Abstract
CD4+CD25+ regulatory T cells contribute to the maintenance of peripheral tolerance by active suppression because their deletion causes spontaneous autoimmune diseases in mice. Human CD4+ regulatory T cells expressing high levels of CD25 are suppressive in vitro and mimic the activity of murine CD4+CD25+ regulatory T cells. Multiple sclerosis (MS) is an inflammatory disease thought to be mediated by T cells recognizing myelin protein peptides. We hypothesized that altered functions of CD4+CD25hi regulatory T cells play a role in the breakdown of immunologic self-tolerance in patients with MS. Here, we report a significant decrease in the effector function of CD4+CD25hi regulatory T cells from peripheral blood of patients with MS as compared with healthy donors. Differences were also apparent in single cell cloning experiments in which the cloning frequency of CD4+CD25hi T cells was significantly reduced in patients as compared with normal controls. These data are the first to demonstrate alterations of CD4+CD25hi regulatory T cell function in patients with MS.
Keywords: tolerance, autoreactive T cells, autoimmune disease, L-selectin
Introduction
Clonal deletion of self-reactive T cells in the thymus and induction of T cell anergy do not alone explain the maintenance of immunologic self-tolerance, as potentially pathogenic auto-reactive T cells are present in the periphery of healthy individuals (1, 2). Thus, other regulatory mechanisms exist to prevent autoreactive T cells from causing immune disorders. Active suppression by regulatory T cells plays a key role in the control of self-antigen–reactive T cells and the induction of peripheral tolerance in vivo (3, 4). Seminal experiments performed by Sakaguchi et al. have shown that depletion of CD4+CD25+ suppressor cells results in the onset of systemic autoimmune diseases in mice (5). Furthermore, cotransfer of these cells with CD4+CD25− cells prevents the development of experimentally induced autoimmune diseases such as colitis, gastritis, insulin-dependent autoimmune diabetes, and thyroiditis (6–10). We and others have recently described a population of CD4+CD25hi regulatory T cells in human peripheral blood and thymus (11–13). Human CD4+CD25hi T cells, similar to the mouse CD4+CD25+ suppressor cells, are anergic to in vitro antigenic stimulation and strongly suppress the proliferation of responder T cells upon coculture (14). CD4+CD25+ T cells are among the best-characterized immunoregulatory subsets shown to prevent activation and effector function of activated responder T cells (15).
Multiple sclerosis (MS) is a chronic inflammatory disease characterized by lymphocyte infiltration and inflammation of the central nervous system (CNS) white matter. T cells recognizing myelin protein peptides are likely involved in the pathogenesis of the disease (16–18). Although autoreactive T cells are present in healthy individuals and patients with autoimmune disorders, autoreactive T cells found in patients with autoimmune disease are more easily activated as compared with those from normal subjects (19–22). This finding led us to hypothesize that either deficient generation or reduced effector function of CD4+CD25hi T cells play a role in regulating the autoimmune response in patients with MS. Thus, we compared the frequency and function of CD4+CD25hi T regulatory cells derived from a group of untreated patients who have relapsing/remitting (RR) MS with those from age matched healthy control subjects.
Materials and Methods
Subjects.
We enrolled 15 patients in this study with definite MS upon informed consent. This work was approved by the Institutional Review Board at the Brigham and Women's Hospital. The patients were between the ages of 25 and 57 yr, and all had RR disease with Kurtzke Expanded Disability Status Scale scores between 0 and 2.5. Patients were not treated with any immunomodulatory drugs and did not receive corticosteroids within 3 mo of study entry. 21 normal healthy donors between the ages of 24 and 57 yr and with no history of autoimmune diseases were also enrolled in the study. Three healthy subjects between the ages of 20–30 received the influenza virus vaccination, and blood was drawn at the time points outlined in the Results section.
Cell Culture Reagents.
Cells were cultured in RPMI 1640 medium supplemented with 5 mM Hepes, 2 nM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (BioWhittaker), 0.5 mM sodium pyruvate, 0.05 mM of nonessential amino acids (GIBCO BRL), and 5% human AB serum (Omega Scientific Inc.) in U-bottom 96-well plates (CoStar).
Cell Purification.
Human CD4+CD25hi and CD4+CD25− T cells were separated on a FACSVantage™ SE (BD Biosciences) cell sorter. PBMCs were isolated by Ficoll-Paque (Amersham Biosciences) gradient centrifugation. Approximately 2 × 108 cells for each experiment were incubated for 1 h in the culture medium, with 300 μl anti–CD4-CyChrome (cat. no. 555348, IgG1; BD Biosciences), anti–CD25-PE (cat. no. IM0479, IgG2a; Immunotech), and a cocktail of anti-CD14 (cat. no. 30544X, IgG2a), anti-CD32 (cat. no. 30934X, IgG2b), and 20 μl anti-CD116 (cat. no. 18774B, IgM), all FITC labeled (BD Biosciences). Control PBMCs (106 cells each) were also stained with the aforementioned antibodies in combination with different mouse IgGs (IgG2a PE-labeled cat. no. 55574; IgG1 CyChrome-labeled cat. no. 555750; IgG1 FITC-labeled cat. no. 555748; IgG2a FITC-labeled cat. no. 555573, and IgG2b FITC-labeled cat. no. 555742; all purchased from BD Biosciences). Lymphocytes were washed and sorted according to their forward and side scatter properties, excluding large activated cells. T cell–depleted accessory cells were obtained by negative selection of PBMCs incubated with anti-CD2–coated beads (111.13; Dynal) and irradiated at 3,300 rad.
