Abstract
To gain insight into the inability of newborns to mount efficient Th1 responses, we analyzed the molecular basis of defective IL-12(p35) expression in human neonatal monocyte-derived dendritic cells (DCs). Determination of IL-12(p35) pre-mRNA levels by real-time RT-PCR revealed that transcriptional activation of the gene in lipopolysaccharide-stimulated neonatal DCs was strongly impaired compared with adult DCs. We next showed that p50/p65 and p65/p65 dimers interact with kB#1 site, a critical cis-acting element of the IL-12(p35) promoter. We found that LPS-induced p65 activation was similar in adult and newborn DCs. Likewise, in vitro binding activity to the Sp1#1 site, previously shown to be critical for IL-12(p35) gene activation, did not differ in adults and newborns. Since the accessibility to this Sp1#1 site was found to depend on nucleosome remodeling, we used a chromatin accessibility assay to compare remodeling of the relevant nucleosome (nuc-2) in adult and neonatal DCs. We observed that nuc-2 remodeling in neonatal DCs was profoundly impaired in response to lipopolysaccharide. Both nuc-2 remodeling and IL-12(p35) gene transcription were restored upon addition of recombinant interferon-γ. We conclude that IL-12(p35) transcriptional repression in neonatal DCs takes place at the chromatin level.
Keywords: cord blood, newborn, nucleosome, NF-κB, Sp1
Introduction
The fetal immune system is deficient in the generation of Th1-type responses. During pregnancy, this deficiency protects the fetus against potentially harmful inflammatory reactions (1). However, after delivery it results in increased susceptibility to intracellular pathogens (2) and can favor the development of allergic disorders (3).
Inherent CD4+ T cell deficiencies certainly contribute to the Th1/Th2 imbalance in early life as indicated by hypermethylation of specific sites in the promoter region of the IFN-γ gene (4). Furthermore, there is evidence that immaturity of the APC compartment also contributes to impaired T cell responses in human newborns (5–7). Indeed, we demonstrated recently that neonatal monocyte-derived DCs are deficient in the production of IL-12(p70), but not of other cytokines, in response to LPS, CD40 ligand, or poly I:C (6). Determination of IL-12(p40) and IL-12(p35) mRNA levels revealed that IL-12(p35) gene expression was highly repressed in LPS-stimulated neonatal DCs, whereas their IL-12(p40) gene expression was not altered (6). The relevance of these findings obtained on monocyte-derived DCs is supported by the observation that IL-12(p70) but not IL-12(p40) production is impaired in LPS-stimulated cord blood (8). The aim of this study was to identify the molecular basis for impaired IL-12(p35) expression in neonatal DCs.
Materials and Methods
Cells and Reagents.
DCs were generated from cord blood mononuclear cells or PBMC as described previously (6). LPS from Escherichia coli (0128: B12), α-amanitin, and mithramycin were obtained from Sigma-Aldrich. Recombinant human and murine IFN-γ were purchased from Biosource Europe and Roche Diagnostics, respectively.
Plasmid Constructs.
The luciferase reporter p35-lucWT plasmid was described previously (9). The plasmid p35κB-luc is a derivative of p35-lucWT in which the κB#1 site was altered by the QuickChange Site-directed Mutagenesis Method (GTCCCGGGAAAGTCCT to GGAGCCTCAAAGGAGT).
RNA Purification and Real-Time RT-PCR for Determination of IL-12(p35) pre-mRNA Levels.
Total RNA was extracted using a MagnaPure LC RNA Isolation Kit (Roche Diagnostics). RT- and real-time PCR reactions were then performed using LightCycler-RNA Master Hybridization Probes (one-step procedure) on a Lightcycler® apparatus (Roche Diagnostics). To ensure specificity for IL-12(p35) pre-mRNA, primers encompassing the first intron-exon boundary were used. Controls were included for all reactions to exclude amplification of contaminating genomic DNA. To correlate the Ct values to copy number, a standard curve was generated using serial dilutions of a plasmid containing nucleotide (nt) −2606/+1308 from the human IL-12(p35) gene. Primer sequences are listed in Table S1 (available at http://www.jem.org/cgi/content/full/jem.20031272/DC1).
