In the absence of an effective vaccine, educational or interventional strategies that decrease sexual transmission of HIV hold the greatest promise for slowing infection rates. Although a safe and effective microbicide could greatly help in this regard, progress in this area has been disappointing with microbicide development often failing to take into account advances in our understanding of how HIV is transmitted and infects cells. In this issue, Hu et al. demonstrate that HIV infection of human cervical tissue ex vivo can be prevented not only by using antibodies that target the virus but by a cocktail of compounds that target the cell surface receptors to which the virus binds, thus providing a basis for the design of microbicides that prevent virus infection in a highly specific manner (1).
Potential microbicides for HIV can be placed into one of three categories: compounds that inhibit virus infection nonspecifically, compounds that specifically target the virus, and compounds that target the cell surface receptors to which the virus binds. Most microbicide candidates tested to date fall squarely into the first category and illustrate the pitfalls of using agents that do not discriminate between pathogen and host. The first candidate microbicide for HIV to reach phase III clinical trials was the spermicidal detergent nonoxynol-9. Although the compound inactivates HIV in vitro by disrupting the outer viral membrane, it failed to prevent sexual transmission of the virus in vivo (2). In fact, women who used nonoxyl-9 containing gels had a higher rate of infection by HIV, most likely because the detergent disrupted the membranes of the epithelial cells in the genital tract which otherwise serve as an important barrier to virus infection. The failure of nonoxynol-9 has increased interest in agents that more specifically target HIV.
That HIV transmission can be prevented has been shown most clearly through the use of neutralizing antibodies. Passive administration of neutralizing antibodies can confer sterilizing immunity to macaques who are vaginally challenged with virus, provided that the antibodies are present within several hours of virus application (3–5). Likewise, a vaginally applied neutralizing antibody prevented infection of macaques (6). Although promising, the greatest drawbacks to the use of monoclonal antibodies is their cost and the structural variability of the viral Env protein to which they bind (7). Only a handful of broadly cross-reactive, neutralizing antibodies have been developed over the past 20 yr, and none of these recognize all virus strains. Even when used in combination, it is not difficult to identify virus strains that are neutralized only at very high concentrations of antibody or that escape neutralization altogether. Still, these results demonstrate that specific antiviral agents can prevent transmission of virus across the genital mucosa.
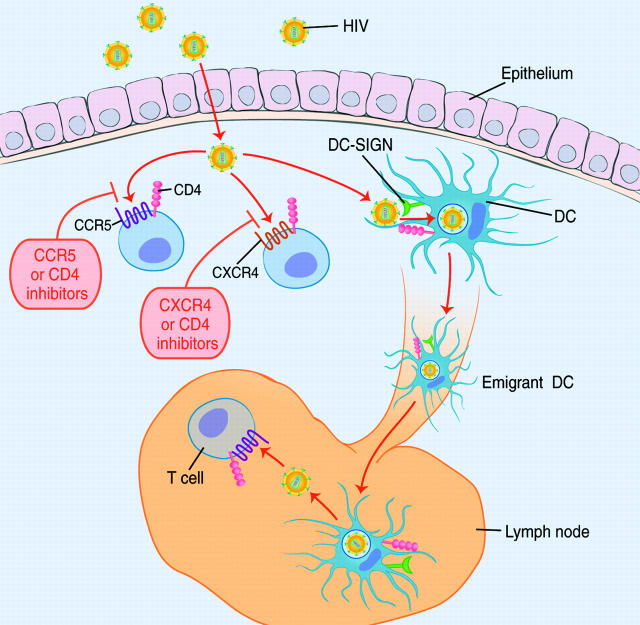
Recently, an impressive array of small molecule inhibitors that prevent HIV entry into cells have been developed, with many in clinical trials and one having been licensed in 2003 (8). Since viral attachment to and entry into host cells is the first step in establishing an infection, this process is a particularly attractive target for microbicides. Because attachment and entry involve interactions between the virus and host cells, it is important to use model systems that recapitulate the cellular environment in which infection is thought to occur as closely as possible. A major strength of the Hu et al. study is the use of human cervical tissue explants to elucidate the pathways by which HIV can establish an infection in genital mucosal tissue (1). Their work suggests two important principles that should guide the formulation of new microbicides. First, there are multiple ways by which HIV can infect target cells in the genital mucosa (Fig. 1). Thus, a successful host-targeted microbicide formulation will have to block all the potential pathways of HIV entry. Second, even if all host receptors for viral entry are blocked, HIV may be capable of evading these inhibitors by hitching a ride on dendritic cells (DCs). This may allow HIV to remain in an infectious state long enough to reach areas where microbicides do not penetrate (Fig. 1). As a result, targeting the molecules on the DC surface to which the virus binds may also be necessary for a microbicide to be as effective as possible.