Proliferation Assay.
CD4+CD25− (responders) and CD4+ CD25hi (suppressors) T cells (2.5 × 103 cells/well) were stimulated with plate-bound anti-CD3 (clone UCHT1; BD Biosciences) used at three different concentrations (0.1, 0.5, and 2.5 μg/ml, coated in PBS for 2–4 h at 37°C), or soluble anti-CD3 (clone HIT3a at 5 μg/ml) together with soluble anti-CD28 (clone 28.2 at 5 μg/ml) in U-bottom 96-well plates. CD4+CD25− T cells were also cocultured with decreasing numbers of CD4+CD25hi T cells (responder/suppressor ratios: 1:1, 1:1/2, 1:1/4, and 1:1/8). All cells were cultured in a final volume of 200 μl in the presence of 104 T cell–depleted accessory cells/well. After 5 d of culture, 100 μl of supernatant was removed from each well and used for cytokine detection, and 1 μCi [3H]thymidine (NEN Life Science Products) was added to each well. The cells were harvested after 16 h; cpm/well was determined by scintillation counting (PerkinElmer). Mixing experiments were performed in the presence of fixed accessory cells autologous to the CD4+CD25− responder T cells.
Measurement of Cytokine Level.
The supernatants removed from each well before [3H]thymidine addition were diluted and analyzed to determine the cytokine profile by ELISA using Immulon 4 ELISA plates (Dynex Technologies). Antibody pairs and standards for IFNγ were purchased from Endogen. Abs for IL-13 and IL-10 were purchased from BD Biosciences. Avidin–peroxidase conjugate (Sigma-Aldrich) and tetramethylbenzidine peroxidase substrate (Kirkegaard and Perry Laboratories) were used to develop the assay. For IL-6 detection, the Human Inflammatory Cytometric Bead Array kit obtained from BD Biosciences was used.
Generation of CD4+CD25hi Clones.
PBMCs were stained with the aforementioned antibodies for flow cytometry. The CD4+CD25hi and CD4+CD25− cells were single cell sorted into 96-well plates. The cells were stimulated (modification of method described by Levings et al.; reference 23) with PHA (Boehringer) at 0.05 μg/ml, autologous PBMCs (6,000 rad) were stimulated at 3 × 104 cells/well, JY cells (10,000 rad) were stimulated at 104 cells/well, and IL-2 (Teceleukin; National Cancer Institute) were stimulated at 100 U/ml. Half the medium was replaced at day 7, and every 3–4 d thereafter it was replaced with fresh medium containing 100 U/ml IL-2. The cultures were restimulated with 0.05 μg/ml PHA, a mixture of irradiated autologous (1–3 × 104 cells/well) and allogeneic (1–3 × 104 cells/well) PBMCs, and 100 U/ml IL-2. After 2–3 wk, growing clones were tested for regulatory activity as defined by suppression of cocultured anti-CD3–mediated T cell proliferation and the cloned cell dependence on exogenous IL-2 for proliferation. With these experiments, we further confirmed the data from Levings et al. showing the possibility of single cell cloning of CD4+CD25hi regulatory cells. Clones were analyzed in cocultures containing 4 × 103 autologous CD4+CD25− T cells or 4 × 103 PBMCs/well and 2 × 103 cloned cells/well. Stimulation was provided by the addition of magnetic beads with covalently attached anti-CD3 (UCHT1, bound to tosyl-activated magnetic beads at 1 μg/107 beads; Dynal Biotech Inc.). The beads were added to the cultures at 1.5 × 104 cells/well. 3[H]Thymidine was added to the cultures after 4 d of stimulation. Percent suppression was determined as 1 − (cpm incorporated in the coculture)/cpm of responder population alone) × 100%.
Statistical Analysis.
The mean ± SE thymidine uptake and cytokine secretion of triplicate cultures was calculated for each experimental condition. The response of CD4+CD25− T cells were normalized to 100 to calculate the percent suppression resulting from the addition of CD4+CD25hi to the cultures. The Mann-Whitney test was used to evaluate possible differences in the CD4+CD25hi function between patients with MS and healthy donors. The number of wells that produced growth from the cloning of cells derived from patients with MS and healthy controls was compared using hierarchical logistic regression analysis.
Results
CD4+CD25hi T Cells Are Present with the Same Frequency in Healthy Donors and Patients with MS.
We stained whole mononuclear cells from freshly drawn human blood with different combination of anti-CD4–CyChrome, anti-CD25–PE, and a cocktail of FITC-labeled anti-CD14, anti-CD32, and anti-CD116 antibodies. The cells were gated on lymphocytes via their forward and side scatter features, and all FITC-labeled cells were negatively selected during sorting. Human peripheral blood contains a heterogeneous population of CD4+CD25+ T cells that express either moderate levels of CD25 consisting of nonregulatory T cells or high levels of CD25 that exhibit regulatory function (24). As there are no other known cell surface markers able to identify regulatory T cells ex vivo, we used CD25 expression to discriminate regulatory T cells in humans. We analyzed the mean fluorescent intensity of the CD25+ population in both patients with MS and control subjects and found no differences between the two groups. Similarly, no differences in the frequency of CD4+CD25hi T cells were found between patients and healthy controls; ∼10% of CD4 T cells express the α-chain of IL-2 receptor CD25, whereas only 1–2% of the CD4 T cells are CD25hi in both subject groups. The mean of frequency was 1.4% ± 0.3 in the healthy control group and 1.2% ± 0.2 in the MS patient group; no statistically significant differences were found between the two populations (P = 0.21).