Electrophoretic Mobility Shift Assays.
κB#1wt probe was generated by end labeling the double stranded oligonucleotide 5′-AGAGTCCCGGGAAAGTCCTGCCGCGCC-3′ (corresponding to nt −68 to −42), and electrophoretic mobility shift assays (EMSAs) were performed as described previously (9). For competition analysis, increasing concentrations (5–80-fold molar excess) of unlabeled κB#1wt, consensus (5′-AGTTGAGGGGACTTTCCCAGGC-3′) or mutated consensus (5′-AGTTGAGGCGACTTTCCCAGGC-3′) competitor DNA was added. For supershift assays, antibodies against p50, p52 (both obtained from Upstate Biotechnology), p65, RelB, c-Rel, or PU-1 (used as a control) (obtained from Santa Cruz Biotechnology, Inc.) were included in the binding-reaction mixture.
Detection of NF-κB DNA-binding Activity.
NF-κB binding activity in nuclear extracts was measured with Trans-AM p65 transcription factor assay kit (Active Motif Europe). 5 μg of nuclear extracts was incubated with plate-coated NF-κB consensus oligonucleotide. Plates were washed before addition of anti-p65 antibody. Antibody binding was detected with a secondary HRP-conjugated antibody and developed with TMB substrate. The intensity of the reaction was measured at 450 nm.
Transient Transfection and Luciferase Assays.
RAW 264.7 cells were transfected using FuGENE™-6 (Roche Diagnostics) and stimulated as described previously (11).
Indirect End Labeling.
Nuclei isolation, in vivo digestion with BstXI, and DNA purification for the indirect end labeling experiments were performed as described previously (10). Purified DNA was incubated with an excess of EcoRI and analyzed by electrophoresis on a 1.5% agarose gel. Samples were then transferred and hybridized with a [32P]dCTP-radiolabeled probe spanning nt –1610 to –890 from the IL-12(p35) gene.
Determination of Chromatin Accessibility by Real-Time PCR (CHART-PCR).
Genomic DNA from purified nuclei was extracted using the MagNa Pure LC DNA Isolation Kit (Roche Diagnostics). Real-time PCR reactions were then performed using LightCycler-DNA Master Hybridization Probes (Roche Diagnostics). The sampling of all components was fully automated to avoid manual sampling errors. To correlate the Ct values to copy number, a standard curve was generated using serial dilutions of a plasmid containing nt –2606/+1308 from the human IL-12(p35) gene. Amplification with primer set A (encompassing BstXI site located at nt –298) is sensitive to remodeling of nuc-2. Increased accessibility of the region results in reduced amplification. To normalize for DNA input amounts and for the efficiency of BstXI digestion, an aliquot of each sample was analyzed with primer set B (encompassing BstXI site located at nt +456). Since the region amplified by this second set of primer does not undergo modification of chromatin structure upon stimulation, it provides an excellent internal control for the experiment. Results were then expressed as a percentage of the accessibility observed in the unstimulated digested samples. Primer sequences are listed in Table S1.
Statistical Analysis.
Statistical significance was evaluated by using two-tailed Student's t test.
Online Supplemental Material.
Table S1 displays primer sequences used for real-time PCR. Table S2 shows TLR4 expression in adult and neonatal DCs. Fig. S1 shows interferon regulatory factor (IRF)-1 activation in adult and neonatal DCs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20031272/DC1.
Results and Discussion
Impaired IL-12(p35) Gene Transcription in LPS-stimulated Neonatal DCs.