Figure 1.
Mucosal transmission of HIV. Mechanisms by which HIV may traverse the epithelium include tears in the epithelium, transcytosis, and interactions with Langerhans cells. In the submucosal space, HIV may infect local T cells or macrophages that express CD4 and either CXCR4 or CCR5. Virus entry can be blocked by agents that prevent binding of the viral Env protein to either CD4 or the viral coreceptor. However, HIV may still be able to establish an infection by binding to CD4, DC-SIGN, or other attachment molecules on the surface of DCs. HIV may be internalized by the DCs, and upon DC maturation (which may be triggered by HIV) and emigration from the submucosa via the lymphatics to regional lymph nodes, be delivered to an area rich in T cells but deficient in topically applied entry inhibitors. Thus, blocking direction infection of cells in the submucosa and interactions with DCs may be required to efficiently inhibit sexual transmission of HIV.
Cell Surface Molecules Involved in Virus Infection.
At the minimum, HIV must interact with two receptors, CD4 and a coreceptor, to activate the membrane fusion potential of the viral Env protein (8). All HIV strains identified to date use the chemokine receptors CCR5 or CXCR4 as coreceptors to infect cells. Since viruses that use CCR5 cause the vast majority of new infections, this coreceptor is of particular interest (9, 10). However, utilization of alternative coreceptors for virus infection, such as CCR2 and CCR3, can sometimes be demonstrated in vitro. Hu et al. confirmed that both CCR5 and CXCR4-tropic HIV strains (R5 and X4, respectively) are capable of replication in cervical tissue stimulated with the T cell mitogen phytohemagluttinin (1). As expected, a small molecule inhibitor that targets CCR5 prevented infection by R5 virus strains but failed to neutralize infection by viruses that are capable of using CXCR4, whereas a CXCR4 inhibitor did the opposite. Importantly, although one of the HIV strains tested by the authors was capable of using the alternative coreceptors CCR3, CCR8, and CXCR6 in vitro, simultaneous blockade of CCR5 and CXCR4 by small molecule inhibitors was sufficient to prevent infection of the explants, suggesting that these alternative coreceptors were not relevant in the setting of this primary tissue. Therefore, a microbicide formulation directed against chemokine receptors will probably need to target only CCR5 and CXCR4 in order to block localized mucosal infection by HIV.
DCs: Another Viral Doorway.
Hu et al. also investigated the relative importance of host proteins involved in the capture of HIV by host DC (1). Cocultures of human DCs and T cells support higher levels of HIV replication than when T cells are cultured alone (11). This effect is partly explained by the ability of DCs to efficiently capture HIV through “attachment factors” such as DC-SIGN, a calcium dependent (C-type) lectin that binds high mannose oligosaccharide groups on the HIV Env glycoprotein (12). Although attachment of HIV to DC-SIGN alone does not cause virus–cell fusion, captured HIV is efficiently routed to sites of contact between DCs and T cells in vitro. CD4, CCR5, and CXCR4 on the T cell are also routed to this site of contact, and this is believed to facilitate viral entry into the T cell (13). To see if this capture and transfer mechanism might play a role in direct infection of cervical explants, the authors used a saturating concentration of the yeast cell wall component mannan, which blocks HIV binding to DC-SIGN and at least some other C-type lectins. Treatment with mannan or antibodies to DC-SIGN failed to affect the levels of infection, even when viral input was varied across a 100-fold range. This suggests that interactions between HIV, DC-SIGN, and other C-type lectins that are competent to bind mannan did not contribute significantly to direct infection of local T cells and macrophages.
