Abstract
Previous studies have indicated that the E2A gene products are required to initiate B lineage development. Here, we demonstrate that E2A+/− B cells that express an autoreactive B cell receptor fail to mature due in part to an inability to activate secondary immunoglobulin (Ig) light chain gene rearrangement. Both RAG1/2 gene expression and RS deletion are severely defective in E2A+/− mice. Additionally, we demonstrate that E2A+/− mice show an increase in the proportion of marginal zone B cells with a concomitant decrease in the proportion of follicular B cells. In contrast, Id3-deficient splenocytes show a decline in the proportion of marginal zone B cells. Based on these observations, we propose that E-protein activity regulates secondary Ig gene rearrangement at the immature B cell stage and contributes to cell fate determination of marginal zone B cells. Additionally, we propose a model in which E-proteins enforce the developmental checkpoint at the immature B cell stage.
Keywords: E-proteins, Id3, RAG, follicular B cells, checkpoint
Introduction
B cell developmental progression requires the assembly of Ig genes from germline gene segments (1, 2, 3). In pro–B cells, Ig heavy chain (IgH) gene rearrangement is initiated by the joining of D and J segments, followed by V(D)J rearrangement. Once a VDJ rearrangement has produced an in-frame joining of V, D, and J DNA segments, the heavy chain together with surrogate light chains is assembled into a signaling complex called the pre–B cell receptor (BCR). Signals emanating from the pre-BCR inhibit further recombinase activity, preventing continued IgH chain gene rearrangement (4, 5). B lineage cells undergo rapid proliferation followed by G1 arrest, reactivation of RAG expression, and initiation of Ig light chain (IgL) gene rearrangement. A productive IgL gene rearrangement permits surface Ig expression and developmental progression toward B cell maturity (6). Immature B cells expressing a unique IgH and IgL gene product are tested for reactivity against self-antigens. At this stage, autoreactive B cells are either deleted, anergized, or modified by continued IgL gene rearrangement, termed receptor editing (7–10). During the receptor editing process, secondary light chain gene rearrangements replace existing IgLs, generating receptors with altered specificities. B cells develop to maturity if the replacement generates a nonself-reactive receptor (11–13).
Several transcription factors that regulate the early stages of B cell development have been identified. Among these are the E2A proteins, early B cell factor (EBF) and Pax-5 (14–17). Both E2A and EBF null mutant mice are arrested at a stage before the onset of IgH DJ rearrangement (14, 15, 17). B cell development in Pax-5 null mutant mice is arrested at the pro–B cell stage (16). The E2A gene encodes for two proteins, designated E12 and E47, which arise by differential splicing. Both E12 and E47 are members of the class I helix-loop-helix proteins, also named E-proteins (18). The E2A proteins are required for cell survival at the pro–B cell stage and act in concert with EBF to regulate λ5 and RAG gene expression (19–21). In vitro experiments have suggested that E2A and EBF also have the ability to promote IgH DJ and Igκ light chain gene rearrangement (22, 23).
Here, we have examined the expression and function of the E2A proteins beyond the pro–B cell stage. E47 protein levels are high in pro–B cells, but are lowered upon maturing through the pre-BCR checkpoint. E47 levels are elevated again at the pre–B cell stage and remain high until the cells reach the mature B cell compartment. We show that the E2A proteins are required to regulate secondary Ig gene rearrangements in B cells that express an autoreactive BCR, due in part to activate RAG expression and to induce RS deletion. Finally, our observations demonstrate that the E2A proteins and their antagonist, Id3, modulate the developmental progression of immature B lineage cells and regulate cell fate determination of marginal zone B cells.
Materials and Methods
Mouse Strains.
C57BL/6 E2A and Id3 mutant mice have been described previously (14, 15, 24). 3-83 BCR transgenic B10.D2nSn/J mice have been described previously (8). All mice were analyzed between 6 and 16 wk of age.
Flow Cytometric Analysis.
Cells were stained in suspension in 0.1 ml FACS® buffer (1× PBS, 0.5% FCS plus 0.02% sodium azide) plus the indicated antibodies on ice for 15 min. Cells stained with biotinylated antibodies were washed once in 2 ml FACS® buffer and incubated with streptavidin-allophycocyanin for 15 min on ice. After staining, cells were washed, fixed in 1× PBS plus 1% formaldehyde and analyzed on a FACScan™ or FACScalibur™ (Becton Dickinson). Cells were gated on the basis of forward side scatter. All antibodies, except the anti–3-83 antibody (S23), were obtained from BD Biosciences. For analysis of intracellular E47 expression, cells were first stained for surface antigens, washed in PBS plus 5% FCS, fixed in 1% paraformaldehyde, permeabilized with Tween-20, and stained with G127-32 (anti–E47-PE) as described previously (25).
Rag, κo Germline Transcript, IgL Rearrangement, and RS Deletion Analyses.
Bone marrow B220+ cells were purified using MiniMACS LS columns (Miltenyi Biotec). RNA was prepared via RNeasy (QIAGEN). Synthesis of cDNA was performed using oligo-dT and Superscript II reverse transcriptase. The template for RT-PCR was cDNA diluted three- or fivefold in water for each serial dilution point. Genomic DNA was prepared by proteinase K treatment. PCR primer sequences used were as follows: Rag1, Rag2, and B29 have been described previously (10, 21); κofor, 5′-CAGTGAGGAGGGTTTTTGTACAGCCAGACAG-3′; Jκ2rev, 5′-TTTCCAGCTTGGTCCCCCCTCCGAA-3′ (26); VκconsFor, 5′-GGCTGCAGSTTCAGTGGCAGTGGRTCWGGRAC-3′; Jκ1Rev, 5′-GCCACAGACATAGACAACGGAAGAA-3′ (26); VλleaderFor, 5′-GTTTGTGAATTATGGCCTGGAT-3′; Jλ1Rev, 5′-CTAGGACAGTCAGTTTGGTTCC-3′ (27); VκD, 5′-GGCTGCAGSTTCAGTGGCAGTGGRTCWGGRAC-3′; and RS2, 5′-CTGCCCACACGACTCCTTCAGGCAGACG-3′ (28). Induction of RS deletion in vitro in the BOSC 23 embryonic kidney cell line was performed as described previously (23). Primer sequences used were as follows: Vκ1, 5′-GTAGGAGACAGAGTCACCATCACT-3′; and Kde, 5′-CCCTTCATAGACCCTTCAGGCAC-3′.
Results
Dynamic Pattern of E47 Expression in B Lineage Development.
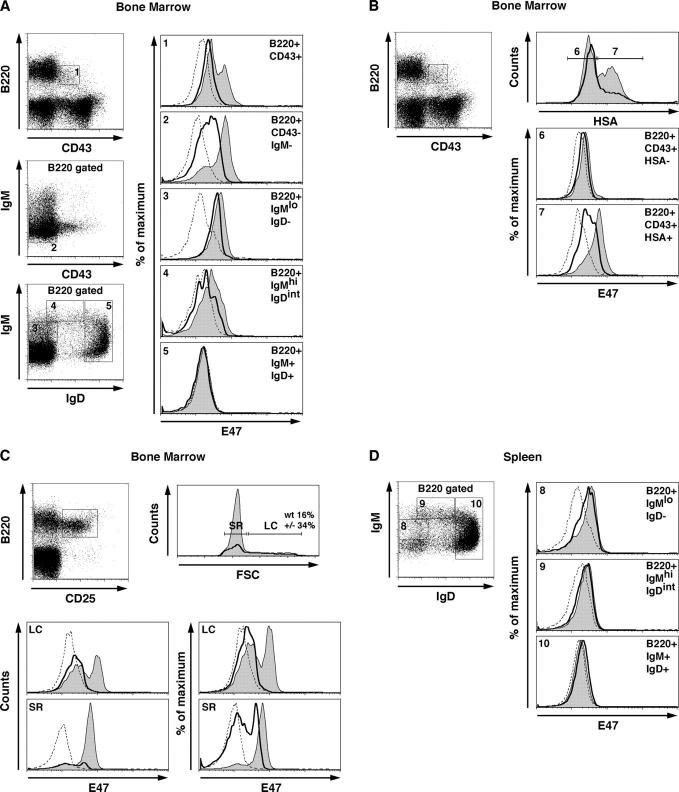
Previous data using cell sorting, immunofluorescence, and Western blotting have indicated that E47 levels are expressed in pro–B and pre–B cells but decreased in immature and mature B lineage cells (29). To examine the expression of E47 more precisely in primary B lineage cells, lymphocytes were isolated from the bone marrow of wild-type and E2A+/− mice and stained for the presence of E47 using flow cytometric analysis of permeabilized cells (Fig. 1). The expression of E47 was examined in five developmental B cell compartments as defined by the expression of B220, CD43, IgM, and IgD. The pro–B cell stage can be characterized by the expression of B220 and CD43. B220+CD43+ B cells showed two distinct populations expressing different levels of E47 (Fig. 1 A). Interestingly, E47 expression was variegated in the pre–B cell compartment (Fig. 1 A, B220+CD43−IgM−). The expression level of E47 was high within virtually all B220+ IgMlo IgD− cells, but was decreased significantly in the IgMhi and IgM+IgD+ subsets (Fig. 1 A).
