Abstract
Identification of cellular factors involved in HIV-1 entry and transmission at mucosal surfaces is critical for understanding viral pathogenesis and development of effective prevention strategies. Here we describe the evaluation of HIV-1 entry inhibitors for their ability to prevent infection of, and dissemination from, human cervical tissue ex vivo. Blockade of CD4 alone or CCR5 and CXCR4 together inhibited localized mucosal infection. However, simultaneous blockade of CD4 and mannose-binding C-type lectin receptors including dendritic cell–specific intercellular adhesion molecule–grabbing integrin was required to inhibit HIV-1 uptake and dissemination by migratory cells. In contrast, direct targeting of HIV-1 by neutralizing mAb b12 and CD4-IgG2 (PRO-542) blocked both localized infection and viral dissemination pathways. Flow cytometric analysis and immunostaining of migratory cells revealed two major populations, CD3+HLA-DR− and CD3−HLA-DR+ cells, with a significant proportion of the latter also expressing dendritic cell–specific intercellular adhesion molecule–grabbing integrin. Bead depletion studies demonstrated that such HLA-DR+ cells accounted for as much as 90% of HIV-1 dissemination. Additional studies using immature monocyte-derived dendritic cells demonstrated that although mannose-binding C-type lectin receptors and CD4 are the principal receptors for gp120, other mechanisms may account for virus capture. Our identification of the predominant receptors involved in HIV-1 infection and dissemination within human cervical tissue highlight important targets for microbicide development.
Keywords: AIDS, mucosa, transmission, dendritic cells, microbicide
Introduction
The majority of HIV-1 infections are acquired by mucosal exposure, with heterosexual transmission as the leading mode of HIV-1 infection worldwide. The HIV-1 epidemic is increasingly affecting women, particularly in Sub-Saharan Africa where they represent over 55% of those infected (http://www.unaids.org). In the absence of a protective vaccine, such women are particularly vulnerable to HIV-1 infection as they often cannot control sexual encounters and/or condom use. Thus, there is an urgent need to develop female-controlled prevention methods, which could be used without partner consent. Although the potential role of microbicides, compounds that could be applied topically to prevent HIV-1 transmission, is clearly recognized, their efficacy may be critically dependent on effective blockade of all potential receptors involved in HIV-1 entry and dissemination within genital mucosa (1).
The lower female genital tract is lined by stratified squamous epithelial cells (vagina and ectocervix) or a single epithelia monolayer (endocervix). It is unclear how HIV-1 particles or cell-associated virus penetrate intact mucosa and initiate infection. Although several mechanisms for HIV-1 transmission across genital epithelium have been proposed, their in vivo relevance remains controversial (2). We have demonstrated previously that intact normal cervical and vaginal epithelium provides a significant barrier to HIV-1 infection (3), suggesting mucosal infection may require some perturbation of epithelial integrity. Although ex vivo studies using human cervical explant tissue have identified subepithelial CD4 T cells and macrophages as the initial target for HIV-1 infection (3, 4), the principal receptors involved have not been defined. HIV-1 isolates from newly infected individuals predominantly use CCR5 (R5), suggesting that they are either selectively transmitted and/or preferentially amplified, whereas CXCR4 using (X4) isolates can become more common later in the disease course (5). We have observed previously preferential replication of the R5 virus HIV-1BaL in cervical tissues. However, R5, X4, and dual-tropic R5X4 HIV-1 isolates can all infect PHA-activated cervical tissues (3), suggesting that immune activation may increase susceptibility to additional HIV-1 phenotypes. Cervical and vaginal coreceptor expression levels are relatively low compared with other tissues (6), perhaps due, at least in part, to high levels of endogenous SDF-1 and β-chemokine expression by stratified epithelium (7). This suggests that coreceptor expression may be a rate-limiting step governing HIV-1 infection.
Studies using macaque models of vaginal transmission have suggested a critical role for DCs in mucosal SIV infection and rapid dissemination to draining lymph nodes (2). Observations that in vitro–derived human DCs can bind HIV-1 without becoming infected and maintain or even enhance HIV-1 infection of T cells have led to the suggestion that these cells may be critical in mucosal transmission of HIV-1 (8–11). The ability of mature DCs to capture HIV-1 in the absence of infection has been linked to the mannose-binding C-type lectin receptor (MCLR) DC-specific intercellular adhesion molecule–grabbing integrin (DC-SIGN) (or CD209) (9). DC-SIGN binds HIV-1 by interaction with high mannose residues expressed on viral gp120 and can enhance infection both in trans and cis (8, 11). The expression of DC-SIGN on subepithelial cervical and vaginal DCs (9, 12) suggests it may have a pivotal role in HIV-1 transmission, either promoting localized mucosal infection, where coreceptor levels may be limiting and/or facilitating dissemination of HIV-1 through migration of DC-SIGN–positive DCs to secondary lymphoid organs. However, recent evidence suggests that other MCLRs like mannose receptor (MR) (13) and non-MCLRs (14) may also contribute to virus capture and dissemination. Although these potential pathways have been the source of much conjecture, to date they have not been tested using human mucosal tissue.