Although DCs may initiate HIV infection of T cells in vitro, they may play an additional role in vivo: dissemination of virus from local sites of infection to proximally located lymphoid organs. In rhesus macaques, DCs bearing simian immunodeficiency virus can be seen in draining lymph tissue as early as 30 min after vaginal simian immunodeficiency virus challenge (14). To model the fate of HIV carried to lymph nodes by DCs, Hu et al. stimulated their infected explants with chemokines to induce DC migration and harvested the emigrating cells. When T cells were added to this emigrating cell population, strong HIV replication was seen, indicating that the emigrating cells carried HIV in an infectious state. Since the emigrating cells were composed of both T cells and DCs, the authors separated the two populations with magnetic beads and found that the majority of the infectious HIV was carried by the DC fraction. If the cervical explants were preincubated with mannan or antibodies against CD4 or DC-SIGN before addition of virus, the amount of HIV carried by the DCs was significantly reduced. Importantly, the DCs carried HIV even when localized infection in the cervical explants was largely blocked by coreceptor inhibitors or antibody to CD4. This is an important result because it indicates that simply blocking host molecules required for localized infection in the cervix may not be enough to prevent sexual transmission of HIV. In fact, a recent study published in this journal showed that a CCR5 inhibitor, when used alone, provided full protection to only 2 of 11 macaques that were vaginally challenged with virus (15).
Conflicting Results on the Role of DCs.
Superficially, it might seem that the results of Hu et al. (1) are somewhat at odds with two recent studies that examined the fate of HIV bound by DCs (16, 17). Findings by Turville et al. question whether DCs really do retain HIV for long periods (16) and argue that direct infection of DCs is responsible for long-term preservation of HIV infectivity. However, this study used DCs that were grown in vitro from blood progenitor cells, whereas the DCs studied by Hu et al. are authentic DCs taken from a tissue that is highly relevant for HIV transmission (1), and may vary in important ways from blood-derived DCs. More importantly, the findings of Turville et al. applied mainly to nonactivated (immature) DCs (16), whereas the emigrating DCs studied by Hu et al. expressed CD83 (1), indicating that they were of a mature phenotype. When Turville et al. studied mature DCs, they found that there was some long term preservation of HIV in mature DCs in the absence of infection (16). In the second study that questions the ability of DCs to capture and retain HIV, skin explants were used to investigate a specialized subset of immature DCs known as Langerhans cells (17). These cells could be infected directly by R5 but not X4 HIV strains. Langerhans cells that emigrated from the skin explants did not carry HIV if direct infection was blocked, and preincubation of the explants with mannan had no effect on the amount of infection observed or the ability of the cells to transmit the HIV to T cells. Again, the difference between this study and that of Hu et al. is in the type of DC examined. The DCs in the explants used by Hu et al. likely included both DC-SIGN–negative Langerhans DCs from the cervical epithelium and DC-SIGN–positive interstitial DCs from the lamina propria. A C-type lectin-dependent pathway of HIV transmission has been established only for the latter DC subset, as most studies have used in in vitro monocyte-derived DCs, which most closely resemble interstitial DCs. In the setting of an intact epithelial barrier, only Langerhans cells would be expected to interact with HIV, whereas breaks in the epithelium would likely allow HIV to reach the lamina propria and be captured by interstitial DCs. These and other studies show that DCs express an array of molecules that can interact with different pathogens (18). Depending on the DC subset studied, these interactions sometimes result in DC infection and other times result in a DC that carries with it an infectious agent that can, at some later point, be transmitted to a susceptible cell. This variability makes studies like Hu et al. that utilize primary human tissues, with their associated resident DCs (1), even more important.
