Abstract
The role of the hematopoietic lineage-restricted minor histocompatibility (H) antigen HA-1 in renal allograft tolerance was explored. We obtained peripheral blood samples from three recipients of histocompatibility leukocyte antigen (HLA)–matched, HA-1–mismatched renal transplants, one of which had discontinued immunosuppression >30 yr ago while sustaining normal kidney function. Peripheral blood mononuclear cells (PBMCs) were injected into the footpads of severe combined immunodeficiency mice to measure human delayed type hypersensitivity (DTH) responses. All three patients manifested regulated DTH responses to HA-1H peptide. By differential tetramer staining intensities, we observed two distinct minor H antigen HA-1–specific CD8+ T cell subsets. The one that stained dimly had the characteristics of a T regulatory (TR) cell and produced interleukin (IL) 10 and/or transforming growth factor (TGF) β. These HA-1–specific TR cells coexisted with bright tetramer-binding CD8+ T effector (TE) cells. The CD8+ TE cells mediated HA-1–specific DTH and produced interferon-γ. Suppression of these TE functions by TR cells was TGFβ, IL-10, and cytotoxic T lymphocyte–associated antigen 4 dependent. In addition, HA-1 microchimerism was detected in two recipients, primarily in the dendritic cell fraction of the PBMCs. This is the first demonstration of coexisting CD8+ memory TR and TE cells, both specific for the same HA-1 antigen, in the context of renal allograft tolerance.
Keywords: regulatory T cells, immunoregulation, peripheral tolerance, kidney transplantation
Introduction
The basis of immunologic tolerance remains largely a mystery >50 yr after its discovery by Owen (1). Inhibition of potentially pathogenic self-reactive T cells by CD4+ T regulatory (TR) cells has been proposed as one of the major mechanisms for the establishment of peripheral tolerance to autoantigens (2, 3) and for the persistence of certain forms of chronic infection (4). Transplantation tolerance studies in rodents have suggested that certain donor-derived alloantigens, which stimulate T cell–dependent graft rejection, could also trigger TR cell responses that promote allograft acceptance (5, 6). However, the T cell receptor specificity of TR cells and what drives their development remain largely unknown.
The hematopoietic-specific minor histocompatibility (H) antigen HA-1 is a nine–amino acid peptide encoded by a diallelic gene on human chromosome 19 (7, 8). The immunogenic HA-1 T cell epitope differs from its allelic counterpart by one amino acid at position 3 (i.e., VLHDDLLEA→VLRDDLLEA; reference 7). Although nonameric peptides of both the HA-1H and the HA-1R alleles bind to HLA-A2, the HA-1R allele fails to be expressed at the cell surface in the context of HLA-A2 (7). The HA-1H peptide is presented at the cell surface and induces strong HLA-A2–restricted CTLs (9, 10). The HA-1 difference between HLA-A2+ bone marrow donor and recipient, when the recipient is H/R or H/H, and the donor is R/R, can lead to the development of acute GVHD (11). HA-1–specific mismatch GVHD occurs early after bone marrow transplant when residual recipient APCs can still provide the target antigen, triggering bystander destruction of skin and other epithelial tissues (12).
HLA-identical siblings and HLA-matched cadaver donors are the ideal renal transplant donors and indeed have the best outcomes long-term. Yet, in the case of a minor H antigen such as HA-1, the efflux of large numbers of “passenger leukocytes” (13) early after transplantation might induce activation of donor-specific cytotoxic and proinflammatory T effector (TE) cells that could trigger bystander destruction of the kidney epithelium. In contrast, long-term persistence of donor-derived microchimerism (14) might lead to chronic suppression of host TE cells (15, 16). Here, we demonstrate the coexistence of CD8+ TR and TE cells, both specific for the same hematopoietic-specific minor H antigen, HA-1, together with dendritic cell microchimerism in the context of solid organ transplant tolerance.
Materials and Methods
Animals.
CB-17 SCID mice were purchased from Harlan Sprague Dawley, Inc. or were bred locally. All animals were housed and treated in accordance with National Institutes of Health guidelines.
Reagents, Antibodies, and Antigens.
All antibodies used in flow cytometry were purchased from BD Biosciences. Neutralizing anti–human CD152 (CTLA-4) mAb was purchased from Antibody Solutions. IL-10– and TGFβ-neutralizing antibodies were purchased from R&D Systems. HA-1H, HA-1R, CMVpp65 (NLVPMVATV), and HY (FIDSYICQV) peptides were all synthesized and purified (purity >94%) at the University of Wisconsin-Madison Biotechnology facility. HA-1A2 tetramers were prepared as described previously (11). All other reagents were purchased from Sigma-Aldrich.
Patients.