CD4+CD25hi Regulatory T Cells Display Impaired Function in Patients with MS.
Because it was critical to examine the regulatory T cell function, we isolated highly pure CD4+CD25hi regulatory and CD4+CD25− responder cell populations by high speed flow cytometric sorting. CD4+ CD25− responder cells from both patients with MS and healthy individuals responded similarly in a dose-dependent fashion to varying concentration of plate-bound anti-CD3 mAb. CD4+CD25hi T cells isolated from both groups were anergic to stimulation at all doses of plate-bound anti-CD3, indicating that CD4+CD25hi T cells isolated from patients with MS do exhibit this regulatory property.
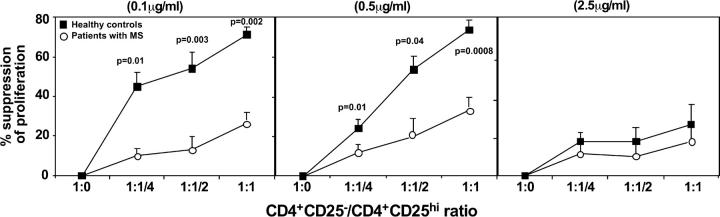
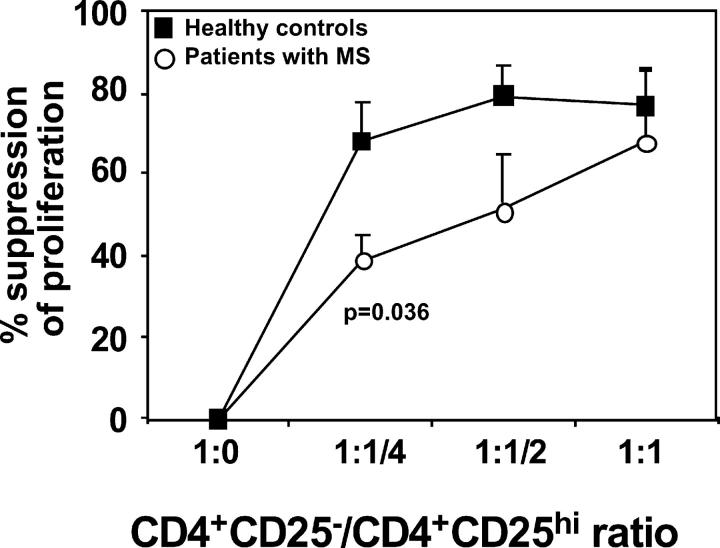
To quantitate their regulatory function, CD4+CD25hi T cells were cocultured with autologous responder cells (2.5 × 103 cells/well) at different ratios (responder/suppressor ratios: 1:1, 1:1/2, 1:1/4, and 1:1/8). As reported previously in healthy individuals, CD4+CD25hi T cells consistently suppressed proliferation at a 1:1 ratio (Figs. 1 A and 2). Increasing the ratio of responder/suppressor T cells resulted in less suppression (Fig. 2). In striking contrast, the regulatory CD4+CD25hi T cells isolated from the circulation of patients with MS (while similar in frequency as compared with healthy controls) poorly inhibited responder CD4+CD25− T cell proliferation (Figs. 1 B and 2). Because we have shown previously that increased strength of signal inhibits regulation (24), we stimulated cocultures of regulatory and responder T cells from patients with MS and healthy controls with a maximal concentration (2.5 μg/ml) of plate-bound anti-CD3 mAb. As predicted, the CD4+ CD25hi regulatory T cells no longer suppressed the proliferation of responder T cells (Fig. 2).
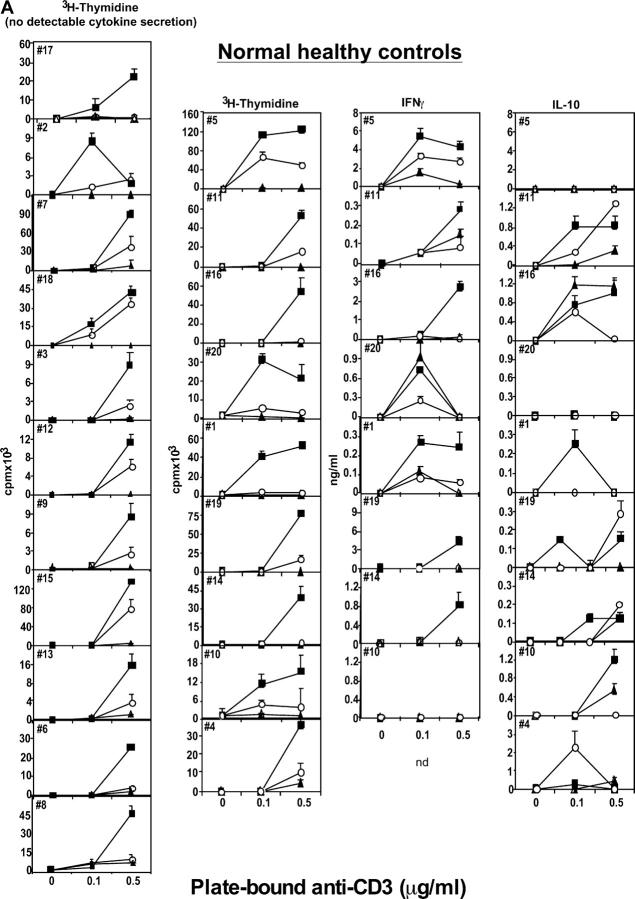
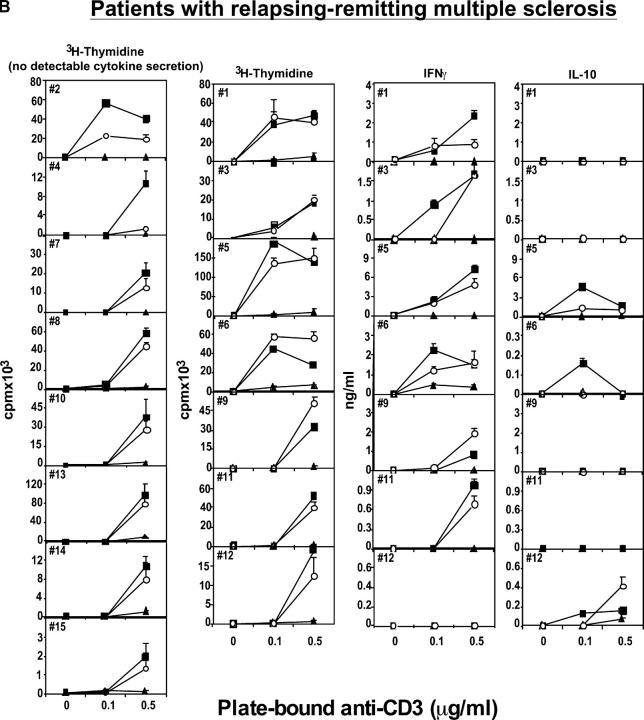
Figure 1.