Materials and Methods
Viruses, Cell Lines, Inhibitors, and Antibodies.
HIV-1 BaL and NL4–3 (obtained from Centralized Facility for AIDS Reagents) were grown in PM1 cells. Low-passage strains (SL2, 2044, and 2076; donated by P. Clapham, University College London, London, UK) were grown in PHA-stimulated PBMCs supplemented with 20 U/ml of recombinant human IL-2. The AT-2–inactivated R5 isolate HIV-1ADA was provided by J.D. Lifson (AIDS Vaccine Program, National Cancer Institute [NCI], Frederick, MD). DC-SIGN–expressing THP1 cell line was provided by V.N. KewalRamani (HIV Drug Resistance Program, NCI, Frederick, MD). HIV-1 inhibitors used included mAbs: anti-CD4 (clone RPA-T4; BD Biosciences), anti-MR (clone19.2; BD Biosciences), anti–galactosyl ceramide (GalCer) (mAb342; Chemicon International), anti–DC-SIGN (a 1:1 mixture of clone507 and clone526; provided by R.W. Doms, University of Pennsylvania, PA), mAb b12 (provided by D.R. Burton, The Scripps Research Institute, La Jolla, CA), unconjugated and biotinylated CD4-IgG2 (bCD4-IgG2) (provided by W.C. Olson, Progenics Pharmaceuticals, Tarrytown, NY), and chemokine antagonists AOP-RANTES (Serono Pharmaceutical Research Institute), TAK-779 (TAKeda Chemical Industries Ltd.), and AMD3100 (AnorMED). Human IgG and mouse IgG were purchased from Serotec. Mannan was purchased from Sigma-Aldrich. Mouse anti–human mAbs used for flow cytometry and immunostaining include: CD11b-PE (Exalpha Biological); CD1a-FITC, CD3-FITC, CD4-FITC, CD83-FITC, DC-SIGN-FITC, HLA-DR-FITC, CD3-PE, CD4-PE, CD8-PE, CD14-PE, CD20-PE, CD25-PE, CD80-PE, CD83-PE, and CD86-PE (BD Biosciences); CD3-FITC, HLA-DR-ECD (PE-Texas red), CD19-PE, CD56-PE, and CD83-PE (Beckman Coulter); and DC-SIGN (DC4, DC28; provided by Robert W. Doms, University of Pennsylvania, PA). Corresponding isotype-matched controls were included in all stainings.
Infection of Human Cervical Tissues.
Cervical tissue was obtained from women undergoing planned therapeutic hysterectomy in the absence of any cervical pathology at St. George's Hospital (written consent was obtained from all tissue donors, according to the Local Research Ethics Committee). 3-mm × 3-mm explants were cultured in 200 μl of supplemented RPMI 1640 as described previously with modifications (3). Explants were preincubated in the presence or absence of inhibitors for 1 h at 37°C before exposure to HIV-1. After a 2-h exposure at 37°C to virus (5 × 103–5 × 105 TCID50), cervical explants were extensively washed to remove unbound virus and inhibitor, and cultured for 14 d at 37°C in fresh plates, with each experimental condition performed in triplicates. For migratory cell experiments, after exposure to HIV-1, explants were cultured in the presence or absence of 100 ng/ml recombinant human MIP-3β (R&D Systems; chemotactic for DCs) for 24–48 h at 37°C; migratory cells that had emigrated out of the explants were then collected and explants cultured for 14 d as above. Migratory cells (e.g., DCs; pooled from three explants per condition) were washed and cocultured with indicator T cells (PM1 cells, 0.5 × 105 cells/well in 96-well plate) at 37°C. In some cases, migratory cells were collected and separated using CD3 or HLA-DR MicroBeads (Miltenyi Biotec) according to the manufacturer's protocol. Negative and positive populations were cocultured with PM1 cells, respectively, for 7 d. For experiments using activated tissue, explants were cultured for 3 d before infection in the presence of PHA (5 μg/ml). Stimulated explants were exposed to virus and washed as above before culture in the presence of 20 U/ml IL-2. In all cases, supernatants were collected every 3–4 d and stored at −80°C before subsequent measurement of p24 antigen by ELISA.
Characterization of Migratory Cells by Flow Cytometry and Immunofluorescent Microscopy.
Migratory cells were harvested from cervical explants after culture in complete RPMI 1640 in the presence or absence of 100 ng/ml MIP-3β for 12–48 h at 37°C. A Cell Dissociation Sieve-Tissue Grinder kit (Sigma-Aldrich) was used to remove tissue debris and big epithelial cells. Cells were monitored by three-color flow cytometry using CD3-PE and HLA-DR-ECD mAbs combined with CD4-FITC or DC-SIGN–FITC mAb. After staining, whole blood erythrocyte Lysing kit (R&D Systems) was used to lyse remaining blood cells. Cells were analyzed on FACStar (Becton Dickinson), and acquired data were analyzed using WinMDI 2.8 software.