Figure 1.
Expression of E47 in B lineage subsets derived from the bone marrow and spleen from E2A wild-type and +/− mice. Bone marrow or spleen cells were stained with the indicated antibodies and analyzed for E47 expression by flow cytometry. (A) E47 expression in developing B lineage cells. Numbered gates indicate the subpopulations examined for E47 expression. Histograms show E47 expression levels in each subset using an E47-specific antibody. Shaded areas indicate E47 expression in wild-type B cells. Solid lines indicate E47 expression in E2A+/− cells. Dotted lines indicate isotype control staining. (B) B220+CD43+ pro–B cells were subdivided based on HSA expression and analyzed for E47 expression. Histograms depict the E47 staining in wild-type (shaded areas), E2A+/− (solid lines), and isotype control (dotted lines) mice within the pro–B cell compartment. (C) E47 expression in large and small pre–B cells. Bone marrow cells were stained for the presence of B220 and CD25 and further subdivided on the basis of cell size. Histograms show the expression of E47 in large cycling (LC) versus small resting (SR) pre–B cells. Histograms depict E47 expression in wild-type (shaded areas), E2A+/− (solid lines), and isotype control staining (dotted lines) mice in each gated subset. (D) E47 expression in peripheral B cells. Splenocytes were stained for the presence of B220, IgM, and IgD. Histograms depict E47 expression in wild-type (shaded areas), E2A+/− (solid lines), and isotype control staining (dotted lines) mice in each gated subset.
To further define the stage in which E47 is increased at the pro–B cell compartment, cells were stained for the presence of B220, CD43, and heat-stable antigen (HSA). HSA negative cells lack significant levels of IgH DJ rearrangements, whereas most cells that express HSA have undergone IgH DJ rearrangements. The majority of the pro–B cells that express HSA express relatively high levels of E47, whereas the HSA null cells contained low levels of E47 (Fig. 1 B). These observations indicate that E47 expression is elevated at a stage in which B cells become committed to the B cell lineage.
As aforementioned, E47 expression was heterogeneous in the B220+CD43−IgM− subset. To more precisely define E47 expression in this population, cells were stained for the expression of B220 and CD25 and further analyzed based on cell size (Fig. 1 C). E47 expression was uniformly high in the small resting pre–B cell population. In contrast, the majority of the large cycling cells expressed relatively low levels of E47 (Fig. 1 C). Interestingly, E2A+/− cells showed a large fraction of cycling cells as compared with wild-type cells (Fig. 1 C). Additionally, we note that the proportion of cells that express significant levels of E47 in E2A+/− cells was significantly decreased in the cycling populations (Fig. 1 C). Together, these data suggest that E47 levels transiently decline in response to pre-BCR signaling. Once the cells reach the pre–B cell stage, E47 expression is elevated to levels similar to that at the pro–B cell stage.
To examine E47 expression in peripheral B lineage cells, splenocytes were stained for the presence of IgM and IgD, permeabilized, stained, and analyzed for E47 expression by flow cytometry. As observed in the bone marrow, transitional B cells (IgMhi IgDint) displayed a marked decrease in E47 protein levels as compared with immature B cells (Fig. 1 D, IgMlo IgD−). The mature B cell compartment (IgM+IgD+) virtually lacked E47 expression (Fig. 1 D). Thus, E47 levels decline as cells mature into the IgM+IgD+ cell stage.
In summary, these data indicate that the pattern of E47 expression is dynamic throughout B lineage development. E47 expression is induced at the point of B lineage commitment, lowered at the pre-BCR checkpoint, elevated in small resting pre–B cells, lowered in transitional B cells, and barely detectable in mature B cells.
E2A Heterozygosity Perturbs B Lineage Development.
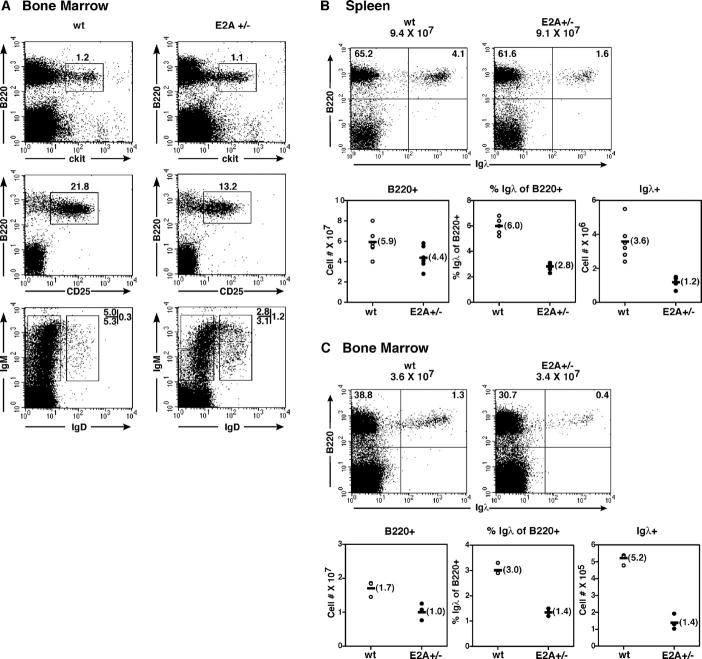
Because B cell development in E2A−/− mice is blocked at a stage before the onset of IgH chain gene rearrangement, we examined E2A+/− mice for abnormalities beyond the pro–B cell stage. Bone marrow cells were isolated from wild-type and E2A+/− mice and analyzed by flow cytometry. The pro–B cell population (B220+c-kit+) was not significantly altered in E2A+/− mice as compared with wild-type mice (Fig. 2 A). However, the fraction of pre–B cells (B220+ CD25+) was reduced almost twofold in E2A+/− mice (Fig. 2 A). Similarly, the IgM+IgD− population was significantly decreased in E2A heterozygous mice (Fig. 2 A). The fraction of IgM+IgD+ mature B cells was elevated in the bone marrow of E2A+/− mice (Fig. 2 A).
Figure 2.
B cell development in E2A+/− mice is perturbed. (A) Comparison of the pro–B, pre–B, and immature B cell compartments from wild-type and E2A+/− mice. Cells were isolated from bone marrow and analyzed for the presence of B220, c-kit, CD25, IgM, and IgD by flow cytometric analysis. Numbers reflect the percentage of cells in each gated population. (B) Igλ expression is perturbed in E2A+/− mice. Splenocytes isolated from E2A wild-type and E2A+/− mice were stained for surface expression of B220 and Igλ and analyzed by flow cytometry. Representative dot plots are shown (top). (bottom) Total number of B220+ cells (left). The percentage and total number of λ+ cells, respectively (middle and right). (C) Flow cytometric analysis of B220 and Igλ expression in bone marrow from E2A wild-type and E2A+/− mice as described in B.