Human CD4+CD25hi T cells mediate suppression of CD4+CD25− responder cell proliferation. (A) The regulatory properties of CD4+CD25hi T cells were examined from 21 healthy individuals or (B) from 15 patients with RR MS. CD4+CD25− responder (▪) and CD4+CD25hi suppressor (▴) cells (2.5 × 103 cells/well) were stimulated with plate-bound anti-CD3 (0.1 and 0.5 μg/ml). CD4+CD25− T cells were cocultured with CD4+CD25hi T cells at a 1:1 ratio (○). T cell–proliferative responses are expressed as the mean ± SE of triplicate cultures. Culture supernatants were diluted and analyzed to determine the cytokine profile. In the series of experiments shown on the left, cytokine secretion was not measurable. In the subsequent series, IFNγ and IL-10 were detected; their values, representing the mean ± SE of triplicate cultures, are expressed as ng/ml.
Figure 2.
Summary of CD4+CD25hi T cell regulatory function that is altered in patients with MS. The mean percent inhibition of the proliferative response by CD4+CD25hi cells derived from 15 MS patients (○) and 21 healthy controls (▪) was calculated. CD4+CD25− and CD4+CD25hi populations were stimulated with plate-bound anti-CD3 mAb, alone or cocultured at varying ratios. The proliferative response was inhibited upon addition of CD4+CD25hi cells to the CD4+CD25− responder cells at a 1:1 ratio in normal controls. Regulatory T cells from patients with MS exhibited significantly less suppressor activity. Decreasing the number of CD4+CD25hi T cells (responder/suppressor ratios: 1:1/2 and 1:1/4) resulted in less suppression in all the conditions examined.
We also examined the production of cytokines in all the cultures and the ability to inhibit their secretion by CD4+CD25hi T cells cocultured with CD4+CD25− responder cells. The secretion of the Th1 cytokine IFNγ, as would result from the activation of destructive, autoreactive T cells, was suppressed in CD4+CD25hi T cell cocultures from healthy controls, but not in cocultures derived from patients with MS (Fig. 1, A and B). Because there was no detectable secretion of IL-13 at the cell numbers used in these experiments, those data are unpublished. IL-10 was variably secreted, predominantly by the CD4+CD25− T cells. The secretion of IL-10 was often reduced upon coculture with CD4+CD25hi T cells, excluding a potential role of this cytokine in mediating this regulatory suppressor function (Fig. 1, A and B). In this ex vivo model of suppression, blocking IL-10 or TGFβ does not result in loss of suppressor function by CD4+CD25+ regulatory T cells (unpublished data).
Recent studies have shown that the presence of IL-6 can contribute to the loss of CD4+CD25+ suppression (25). We measured the levels of IL-6 secretion in the cultures of five healthy individuals and four patients with MS to investigate its potential role in the lack of suppression observed with patient-derived cells. We found that IL-6 levels did not correlate with the presence or absence of CD4+CD25hi T cell–mediated suppression.
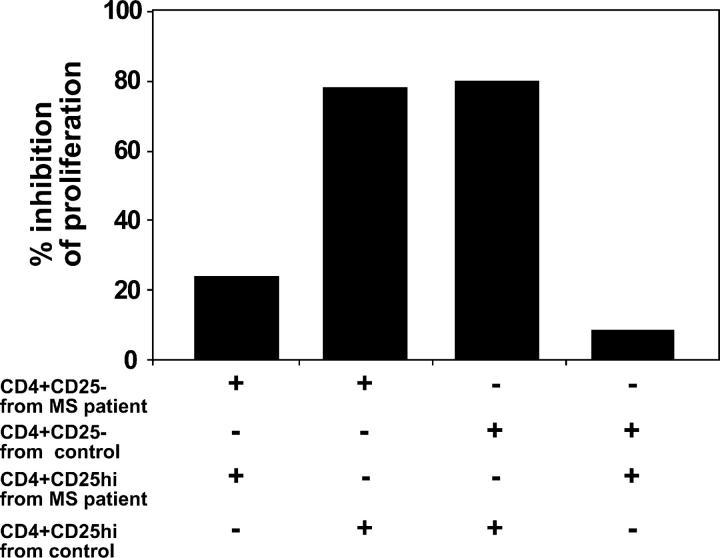
It was also important to examine whether the loss of regulatory function was due to a decrease in CD4+CD25hi T cell function or an increase in the resistance of activated CD4+CD25− responder T cells to inhibition. Thus, we performed mixing experiments in which patient and control regulatory CD4+CD25hi T cells were cocultured with the autologous and the converse target cells isolated from either healthy subjects or patients with MS. Regulatory T cells from patients with MS could not suppress the proliferative response of target responder T cells from either patients or healthy controls (suppression ≤23%). In contrast, in the reciprocal experiments, regulatory CD4+CD25hi T cells from healthy controls suppressed the proliferative response of target CD4+CD25− T cells derived from both controls and patients with MS (suppression ≥78%; Fig. 3). These data indicate that the primary regulatory defect is in the function of CD4+CD25hi T cells isolated from the circulation of patients with MS.
