the health infrastructure is inadequate. These are important concerns that will need to be addressed to develop a PAM vaccine that can protect the health of mother and fetus.
Prospects for Syndrome-specific Antimalaria Vaccines.
Researchers have strived for decades to develop an effective vaccine to combat malaria morbidity and mortality. Considering the variability of malaria surface antigens and the fact that no subunit vaccine against a parasite or bacterial pathogen has ever been successfully developed, the prospect of a vaccine that can prevent or limit parasite infection is indeed daunting. However, the study of Salanti et al. (17) and other recent advances hint that it may be possible to develop vaccines that target disease-specific erythrocyte surface variants rather than infection in general. Similar to PAM, there is evidence that other disease phenotypes may be associated with a more limited subset of variant antigens. For instance, infected erythrocyte antigenic types associated with severe childhood malaria appear to be more common between parasite strains than is typical (27). In addition, immunity to noncerebral, severe malaria infections may be acquired after one or two infections (28). These observations could be explained if particular antigenic types were responsible for severe childhood malaria. Indeed, a var gene common to different parasite strains was recently associated with severe childhood malaria (29). Whether it can be established that a limited and specific subset of PfEMP1 variants cause disease is still an open question. However, the discovery of strain-transcendent loci has significant implications for parasite biology and possibly for human disease and needs to be explored more fully. With our increasing understanding of the interactions between host and parasite and the pioneering studies of PAM, there is renewed hope that antimalaria vaccines will finally have an impact on the toll that malaria takes on the populations of the developing world.
References
- 1.Brabin, B.J., C. Romagosa, S. Abdelgalil, C. Menendez, F.H. Verhoeff, R. McGready, K.A. Fletcher, S. Owens, U. D'Alessandro, F. Nosten, et al. 2004. The sick placenta—the role of malaria. Placenta. 25:359–378. [DOI] [PubMed] [Google Scholar]
- 2.Steketee, R.W., B.L. Nahlen, M.E. Parise, and C. Menendez. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64:28–35. [DOI] [PubMed] [Google Scholar]
- 3.Duffy, P.E., and M. Fried. 2003. Plasmodium falciparum adhesion in the placenta. Curr. Opin. Microbiol. 6:371–376. [DOI] [PubMed] [Google Scholar]
- 4.Beeson, J.G., J.C. Reeder, S.J. Rogerson, and G.V. Brown. 2001. Parasite adhesion and immune evasion in placental malaria. Trends Parasitol. 17:331–337. [DOI] [PubMed] [Google Scholar]
- 5.Rogerson, S.J., E. Pollina, A. Getachew, E. Tadesse, V.M. Lema, and M.E. Molyneux. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am. J. Trop. Med. Hyg. 68:115–119. [PubMed] [Google Scholar]
- 6.Fried, M., and P.E. Duffy. 1996. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 272:1502–1504. [DOI] [PubMed] [Google Scholar]
- 7.Beeson, J.G., S.J. Rogerson, B.M. Cooke, J.C. Reeder, W. Chai, A.M. Lawson, M.E. Molyneux, and G.V. Brown. 2000. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat. Med. 6:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried, M., F. Nosten, A. Brockman, B.J. Brabin, and P.E. Duffy. 1998. Maternal antibodies block malaria. Nature. 395:851–852. [DOI] [PubMed] [Google Scholar]
- 9.Baruch, D.I. 1999. Adhesive receptors on malaria-parasitized red cells. Baillieres. Best Pract. Res. Clin. Haematol. 12:747–761. [DOI] [PubMed] [Google Scholar]
- 10.Miller, L.H., D.I. Baruch, K. Marsh, and O.K. Doumbo. 2002. The pathogenic basis of malaria. Nature. 415:673–679. [DOI] [PubMed] [Google Scholar]