In some cases, migratory cells from cervical tissue were adhered to alcian blue–pretreated microscope slides as previously described (15). To identify DC subsets and T cells, adherent cells were immunostained with the following mAbs: anti–HLA-DR (clone 9.3C9, HB180; hybridoma supernatant; provided by Ralph Steinman, The Rockefeller University, New York, NY), anti–DC-SIGN (5 μg/ml; clone 507), anti-MR (5 μg/ml; clone 19.2), anti-CD3 (5 μg/ml; clone Leu-4; BD Bioscience), anti-CD83 (1:50; clone PN IM2218; Beckman Coulter) including isotype controls. To reduce nonspecific signals, cells were incubated with blocking reagent provided with the TSA™ Kit (NEN Life Science) supplemented with 0.05% saponin for permeabilization. Saponin-containing blocking buffer was also used to dilute Ab. Between incubation steps, cells were washed with PBS. All steps were performed at room temperature. After blocking, Ab including isotype controls were added for 30 min, followed by a 30-min incubation with a goat anti–mouse (GAM) Alexa Fluor594 IgG (10 μg/ml; Molecular Probes) before cell nuclei were stained with DAPI (1.75 ng/ml D-1306; Molecular Probes). Mounted slides were examined by fluorescence microscopy.
Preparation and Culture of MDDCs and CD4+ T Cells.
Human monocyte-derived DCs (MDDCs) were generated from a highly enriched population of CD14+ monocytes. Briefly, PBMCs were isolated from buffy coats obtained from the National Blood Transfusion Service at London or the New York City Blood Bank using a Ficoll-Hypaque density gradient followed by negative selection using the AutoMACS (Miltenyi Biotec) and the Monocyte Isolation kit according to the manufacturer's protocol, or positive selection for CD14+ cells using the Magnetic Cell Sorting System. The purity of the isolated CD14+ cells was >95% as assessed by flow cytometry. Immature MDDCs (iMDDCs) were cultured from monocytes in the presence of IL-4 (500 U/ml; R&D Systems) and GM-CSF (800 U/ml; R&D Systems), respectively. At day 7, the phenotype of the cultured iMDDCs was confirmed by flow cytometric analysis. The iMDDCs expressed high levels of DC-SIGN, MR, and MHC class II (HLA-DR) as assessed by flow cytometry.
CD4+ T cells were separated from PBMCs using the CD4+ T Cell Isolation kit according to the manufacturer's protocol (Miltenyi Biotec). Alternatively, PBMCs (which were first depleted of the CD14+ cells used to generate iMDDCs) were kept in culture, and on the day of the experiment, the CD4+ T cell population was obtained by adding anti–HLA-DR, -CD8, and -CD11b MACS beads to the CD14-depleted PBMC suspension. The purity of CD4+ T cells was >95% as confirmed by flow cytometry.
Visualization of HIV-1 Captured by iMDDCs or CD4+ T Cells.
5 × 105 iMDDCs or CD4+ T cells were placed into 1.5-ml Eppendorf tubes and resuspended in 45 μl RPMI containing 10% FCS before the inhibitors (5 μl of each per tube) and/or PBS (5 μl per tube) were added to the cells. TAK-779 (1 μM) was added to each tube (except for the controls) combined with a second drug as listed in the following: PBS, anti-CD4 RPA-T4 (20 μg/ml), mannan (200 μg/ml), DC-SIGN mAb (1:1 mixture of clone 507 and 526; 20 μg/ml), mAb b12 (20 μg/ml), and CD4-IgG2 (20 μg/ml). Two tubes of iMDDCs and CD4+ T cells were incubated with PBS only. After 1 h at 37°C, drug pretreated cells were exposed to AT-2 HIV-1ADA (300 μg p24/1× 105 cells in 50 μl per tube) except for one tube of each cell type, which served as negative control and received the equivalent amount of buffer (1% BSA in PBS). After a further 2-h incubation at 37°C, cells were placed onto ice and washed using a refrigerated microfuge (3,000 rpm for 3 min). This was repeated four times, each time resuspending the cells in 1 ml of cold serum-free RPMI to remove cell-free virus and/or drugs before viable cells were recounted by trypan blue exclusion and monitored for the presence of viral protein by immunostaining (15).
Immunofluorescent staining was applied in order to assert the potency of several inhibitors (directed either against the envelope protein gp120 or cell surface markers) to block virus uptake. To identify iMDDCs or CD4+ T cell–associated virus, either the biotinylated tetravalent human CD4-IgG2 recognizing the CD4-binding epitope of conformationally intact gp120 (16) or a mouse mAb (clone 183-H12–5C) that reacts with HIV-1 p24 gag protein was used. For signal amplification of labeled cells, the TSA™ kit carrying the fluorochrome FITC was used. The immunostaining for viral envelope (Env) glycoprotein and gag protein was performed as previously described except that an anti-p24 Ab was used instead of an anti-p27 Ab (15).
HIV-1 Capture and Transfer Assay.