Figure 3.
CD4+CD25hi T cells from patients with MS do not inhibit proliferation of responder T cells isolated from either the autologous individual or healthy donors. CD4+CD25− responder T cells and CD4+CD25hi cells from MS patients and normal controls were stimulated with plate-bound anti-CD3 at 0.5 μg/ml. 2.5 × 103 cells/well responder T cells from MS patients were cocultured with the same number of autologous CD4+CD25hi regulatory T cells (1st bar) or with regulatory cells isolated from healthy donors (2nd bar). Conversely, responder T cells from healthy donors were cocultured either with CD4+CD25hi cells derived from the same subject (3rd bar), or with regulatory cells isolated from patients with MS (4th bar).
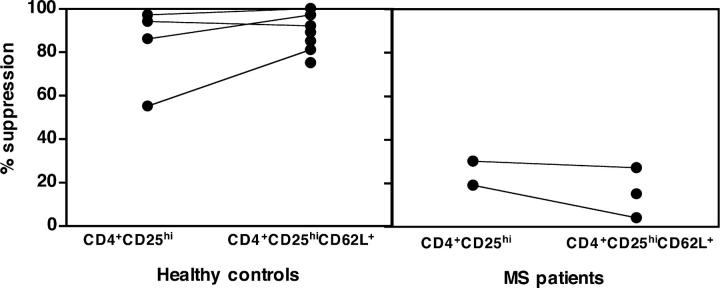
CD62L Expression on CD4+CD25hi Regulatory T Cells.
Although there were no differences in the frequency of CD4+CD25hi T cells or in their proliferation or cytokine secretion in response to different stimuli between healthy subjects and patients with MS, it was important to determine whether an increase in the frequency of activated CD4+ T cells in the circulation of patients with MS may be diluting the regulatory CD4+CD25hi T cells. Therefore, we further restricted the isolation of T regulatory cells by sorting only those CD4+CD25hi T cells that expressed CD62L because L-selectin expression is down-regulated upon activation. We isolated CD4+CD25hiCD62L+ and total CD4+CD25hi regulatory T cells from healthy subjects and patients with MS and found that, whereas in the healthy controls both populations were able to suppress the proliferative response to anti-CD3 stimulation, neither population of regulatory cells isolated from patients with MS was able to inhibit the proliferation of the CD4+ CD25− responder population (Fig. 4). The fact that the CD4+CD25hiCD62L+ T cells isolated from patients are depleted of the potentially activated CD62L− T cells yet still unable to suppress indicates that the impaired regulatory T cell function in patients with MS is not due to contamination by activated T cells.
Figure 4.
The CD62L+ subset of CD4+CD25hi regulatory T cells exhibits decreased suppressive function in patients with MS. CD4+CD25hiCD62L+ T cells were isolated from seven healthy individuals (left) and three patients with MS (right). Each symbol is representative of a different individual. Total CD4+CD25hi T cells were also isolated from four out of seven healthy subjects and two out of three patients. The percent suppression of coculture proliferation in response to plate-bound anti-CD3 was calculated in each individual. The CD4+CD25hi regulatory T cells derived from healthy controls were able to induce strong inhibition of the proliferative response (55–97% suppression) as shown previously. The CD62L+ subset of CD4+CD25hi T cells derived from the same individuals exhibit enhanced suppressive capacity. In contrast, the regulatory cells derived from patients, although depleted of CD62L− activated T cells, show decreased inhibitory function (4–29% suppression) as compared with the suppression observed in healthy individuals.
In Vivo Immunization Did Not Alter CD4+CD25hi Regulatory T Cell Function.
To further rule out the possibility that impaired suppressive function observed in patients with MS was due to an increase in the frequency of activated CD4+ T cells in the circulation or due to an altered activation state of the responder cells, we examined the regulatory function of CD4+CD25hi T cells derived from healthy subjects recently immunized with the influenza virus vaccine. The regulatory T cells from three subjects examined after vaccination showed intact suppressive ability (93, 84, and 65% suppression of proliferation). One individual was examined both before and after vaccination and demonstrated no differences in the suppressive ability (68% suppression before and 65% after vaccination). These data provide evidence that an ongoing immune response does not affect the intrinsic ability of CD4+CD25hi regulatory T cells to function.
CD4+CD25hi Regulatory T Cells in Other Autoimmune Diseases.