In vitro and in vivo observations have indicated that the E2A proteins bind to sites present in the Igκ and λ enhancers (30–32). Furthermore, overexpression of E2A in conjunction with the RAG gene products readily activates Igκ and λ gene rearrangement in embryonic kidney cells (23, 33). These data suggested that the E2A proteins function in B cell development by promoting accessibility of the recombination signal sequences to the recombination machinery. These findings also raised the possibility that in immature B cells, the E2A proteins function to promote secondary IgL gene rearrangements. As a first approach to address this possibility, we examined the expression of Igλ on mature B cells derived from wild-type and E2A+/− mice. Splenocytes were isolated and examined for the expression of B220 and Igλ (Fig. 2 B). The total number of B220+ cells was slightly reduced in E2A+/− mice as compared with wild-type mice (Fig. 2 B). Interestingly, the fraction of B cells that expressed Igλ was significantly reduced in E2A+/− mice (Fig. 2 B). On average, the total number of Igλ-expressing cells was threefold lower in E2A+/− mice as compared with wild-type mice (Fig. 2 B). To determine whether the proportion of Igλ-expressing cells in the bone marrow was also altered in E2A+/− mice, bone marrow cells were isolated and analyzed for the expression of Igλ. As expected, the proportion and the total number of Igλ positive cells were significantly lower in the bone marrow of E2A heterozygous mice (Fig. 2 C). These data demonstrate that E2A dosage regulates the κ/λ ratio and establish a role for E2A proteins in B cell development beyond the pro–B cell stage.
Self-antigen Promotes B Cell Depletion in E2A Heterozygous Mice.
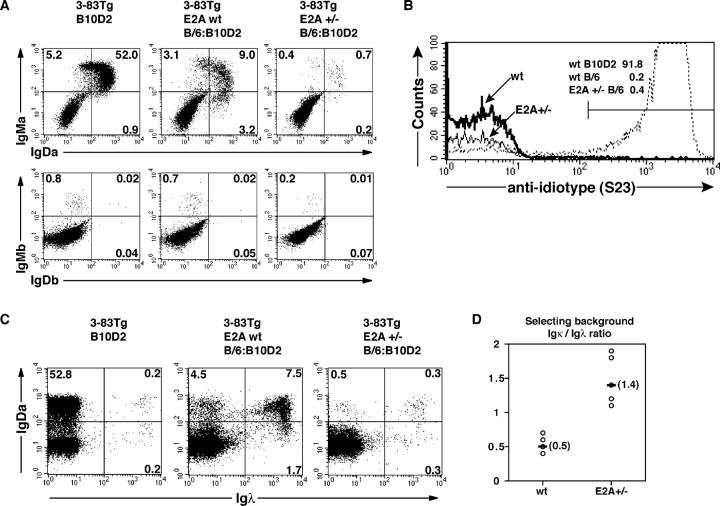
The observation that E2A heterozygosity results in decreased Igλ usage suggests a potential role for E2A in promoting secondary IgL chain gene rearrangements. To test this possibility, E2A+/− mice were crossed with mice that carry the 3-83 transgene (8). 3-83 B cells express a BCR of defined specificity that recognizes epitopes present on the H-2Kk,b major histocompatibility complex antigen. In a nondeleting H-2Kd background, 3-83 mice contain a virtual monoclonal B cell compartment expressing the 3-83 transgene. However, in the presence of autoantigen, in the H-2Kb background, B cells undergo receptor editing. Such B cells contain secondary Igκ or Igλ rearrangements and lack 3-83 reactivity (10). Consistent with previous observations, peripheral B cells from 3-83 transgenic mice in both nonediting and editing backgrounds were readily detectable (Fig. 3, A and C, left and middle, respectively). In contrast, mature B cells were virtually absent in splenocyte populations derived from E2A+/− mice expressing the 3-83 transgene in the presence of self-antigen (Fig. 3, A and C; right). As expected, the Igκ/λ ratio was severely affected in the few B cells that developed in E2A+/− mice (Fig. 3, C and D). We also examined whether E2A heterozygosity affected allelic exclusion by staining with antibodies specific for the endogenous Ig allotypes. Expression of the endogenous IgH remained excluded by the presence of the 3-83 transgene, indicating that allelic exclusion was not affected by E2A heterozygosity (Fig. 3 A, bottom). Additionally, tolerance was maintained in E2A+/−;3-83 mice because the few B cells that did develop lacked 3-83 specificity (Fig. 3 B). Together, these data suggest that E2A heterozygosity affects the ability of B lineage cells to undergo receptor editing in response to self-antigen.
Figure 3.
The E2A proteins are required to promote developmental progression of autoreactive B lineage cells. (A) Flow cytometric analysis of splenocytes from E2A wild-type 3-83 transgenic nonediting (B10D2) and editing (B/6:B10D2) mice and E2A+/−;3-83 transgenic editing mice. (top) Staining for the 3-83 transgenic heavy chain. (bottom) Expression of endogenous heavy chain. (left) B cells from E2A wild-type transgenic mice in a nonediting B10D2 background. (middle and right) B cells from wild-type and E2A+/− transgenic mice in an editing background, respectively. Percentages in each quadrant are indicated. (B) Tolerance is maintained in E2A+/−;3-83 transgenic editing mice. Anti-idiotype staining for 3-83 BCR expression in splenocytes from transgenic wild-type and E2A+/− mice. (bold and solid lines) 3-83 expression in mature B cells from wild-type and E2A+/− editing mice, respectively. (dotted line) Positive 3-83 expression in B cells derived from transgenic mice in the nonediting background (B10D2). The percentage of 3-83 positive mature B cells is indicated. (C) Both secondary Igκ and Igλ rearrangements are perturbed in E2A+/−;3-83 editing B cells. Splenocytes derived from wild-type and E2A+/−;3-83 mice as in A were analyzed for the expression of Igλ and IgDa by flow cytometry. As a comparison, the percentage of Igλ+ B cells in a nonediting background is shown (left). (middle and right) The expression of secondary Igκ (top left) and secondary Igλ (top right) chains on edited B cells from E2A wild-type and E2A+/− mice. Percentages in each quadrant are indicated. (D) The Igκ versus Igλ ratio in B lymphocytes derived from E2A wild-type and E2A+/−;3-83 editing mice.
RAG Gene Expression, Igκ and λ Gene Rearrangement, and RS Deletion Are Affected in E2A+/− B Lineage Cells.
Because proper receptor editing requires the expression of the RAG gene products, we analyzed E2A+/−;3-83 B cells for RAG expression using mice carrying a transgene in which the green fluorescent protein (GFP) coding sequence was inserted into the RAG2 locus (34). RAG-GFP mice were crossed into E2A+/− mice carrying an autoreactive BCR (3-83) and analyzed for GFP expression in B220+CD43− cells. RAG expression as measured by fluorescence was decreased by a factor of approximately twofold in E2A+/− cells carrying an autoreactive BCR (unpublished data). To further analyze RAG transcript levels, RNA was isolated from purified B220+CD43− bone marrow cells and analyzed by semi-quantitative RT-PCR (Fig. 4 A). As expected, both RAG-1 and RAG-2 transcripts were readily detectable in mRNA derived from wild-type 3-83 transgenic B cells receiving the autoantigen signal. In contrast, both RAG-1 and RAG-2 transcripts were severely reduced in E2A+/−;3-83 transgenic B cells. Interestingly, RAG-1 transcript levels were more perturbed than RAG-2 levels, ∼25 versus 5-fold, respectively.
Figure 4.