HIV-1 capture and transfer assay was performed as described previously with minor modifications (17, 18). iMDDCs or DC-SIGN–expressing THP1 cells were seeded in triplicates into wells of round-bottom 96-well plates (2 × 105 cells/well). Cells were preincubated in the presence or absence of inhibitors for 1 h at 37°C before exposure to HIV-1 for 2 h at 37°C. Cells were extensively washed to remove unbound virus and inhibitors and lysed in 1% Triton X-100. Virus capture was measured by p24 ELISA. For the virus transfer assay, cells pretreated with inhibitors and pulsed with virus were extensively washed and removed to fresh plates to coculture with PM1 cells (0.5 × 105 cells/well) at 37°C. The supernatants were collected every 3–4 d and stored at −80°C until virus replication was monitored by p24 ELISA.
p24 ELISA and Multiplex Quantitative PCR.
p24 antigen was measured by ELISA at day 14 for explants and day 7 for cells (unless indicated) according to the manufacturer's protocol (Beckman Coulter). Where indicated, multiplex quantitative PCR for HIV-1 DNA and β-actin was performed on extracted DNA as described previously (3).
gp120 Binding Assay.
The gp120 binding assay was performed as described previously with minor modifications (19). In brief, HeLa cells were infected for 1 h at 37°C with the vTF7–3 vaccinia virus and transfected for 5 h with the BaL gp120/pcDNA3 construct using Lipofectamine 2000 (Invitrogen). After a 16-h incubation at 37°C, cultures were metabolically labeled for 20 h at 37°C in Met-free/Cys-free DMEM (Sigma-Aldrich) supplemented with 100 μCi/ml of 35S Met/Cys (ICN Biomedicals). Labeled cell supernatants were harvested and stored at 4°C until used for binding. iMDDCs (5 × 106) were incubated in the presence or absence of inhibitors for 1 h at room temperature. Then, cells were resuspended in 500 μl of supernatant containing 35S-labeled gp120 and rocked gently for 2 h at room temperature. Cells were pelleted, washed with PBS, and lysed in 0.5% NP-40 for 4 h at 4°C. Lysates of cells were clarified by centrifugation and subsequently incubated with HIVIG and protein G–Sepharose (Sigma-Aldrich) rotating overnight at 4°C. Immune complexes bound by the protein G–Sepharose were extensively washed, resuspended in SDS loading buffer, and resolved by 7.5% SDS-PAGE (Bio-Rad Laboratories). Autoradiographed gels were analyzed and quantitated using the PhosphorImager (Molecular Dynamics Storm 840; Amersham Bioscience).
Results
HIV-1 Infection of Unstimulated Cervical Explants Is Inhibited by Either CD4 mAb or CCR5 Inhibitor.
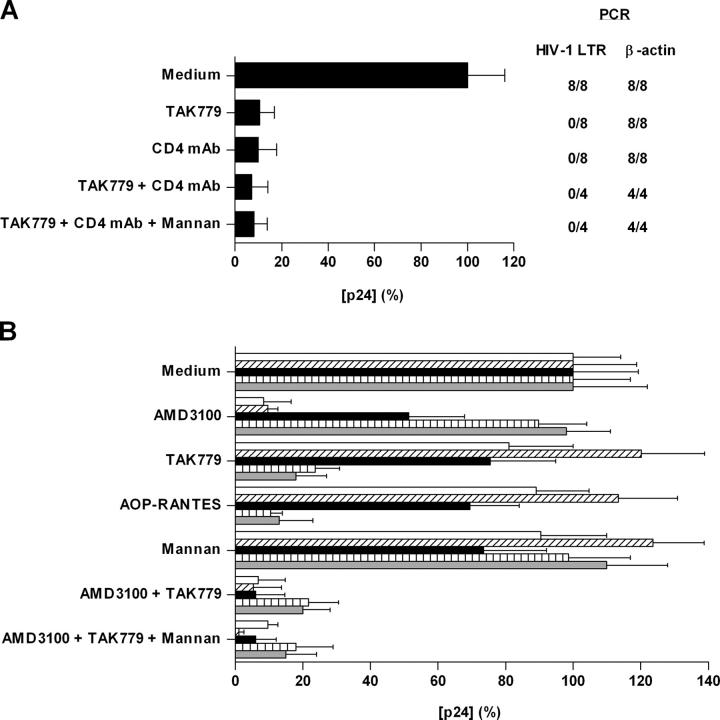
We have demonstrated previously preferential replication of the R5 virus HIV-1BaL in cervical tissues that were not exogenously stimulated or activated. To identify the predominant receptors mediating infection of cervical tissue, we used inhibitors of HIV-1BaL attachment or entry. Cervical explants were incubated with or without inhibitors specific for CD4 and CCR5 for 1 h before virus addition for 2 h. After incubation, the cervical explants were then extensively washed to remove unbound virus and inhibitors and cultured for 14 d. Both the anti-CD4 mAb RPA-T4 (20 μg/ml) (9) and the CCR5 inhibitor TAK-779 (1 μM) (20) efficiently (∼90%) inhibited HIV-1BaL infection (Fig. 1 A). There was no additional inhibition when mannan (200 μg/ml) was added to block any potential interactions of HIV-1BaL with MCLRs. Hence, blockade of either CD4 or CCR5 is sufficient to inhibit HIV-1BaL infection of nonstimulated human cervical tissue ex vivo.
Figure 1.