We examined the regulatory activity of CD4+ CD25hi T cells isolated from a small series of patients with other autoimmune diseases to confirm our hypothesis that the changes in function of these regulatory CD4+CD25hi T cells would not be specific to a single autoimmune disease. The frequency of CD4+CD25hi T cells in this control group was 1.4% ± 0.4; no differences were found between these patients and the subjects with MS (P = 0.44) or the healthy individuals (P = 0.35). The regulatory cells derived from patients with thyroiditis and psoriasis showed reduced suppressive capacity (7 and 19%, respectively), whereas the regulatory activity of cells from the patients with inflammatory bowel disease and ankylosing spondylitis showed strong suppressive activity (97 and 68%, respectively). These preliminary data suggest the need for a more extensive analysis of the role of the regulatory T cell subset in other autoimmune diseases and are consistent with our hypothesis that dysfunctions in regulatory T cells may be viewed as one of many risk factors in human autoimmune disorders.
The Nature of TCR Signaling Determines Suppressor Function.
We have shown previously that a qualitatively different stimulus, such as soluble anti-CD3 and anti-CD28, induced lower levels of responder T cell proliferation as compared with plate-bound anti-CD3 and, thus, was more significantly inhibited upon coculture with CD4+CD25hi. It was of interest to examine whether differences in suppressor function by CD4+CD25hi cells between patients with MS and control subjects would be observed with qualitative changes in TCR signaling. Cocultures of regulatory and responder T cells from patients and controls were stimulated with soluble anti-CD3 and anti-CD28. As expected, the magnitude of the proliferative response was significantly lower than that obtained with plate-bound anti-CD3 from the same individual. The CD4+CD25− T cells isolated from patients and controls responded similarly to this stimulus. Yet, in contrast to plate-bound anti-CD3, the qualitatively different TCR signal delivered by soluble anti-CD3 and anti-CD28 allowed the appearance of suppressor function by cocultured CD4+CD25hi regulatory T cells derived from patients with MS, particularly at higher ratio of regulatory T cells in the culture (Fig. 5).
Figure 5.
The proliferative response in both patients and healthy controls is suppressed in cultures receiving T cell receptor signals of different natures. CD4+CD25− responder T cells and CD4+CD25hi cells from six MS patients and six normal controls were stimulated with soluble anti-CD3 and anti-CD28 (5 μg/ml) alone or cocultured at different responder/suppressor ratios (1:1, 1:1/2, and 1:1/4). The average proliferative response was 13,494 cpm in the patient group and 15,974 cpm in the control group. The percent inhibition of coculture proliferation was calculated in patients with MS (○) and healthy controls (▪).
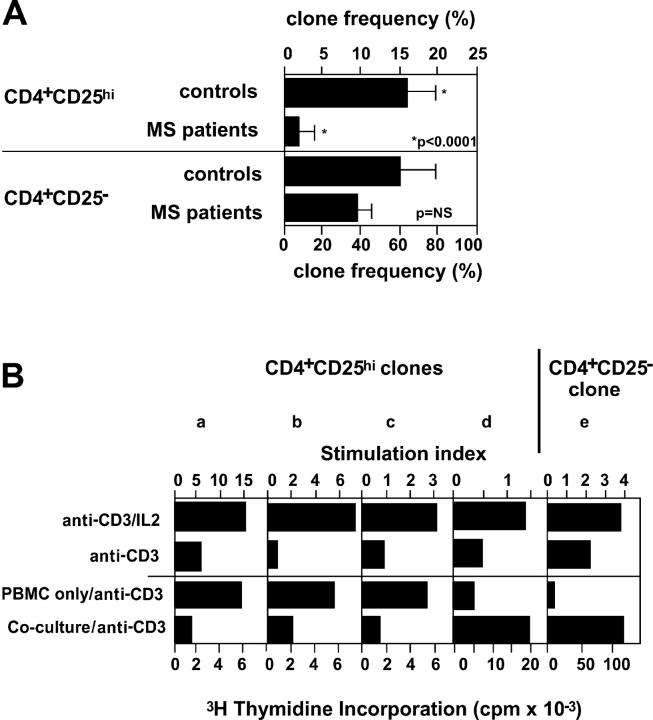
Low CD4+CD25hi Cloning Frequency in Patients with MS.
To examine potential mechanisms for the loss of regulatory T cell function, we directly single cell sorted CD4+CD25hi and CD4+CD25− cells from patients and healthy subjects and analyzed their frequency and functional activity. Although the cloning frequency of CD4+CD25− T cells was similar in patients with MS and control subjects, the cloning frequency of CD4+CD25hi cells was significantly lower in patients with MS as compared with normal control subjects (Fig. 6 A). Due to the slow growth of the clones, we tested their regulatory activity after a second round of PHA/IL-2 stimulation. We examined IL-2–dependent TCR-mediated proliferation and inhibition of T cell proliferation as two major characteristics of regulatory T cells (Fig. 6 B). By these criteria, 60% of the CD4+CD25hi clones generated from healthy controls and 17% of the clones derived from patients with MS exhibited regulatory activity.
Figure 6.
Reduced ability to generate CD4+CD25hi clones from blood of MS patients. (A) CD4+CD25hi or CD4+CD25− T cells from patients with MS and healthy controls were cloned as described in Materials and Methods. The difference in the cloning frequency of the regulatory CD4+CD25hi cells between patients with MS and healthy subjects was highly significant (P < 0.0001), whereas the ability to generate clones from CD4+CD25− cells was not different (P = 0.21). (B) T cell clones were tested for dependency on exogenous IL-2 by stimulation with anti-CD3 beads ± IL-2 (data are presented as stimulation indices). The same clones were also tested for their ability to inhibit the proliferation of cocultured responder T cells upon stimulation with anti-CD3 beads. The three representative CD4+CD25hi clones (a–c) shown suppress the proliferation of autologous CD4+CD25− T cells, whereas a T cell clone (d) representing a minor population of the clones derived from the CD4+CD25hi population is shown, and all of the CD4+CD25− clones (e) did not inhibit proliferation of cocultured T cells.