Patient I (tolerant; 32 yr off immunosuppressive drugs; HLA A 2, 3; B7, 12/44; DR1, 4; and HA-1 R/R) received a kidney transplant from her HLA-identical sister (HA-1H/H) in 1967. Patient II (HLA: A1, 2; B8, 27; DR4, 17; and HA-1R/R) received a kidney transplant from her HLA-identical HA-1–mismatched brother (HA-1H/R) in 1996. Patient III (HLA: A2, 24; B35, 57; DR4, 11; and HA-1R/R) received an HLA-identical, HA-1–mismatched (HA-1: H/R) kidney transplant from a sister in 1988, lost the graft 10 yr later because of chronic allograft nephropathy, and received a second HA-1–mismatched transplant from a 5-HLA antigen-matched sister (HLA: A2, 24; B44, 57; DR4, 11; and HA-1H/R) in 2000. Control patient IV (HLA: A2, 24, B13, 35; DR7, 8; and HA-1: R/R) received a kidney transplant from her HLA- and HA-1(R/R)–identical brother in 1997. Patients II and IV are taking azathioprine and cyclosporine, and patient III is taking prednisone, tacrolimus, and mycophenolate mofetil. All patients currently have excellent graft function, with serum creatinine ≤1.4 mg/dL.
Flow Cytometric Analysis and Cell Sorting.
For flow cytometric analysis, we used a FACSCalibur™ instrument with CELLQuest™ software (BD Biosciences). Flow sorting was performed at 4°C using FACSVantage™ (Becton Dickinson). After sorting, tetramer-positive cells were incubated at 37°C, 5% CO2 in 10% FCS-containing RPMI 1640 for 1–2 h, washed with PBS, and used in DTH or ELISPOT assay.
Trans Vivo DTH Analysis.
The trans vivo DTH assay was used as described previously (17) to detect donor antigen-linked suppression of human recall antigen responses to tetanus toxoid (TT), EBV antigen, or to EBV-transformed B-lymphoblastoid cell lines (B-LCLs). To test for regulated DTH responses to HA-1, 7–9 × 106 cryopreserved PBMCs were coinjected into a CB17 SCID mouse footpad with 1 μg HA-1H peptide and 25 μg of either control or anticytokine (TGFβ or IL-10) neutralizing antibodies. Swelling response was measured 24 h later by using a thickness gauge.
Detection of IFN-γ–secreting Cells by ELISPOT Assays.
IFN-γ ELISPOT was performed as described previously (18), except that: (a) tetramer-binding cell-depleted PBMCs (TDPs, 125,000) were used as APCs; (b) 1,000 flow-sorted HA-1A2-high or 30,000 HA-1A2-low cells were used as responders; and (c) 1 μg/well HA-1H peptide was used as antigen. In some wells, 10 μg anti-TGFβ, anti–IL-10, or anti–CTLA-4 blocking antibodies were added.
Detection of Microchimerism.
Genomic DNA from PBMCs was amplified by PCR with HA-1 H sequence-specific primers (SSPs); the products were separated and transferred to nylon membrane. After hybridization with a digoxigenin-11-ddUTP–labeled oligonucleotide probe, HA-1 H–specific signal was detected by chemiluminescence. Microchimerism in leukocyte subsets was determined by nested PCR using HA-1 allele-specific primer sets (unpublished data).
Online Supplemental Material.
The online supplemental material contains three figures, as well as additional Materials and Methods and Results. Fig. S1 shows TGFβ response of CD8+ tetramer-low T cells in overnight culture with APCs and peptides. Fig. S2 shows surface phenotype and cytotoxic function of CD8 T cell lines derived by in vitro culture of HA-1/A2 tetramer-low and HA-1/A2 tetramer-high T cells. Fig. S3 shows surface phenotype of HA-1/A2 tetramer-low and HA-1/A2 tetramer-high CD8 T cells in flow cytometry histograms. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20031012/DC1.