Receptor blockade inhibits HIV-1 infection of cervical explant tissue. (A) Unstimulated or (B) PHA-activated cervical explants were incubated with inhibitors for 1 h at 37°C before exposure to (A) HIV-1BaL or (B) NL4–3, 2044, 2076, SL2, and BaL for 2 h at 37°C. After incubation, explants were extensively washed and cultured at 37°C for 14 d. Virus production in the absence of inhibitor was defined as 100% and was (A) 1.92 ng/ml or (B) 1.39 (NL4.3, open bar), 1.04 (2044, hatched bar), 0.94 (2076, black bar), 0.79 (SL2, vertical hatched bar), and 1.70 ng/ml (BaL, gray bar). The data shown are representative of four independent experiments from separate donors yielding similar results, with each p24 determination shown as the mean (± SD) of triplicate determinations. Proviral DNA accumulation (A) at day 14 was determined by quantitative real-time PCR using LTR primers. β-Actin primers were used to control for total amount of DNA.
CCR5 and CXCR4 Are Predominant HIV-1 Coreceptors in Activated Cervical Tissue.
To determine the coreceptors used by HIV-1 in activated tissue (PHA stimulated), we used coreceptor inhibitors and R5-, X4-, or R5X4-tropic isolates (Fig. 1 B). AOP-RANTES (inhibitor targeted at CCR5 and CCR3; 100 nM) (21), and TAK-779 (CCR5 inhibitor with a limited effect on CCR2b; 1 μM) (20) inhibited infection by two different R5 isolates (SL2 and BaL). Since neither isolate can utilize CCR2b, CCR5 is considered to be their principal coreceptor in activated cervical tissue. As expected, the CXCR4 inhibitor AMD3100 (1 μM) (22) inhibited X4 viruses NL4–3 and 2044 only. Cell entry of the primary virus isolate 2076, which can utilize CCR5, CXCR4, CCR3, CCR8, and CXCR6 in coreceptor-transfected cells in vitro (23), was only inhibited when activated cervical tissue was pretreated with both AMD3100 and TAK-779, but inhibition was complete when both inhibitors were present. Hence, coreceptors other than CCR5 or CXCR4 appear unimportant for infection, but protection against dual-tropic viruses is likely to require blockade of both coreceptors, CCR5 and CXCR4. Mannan (200 μg/ml) had no effect on HIV-1 infection of activated cervical tissue.
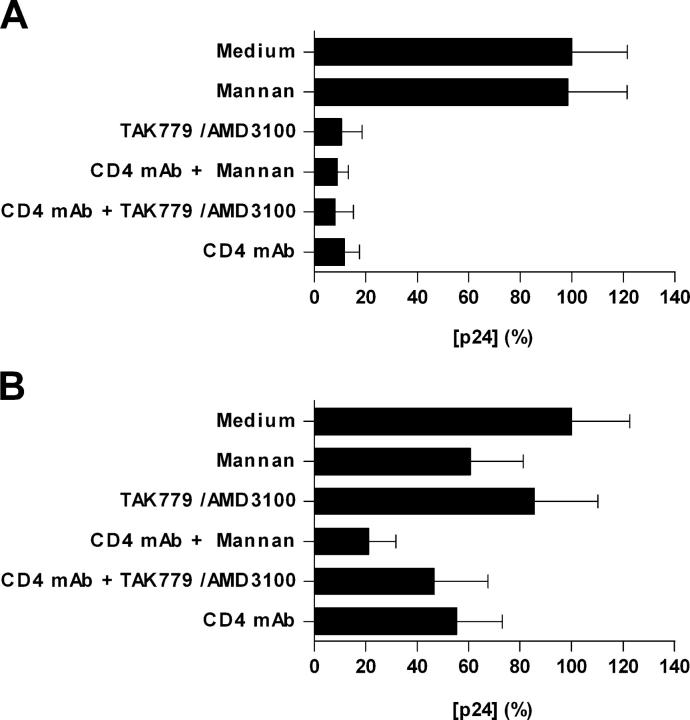
Migratory Cells Emigrating from Cervical Tissue Efficiently Capture and Transmit HIV-1 Infection In Trans.
Although the above studies show that blockade of CD4 alone or of CCR5 and/or CXCR4 can efficiently inhibit HIV-1 infection of cells within cervical tissue, they do not address whether whole HIV-1 can be taken up by migratory cells in the absence of infection. Here, the role of MCLR+ DCs may be particularly important (8, 10, 11, 24). To examine this pathway, we collected migratory cells emigrating from cervical explants 48 h after HIV-1 inoculation in the presence or absence of inhibitors and then cocultured them with indicator cells (PM1) to determine whether they contained infectious virus. Additionally, after removal of the migratory cells the remaining explants were cultured for another 14 d before measurement of HIV-1 replication. As found previously in cervical tissue, the CD4 mAb RPA-T4 alone (20 μg/ml) or TAK-779 and AMD3100 together (1 μM) completely blocked HIV-1 infection with either R5 HIV-1BaL (Fig. 2 A) or HIV-1BaL in combination with X4 HIV-1NL4.3 (not depicted), whereas mannan (200 μg/ml) had no effect. In marked contrast, the combination of TAK-779 with AMD3100 almost completely failed to inhibit the uptake of infectious HIV-1 by migratory cells, whereas the CD4 mAb RPA-T4 and mannan were each partially inhibitory (∼45 and ∼40%, respectively) (Fig. 2 B). Substantial inhibition (∼80%) of the uptake of infectious HIV-1 by migratory cells was only achieved when RPA-T4 was combined with mannan. Hence, these data suggest that migratory cells can capture infectious HIV-1 by processes involving both CD4 and MCLRs even in the presence of coreceptor inhibitors.