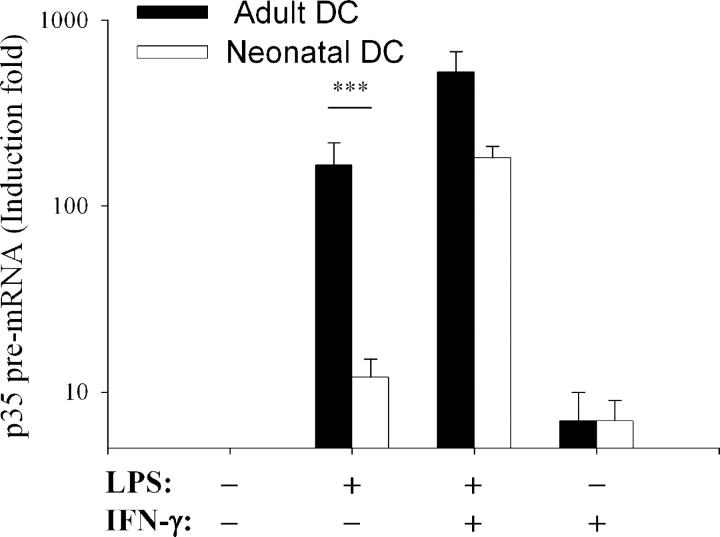
As shown in Fig. 1, induction of IL-12(p35) pre-mRNA in response to LPS alone was significantly lower in neonatal DCs compared with adult DCs. IL-12(p35) pre-mRNA levels were strongly up-regulated by the addition of recombinant IFN (rIFN)-γ to LPS both in neonatal and adult DCs. rIFN-γ alone led to a weak increase in IL-12(p35) pre-mRNA levels, which was comparable in both populations. These results indicate that deficient LPS-induced IL-12(p70) synthesis in neonatal DCs is related to impaired IL-12(p35) gene transcription and can be compensated by rIFN-γ addition.
Figure 1.
Deficient transcriptional activation of the IL-12(p35) gene in LPS-stimulated neonatal DCs. Adult (black bars) or neonatal DCs (white bars) were either incubated with medium alone or stimulated with LPS (1 μg/ml) and/or rIFN-γ (100 U/ml) for 5 h. IL-12(p35) pre-mRNA levels were normalized using β-actin mRNA levels and compared with unstimulated conditions. Data are shown as mean ± SEM of at least eight independent experiments on different donors. ***P < 0.001 compared with adult DCs.
As TLR4 represents the main signaling receptor for LPS, we compared its expression in adult and neonatal DCs. TLR4 mRNA and surface expression was comparable in both groups (Table S2, available at http://www.jem.org/cgi/content/full/jem.20031272/DC1). Together with previous reports (6, 7), these data indicate that the deficient IL-12(p35) production by neonatal DCs cannot be attributed to a global defect in TLR4 signaling.
p50/p65 and p65/p65 Dimers Interact with κB#1 Site, a Critical cis-acting Element of the IL-12(p35) Promoter.
Since IL-12(p35) expression is impaired in c-Rel knockout mice (11), we analyzed the nucleotide sequence of the p35 promoter region for the presence of putative κB elements. We designed a double stranded oligonucleotide, designated κB#1wt, encompassing a potential binding site at position nt −50/−64. Competition EMSAs illustrated in Fig. 2 A indicated that protein binding in complexes A and B was specific to NF-κB, since a consensus oligonucleotide (NF-κB cons) specifically inhibited their formation although they were not affected by a mutated version of the NF-κB consensus oligonucleotide (NF-κB mut). As shown in Fig. 2 B, specific binding to the κB#1 site (complexes A and B) was inducible since it required LPS stimulation. Addition of anti-p50 antibody interfered with the formation of complex B and generated a supershift complex. The anti-p65 antibody also generated a strong supershift and inhibited the formation of both complexes A and B. No supershifted complex was observed with anti-RelB, -cRel, -p52 or a control antibody (Fig. 2 B). Overall, these results demonstrate that the induction of complexes A and B upon LPS stimulation corresponds to the formation of p65/p65 homodimers and p50/p65 heterodimers, respectively.
Figure 2.