Results and Discussion
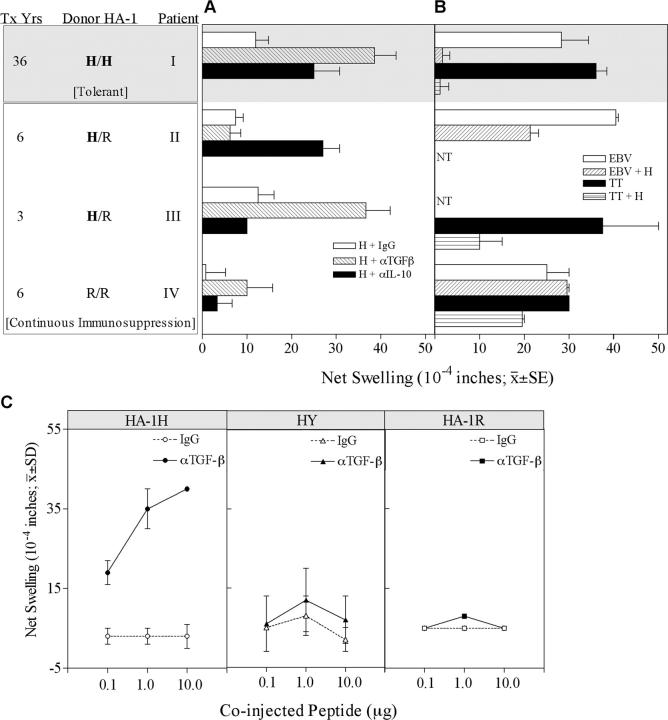
We tested DTH responses to the HA-1H antigen in the footpads of SCID mice. PBMCs were obtained from 4 HLA-A2+, HA-1-R/R recipients of primary renal transplants from HLA-identical sibling donors, including one (patient III), who was tested 3 yr after a second transplant from a sibling matched for HLA-A, -DR, and for 1 (out of 2) HLA-B alleles. Patient I stopped taking azathioprine and prednisone 5 yr after transplant (19) and remained off immunosuppressive drugs with excellent graft function for >30 yr (17). The other three allograft recipients are still taking immunosuppressive drugs. As shown in Fig. 1 A, all PBMCs tested had weak swelling responses to the HA-1H peptide, either in the presence of PBS (unpublished data) or a control IgG. In the three patients who received a HA-1H–mismatched renal transplant, DTH responses to HA-1H peptide were observed when neutralizing antibodies to either IL-10 (patient II), TGFβ (III), or both (I) were coinjected. The control recipient, patient IV, who received a HA-1–matched renal transplant, remained unresponsive to HA-1H after cytokine neutralization (Fig. 1 A). The HA-1H–responsive TR cells also demonstrated “linked suppression” of DTH response to a recall antigen (Fig. 1 B). Although PBMCs of patients I–III had strong DTH responses to TT or EBV antigen alone, these responses were markedly suppressed in the presence of coinjected HA-1H peptide. In contrast, patient IV's PBMCs retained a strong response to recall antigens in the presence of HA-1H (Fig. 1 B).
Figure 1.
Minor H antigen HA-1–mismatched renal transplant recipients have regulated DTH responses to HA-1H peptide. (A) PBMCs were tested for DTH responses to H peptide with control IgG, anti-TGFβ, and anti–IL-10 antibodies. Donor HA-1 typing, years after transplant, and patient status are indicated (gray background, tolerant; white background, continuous immunosuppression). Bars represent mean ± SE of 2–3 determinations. (B) PBMCs were injected with a recall antigen (TT or EBV) alone, or with recall antigen plus H peptide. NT, not tested. (C) Patient I PBMCs were tested for DTH responses in the presence or absence of anti-TGFβ1 neutralizing antibody, with varying doses of HA-1H (left), HY (center), and HA-1R peptide (right).
The DTH response of tolerant patient I to HA-1H was donor specific and dose dependent, with no anti-HY (third party) or anti-HA-1R (self) peptide responses seen in the presence of neutralizing anti-TGFβ antibodies (Fig. 1 C).
Regulator and Effector HA-1–specific T Cells Can Be Distinguished by HLA Tetramer Staining Intensity.
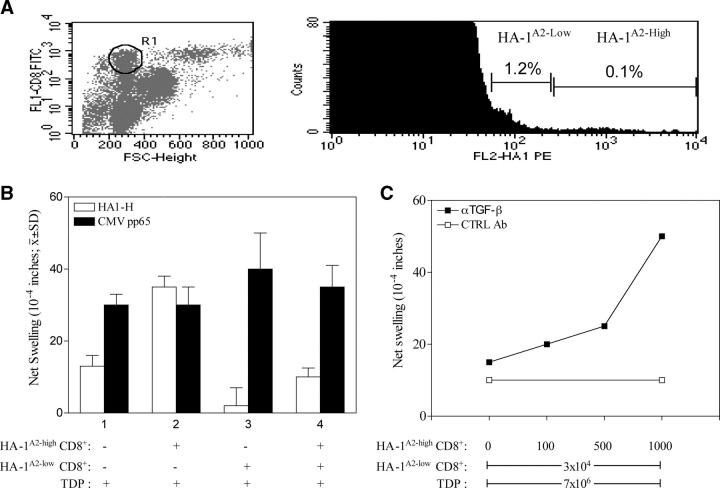
The recovery of a strong DTH response to HA-1H by cytokine neutralization suggested that PBMCs obtained 36 yr after renal transplant contained not one, but two distinct functional subtypes of HA-1H–specific T cells as follows: one mediating DTH, the other producing TGFβ and/or IL-10, thereby suppressing DTH. Two types of minor H antigen-specific T cells were distinguishable by HA-1A2 tetramer (11) staining intensity. Fig. 2 A shows that the majority of the small CD8+ T lymphocytes that stained positively (1.22 ± 0.46%; n = 5) showed low tetramer staining (HA-1A2-low; mean fluorescent index = 58 ± 25). A small portion (0.056 ± 0.027%; n = 5) of the CD8+ T cells showed bright staining (HA-1A2-high; mean fluorescent index = 720 ± 160). Next, sorted HA-1A2-high and HA-1A2-low CD8+ T cells were tested for DTH response to HA-1H peptide. As shown in Fig. 2 B, control mouse footpad injections of PBMCs depleted of tetramer-binding CD8+cells (TDPs) plus HA-1H peptide caused a weak swelling. In the presence of TDP and peptide, 103 HA-1A2 high CD8+ T cells caused a strong DTH response, whereas 3 × 104 HA-1A2-low CD8+ T cells failed to mediate a detectable swelling response. When combined with the HA-1A2-low cells, the DTH response of HA-1A2-high cells was suppressed. TDP still contained CMV-reactive CD8+ T cells, as indicated by a strong DTH response to CMVpp65 (Fig. 2 B). Addition of tetramer-sorted, HA-1A2-low CD8+ T cells had no effect on the DTH response to CMVpp65 (Fig. 2 B), which confirmed that, in the absence of cognate ligand for the CD8+ TR cell, there is no linked suppression of a third party antiviral CD8 TE cell response.