Figure 2.
Inhibition of localized mucosal HIV-1 infection and dissemination pathways. Human cervical explants were incubated with inhibitors for 1 h at 37°C before exposure to HIV-1BaL for 2 h at 37°C. After incubation, explants were extensively washed and cultured in the presence of 100 ng/ml of MIP-3β for 48 h. Emigrated wells were collected, washed, and cocultured with PM1 cells. The explants were cultured in separate wells. The data shown represent mean p24 antigen release (± SD) from both (A) cultured explants and (B) cocultured migratory cells and were derived from three separate donors with each determination performed in triplicates. Virus production in the absence of inhibitor was defined as 100% and was 0.99 and 1.26 ng/ml for the cervical explants and migratory cells, respectively.
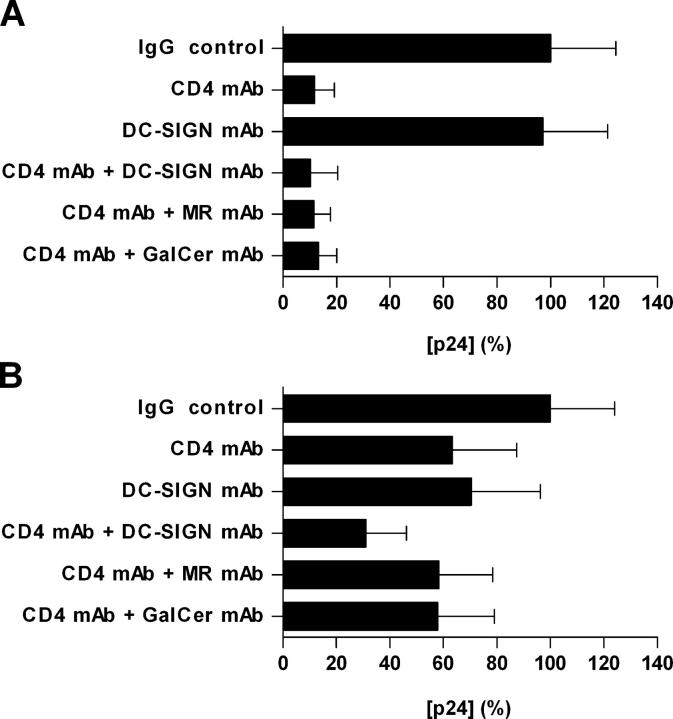
Multiple Receptors Are Involved in HIV-1 Uptake by Migratory Cells.
Since mannan is not specific for any individual MCLR, we next used blocking Abs against DC-SIGN (18), MR (25), and the mannose-independent HIV-1 ligand, GalCer (26). Consistent with the lack of inhibition we saw using mannan, mAbs to DC-SIGN, MR, and GalCer (each at 20 μg/ml) neither affected HIV-1 infection of cervical tissue nor enhanced the protective effect of the anti-CD4 mAb RPA-T4 (20 μg/ml) (Fig. 3 A and not depicted). However, when migrated cells from these explants were collected and cocultured with PM1 cells the DC-SIGN mAb modestly but detectably (∼30%) inhibited HIV-1 uptake and significantly (∼70%) inhibited uptake when combined with RPA-T4 (Fig. 3 B), albeit to a lesser extent than mannan (Fig. 2 B). The mAbs against MR (partial inhibitor of gp120 binding to MR-expressing transfectants [25]) and GalCer did not inhibit HIV-1 uptake (not depicted) and when combined with RPA-T4 did not increase its inhibition provided by RPA-T4 alone (Fig. 3 B). No inhibition was observed when control mouse IgG (20 μg/ml) was used. These experiments suggest that although DC-SIGN does not play a major role in potentiating localized mucosal infection by HIV-1 within cervical tissue, it is involved in the uptake of virus by migratory cells.
Figure 3.
Multiple receptors are involved in HIV-1 uptake by migratory cells. Experiments were performed as described in the legend for Fig. 2. The data shown represent mean p24 antigen release (± SD) from both (A) cultured explants and (B) cocultured migratory cells and were derived from two separate donors with each determination performed in triplicates. Virus production in the absence of inhibitor was defined as 100% and was 1.62 and 2.11 ng/ml for the cervical explants and migratory cells, respectively.