Discussion
It has become clear that defects in different populations of regulatory T cells contribute to the induction of autoimmune diseases in animal models. Here, we examined whether a similar defect in regulatory CD4+CD25hi T cells occurs in humans with MS. We observed a significant reduction in the effector functions of this regulatory T cell population in subjects with MS compared with healthy donors. These data are among the first clear demonstrations of the functional defect in CD4+CD25hi regulatory T cells in a human autoimmune disease.
An important aspect of these investigations was the measurement of regulatory T cell function as opposed to simple phenotypic measurement of CD4+CD25+ T cell frequency. Unlike 6-wk-old mice raised in clean facilities, whose total CD4+CD25+ T cell population manifests regulatory properties, humans are exposed to myriad infections and show a significant population of CD4+ CD25medium/low T cells that do not exhibit regulatory function (24). Thus, it was critical to examine the functional state of the regulatory T cells expressing high levels of CD25. We have demonstrated previously that the strength of signal delivered through the TCR of target T cells is one factor determining whether regulatory CD4+CD25hi T cells can suppress the responder T cell proliferation (24). Thus, to properly examine the function of regulatory CD4+CD25hi T cells, we used several different strengths of stimulatory signals in these experiments. We observed that the strong signal provided by maximal concentration of plate-bound anti-CD3 mAb similarly abrogated suppression in both patient and control cocultures. In contrast, lower concentrations of plate-bound anti-CD3 delivered a signal that resulted in the appearance of a significant defect in the suppressive function of this subset of regulatory cells derived from patients with MS.
The use of different stimulatory conditions allowed us to reveal alterations in the regulatory function of CD4+CD25hi T cells while still demonstrating that they are CD25+ regulatory T cells as opposed to activated responder cells expressing CD25. Stimulation of cultures with soluble anti-CD3 and anti-CD28, which have previously shown to be the most permissive for enabling coculture suppression, gave equivalent levels of suppression in patients and control subjects when cocultured at 1:1 ratios. In contrast, the stimulation provided by plate-bound anti-CD3 at 0.1 and 0.5 μg/ml resulted in a threefold decrease of suppression by CD4+CD25hi cells derived from patients with MS as compared with normal controls. Previous experiments showed that delivering qualitatively different T cell receptor signals to responder T cells, such as that provided by self-antigens as compared with microbial antigens, results in a greater sensitivity to suppression. Thus, the present findings may help explain defects in suppression of autoreactive T cells in autoimmune patients as compared with T cells stimulated by microbial antigens during infections.
Because it was of interest to examine the regulatory function of CD4+CD25high T regulatory cells in an antigen-specific system, we attempted to induce antigen-specific responses to myelin basic protein (MBP) in this in vitro system. However, as expected with the low frequency of MBP-specific CD4+CD25− effector T cells and the low number of CD4+CD25high T regulatory cells that can be isolated by FACS®, it was not possible to directly measure suppression of autoantigen-specific T cells in patients. As the frequency of MBP-specific T cells appears to be in the range of 1/106 cells, the majority of the effector T cells that in our assay are cultured at 1–2 × 104 cells/well do not respond to the antigen.
An important control to note in all these experiments is the anergy or lack of thymidine incorporation resulting from stimulation of CD4+CD25hi T cells cultured alone. This anergy indicates that CD4+CD25hi T cells isolated from patients with MS are not CD25+-activated T cells because such cells would not exhibit regulatory activity, but rather enhance proliferation. It was critical to determine whether the decrease in T cell regulatory function observed in patients with MS was due to a defect in the CD4+CD25hi T cell subset or whether the responder CD4+CD25− T cells were refractory to suppression. By performing comixing experiments, we could clearly demonstrate that the defect lies in the CD4+CD25hi T cell function, as opposed to enhanced responder T cell resistance in patients with MS.
In mice thymectomized on day 3 of life, a spontaneous autoimmune disease develops that does not involve inflammation in the CNS (5). This raises the question as to whether CD4+CD25+ T cells can be involved in murine models of CNS autoimmunity, reflecting the observations made here in patients with MS. Thus, it is of interest that in a mouse model of MS, MOG35–55-specific experimental autoimmune encephalomyelitis, a similar population of CD4+CD25+ T cells, has been shown able to protect from both the onset and the progression of autoimmune demyelination induced by either active MOG35–55 immunization or adoptive transfer of autoreactive T cells (26). These data provide further evidence that CD4+CD25+ regulatory T cells can migrate into the CNS to mediate immune responses.
The cell surface marker CD62L was used to further characterize the CD4+CD25hi regulatory subset derived from both healthy individuals and patients with MS. In mice, the expression of this molecule on CD4+CD25+ T cells has been shown to confer a greater suppressive ability (27, 28). We demonstrated that in healthy subjects, both CD4+CD25hiCD62L+ and total CD4+CD25hi regulatory T cell populations exhibit similar abilities to suppress the proliferative response with anti-CD3 stimulation. In contrast, the regulatory T cells isolated from patients with MS, although expressing high levels of CD62L, were still unable to inhibit the responder cell proliferation, thus confirming a defect in the function of regulatory T cells derived from patients with MS.