Figure 2.
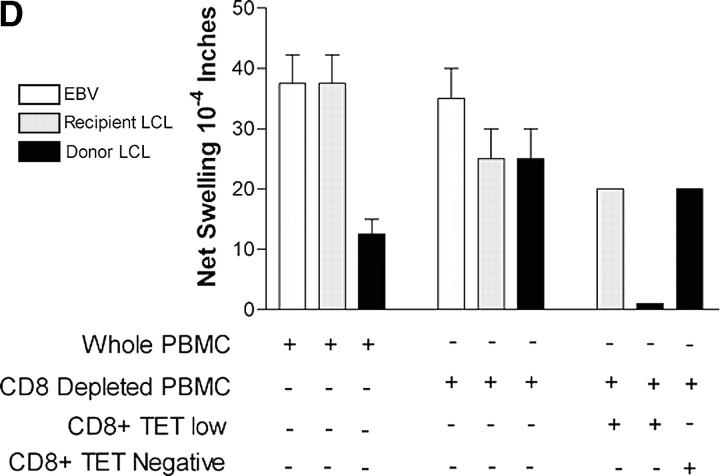
Antagonistic effects of HA-1 tetramer-high and -low binding CD8 T cells on the HA-1H–specific DTH response of patient I and induction of linked suppression by tetramer-low CD8 T cells. (A, left) Forward scatter versus CD8 staining. R1 indicates gating on CD8+ small lymphocytes. (right) Detection of HA-1H–specific CD8+ T cells in the R1 gate using HLA tetramers. The percentage of total CD8+ T cells in each subset, HA-1A2–low and HA-1A2–high, is indicated. This histogram is representative of five experiments. (B) Flow-sorted HA-1A2–high (high, 103) and HA-1A2–low (low, 3 × 104) CD8+ tetramer-binding T cells plus tetramer-depleted PBMCs (TDPs, 7 × 106) were injected separately or along with either HA-1H or CMVpp65 control peptide. The results shown are mean ± SD of three separate experiments. (C) Varying numbers of HA-1A2–high tetramer-binding CD8+ T cells (0 ∼ 103 cells) were tested for DTH response to HA-1H in the presence of 3 × 104 low tetramer-binding cells and 7 × 106 TDP. Either rabbit IgG (open squares) or anti–TGF-β1 antibody (closed squares) was added before footpad injection. (D) Unfractionated PBMCs (8 × 106) or PBMCs depleted of CD8+ T cells (8 × 106) were injected along with EBV antigen (EBV, white bars), recipient (gray bars), or donor (black bars) B-LCLs (2 × 10 5cells). Shown are the mean ± SE of n = 3 DTH determinations. To restore donor-specific DTH suppression in CD8-depleted PBMCs, 9 × 103 HA-1A2–low CD8+ T cells or 9 × 103 HA-1A2–negative CD8+ T cells were added along with either recipient or donor B-LCL.
The suppression by HA-1A2-low CD8+ T cells of the HA-1–specific DTH response of HA-1A2-high CD8+ T cells was reversed by addition of TGFβ neutralizing antibody, but not by a control IgG. The magnitude of the recovered DTH response was dependent on the dose of added HA-1A2-high T cells (Fig. 2 C). Furthermore, the HA-1H peptide, but not CMVpp65, induced approximately twofold increased expression of intracellular TGFβ1 over the background level in sorted HA-1A2-low CD8+ T cells during overnight culture with autologous APCs (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20031012/DC1). To see if the CD8+ TR cells could respond to endogenous HA-1 antigen at levels normally expressed by donor leukocytes, we used a linked suppression system similar to that shown in Fig. 1 B, except that B-LCLs were used as a source of both EBV and endogenous minor H antigen. We found similarly strong DTH responses to autologous B-LCLs and to EBV antigen; however, a markedly reduced response to donor B-LCLs was observed (Fig. 2 D, left). This result was likely due to donor-specific CD8 T cell–mediated suppression because CD8-depleted PBMCs made equally strong DTH responses to both donor and autologous B-LCLs. Addition of flow-sorted HA-1A2-low CD8+ T cells completely suppressed the DTH response to donor, but not autologous, B-LCLs. Similar numbers of tetramer-negative CD8+ T cells failed to mediate significant suppression of DTH (Fig. 2 D, right).