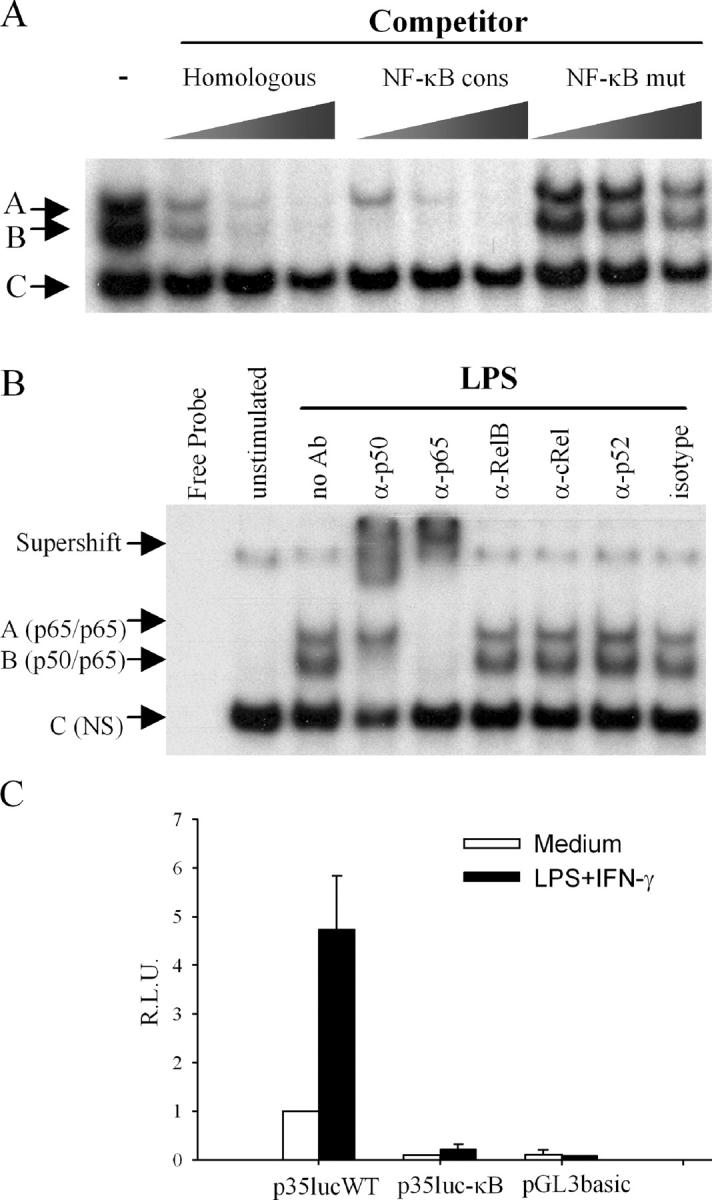
Physical and functional characterization of a κB site within the p35 proximal promoter region. (A) Competition assays. Nuclear extracts from LPS-stimulated adult DCs (10 μg) were incubated with radiolabeled κB#1wt probe in the absence or the presence of increasing concentrations of the indicated unlabeled competitor. Three protein–DNA complexes (A–C) were observed. (B) Supershift assays. DCs were incubated with medium alone or activated for 2 h with LPS. Nuclear extracts were incubated with the indicated specific antibody before adding the radiolabeled κB#1wt probe. NS, nonspecific. (C) κB#1 site is necessary for basal and inducible p35 promoter activity. RAW 264.7 cells were transiently transfected with 2 μg p35-lucWT or p35-mutκB#1 reporter plasmid and 20 ng pRL-TK as an internal control. Promoter activities were normalized using Renilla luciferase activities. Values represent the means ± SEM of three independent experiments performed in triplicates.
To examine the functional role of the κB#1 site in the transcriptional activity of the p35 promoter, GGG (or GGA) to CTC mutations were introduced by site-directed mutagenesis in the context of a reporter plasmid containing the −1121/+66 region of the human IL-12(p35) gene. In RAW cells transiently transfected with either the wild-type or the mutated reporter construct, we found that both basal and inducible p35 promoter activities were strongly reduced by mutation of the κB1#1 site. These results indicate that the κB1#1 site is a key cis-acting element for p35 promoter activity.
NF-κB and Sp1 Binding Activities Are Comparable in Adult and Neonatal DCs.
To explore the molecular mechanisms responsible for deficient IL-12(p35) gene expression in neonatal DCs, we quantified Rel protein p65 activation in LPS-stimulated adult and neonatal DCs. As shown in Fig. 3 A, p65 activation was comparable in both groups, suggesting that TLR4-triggered events leading to NF-κB activation are functional in early life. This is consistent with the maintained production of NF-κB–dependent proinflammatory cytokines (6).
Figure 3.