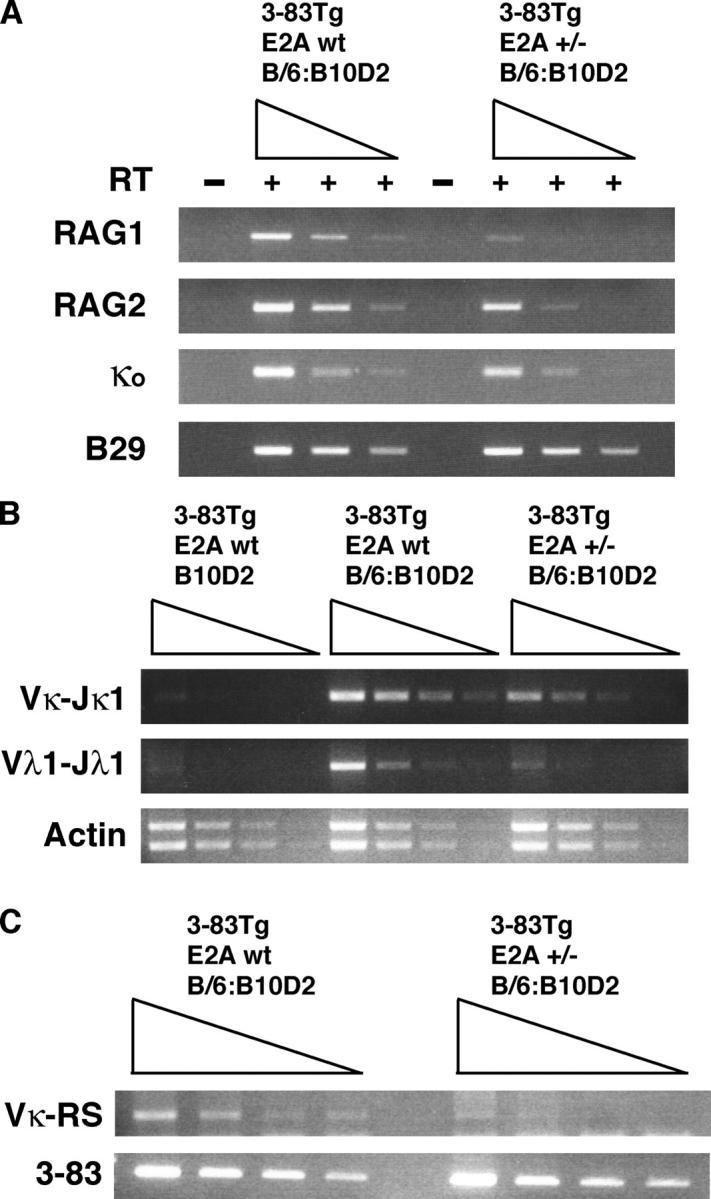
RAG gene expression, IgL chain rearrangements, and RS deletions are severely affected in E2A+/− mice expressing a self-reactive BCR. (A) RNA was isolated from purified B220+CD43− bone marrow cells from E2A wild-type and E2A+/−;3-83 transgenic mice. Fivefold serial dilutions were analyzed for RAG1, RAG2, and κo germline transcripts by RT-PCR. PCR products were separated by DNA gel electrophoresis and visualized by ethidium bromide staining. B29 transcripts are shown to indicate equivalent loading and integrity of the cDNAs. (B) Genomic DNA was isolated from purified B220+ bone marrow cells, and threefold serial dilutions were analyzed for secondary Vκ-Jκ1 and Vλ1-Jλ1 rearrangements by PCR. PCR products were separated by DNA gel electrophoresis and visualized by ethidium bromide staining. (left) Absence of rearrangements in nonselecting (B10D2) background. (middle) Igκ and Igλ rearrangements in E2A+/+;3-83 B cells. (right) Igκ and Igλ rearrangements in E2A+/−;3-83 B cells. (bottom) Equivalent loading and DNA integrity using actin primers. (C) RS deletion is perturbed in E2A+/− mice carrying an autoreactive BCR. DNA isolated from B220+ bone marrow cells was analyzed by PCR for RS deletion. PCR products were analyzed by DNA gel electrophoresis and visualized by ethidium bromide staining. (bottom) Equivalent DNA loading using primers specific for the 3-83 transgene.
To determine whether E2A heterozygosity also affects Igκ locus accessibility, the same samples were analyzed for κo germline transcripts (Fig. 4 A). Both wild-type and E2A heterozygous B cells express κo germline transcripts, albeit a modest decrease was observed in the E2A+/− B cells (Fig. 4 A).
In vitro studies have indicated that the E2A proteins have the ability to promote both Igκ and λ VJ rearrangement (23, 33). Chromatin immunoprecipitation assays have demonstrated that the E2A proteins directly bind to sites present in the Igκ light chain gene enhancer (32). Furthermore, it has been demonstrated that mice harboring a deletion of the Igκ enhancers show a defect in the κ/λ ratio similar to that observed here in E2A+/− B lineage cells (35). These observations raised the possibility that E2A+/− B cells failed to undergo receptor editing upon encountering self-antigen because of an inability to undergo efficient IgL gene rearrangement. To determine whether wild-type levels of E2A are required to activate Igκ and λ secondary rearrangements, DNA was isolated from purified B220+ bone marrow cells, and VJ rearrangements were analyzed by PCR. As expected, in wild-type mice that carry the 3-38 BCR in the presence of self-antigen, secondary Igκ and λ rearrangements were readily detectable. In contrast, both Igκ and λ VJ rearrangements were severely impaired in E2A+/− B lineage cells (Fig. 4 B). These data indicate that secondary gene rearrangements involving the Igκ and λ loci are significantly affected in E2A+/− mice.
The observed skewing of the Igκ/λ ratio in E2A+/− mice raised the possibility that Igλ rearrangement is particularly sensitive to E2A dosage. Secondary rearrangements involving the Igλ locus are frequently preceded by deletion of the Igκ enhancer and constant regions through a specific rearrangement event involving an upstream RSS and the 3′ RS element, also named the κ-deleting element. To determine whether RS deletion is affected in E2A+/−;3-83 B lymphocytes, DNA was isolated and analyzed for the presence of RS rearrangement. As expected, in wild-type B cells, V-RS rearrangements were readily detectable (Fig. 4 C). In striking contrast, RS deletion was severely affected in E2A+/−;3-83 B cells (Fig. 4 C). Together, these data indicate that E2A heterozygous B cells that express an autoreactive BCR are severely impaired in their ability to undergo secondary IgL chain gene rearrangements.
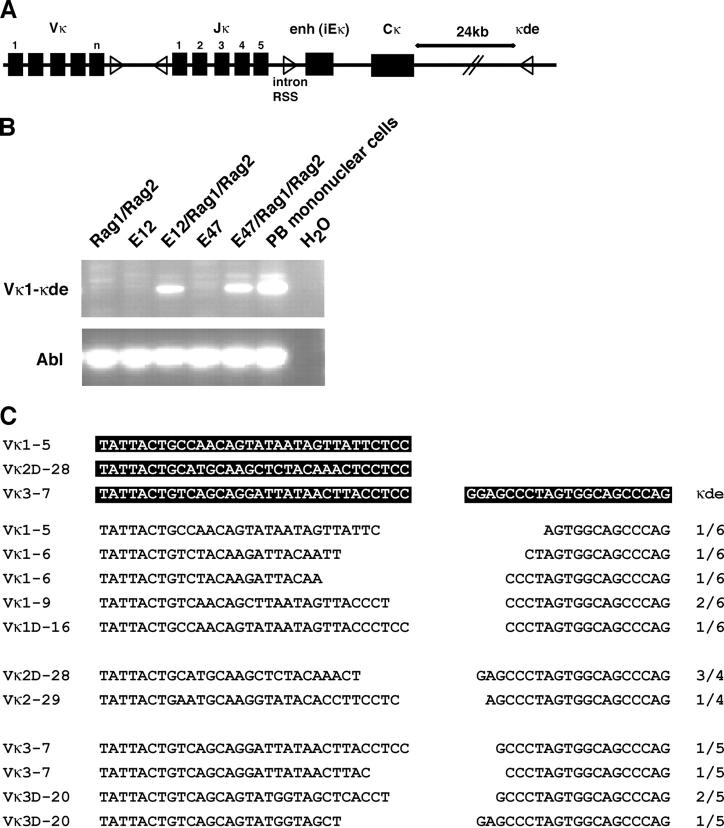
Induction of RS Deletion by the Expression of E2A and RAG1/2 in Nonlymphoid Cells.