Tetramer-sorted CD8+ T cells from patient I were placed in long-term culture with autologous HA-1H–pulsed DCs. Tetramer-low CTL lines expressed normal membrane levels of TCRαβ and CD8, but required a 100-fold higher HA-1H peptide level to sensitize autologous B-LCLs for lysis, as compared with tetramer-high CTL (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20031012/DC1).
Suppression of In Vitro IFN-γ Production by Addition of TR to TE Cells.
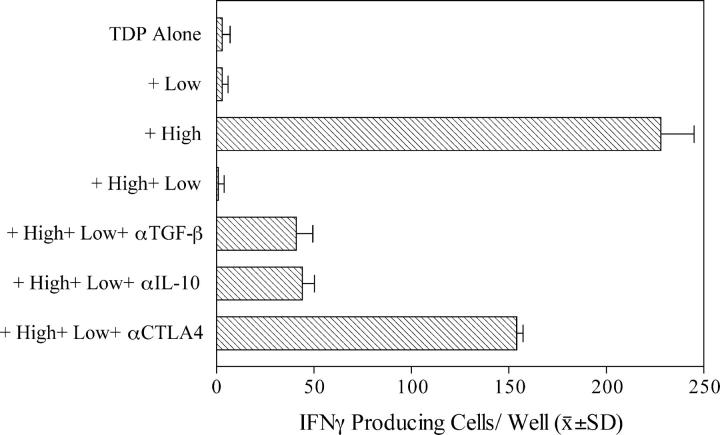
We questioned whether the HA-1A2-high T cells contained IFN-γ producers and whether the HA-1A2-low cells could suppress the IFN production. Tetramer-based flow sorting of PBMCs was performed and in vitro IFN-γ responses of the sorted cells were measured ELISPOT. In wells plated with 1.25 × 105 TDP as a source of APCs or with tetramer-sorted T cells alone (unpublished data), <5 IFN-γ spots/well were detected in the presence of 10 μg/ml of HA-1H peptide. When 103 HA-1A2-high CD8+ T cells were stimulated with HA-1 peptide in the presence of APCs, substantial production of IFN-γ (Fig. 3, >200 spots/well) was observed. In contrast, 3 × 104 HA-1A2-low T cells failed to produce IFN-γ upon HA-1 peptide–specific stimulation. The same TR cells completely inhibited HA-1–specific IFN-γ production by cocultured HA-1A2-high CD8 T cells (Fig. 3). This in vitro inhibition effect, unlike the suppression of the in vivo DTH response, was largely resistant to antibodies neutralizing TGFβ or IL-10 (10% recovery of response), but was sensitive to CTLA-4 antibody blockade (Fig. 3, 70% recovery). Anti–CTLA-4 blocking antibody could also reverse DTH unresponsiveness of whole PBMCs to HA-1H (unpublished data), indicating an important role of CTLA-4 in suppressor cell function both in vivo and in vitro.
Figure 3.
Suppression of IFN-γ response of HA-1A2–high by HA-1A2–low tetramer-binding CD8+ T cells. TDP, flow-sorted HA-1 tetramer-low (low, 3 × 104 cells/well), and tetramer-high (high, 103 cells/well) CD8+ T cells were tested separately or in combination for response to HA-1H (n = 3 wells). Control responses of sorted T cells alone to H peptide (no TDP added) were <5 spots/well.
HA-1H–specific T Cells Are Predominantly of the Effector Memory Phenotype.
Further phenotype analysis of the HA-1–specific TR and TE cells is shown in Fig. S3 (available at http://www.jem.org/cgi/content/full/jem.20031012/DC1) and Table I. Although the tetramer-negative CD8+ T cells from the tolerant patient were predominantly CD28−, 78% of the TR cells and 68% of the TE cells were CD28+. The TR cells consisted mainly of CD45RO+ CD62L− CCR7− T cells of the memory effector type (20). The tetramerhigh CD8+ TE subset did contain a significant component of central memory T cells (Table I, 27%), but like the TR cells, were predominantly memory effector type.
Table I.
Phenotyping of Tetramer HA-1A2 Low, High, and Negative CD8+ T Cells by Flow Cytometric Analysis
| CD8 T cell subset
|
CD45 RO+ CD8 T cells
|
|||||
|---|---|---|---|---|---|---|
| HA-1A2 subsets | CTLA-4+ | CD28+ | CD45RO+ | CD62L− | CCR-7+ | CCR-7− |
| % | % | % | % | % | % | |
| Low | 77 | 78 | 96 | 92 | 4 | 96 |
| High | 50 | 68 | 90 | 82 | 27 | 73 |
| Negative | 24 | 24 | 49 | 92 | 1 | 99 |
Cell surface expression was analyzed for all markers except CTLA-4 (intracellular). Data are summarized from the flow histograms (Fig. S3). Values shown are representative of two separate experiments.