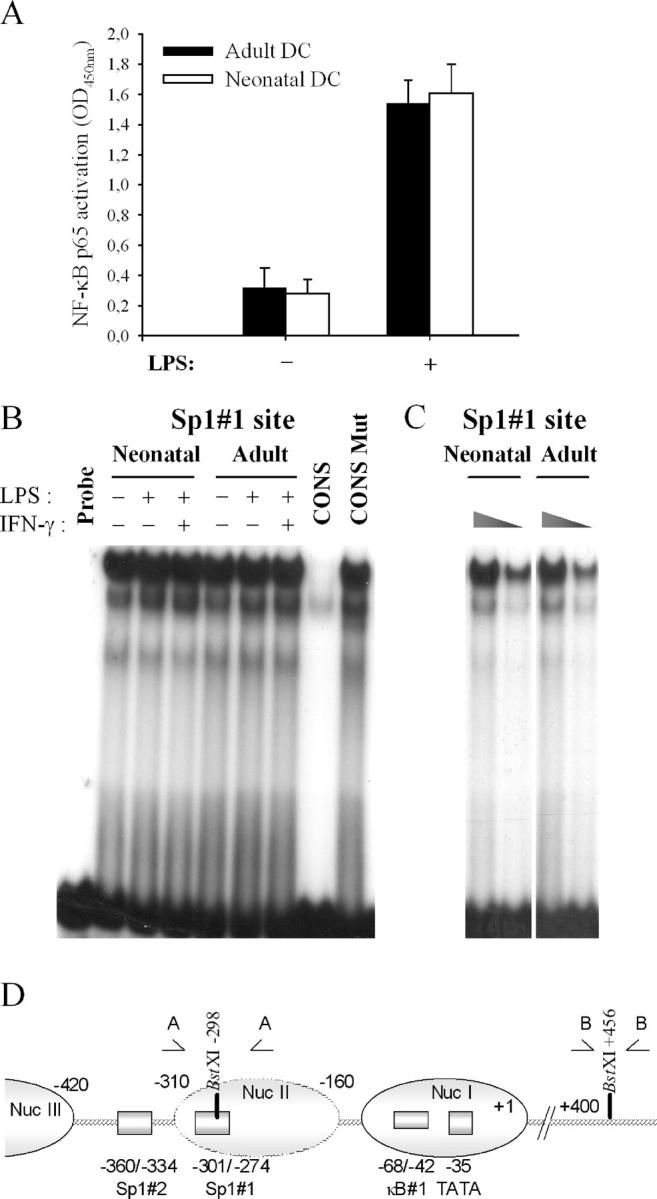
Comparison of NF-κB and Sp1 binding activities in adult and neonatal DCs. (A) Assessment of NF-κB p65 activation in adult and neonatal DCs. DCs were left untreated or stimulated for 2 h with LPS. Results are presented as mean ± SEM from at least four different donors. (B) Constitutive binding activity to the Sp1#1 site in neonatal and adult DCs. DCs were left untreated or stimulated with LPS or LPS + IFN-γ for 2 h. Nuclear extracts (10 μg protein) were then isolated and incubated with radio-labeled Sp1#1wt probe from the p35 promoter (9). To ensure specificity of the binding, nuclear extracts from LPS + IFN-γ–stimulated adult DCs were incubated with radiolabeled Sp1#1wt probe in the presence of a 50-fold molar excess of unlabeled Sp1 consensus (CONS) or mutated consensus (CONS Mut) (9). The results are representative of three independent experiments. (C) Nuclear extracts (5 and 2.5 μg protein) from adult and neonatal LPS-stimulated DCs were incubated with radiolabeled Sp1#1wt probe from the p35 promoter. (D) Schematic representation of the nucleosome organization of the p35 promoter. Positioned nucleosomes (ovals) are indicated. nuc-2 (−310/−160, broken lined oval) is selectively remodeled on p35 transcriptional activation. Locations of the Sp1#1, Sp1#2, and the κB#1 sites are shown. The BstXI cleavage sites and the relative position of primer sets A and B, used for the CHART-PCR experiments, are also represented.