Because RS deletion is perturbed in E2A+/− mice that carry the 3-83 transgene, we considered the possibility that E2A proteins directly regulate secondary IgL gene rearrangements by promoting accessibility of the RSSs to the recombination machinery. Previous data showed that the E2A proteins have the ability to regulate rearrangement of the light chain gene loci in nonlymphoid cells (23, 27). However, it remained to be determined whether the E2A proteins also have the ability to activate RS deletion. Two distinct elements containing an RSS involved in RS deletion are located in the human Igκ locus (36). An isolated RSS is located between the joining and constant regions, whereas another RSS, designated as the Kde element is located 3′ of the constant region (Fig. 5 A). The Igκ enhancer and the constant region are deleted upon rearrangements that involve the Kde element (Fig. 5 A). To determine whether E2A proteins have the ability to directly promote RS deletion, an embryonic kidney cell line was transfected with expression vectors encoding either E12 or E47 and RAG1 and RAG2. 3 d after transfection, DNA was harvested and analyzed by PCR for RS deletion. As expected, neither E12 nor E47 alone had the ability to promote RS deletion. Similarly, expression of RAG1 and RAG2 by themselves did not promote V-Kde joining (Fig. 5 B). In contrast, in the presence of RAG1 and -2, both E12 and E47 were able to activate RS deletion upon overexpression (Fig. 5 B). To examine the nature of the V-Kde joints, PCR products were isolated, cloned, and analyzed by DNA sequencing (Fig. 5 C). The RS deletions showed significant diversity. Distinct Vκ regions interspersed throughout the human Igκ locus were joined with the Kde element (Fig. 5 C). All RS rearrangements showed nucleotide deletions of variable sizes (Fig. 5 C). As expected nucleotide additions were absent due to the lack of TdT expression. Together, these data demonstrate that the E2A proteins have the ability to directly promote RS deletion.
Figure 5.
E12 and E47 act in concert with RAG1 and RAG2 to promote RS deletion in an embryonic kidney cell line, BOSC 23. (A) Schematic representation of the Igκ locus. Triangles represent the recombination signal sequences (RSSs). The intron RSS and κde element are indicated. (B) BOSC 23 cells were transfected with expression vectors for E12, E47, RAG1, and RAG2 and analyzed by PCR for Vκ-κde recombination using Vκ and κde consensus primers. PCR products were separated on a 1.5% agarose gel and visualized by staining with ethidium bromide. The integrity of the DNA was verified using PCR primers corresponding to the human Abelson locus (bottom). (C) Diverse repertoire of RS deletion generated in nonlymphoid cells. PCR fragments obtained from transfectants were isolated, cloned, and sequenced. Sequences of the rearranged segments are indicated. Numbers reflect frequency of identical clones.
E2A and Id3 Regulate Marginal Zone B Cell Development.
The decline in E47 expression in the transitional B cell populations raised the possibility that lower levels of E2A proteins promote the development toward a mature B lineage phenotype. To determine whether lowering the dosage of E2A promotes B cell maturation in the periphery, we analyzed the splenic compartments from 3-83 transgenic mice in the nonediting background or hen egg lysozyme (HEL) transgenic mice in the absence of selecting antigen. E2A+/− BCR transgenic mice revealed strong reductions in percentages of both IgMhi IgD−/lo and IgMhi IgD+ transitional populations, whereas the proportion of IgMlo IgD+ mature B cells was elevated in E2A heterozygous mice (Fig. 6, A and E). However, we note that nontransgenic E2A+/− mice did not show an increase in the fraction of IgMlo IgD+ cells (Fig. 6 A).
Figure 6.
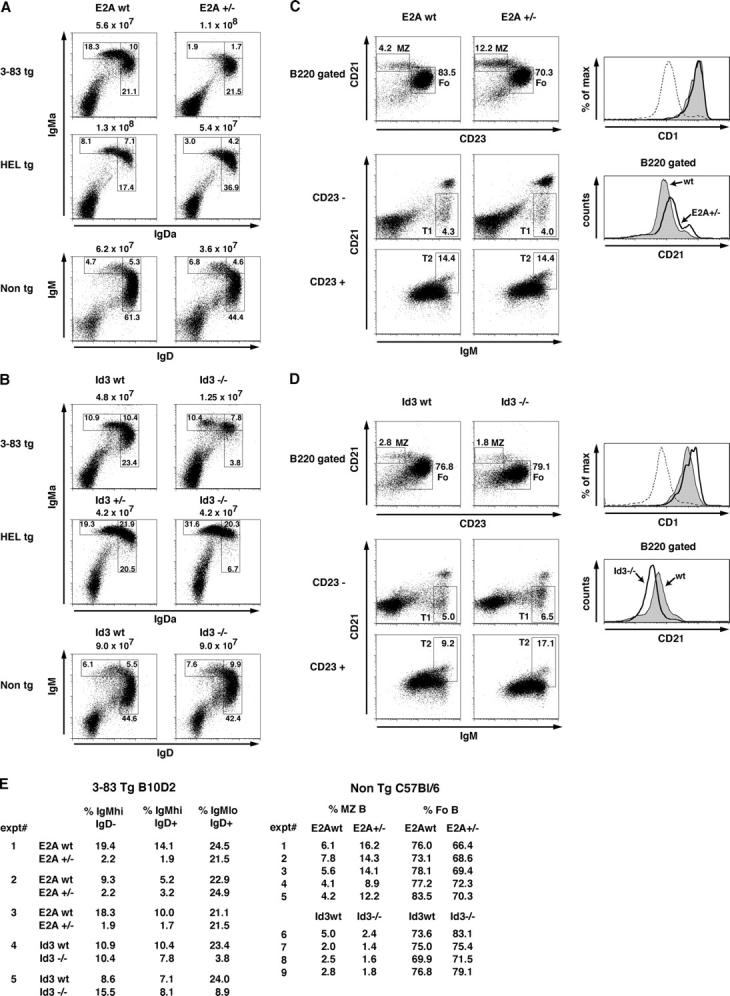
Marginal zone (MZ) B cell development is perturbed in E2A+/− and Id3-deficient mice. (A) Flow cytometric analysis of IgM and IgD expression of splenocytes derived from E2A wild-type and E2A+/− mice carrying either 3-83 (nonautoreactive background B/6; B10D2) or HEL transgenes. The total number of cells per spleen is indicated, and the percentage of each gated population is shown. (B) Flow cytometric analysis of Id3-deficient B lineage cells in the spleen as described in A. (C) Flow cytometric analysis of marginal zone B and follicular B cells from E2A wild-type and E2A+/− splenocytes. MZ B cells and follicular B cells were defined as B220+ CD21hi CD23lo and B220+ CD21int CD23hi, respectively. The percentage of each gated population is shown. The top histogram shows CD1 expression on MZ B gated cells from E2A wild-type (shaded area) and E2A+/− (solid line) mice as shown. For reference, the dotted line shows CD1 expression on B220+ gated cells. MZ B cells express higher levels of CD1 relative to follicular B cells. The lower histogram shows CD21 expression on B220+ cells from E2A wild-type (shaded area) versus E2A+/− (solid line) splenocytes. (bottom) Dot plots indicate flow cytometric analysis of T1 and T2 transitional B cell populations. T1 and T2 cells are defined as B220+CD23−CD21−/lo IgMhi and B220+CD23+CD21hi IgMhi, respectively. Because CD21 levels are modulated by E-protein activity, the median change in overall B cell CD21 levels was taken into account when gating the T2 population to reflect the proportion of CD21hi cells. This was consistent with T2 estimates using the alternative markers IgM and IgD. Numbers indicate the percentage of the B220+ population. (D) Analysis of Id3 wild-type and Id3−/− splenocytes as described in C. (E) Individual experiments showing percentages of transitional and mature B cell populations as shown in A and B. (right) Percentages of marginal zone (MZ) and follicular (Fo) B populations as shown in C and D.