The relative inability to generate CD4+CD25hi clones from patient blood may suggest a mechanism underlying the defective regulatory T cell function. One possibility is that CD4+CD25hi cells have undergone clonal exhaustion in vivo in the attempt to down-modulate the ongoing autoimmune response in patients. Another possibility is that their expansion is abnormally inhibited by other factors present in the single-cell cloning cultures. We can speculate that altered sensitivity of these cells to stimulation could also negatively affect their ability to exert regulatory functions. Although other factors, such as IL-6 secretion, may abrogate the regulatory function of CD4+CD25+ T cells, they may also adversely affect the growth of these cells. However, because there was no correlation between IL-6 cytokine secretion and the loss of T cell regulatory function, alterations in IL-6 secretion in patients with MS does not appear to be the underlying mechanism for the loss of suppressor function by CD4+CD25hi T cells. These and other possibilities are currently under investigation.
Although in vitro measurement of biologic function in patients with autoimmune diseases will always be correlative, these in vitro experiments, based on in vitro and in vivo experimentation in mouse models of autoimmune disease, provide the first definitive evidence for a defect in regulatory T cell function in a human autoimmune disease. Ultimately, monitoring the effects of immunomodulatory drugs on this regulatory T cell subset will help defining their pathogenic role in MS and other human autoimmune diseases.
Acknowledgments
The authors would like to dedicate this work to the memory of Professor U. Di Mario, whose vision was fundamental to the scientific education of Dr. Viglietta at the University of Rome “La Sapienza.”
This work was supported by the National Institutes of Neurological Disorders and Stroke Javits Investigator Award (to D.A. Hafler) and grants from the National Institutes of Health (RO1 NS24247, P01 AI39671, and R01 AI44447-01), and the National Multiple Sclerosis Society (2172C9-1 and 2949A-8 to D.A. Hafler).
V. Viglietta and C. Baecher-Allan contributed equally to this work.
Abbreviations used in this paper: CNS, central nervous system; MBP, myelin basic protein; MS, multiple sclerosis; RR, relapsing/remitting.
References
- 1.Ota, K., M. Matsui, E.L. Milford, G.A. Mackin, H.L. Weiner, and D.A. Hafler. 1990. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 346:183–187. [DOI] [PubMed] [Google Scholar]
- 2.Kappler, J.W., N. Roehm, and P. Marrack. 1987. T cell tolerance by clonal elimination in the thymus. Cell. 49:273–280. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. [DOI] [PubMed] [Google Scholar]
- 4.Shevach, E.M., R.S. McHugh, C.A. Piccirillo, and A.M. Thornton. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 182:58–67. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi, S., K. Fukuma, K. Kuribayashi, and T. Masuda. 1985. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 161:72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri-Payer, E., A.Z. Amar, A.M. Thornton, and E.M. Shevach. 1998. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160:1212–1218. [PubMed] [Google Scholar]
- 8.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 10.Powrie, F., S. Mauze, and R.L. Coffman. 1997. CD4+ T-cells in the regulation of inflammatory responses in the intestine. Res. Immunol. 148:576–581. [DOI] [PubMed] [Google Scholar]
- 11.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 12.Stephens, L.A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247–1254. [DOI] [PubMed] [Google Scholar]
- 13.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shevach, E.M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423–449. [DOI] [PubMed] [Google Scholar]
- 16.Martin, R., B. Gran, Y. Zhao, S. Markovic-Plese, B. Bielekova, A. Marques, M.H. Sung, B. Hemmer, R. Simon, H.F. McFarland, and C. Pinilla. 2001. Molecular mimicry and antigen-specific T cell responses in multiple sclerosis and chronic CNS Lyme disease. J. Autoimmun. 16:187–192. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor, K.C., A. Bar-Or, and D.A. Hafler. 2001. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 21:81–92. [DOI] [PubMed] [Google Scholar]
- 18.Steinman, L., R. Martin, C. Bernard, P. Conlon, and J.R. Oksenberg. 2002. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 25:491–505. [DOI] [PubMed] [Google Scholar]
- 19.Scholz, C., K.T. Patton, D.E. Anderson, G.J. Freeman, and D.A. Hafler. 1998. Expansion of autoreactive T cells in multiple sclerosis is independent of exogenous B7 costimulation. J. Immunol. 160:1532–1538. [PubMed] [Google Scholar]
- 20.Viglietta, V., S.C. Kent, T. Orban, and D.A. Hafler. 2002. GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes. J. Clin. Invest. 109:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovett-Racke, A.E., J.L. Trotter, J. Lauber, P.J. Perrin, C.H. June, and M.K. Racke. 1998. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J. Clin. Invest. 101:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reijonen, H., E.J. Novak, S. Kochik, A. Heninger, A.W. Liu, W.W. Kwok, and G.T. Nepom. 2002. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 51:1375–1382. [DOI] [PubMed] [Google Scholar]
- 23.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baecher-Allan, C., V. Viglietta, and D.A. Hafler. 2002. Inhibition of human CD4(+)CD25(high) regulatory T cell function. J. Immunol. 169:6210–6217. [DOI] [PubMed] [Google Scholar]
- 25.Pasare, C., and R. Medzhitov. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036. [DOI] [PubMed] [Google Scholar]
- 26.Kohm, A.P., P.A. Carpentier, H.A. Anger, and S.D. Miller. 2002. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712–4716. [DOI] [PubMed] [Google Scholar]
- 27.Szanya, V., J. Ermann, C. Taylor, C. Holness, and C.G. Fathman. 2002. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J. Immunol. 169:2461–2465. [DOI] [PubMed] [Google Scholar]
- 28.Kohm, A.P., and S.D. Miller. 2003. Role of ICAM-1 and P-selectin expression in the development and effector function of CD4+CD25+regulatory T cells. J. Autoimmun. 21:261–271. [DOI] [PubMed] [Google Scholar]