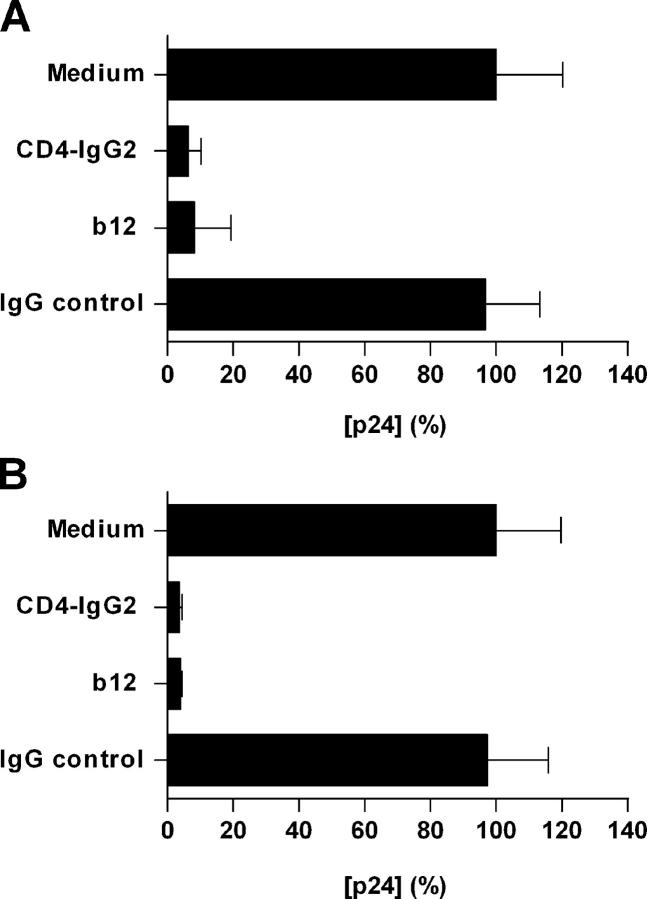
HIV-1 Neutralizing mAb b12 and the CD4-IgG2 Protein Inhibit Both Localized Mucosal Infection and Dissemination Pathways.
We also studied the effect of directly targeting HIV-1 gp120 rather than its cognate receptors using the broadly neutralizing human mAb b12 (27) and CD4-IgG2 (or PRO542), a tetrameric fusion protein between CD4 and IgG to block the CD4 interactions of gp120 (16). Similar to what was observed using RPA-T4, b12 (20 μg/ml) and CD4-IgG2 (20 μg/ml) potently inhibited HIV-1 infection of cervical tissue (Fig. 4 A), but in contrast to RPA-T4 and coreceptor inhibitors, b12 (20 μg/ml) and CD4-IgG2 (20 μg/ml) also inhibited transfer of infectious virus from migratory cells to susceptible indicator cells (Fig. 4 B). No inhibition was observed when control human IgG (20 μg/ml) was used. It is possible that enough Env-bound b12 or CD4-IgG2 remains associated with the virus taken up by migratory cells for the virus to remain neutralized. However, insufficient coreceptor inhibitor remains associated with cells for fusion to be blocked when virus is later transferred to susceptible cells.
Figure 4.
mAb b12 and CD4-IgG2 inhibit localized mucosal infection and dissemination pathways. Experiments were performed as described in the legend for Fig. 2. The data shown represent mean p24 antigen release (± SD) from both (A) cultured explants and (B) cocultured migratory cells and were derived from two separate donors with each determination performed in triplicates. Virus production in the absence of inhibitor was defined as 100% and was 1.73 and 1.88 ng/ml for the cervical explants and migratory cells, respectively.
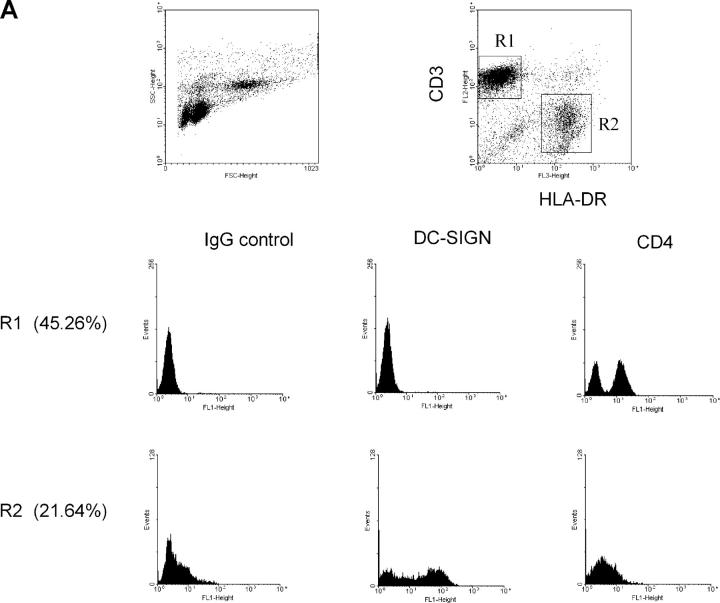
Characterization of Migratory Cells from Cervical Explants.