We showed previously that Sp1 binding to the Sp1#1 and #2 sites of the p35 promoter was critical for both basal and inducible expression of the gene (9). As assessed by EMSA experiments (Fig. 3 B), in vitro binding activity to the Sp1#1 site was found to be constitutive in adult DCs and was not enhanced upon activation. Importantly, no difference in binding to the Sp1#1 site was observed between adult and neonatal DCs, even when nuclear extracts were diluted to ensure that the assay was not saturated (Fig. 3 C).
Chromatin Remodeling in Adult and Neonatal DCs.
Since association of transcription factors with promoter or enhancer regions occurs in the context of chromatin, factors which have been identified on the basis of their in vitro binding activities might not be able to interact with their DNA target in vivo. Indeed, we showed recently that the Sp1#1 binding site is located into a positioned nucleosome (nuc-2), which is selectively and rapidly remodeled upon activation of the p35 gene (Fig. 3 D) (9). Therefore, we compared nuc-2 remodeling in adult and neonatal DCs. For this purpose, we first performed indirect end labeling experiments to monitor the accessibility of the IL-12(p35) promoter region to BstXI digestion in adult and neonatal DCs. A BstXI restriction site (nt –298) is located within the region protected by nuc-2, close to the Sp1#1 site (nt −301/−274). Two other BstXI recognition sites (nt +117 and +456) are located in regions of the p35 locus, which do not undergo chromatin remodeling, therefore providing internal controls in the experiment. As seen in Fig. 4 A, accessibility to recognition site nt –298 was markedly up-regulated upon stimulation with LPS in adult DCs but not in neonatal DCs.
Figure 4.
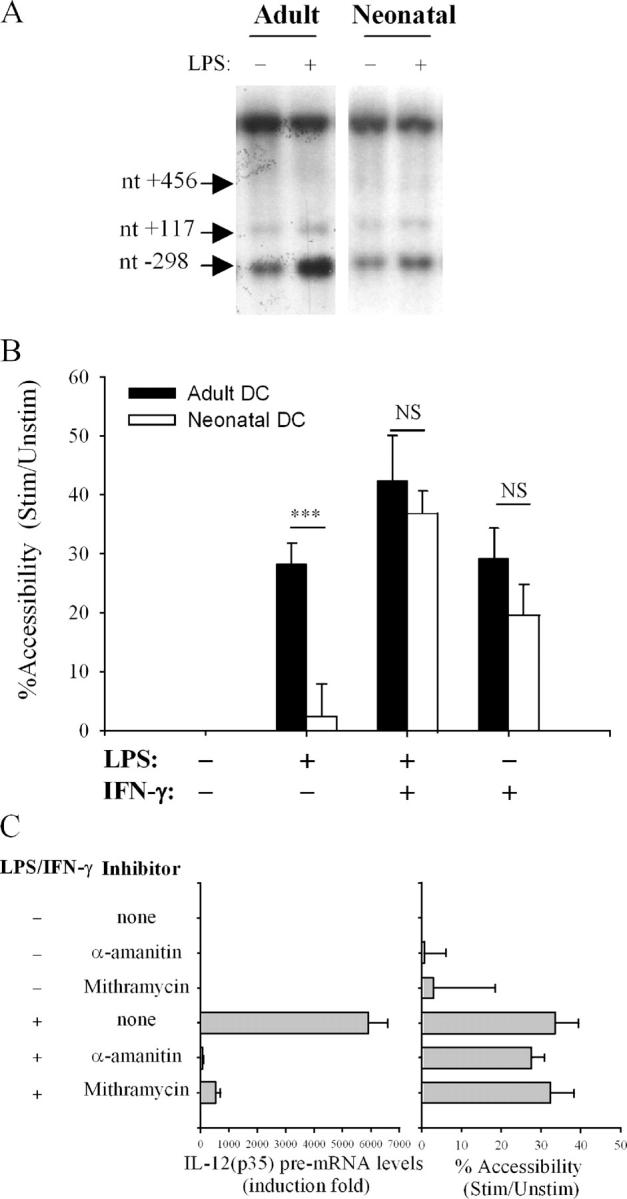
Impaired nuc-2 remodeling in LPS-stimulated neonatal DCs. Accessibility of the IL-12(p35) promoter region protected by nuc-2 to BstXI was assessed by indirect end labeling and CHART-PCR. Adult and neonatal DCs were incubated with medium alone or stimulated with LPS and/or IFN-γ for 3 h. Intact nuclei were incubated with BstXI. (A) After genomic DNA purification and in vitro digestion with EcoRI, DNA samples were analyzed by Southern blotting and the indirect end labeling technique. The results are representative of five independent experiments performed with different blood donors. The different BstXI restriction sites located in regions of the p35 locus (nt –298, +117, +456) are indicated by arrows. (B) For CHART-PCR assays, genomic DNA samples were subjected to real-time analysis using primer sets A and B as described in Materials and Methods. Results are expressed as a percentage of the accessibility observed in the unstimulated digested samples. Data shown are the mean ± SEM obtained from 9 neonatal and 15 adult samples. ***P < 0.001 compared with adult DCs. NS, not significant. (C) nuc-2 remodeling is insensitive to α-amanitin or mithramycin. Adult DCs were pretreated or not with α-amanitin (5 μg/ml) or mithramycin (20 nM) for 1 h. Cells were then incubated with medium alone or stimulated with LPS and IFN-γ for 5 h (real-time RT-PCR experiments, left) or 3 h (CHART-PCR experiments, right).