Previous data have demonstrated that the antagonist helix-loop-helix protein, Id3, is required to promote positive selection in developing thymocytes by inactivating E2A/HEB DNA binding activity (24). To determine whether a deficiency in Id3 also perturbs B cell maturation, Id3−/− mice were generated that carry the 3-83 transgene in a nonediting H-2Kd background or the HEL transgene in the absence of selecting antigen. Interestingly, the Id3 deficiency led to a significant block at the transition from the IgMhi IgD+ to the IgMlo IgD+ cell stage, resulting in a decline of the percentages of mature B cells and in mice that carried the 3-83 and HEL transgenes (Fig. 6, B and E). Similarly, Id3-deficient mice that did not carry a BCR transgene showed overall higher levels of IgM expression as compared with wild-type B cells (Fig. 6 B).
To further delineate the developmental defects observed in splenic B cells, transitional, marginal zone, and follicular B cells were examined in wild-type, E2A+/−, and Id3-deficient mice. In brief, splenocytes were stained for the expression of IgM, IgD, CD21, and CD23. T1 cells are characterized by the expression of surface markers IgMhi IgD−/lo CD21−/lo CD23−; T2 cells by the expression of IgMhi IgDhi CD21hi CD23+; marginal zone cells by the expression of IgMhi IgD−/lo CD21hi CD23lo; and follicular cells by the expression of IgMint IgDhi CD21int CD23hi cells. E2A+/− splenocytes showed a significant increase in the marginal zone B population with a correlative decrease in the follicular compartment (Fig. 6, C and E). On the other hand, Id3−/− splenocytes showed a complementary phenotype with decreased percentages of marginal zone B cells relative to wild-type splenic B cells (Fig. 6, D and E). The data also indicate that CD21 expression in B cells is regulated by E-protein activity. CD21 levels were increased in E2A+/− B cells (Fig. 6 C). In contrast, in Id3−/− B cell CD21 levels were significantly decreased (Fig. 6 D). To confirm that the perturbed CD21hi CD23lo marginal zone compartments were genuine marginal zone B cells, splenocytes were further analyzed for the expression of two additional marginal zone markers, CD1 and CD9. Indeed, both E2A+/− and Id3−/− marginal zone B cells expressed high levels of CD1 and CD9 (Fig. 6, C and D, and not depicted). Although the T1 and T2 populations appeared normal in E2A+/− splenocytes, the fraction of T2 positive cells was significantly altered in Id3−/− splenic B cells (Fig. 6, C and D). Together, the data reveal that E-protein levels play a key role in promoting B lineage maturation. Relative low levels of E2A promote differentiation toward the marginal zone phenotype, whereas loss of Id3 expression affects development at the T2 cell stage and perturbs development toward the marginal zone B cell.
Discussion
E2A in Developing B Cells.
Previous studies have demonstrated a key role for E2A proteins at the earliest stages of B cell development and before or at the stage of IgH DJ rearrangement (14, 15). Here, we have analyzed E-protein activity beyond the pro–B cell stage. We demonstrate that E47 protein levels are expressed at high levels in pro–B cells, but decline upon maturing through the pre-BCR checkpoint. E47 levels are increased again at the pre–B cell stage and continue to be high until the cells reach the mature B cell stage. Previous studies have described E47 expression in B lineage development that is clearly different from the results obtained in our work (29). Relative high levels of E47 expression were detected in pre–B cells, whereas in immature B and mature B lineage cells, E47 protein levels were barely detectable by immunofluorescent staining (29). Additionally, mixtures of populations were used to assess the level of E47 by Western blotting (29). The ability to measure E47 expression using a monoclonal antibody with specificity for E47 and intracellular staining has allowed us to more precisely determine E47 protein levels at specific stages during B lineage maturation.
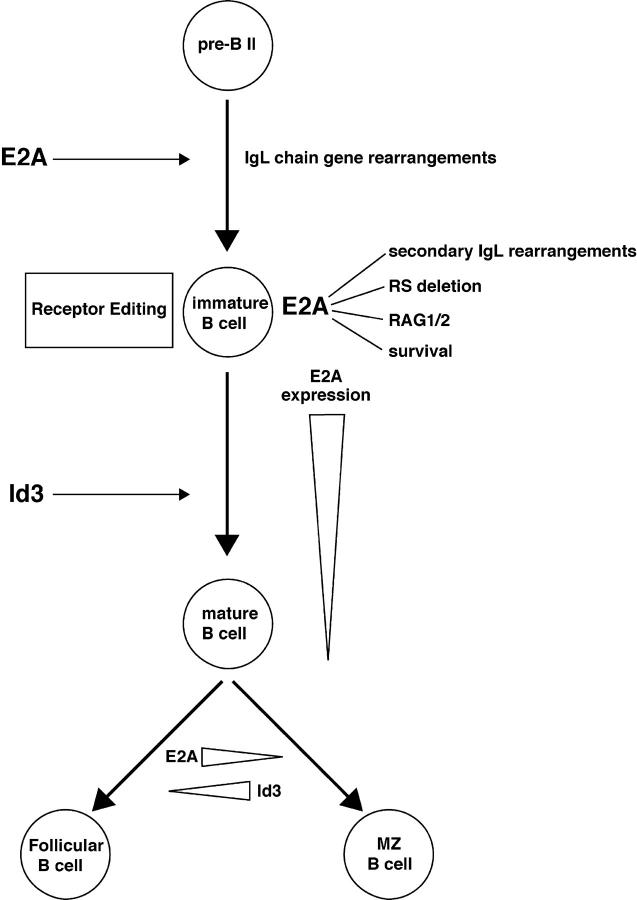
Our studies also demonstrate that receptor editing and developmental progression requires the activities of E2A proteins. Specifically, IgL secondary rearrangements in E2A+/− mice are virtually absent, likely in part due to significant lower levels of RAG1 transcripts. Recent observations have indicated that E2A binding sites are present in an enhancer element that controls the expression of RAG in developing B cells (37). Interestingly, deletion of this enhancer resulted in significant decreases in RAG1 transcript levels, whereas RAG2 levels were only slightly affected, similar to the data described here (37). Thus, it is likely that E2A proteins contribute to receptor editing by directly modulating RAG expression (Fig. 7). Finally, our observations indicate that the differentiation fate of follicular versus marginal zone B cells is affected by the activities of E2A and Id3. Together, it appears that E-proteins act at multiple stages to promote proper B lineage development.
Figure 7.
Model indicating the potential roles of E-proteins during B cell maturation.
E2A at the Pre-BCR Checkpoint.
Our data indicate that B cells begin to express high levels of E47 at a stage in which their developmental potential becomes restricted toward a B cell fate. The pattern of E47 expression is consistent with the phenotype of E2A-deficient B cells, which are blocked in their development before the onset of IgH DJ rearrangement (14, 15). E47 levels decline once a pre-BCR has formed but increase again in small resting pre–B cells and remain high at the immature B cell stage. This expression pattern shows similarities with that observed in thymocyte development (25). During thymocyte maturation, E47 levels increase upon commitment to the T cell lineage and decline beyond the pre-TCR checkpoint, similar to the pro–B cell compartment as documented here. However, we note there are also significant differences. E47 levels remain low beyond the pre-TCR checkpoint (25). On the other hand, E47 levels are elevated beyond the pre-BCR checkpoint. We also note that the expression pattern of Id3 also shows similarities in developing B and T lineage cells. Id3 levels increase upon pre-TCR–mediated signaling (25, 38). Signals emanating from the pre-BCR also induce Id3 transcription (39). Thus, the pre-BCR and pre-TCR checkpoints act similarly to modulate E47 and Id3 expression.