Prospects for Therapy.
Will entry inhibitors or agents that directly and specifically target HIV prevent HIV transmission? The study by Hu et al. (1) as well as the work of others (3–6, 15, 18) certainly suggests that this general approach should be carefully examined. With an array of entry inhibitors moving through clinical development, including CCR5 inhibitors, CXCR4 inhibitors, fusion inhibitors, and compounds that prevent CD4 binding (8), options for microbicide formulations based on knowledge of how HIV infects cells will expand in the coming years. Targeting cell surface receptors has some advantages, specifically the lack of variability in receptor structure and distribution between individuals compared with the impressive variability of the viral Env protein. However, it is likely that several different inhibitors will have to be employed in order to close all of the doors through which HIV can enter.
References
- 1.Hu, Q., I. Frank, V. Williams, P. Watts, J.P. Moore, M. Pope, and R.J. Shattock. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, et al. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 360:971–977. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura, Y., T. Igarashi, N.L. Haigwood, R. Sadjadpour, O.K. Donau, C. Buckler, R.J. Plishka, A. Buckler-White, and M.A. Martin. 2003. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. USA. 100:15131–15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M.W. Cho, and M.A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204–210. [DOI] [PubMed] [Google Scholar]
- 5.Mascola, J.R., M.G. Lewis, G. Stiegler, D. Harris, T.C. VanCott, D. Hayes, M.K. Louder, C.R. Brown, C.V. Sapan, S.S. Frankel, et al. 1999. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veazey, R.S., R.J. Shattock, M. Pope, J.C. Kirijan, J. Jones, Q. Hu, T. Ketas, P.A. Marx, P.J. Klasse, D.R. Burton, and J.P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D.R., R.C. Desrosiers, R.W. Doms, W.C. Koff, P.D. Kwong, J.P. Moore, G.J. Nabel, J. Sodroski, I.A. Wilson, and R.T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233–236. [DOI] [PubMed] [Google Scholar]
- 8.Moore, J.P., and R.W. Doms. 2003. The entry of entry inhibitors: a fusion of science and medicine. Proc. Natl. Acad. Sci. USA. 100:10598–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samson, M., F. Libert, B.J. Doranz, J. Rucker, C. Liesnard, C.M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, et al. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 382:722–725. [DOI] [PubMed] [Google Scholar]
- 10.Liu, R., W.A. Paxton, S. Choe, D. Ceradini, S.R. Martin, R. Horuk, M.E. MacDonald, H. Stuhlmann, R.A. Koup, and N.R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 86:367–377. [DOI] [PubMed] [Google Scholar]
- 11.Cameron, P.U., P.S. Freudenthal, J.M. Barker, S. Gezelter, K. Inaba, and R.M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 257:383–387. [DOI] [PubMed] [Google Scholar]
- 12.Geijtenbeek, T.B., D.S. Kwon, R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. KewalRamani, D.R. Littman, C.G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587–597. [DOI] [PubMed] [Google Scholar]
- 13.McDonald, D., L. Wu, S.M. Bohks, V.N. KewalRamani, D. Unutmaz, and T.J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 300:1295–1297. [DOI] [PubMed] [Google Scholar]
- 14.Miller, C.J., and R.J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59–67. [DOI] [PubMed] [Google Scholar]
- 15.Veazey, R.S., P.J. Klasse, T.J. Ketas, J.D. Reeves, M. Piatak, Jr., K. Kunstman, S.E. Kuhmann, P.A. Marx, J.D. Lifson, J. Dufour, et al. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turville, S.G., J.J. Santos, I. Frank, P.U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., et al. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 103:2170–2179. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura, T., F.O. Gulden, M. Sugaya, D.T. McNamara, D.L. Borris, M.M. Lederman, J.M. Orenstein, P.A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA. 100:8401–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turville, S.G., P.U. Cameron, A. Handley, G. Lin, S. Pohlmann, R.W. Doms, and A.L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975–983. [DOI] [PubMed] [Google Scholar]