Since the indirect end labeling technique does not allow precise quantification of the remodeling process, we adapted the chromatin accessibility (CHART)-PCR technique described by Rao et al. (12) to measure nuc-2 remodeling in the p35 promoter region. In these experiments, purified genomic DNA from BstXI-digested nuclei was subjected to real-time PCR amplification with primers encompassing the enzyme restriction site. Calculation of the copy numbers and normalization of the results are described in Materials and Methods. As seen in Fig. 4 B, stimulation of adult DCs by LPS or IFN-γ alone led to an increase in chromatin accessibility of the region protected by nuc-2 of ∼30%. When rIFN-γ was added to LPS, mean accessibility increased by up to 45%. In neonatal DCs, nuc-2 remodeling in response to LPS was strongly impaired, confirming the data obtained using the indirect end labeling technique. Upon stimulation with rIFN-γ alone or in combination with LPS, the response of neonatal DCs was not significantly different from that of adult DCs. To exclude that impaired nuc-2 remodeling in neonatal DCs was the result rather than the cause of reduced p35 gene expression, we suppressed RNA polymerase II activity using α-amanitin. Transcription in adult DCs pretreated with this reagent was inhibited by >95% (Fig. 4 C). However, this treatment had no effect on nuc-2 remodeling induced by LPS + IFN-γ. To further determine whether nuc-2 remodeling requires Sp1 binding, DCs were incubated with mithramycin, an agent that competes with Sp1 binding to DNA (13). As shown in Fig. 4 C, mithramycin did not prevent chromatin remodeling although it strongly inhibited IL-12(p35) transcription in response to LPS + IFN-γ stimulation, confirming that Sp1 binding is required for inducible p35 gene expression. Together with previous findings showing that remodeling precedes p35 mRNA synthesis (9), these data strongly suggest that nuc-2 remodeling is a primary event during p35 gene activation. Our results further indicate that (a) impaired IL-12(p35) gene expression by LPS-stimulated neonatal DCs is directly related to impaired nuc-2 remodeling and (b) this deficiency can be overcome by IFN-γ-induced signals.
To the best of our knowledge, these observations represent the first evidence that a defect in chromatin remodeling contributes to the immaturity of the newborn immune system. Restriction of Th1 responses in utero might be essential for the normal development of the fetus (1). Together with hypermethylation of the IFN-γ promoter in neonatal CD4+CD45RA+ T cells (4), depressed nucleosomal remodeling at the IL-12(p35) promoter in the neonatal DC compartment might represent an important control mechanism to prevent detrimental Th1 lineage development in early life. Interestingly, depressed IFN-γ production by neonatal CD4+ T cells could contribute to limit IL-12 synthesis by DCs as the addition of IFN-γ to LPS-stimulated neonatal DCs strongly activates their expression of the IL-12(p35) gene (Fig. 1). IFN-γ stimulation of adult or neonatal DCs readily induces chromatin remodeling of the p35 gene without activating its transcription. Therefore, one might speculate that IFN-γ restores LPS-induced p35 production in neonatal DCs through its action on chromatin remodeling. It was shown recently that IRF-1 is recruited to the p35 promoter in response to IFN-γ (14). Interestingly, this IRF-E site is located within the region protected by nuc-2. We found no significant difference in IRF-1 activation between neonatal and adult DCs (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20031272/DC1). In the absence of TLR4 activation, IFN-γ–induced nuc-2 remodeling could be mediated by STAT-1, as described for CIITA promoter IV (15) or by IRF-1 itself. We conclude that understanding the mechanisms involved in nuc-2 remodeling might be crucial to our knowledge of the immune status of the human newborn and for the development of new vaccine strategies dependent on the production of IL-12 during the neonatal period.