What is the role of E-proteins in early T and B lineage development? In developing thymocytes, E2A activity is required to activate TCR β chain V(D)J gene rearrangement and to regulate RAG2 gene expression (40 and unpublished data). In vitro studies have demonstrated that in developing B cells, the E-proteins have the ability to promote IgH DJ rearrangement and to regulate RAG gene expression (22, 23). Thus, in both B and T lineage cells, the E2A proteins regulate distinct aspects of antigen receptor assembly. Finally, in developing thymocytes, high levels of E47 prevent developmental progression beyond the pre-TCR checkpoint (25). Although it remains to be proven, it is conceivable that, given the expression pattern of E47 and Id3, the E-proteins also enforce developmental arrest at the pro–B cell stage.
E2A at the Immature B Cell Stage.
The data presented here show that in the pre–B and immature B cell compartments, E47 expression is increased to levels comparable to that of pro–B cells. What is the role of the E2A proteins at the pre–B and immature B cell stages? E12 and E47 were originally identified as factors that bind to sites present in the Igκ enhancer (41). The E2A proteins have also been shown to promote Igκ VJ rearrangement in nonlymphoid cells (23, 33). Here, we show that in E2A heterozygous mice, B cells that carry an autoreactive receptor fail to undergo efficient secondary Ig gene rearrangement. Our observations indicate that RAG gene expression is perturbed in E2A+/− B cells. We also demonstrate that secondary Ig gene rearrangements, including RS deletion, are affected in E2A+/− B cells. Consistent with a direct involvement of E2A in secondary gene rearrangement, we demonstrate here that the E2A proteins have the ability to induce RS deletion in nonlymphoid cells. Thus, it is likely that the E2A proteins have a direct role in regulating recombinase activity in response to self-antigen. However, we note that we cannot exclude a role for E2A in promoting cell survival at the stage in which the immature B cell is undergoing receptor editing. Previous studies have demonstrated that lower levels of E2A affect B cell survival (21). Consistent with these observations, we noticed that E2A+/− B cells expressing the 3-83 BCR showed a significant decrease in viability in vitro (unpublished data). Together, we favor a model in which the E2A proteins play multiple roles in immature B cells that express an autoreactive BCR (Fig. 7). They are required to allow B cell survival and to directly regulate RAG gene expression, Ig gene rearrangement, and RS deletion in response to self-antigen (Fig. 7).
E2A and the Developmental Progression of Immature B Cells.
Our findings indicate that E47 expression declines in the transitional B cell populations. E47 levels in peripheral B cells further decline upon maturing into the IgMlo IgD+ cells. As aforementioned, high levels of E47 allow immature B cells to undergo receptor editing. We would like to consider a model in which high levels of E47 enforce the developmental block at this stage (Fig. 7). In such a model, E47 levels remain high until a BCR has formed that lacks the ability to recognize self-antigen. High levels of E47 would ensure that RAG levels remain high and that the Igκ locus continues to be accessible to the recombination machinery. In the absence of signaling mediated by self-antigen, E47 levels would eventually decline and promote developmental progression. We note that such a scenario is reminiscent of that observed in thymocyte development, where E47 protein levels decline during thymocyte positive selection and Id3 levels increase (25). Consequently, Id3 deficiency results in a block in thymocyte maturation and defects in E2A lead to accelerated positive selection (24, 42). Thus, the strategies that have evolved to regulate developmental progression at distinct checkpoints may be conserved in both the B and T cell lineages.
E2A and Id3 and Follicular versus Marginal Zone B Cell Development.
Our observations also indicate that in E2A+/− mice the proportion of marginal zone B cells is increased with a concomitant decrease in the proportion of follicular B cells. On the other hand, Id3-deficient B cells exhibit a decrease in the fraction of marginal zone B cells. The abnormalities in the fraction of marginal zone and follicular B cells in E2A and Id3-deficient mice can be caused by either a defect in lineage commitment or alternatively abnormal maintenance of marginal zone B cells. A defect in maintenance cannot be excluded because E-protein activity has been demonstrated to regulate B cell survival and proliferation (21). However, because the changes in the proportion of marginal zone B cells are accompanied by changes in the proportion of follicular B cells, we favor a model in which E2A and Id3 proteins regulate marginal zone versus follicular B cell development (Fig. 7). In such a scenario, high levels of E2A would facilitate development toward the follicular cell fate, whereas Id3 activity would contribute to the differentiation of marginal zone B cells (Fig. 7).
Marginal zone B cell development is also severely affected in Aiolos, CD19, Pyk-2 kinase, and RBP-J null mutant mice (43). The loss of marginal zone B cells in CD19 and Aiolos mutant mice was explained by abnormalities in chemotactic migration or by hypersensitive BCR-mediated signaling (43, 44). Because RBP-J–deficient B cells show an increase in follicular B cells and a concomitant decline of marginal zone B cells, it was proposed that Notch signaling regulates cell fate determination of marginal zone B cells (45). The abnormalities in marginal zone and follicular B cells in E2A and Id3-deficient mice are similar to that described for the RBP-J deficiency. This raises the intriguing question as to whether Notch-mediated signaling regulates E-protein activity. Previous studies have demonstrated that E47 activity is regulated by Notch signaling by modulating transactivation or alternatively protein stability (46, 47).
Because ras signaling regulates Id3 transcription, it is conceivable that Pyk-2 regulates marginal zone B cell development by modulating Id3 gene expression (34, 48). It will be important to establish if Notch and/or Pyk2 signaling in developing B cells regulate E47 and Id3 levels to promote follicular versus marginal zone B cell development.
Acknowledgments
We thank I. Engel and C. Sayegh for carefully reading the manuscript. We thank Dr. M. Nussenzweig for providing us with RAG-GFP mice.
This work was supported by grants from the National Institutes of Health to D. Nemazee and C. Murre. M. Quong and R. Rivera were supported by training grants from the National Institutes of Health.
Abbreviations used in this paper: BCR, B cell receptor; EBF, early B cell factor; GFP, green fluorescent protein; HEL, hen egg lysozyme; HSA, heat-stable antigen; IgH, Ig heavy chain; IgL, Ig light chain; RSS, recombination signal sequence.