Microchimerism as a Possible Source of Ongoing HA-1 Antigen Stimulation.
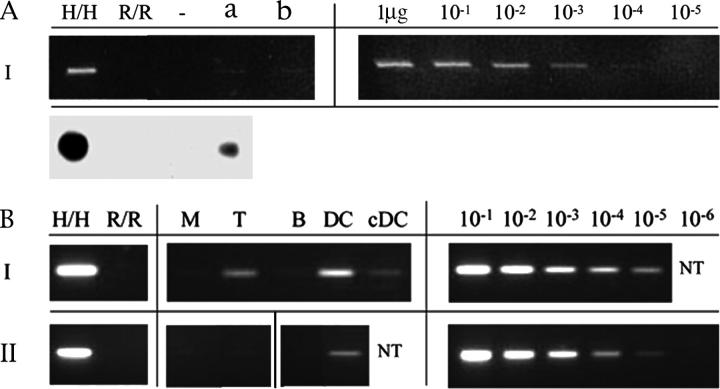
We hypothesized that a source of ongoing antigen stimulation in the HA-1H–regulated patients would account for the HA-1H–specific CD8+ T cells that regulate the DTH response. To identify a continuous source of HA-1 antigen in vivo, we tested for HA-1 H microchimerism. DNA extracted from the PBMCs of patients I and II were analyzed for the presence of HA-1H genomic DNA. Microchimerism corresponding to the lower limit of detection of a PCR/SSP assay (a donor cell frequency of 1/104) was confirmed by sequence-specific oligonucleotide probe in patient I (Fig. 4 A). Next, a nested PCR assay using genomic DNA from flow-sorted T cells, B cells, monocytes, DCs (CD11c+ plus CD123+), and from in vitro–cultured monocyte-derived DCs was performed (Fig. 4 B). The strongest positive signals for HA-1H genomic DNA were detected in the DC-enriched subsets of both patients, despite the low cell recovery in this fraction. A weak positive signal was also detected in T cells and in cultured DCs from tolerant patient I. The HA-1H DNA signals in B cell and monocyte subsets were below the level of detection (1 in 105 cells) in both patients.
Figure 4.
PCR analysis reveals HA-1 microchimerism in the dendritic cell subset of PBMCs. (A) HA-1H+ DNA was detected by PCR/SSP (top) and sequence-specific oligonucleotide probe/Southern Blot assay (bottom left) in whole PBMCs of patient I. DNA from HA-1 H/H and R/R individuals were used as controls. A titration of H/H into R/R genomic DNA (right lanes) is shown. Lanes a and b represent DNA samples derived from 80 and 40 × 106 PBMCs of patient I. (B) Cell subsets were isolated from 300 × 106 PBMCs of patients I and II. HA-1H+ DNA in each cell fraction was detected by nested PCR. Cell fractions are as follows: M, monocytes (6.0 × 106); T, T lymphocytes (15.0 × 106); B, B lymphocytes (1.6 × 106); DC, dendritic cells (0.33 × 106); and cDC, in vitro cultured monocyte-derived DC (0.75 × 106). Right panels indicate the standards (H/H→R/R DNA) for each experiment. NT, not tested.
Here, we describe in a patient with >30 yr tolerance to a renal transplant the coexistence of CD8+ TR cells with TE cells of identical specificity, but differing in strength of binding to cognate peptide/MHC ligand. These findings provide an explanation for the retention of strong but tightly regulated T cell memory responses to the donor minor H antigen, HA-1. Coexistence of TGFβ-producing CD8+ TR cells with IFN-γ–producing CD8+ TE cells, each specific for different HIV epitopes, has been described in chronic HIV infection (21). A similar homeostatic equilibrium, involving CD4+CD25+ TR cells and CD4+ CD25− TE cells has been described for the chronic phase of Leishmania major infection in mice (4).
The in vivo expansion of antigen-specific memory CD8+ T cells with low binding to cognate peptide/MHC ligand and regulatory function is a novel finding. Chronic low avidity engagement of TCR with agonist peptide/MHC complexes in vivo has been shown to render memory CD4+ T cells anergic (22). Indeed, we found that HA-1A2-low CD8+ T cells proliferated poorly in response to HA-1 peptide-pulsed autologous DC, IL-7, and IL-2 stimulation in vitro. In contrast, HA-1A2-high CD8+ cytotoxic TE cell lines showed excellent in vitro growth (unpublished data). These results argue that the TR cells are anergic memory effector T cells. Why antigen restimulation in vivo does not give a selective advantage to high avidity TE cells is not clear. CTLA-4 has been shown to attenuate strong signals generated through the TCR, while permitting the generation of weaker TCR signals (23). This regulatory role of CTLA-4 at the immune synapse has been proposed as a means of broadening the TCR repertoire recruited in response to antigen stimulation by limiting the selective advantage of high affinity over low affinity TCR+ clones (24). Indeed, the majority (>75%) of the HA-1A2-low (TR) cells, and half of the TE cells coexpressed CTLA-4 (Table I and Fig. S3). Because anti–CTLA-4 blocking antibody could reverse TR cell–mediated suppression both in vivo and in vitro, we propose that CTLA-4 plays a dual role in HA-1H–specific CD8+ cell responses, favoring growth and function of TR cells, while restraining that of TE cells.