Acknowledgments
The authors thank the obstetric staff of the Erasme Hospital for their kind support.
This study was supported by the Région Wallonne and GlaxoSmithKline Biologicals, the NEOVAC program of the European Commission (contract no. QLK2-CT-1999-00429), the Fonds National de la Recherche Scientifique (FNRS), and the Belgian Federal Science Policy Office. S. Goriely is a research fellow of the FNRS. C.V. Lint is senior research associate of the FNRS. D. Demonté is supported by a postdoctoral fellowship from the Région Wallonne.
The online version of this article contains supplemental material.
References
- 1.Wegmann, T.G., H. Lin, L. Guilbert, and T.R. Mosmann. 1993. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today. 14:353–356. [DOI] [PubMed] [Google Scholar]
- 2.Siegrist, C.A. 2000. Vaccination in the neonatal period and early infancy. Int. Rev. Immunol. 19:195–219. [DOI] [PubMed] [Google Scholar]
- 3.Holt, P.G., and C. Macaubas. 1997. Development of long-term tolerance versus sensitisation to environmental allergens during the perinatal period. Curr. Opin. Immunol. 9:782–787. [DOI] [PubMed] [Google Scholar]
- 4.White, G.P., P.M. Watt, B.J. Holt, and P.G. Holt. 2002. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J. Immunol. 168:2820–2827. [DOI] [PubMed] [Google Scholar]
- 5.Hunt, D.W., H.I. Huppertz, H.J. Jiang, and R.E. Petty. 1994. Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood. 84:4333–4343. [PubMed] [Google Scholar]
- 6.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141–2146. [DOI] [PubMed] [Google Scholar]
- 7.Langrish, C.L., J.C. Buddle, A.J. Thrasher, and D. Goldblatt. 2002. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin. Exp. Immunol. 128:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Wit, D., S. Tonon, V. Olislagers, S. Goriely, M. Boutriaux, M. Goldman, and F. Willems. 2003. FOCIS: impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 3:272–281. [DOI] [PubMed] [Google Scholar]
- 9.Goriely, S., D. Demonte, S. Nizet, D. De Wit, F. Willems, M. Goldman, and C. Van Lint. 2003. Human IL-12(p35) gene activation involves selective remodeling of a single nucleosome within a region of the promoter containing critical Sp1-binding sites. Blood. 101:4894–4902. [DOI] [PubMed] [Google Scholar]
- 10.Van Lint, C., S. Emiliani, M. Ott, and E. Verdin. 1996. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 11.Grumont, R., H. Hochrein, M. O'Keeffe, R. Gugasyan, C. White, I. Caminschi, W. Cook, and S. Gerondakis. 2001. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J. Exp. Med. 194:1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao, S., E. Procko, and M.F. Shannon. 2001. Chromatin remodeling, measured by a novel real-time polymerase chain reaction assay, across the proximal promoter region of the IL-2 gene. J. Immunol. 167:4494–4503. [DOI] [PubMed] [Google Scholar]
- 13.Ray, R., R.C. Snyder, S. Thomas, C.A. Koller, and D.M. Miller. 1989. Mithramycin blocks protein binding and function of the SV40 early promoter. J. Clin. Invest. 83:2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J., S. Cao, L.M. Herman, and X. Ma. 2003. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-γ-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 198:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris, A.C., G.W. Beresford, M.R. Mooney, and J.M. Boss. 2002. Kinetics of a gamma interferon response: expression and assembly of CIITA promoter IV and inhibition by methylation. Mol. Cell. Biol. 22:4781–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]