- 11.Robinson, B.A., T.L. Welch, and J.D. Smith. 2003. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 47:1265–1278. [DOI] [PubMed] [Google Scholar]
- 12.Buffet, P.A., B. Gamain, C. Scheidig, D. Baruch, J.D. Smith, R. Hernandez-Rivas, B. Pouvelle, S. Oishi, N. Fujii, T. Fusai, et al. 1999. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc. Natl. Acad. Sci. USA. 96:12743–12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig, A.G. 2004. Pregnancy-associated malaria—on the brink? Trends Parasitol. 20:201–204. [DOI] [PubMed] [Google Scholar]
- 14.Rowe, J.A., and S.A. Kyes. 2004. The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol. Microbiol. 53:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa, F.T., T. Fusai, D. Parzy, Y. Sterkers, M. Torrentino, J.B. Douki, B. Traore, S. Petres, A. Scherf, and J. Gysin. 2003. Immunization with recombinant duffy binding-like-gamma3 induces pan-reactive and adhesion-blocking antibodies against placental chondroitin sulfate A-binding Plasmodium falciparum parasites. J. Infect. Dis. 188:153–164. [DOI] [PubMed] [Google Scholar]
- 16.Salanti, A., T. Staalsoe, T. Lavstsen, A.T. Jensen, M.P. Sowa, D.E. Arnot, L. Hviid, and T.G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49:179–191. [DOI] [PubMed] [Google Scholar]
- 17.Salanti, A. M. Dahlbäck, L. Turner, M.A. Nielsen, L. Barfod, P. Magistrado, A.T.R. Jensen, T. Lavstsen, M.F. Ofori, K. Marsh, et al. 2004. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 200:1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricke, C.H., T. Staalsoe, K. Koram, B.D. Akanmori, E.M. Riley, T.G. Theander, and L. Hviid. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165:3309–3316. [DOI] [PubMed] [Google Scholar]
- 19.Staalsoe, T., R. Megnekou, N. Fievet, C.H. Ricke, H.D. Zornig, R. Leke, D.W. Taylor, P. Deloron, and L. Hviid. 2001. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J. Infect. Dis. 184:618–626. [DOI] [PubMed] [Google Scholar]
- 20.Kraemer, S.M., and J.D. Smith. 2003. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 50:1527–1538. [DOI] [PubMed] [Google Scholar]
- 21.Duffy, P.E., and M. Fried. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71:6620–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staalsoe, T., C.E. Shulman, J.N. Bulmer, K. Kawuondo, K. Marsh, and L. Hviid. 2004. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 363:283–289. [DOI] [PubMed] [Google Scholar]
- 23.Mount, A.M., V. Mwapasa, S.R. Elliott, J.G. Beeson, E. Tadesse, V.M. Lema, M.E. Molyneux, S.R. Meshnick, and S.J. Rogerson. 2004. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet. 363:1860–1867. [DOI] [PubMed] [Google Scholar]
- 24.Berendt, A.R., D.J. Ferguson, and C.I. Newbold. 1990. Sequestration in Plasmodium falciparum malaria: sticky cells and sticky problems. Parasitol. Today. 6:247–254. [DOI] [PubMed] [Google Scholar]
- 25.Galinski, M.R., and V. Corredor. 2004. Variant antigen expression in malaria infections: posttranscriptional gene silencing, virulence and severe pathology. Mol. Biochem. Parasitol. 134:17–25. [DOI] [PubMed] [Google Scholar]
- 26.Carlton, J.M., S.V. Angiuoli, B.B. Suh, T.W. Kooij, M. Pertea, J.C. Silva, M.D. Ermolaeva, J.E. Allen, J.D. Selengut, H.L. Koo, et al. 2002. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 419:512–519. [DOI] [PubMed] [Google Scholar]
- 27.Bull, P.C., M. Kortok, O. Kai, F. Ndungu, A. Ross, B.S. Lowe, C.I. Newbold, and K. Marsh. 2000. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J. Infect. Dis. 182:252–259. [DOI] [PubMed] [Google Scholar]
- 28.Gupta, S., R.W. Snow, C.A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340–343. [DOI] [PubMed] [Google Scholar]
- 29.Jensen, A.T., P. Magistrado, S. Sharp, L. Joergensen, T. Lavstsen, A. Chiucchiuini, A. Salanti, L.S. Vestergaard, J.P. Lusingu, R. Hermsen, et al. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]