As yet, no MHC class II–restricted T cell epitopes encoded by the HA-1 gene have been defined. Thus, we cannot rule out the possible involvement of HA-1–specific CD4+ CD25+ TR cells at some phase of the host regulatory T cell response. The fact that CD8 T cell–depleted PBMCs of the tolerant patient failed to manifest linked suppression of DTH to donor B-LCLs would suggest that CD4+ TR cells were not required. However, it is clear that CD4+ TR cells do mediate regulation of DTH and skin allograft rejection in MHC-mismatched, tolerant renal allograft recipients (6, 25, 26).
The hematopoietic-specific HA-1 antigen is not expressed by kidney parenchymal cells. This suggests an indirect role for HA-1–specific TR cells in maintaining renal transplant tolerance. All three HA-1–mismatched patients displayed linked suppression of DTH responses to a third party antigen when HA-1H was present (Fig. 1 B). Therefore, we speculate that the colocalization of HA-1–specific TR cells with donor-derived DCs (Fig. 4 B) may propagate infectious tolerance to antigens shed by the kidney parenchyma and presented by the same DCs to other minor H-specific T cells. The fact that low numbers of HA-1–specific CD8+ TR cells could mediate linked suppression when donor leukocytes were used as the source of endogenous antigen (Fig. 2 D) supports this speculation. Although we cannot rule out the “null” hypothesis (i.e., that HA-1 microchimerism in blood DC precursors is epiphenomenal), we also cannot exclude the alternative possibility that microchimerism sustains minor H antigen-specific CD8+ T memory cells as has been suggested recently (27). Based on the HA-1 typing of patient I's daughter born 20 yr before transplant, as well as the deduced HA-1 heterozygosity of her parents, either the transplant donor (H/H), maternal (H/R), or fetal (H/R) exposures may have contributed to her HA-1 microchimerism.
If the HA-1 H+ DCs do contact the recipient's HA-1–specific T cells in vivo, it is noteworthy that the CD8+ TR cells were found in the small lymphocyte population (Fig. 2 A), and thus by scatter profile were not typical of effector memory T cells that have recently encountered antigen. In this regard, the encounter of plasmacytoid DC with allogeneic CD8+ T cells has been found to induce an IL-10–producing TR cell with a smaller size (based on forward scatter) than that of antigen-activated TE cells (28).
In conclusion, our findings indicate that lifelong peripheral tolerance to an organ allograft can be achieved without the loss of immunologic memory to donor antigen. If these results can be confirmed in other tolerant transplant recipients, it suggests a new rationale for clinical tolerance strategies, taking advantage of the inhibition of memory CD8 TE cells by regulatory T cells with low avidity for the same cognate antigen.
Acknowledgments
The authors would like to thank K. Schell, R. Rigden, D. Rodriguez, L. Haynes, and D. Holte for technical assistance, and Drs. M. Suresh, S. O'Herrin, M. Sandor, G. Splitter, and R.I. Demars for helpful suggestions.
This work was supported by National Institutes of Health grants R21-AI49900, R01-AI44077, and K02-AI01452 (to W.J. Burlingham), P01-CA14520 (University of Wisconsin Clinical Cancer Center), and by the Leiden University Medical Center (to J. Pool and E. Goulmy).
J. Cai and J. Lee contributed equally to this work.
The online version of this article contains supplemental material.
The present address of J. Cai is Terasaki Foundation, Los Angeles, CA 90064.
The present address of J. Lee is Yonsei University Medical School, Seoul 110-744 Korea.
The present address of T. Mutis is University Medical Center, Utrecht, 3584 CX, Netherlands.