References
- 1.Alt, F.W., G.D. Yancopoulos, T.K. Blackwell, C. Wood, E. Thomas, M. Boss, R. Coffman, N. Rosenberg, S. Tonegawa, and D. Baltimore. 1984. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 3:1209–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajewsky, K. 1996. Clonal selection and learning in the antibody system. Nature. 381:751–758. [DOI] [PubMed] [Google Scholar]
- 3.Cedar, H., and Y. Bergman. Developmental regulation of immune system gene rearrangement. 1999. Curr Opin. Immunol. 11:64–69. [DOI] [PubMed] [Google Scholar]
- 4.Nussenzweig, M.C., A.C. Shaw, E. Sinn, D.B. Danner, H.C. Hohnes, C. Morse III, and P. Leder. 1988. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin μ. Science. 236:816–819. [DOI] [PubMed] [Google Scholar]
- 5.Manz, J., K. Denis, O. Witte, R. Brinster, and U. Storb. 1988. Feedback inhibition of immunoglobulin gene rearrangement by membrane but not by secreted μ heavy chains. J. Exp. Med. 168:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleckman, B.P., J.R. Gorman, and F.W. Alt. 1996. Accessibility control of antigen-receptor variable-region gene assembly: role of cis-acting element. Annu. Rev. Immunol. 14:459–481. [DOI] [PubMed] [Google Scholar]
- 7.Goodnow, C.C., J. Crosbie, S. Adelstein, T.B. Lavoie, S.J. Smith-Gill, R.A. Brink, H. Pritchard-Briscoe, J.S. Wotherspoon, R.H. Loblay, and K. Raphale. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 334:676–682. [DOI] [PubMed] [Google Scholar]
- 8.Nemazee, D.A., and K. Burki. 1989. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 337:562–566. [DOI] [PubMed] [Google Scholar]
- 9.Gay, D., T. Saunders, S. Camper, and M. Weigert. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiegs, S.L., D.M. Russell, and D. Nemazee. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radic, M.Z., J. Erikson, S. Litwin, and M. Weigert. 1993. B lymphocytes may escape tolerance by revising their antigen receptors. J. Exp. Med. 177:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casellas, R., T. Shih, M. Kleinewietfeld, J. Rakonjac, D. Nemazee, K. Rajewsky, and M.C. Nussenzweig. 2001. Contribution of receptor editing to the antibody repertoire. Science. 291:1541–1544. [DOI] [PubMed] [Google Scholar]
- 13.Melamed, D., R.J. Benschop, J.C. Cambier, and D. Nemazee. 1998. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 92:173–182. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang, Y., P. Soriano, and H. Weintraub. 1994. The helix-loop-helix gene E2A is required for B cell formation. Cell. 79:875–884. [DOI] [PubMed] [Google Scholar]
- 15.Bain, G., E. Robanus Maandag, D. Izon, D. Armsen, A. Kruisbeek, B.C. Weintraub, I. Krop, M.S. Schlissel, A. Feeney, M. van Roon, M. van der Valk, H.P.J. te Riele, A. Berns, and C. Murre. 1994. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 79:885–892. [DOI] [PubMed] [Google Scholar]
- 16.Urbanek, P., Z. Wang, I. Fetka, E.F. Wagner, and M. Busslinger. 1994. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 79:901–912. [DOI] [PubMed] [Google Scholar]
- 17.Lin, H., and R. Grosschedl. 1995. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 376:263–267. [DOI] [PubMed] [Google Scholar]
- 18.Engel, I., and C. Murre. 2001. The function of E- and Id proteins in lymphocyte development. Nat. Rev. Immunol. 1:193–200. [DOI] [PubMed] [Google Scholar]
- 19.O'Riordan, M., and R. Grosschedl. 1999. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 11:21–31. [DOI] [PubMed] [Google Scholar]
- 20.Kee, B.L., and C. Murre. 1998. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J. Exp. Med. 188:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kee, B.L., R.R. Rivera, and C. Murre. 2001. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-β. Nat. Immun. 2:242–247. [DOI] [PubMed] [Google Scholar]
- 22.Schlissel, M., A. Voronova, and D. Baltimore. 1991. Helix-loop-helix transcription factor E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 5:1367–1371. [DOI] [PubMed] [Google Scholar]
- 23.Romanow, W., A.W. Langerak, P. Goebel, I.L.M. Wovers-Tettero, J.J.M. van Dongen, A.J. Feeney, and C. Murre. 2000. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol. Cell. 5:343–553. [DOI] [PubMed] [Google Scholar]
- 24.Rivera, R.R., C.P. Johns, J. Quan, R.S. Johnson, and Murre, C. 2000. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 12:17–26. [DOI] [PubMed] [Google Scholar]
- 25.Engel, I., C. Johns, G. Bain, R.R. Rivera, and C. Murre. 2001. Early thymocyte development is regulated by modulation of E2A protein activity. J. Exp. Med. 194:733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlissel, M.S., and D. Baltimore. 1989. Activation of immunolobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 58:1001–1007. [DOI] [PubMed] [Google Scholar]
- 27.Engel, H., A. Rolink, and S. Weiss. 1999. B cells are programmed to activate kappa and lambda for rearrangement at consecutive developmental stages. Eur. J. Immunol. 29:2167–2176. [DOI] [PubMed] [Google Scholar]
- 28.Pongers-Willemse, M.J., O.J. Verhagen, G.J. Tibbe, A.J. Wijkhuis, V. de Haas, E. Roovers, C.E. van der Schoot, and J.J. van Dongen. 1998. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 12:2006–2014. [DOI] [PubMed] [Google Scholar]
- 29.Herblot, S., P.D. Aplan, and T. Hoang. 2002. Gradient of E2A activity in B cell development. Mol. Cell. Biol. 22:886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bain, G., S. Gruenwald, and C. Murre. 1993. E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol. Cell. Biol. 13:3522–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen, C.P., and T. Kadesch. 1995. B-cell-specific DNA binding by an E47 homodimer. Mol. Cell. Biol. 15:4518-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenbaum, S., and Y. Zhuang. 2002. Identification of E2A target genes in B lymphocyte development by using a gene tagging-based chromatin immunoprecipitation system. Proc. Natl. Acad. Sci. USA. 99:15030–15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goebel, P., N. Janney, J.R. Valenzuela, W.J. Romanow, C. Murre, and A. Feeney. 2001. Localized gene-specific induction of accessibility to V(D)J recombination induced by E2A and EBF in nonlymphoid cells. J. Exp. Med. 194:645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu, W., H. Nagaoka, M. Jankovic, Z. Misulovin, H. Suh, A. Rolink, F. Melchers, E. Meffre, and M.C. Nussenzweig. 1999. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 400:682–687. [DOI] [PubMed] [Google Scholar]
- 35.Xu, Y., L. Davidson, F.W. Alt, and D. Baltimore. 1996. Deletion of the Igκ light chain intronic enhancer/matrix attachment region impairs but does not abolish VκJκ rearrangement. Immunity. 4:377–385. [DOI] [PubMed] [Google Scholar]
- 36.Siminovitch, K.A., A. Bakhshi, P. Goldman, and S.J. Korsmeyer. 1985. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 316:260–262. [DOI] [PubMed] [Google Scholar]
- 37.Hau, L, J. Lauring, H. Liang, S. Greenbaum, D. Cado, Y. Zhuang, and M.S. Schlissel. 2003. A conserved transcriptional enhancer regulates RAG gene expression in developing B cells. Immunity. 19:105–117. [DOI] [PubMed] [Google Scholar]
- 38.Bain, G., C.B. Cravatt, C. Loomans, J. Alberola-Ila, S.M. Hedrick, and C. Murre. 2001. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat. Immun. 2:165–171. [DOI] [PubMed] [Google Scholar]
- 39.Schebesta, M., P.L. Pfeffer, and M. Busslinger. 2002. Control of pre-BCR signaling by Pax-5 dependent activation of the BLNK gene. Immunity. 17:473–485. [DOI] [PubMed] [Google Scholar]
- 40.Barndt, R.J., M. Dai, and Y. Zhuang. 2000. Functions of E2A/HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol. Cell. Biol. 20:6677–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murre, C., P.S. McCaw, and D. Baltimore. 1989. A new DNA binding and dimerization motif in immunoglobulin enhancer, daughterless, MyoD and myc proteins. Cell. 56:777–783. [DOI] [PubMed] [Google Scholar]
- 42.Bain, G., M.W. Quong, R.S. Soloff, S.M. Hedrick, and C. Murre. 1999. Thymocyte maturation is regulated by the activity of the helix-loop-helix protein, E47. J. Exp. Med. 190:1605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin, F., and J.F. Kearney. 2002. Marginal zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 44.Wang, J.H., N. Avitahl, A. Cariappa, C. Friedrich, T. Ikeda, A. Renold, K. Andrikopoulos, L. Liang, S. Pillai, B.A. Morgan, and K. Georgopoulos. 1998. Aiolos regulates B cell activation and maturation to effector state. Immunity. 9:543–553. [DOI] [PubMed] [Google Scholar]
- 45.Tanigaki, K., H. Han, N. Yamamoto, K. Tashiro, M. Ikegawa, K. Kuroda, A. Susuki, T. Nakano, and T. Honjo. 2002. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat. Immun. 3:443–450. [DOI] [PubMed] [Google Scholar]
- 46.Ordentlich, P., A. Lin, C.P. Shen, C. Blaumueller, K. Matsuno, S. Artavanis-Tsakonas, and T. Kadesch. 1998. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol. Cell. Biol. 18:2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie, L., M. Xu, A. Vladimirova, and X.H. Sun. 2003. Notch-induced E2A ubiquitination and activities. EMBO J. 3:5780–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guinamard, R., M. Okigaki, J. Schlessinger, and J.V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immun. 1:31–36. [DOI] [PubMed] [Google Scholar]