References
- 1.Owen, R.D. 1945. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 102:400–404. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 3.Powrie, F., M.W. Leach, S. Mauze, S. Menon, L.B. Caddle, and R.L. Coffman. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1:553–562. [DOI] [PubMed] [Google Scholar]
- 4.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]
- 5.Hall, B.M., N.W. Pearce, K.E. Gurley, and S.E. Dorsch. 1990. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J. Exp. Med. 171:141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara, M., C.I. Kingsley, M. Niimi, S. Read, S.E. Turvey, A.R. Bushell, P.J. Morris, F. Powrie, and K.J. Wood. 2001. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166:3789–3796. [DOI] [PubMed] [Google Scholar]
- 7.den Haan, J.M., L.M. Meadows, W. Wang, J. Pool, E. Blokland, T.L. Bishop, C. Reinhardus, J. Shabanowitz, R. Offringa, D.F. Hunt, V.H. Engelhard, and E. Goulmy. 1998. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science. 279:1054–1057. [DOI] [PubMed] [Google Scholar]
- 8.de Bueger, M., A. Bakker, J.J. Van Rood, F. Van der Woude, and E. Goulmy. 1992. Tissue distribution of human minor histocompatibility antigens. Ubiquitous versus restricted tissue distribution indicates heterogeneity among human cytotoxic T lymphocyte-defined non-MHC antigens. J. Immunol. 149:1788–1794. [PubMed] [Google Scholar]
- 9.Goulmy, E., J.W. Gratama, E. Blokland, F.E. Zwaan, and J.J. van Rood. 1983. A minor transplantation antigen detected by MHC-restricted cytotoxic T lymphocytes during graft-versus-host disease. Nature. 302:159–161. [DOI] [PubMed] [Google Scholar]
- 10.Mutis, T., R. Verdijk, E. Schrama, B. Esendam, A. Brand, and E. Goulmy. 1999. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 93:2336–2341. [PubMed] [Google Scholar]
- 11.Mutis, T., G. Gillespie, E. Schrama, J.H. Falkenburg, P. Moss, and E. Goulmy. 1999. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat. Med. 5:839–842. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson, A.M., X.N. Wang, L. Sviland, F.A. Vyth-Dreese, G.H. Jackson, T.N. Schumacher, J.B. Haanen, T. Mutis, and E. Goulmy. 2002. In situ dissection of the graft-versus-host activities of cytotoxic T cells specific for minor histocompatibility antigens. Nat. Med. 8:410–414. [DOI] [PubMed] [Google Scholar]
- 13.Steinmuller, D. 1967. Immunization with skin isografts taken from tolerant mice. Science. 158:127–129. [DOI] [PubMed] [Google Scholar]
- 14.Starzl, T.E., A.J. Demetris, M. Trucco, H. Ramos, A. Zeevi, W.A. Rudert, M. Kocova, C. Ricordi, S. Ildstad, and N. Murase. 1992. Systemic chimerism in human female recipients of male livers. Lancet. 340:876–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burlingham, W.J., A.P. Grailer, J.H. Fechner, Jr., S. Kusaka, M. Trucco, M. Kocova, F.O. Belzer, and H.W. Sollinger. 1995. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 59:1147–1155. [PubMed] [Google Scholar]
- 16.Anderson, C.C., and P. Matzinger. 2001. Immunity or tolerance: Opposite outcomes of microchimerism from skin grafts. Nat. Med. 7:80–87. [DOI] [PubMed] [Google Scholar]
- 17.VanBuskirk, A.M., W.J. Burlingham, E. Jankowska-Gan, T. Chin, S. Kusaka, F. Geissler, R.P. Pelletier, and C.G. Orosz. 2000. Human allograft acceptance is associated with immune regulation. J. Clin. Invest. 106:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tary-Lehmann, M., D.E. Hricik, A.C. Justice, N.S. Potter, and P.S. Heeger. 1998. Enzyme-linked immunosorbent assay spot detection of interferon-gamma and interleukin 5-producing cells as a predictive marker for renal allograft failure. Transplantation. 66:219–224. [DOI] [PubMed] [Google Scholar]
- 19.Uehling, D.T., J.L. Hussey, A.B. Weinstein, R. Wank, and F.H. Bach. 1976. Cessation of immunosuppression after renal transplantation. Surgery. 79:278–282. [PubMed] [Google Scholar]
- 20.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 21.Garba, M.L., C.D. Pilcher, A.L. Bingham, J. Eron, and J.A. Frelinger. 2002. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J. Immunol. 168:2247–2254. [DOI] [PubMed] [Google Scholar]
- 22.Mirshahidi, S., C.T. Huang, and S. Sadegh-Nasseri. 2001. Anergy in peripheral memory CD4+ T cells induced by low avidity engagement of T cell receptor. J. Exp. Med. 194:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egen, J.G., and J.P. Allison. 2002. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 16:23–35. [DOI] [PubMed] [Google Scholar]
- 24.Egen, J.G., M.S. Kuhns, and J.P. Allison. 2002. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 3:611–618. [DOI] [PubMed] [Google Scholar]
- 25.Torrealba, J., M. Katayama, J.H. Fechner, E. Jankowska-Gau, S. Kusaka, Q. Xu, J.M. Schultz, T.D. Oberley, H. Hu, M.M. Hamawy, et al. 2004. Metastable tolerance to Rhesus monkey renal transplants is correlated with allograft TGFβ1+CD4+ T regulatory cell infiltrates. J. Immunol. In press. [DOI] [PubMed] [Google Scholar]
- 26.Lin, C.Y., L. Graca, S.P. Cobbold, and H. Waldmann. 2002. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat. Immunol. 3:1208–1213. [DOI] [PubMed] [Google Scholar]
- 27.Verdijk, R.M., A. Kloosterman, J. Pool, M. Van De Keur, A.M. Naipal, A.G. Van Halteren, A. Brand, T. Mutis, and E. Goulmy. 2004. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 103:1961–1964. [DOI] [PubMed] [Google Scholar]
- 28.Gilliet, M., and Y.J. Liu. 2002. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]