To characterize cells migrating from cervical tissues, cervical explants were cultured for 12–48 h to collect emigrant cells. The majority of DCs emigrated out quickly during the first 24 h; the number of smaller lymphocytes emigrating out of tissue increased with time. Migratory cells were stained with specific mAbs for FACS® or prepared on slides for immunostaining. As shown in Fig. 5 A, two major populations, CD3+HLA-DR− (R1: 45.26%) and CD3−HLA-DR+ (R2: 21.64%), were identified within the migratory cells. A population of CD3+HLA-DR+ DC-T cell conjugates (28) was also apparent. Expression of additional markers on the surface of CD3+HLA-DR− and CD3−HLA-DR+ was assessed using three-color flow cytometry. CD4+ cells were mainly found in the CD3+HLA-DR− population, although the expression was low. DC-SIGN+ cells were only identified among CD3−HLA-DR+ cells. However, some of the CD3−HLA-DR+ cells were negative for DC-SIGN, possibly due to the down-regulation of DC-SIGN expression after DC emigration or to the presence of an existing DC-SIGN− DC population (e.g., Langerhans cells).
Figure 5.
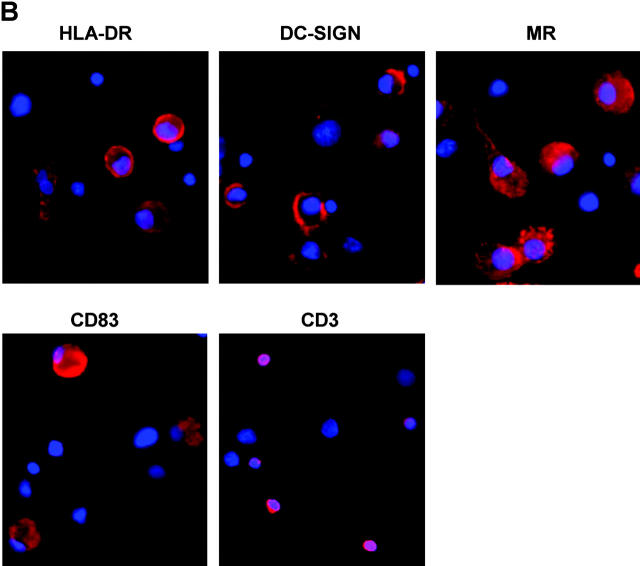
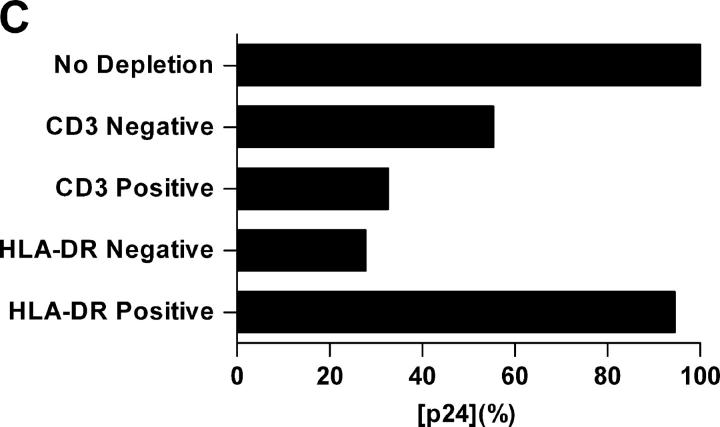
Characterization of cells emigrated from cervical tissue. (A) Three-color FACS® analyses showed a typical scatterplot distinguishing between large cells with high granularity (CD3−HLA-DR+) and small cells with low granularity (CD3+HLA-DR−). CD3+HLA-DR− and CD3−HLA-DR+ populations were gated to analyze their CD4 and DC-SIGN expression. (B) Emigrated cells were adhered to alcian blue–pretreated microscope slides and stained with the indicated mAbs followed by incubation with GAM Alexa Fluor594. Cell nuclei were stained using DAPI. (C) Emigrated cells from HIV-1BaL–infected cervical explants were separated using CD3 or HLA-DR MicroBeads. Negative and positive populations were cocultured with PM1 cells. The data shown are representative of two independent experiments derived from two separate donors. Virus production in the coculture with nondepletion population was defined as 100% and was 8.9 ng/ml.
Migratory DCs were large and irregular in shape and expressed high level of HLA-DR, MR, DC-SIGN, and the mature DC marker CD83 (Fig. 5 B). CD3+ lymphocytes were round and much smaller than DCs. Although DCs were consistently present in migratory cells, the number of DC-SIGN+ cells was highly variable, suggesting down-regulation after emigration or interdonor variability. The morphological and phenotypic features of cervical DCs were similar to those emigrated from macaque cervical explants as demonstrated previously (29). Within all preparations, only a few langerin-positive cells were detected (<1%; not depicted).
Subsequent experiments were performed to determine relative contributions of CD3+HLA-DR− and CD3−HLA-DR+ populations to HIV-1 dissemination. Cervical explants were exposed to HIV-1BaL in the presence of 1 μM TAK-779 to block productive infection. After 2 h, unbound HIV-1 and TAK-779 were removed by extensive washes, and explants were cultured for 48 h. Migratory cells were collected and separated using CD3+ or HLA-DR+ MicroBeads, respectively; CD3− or HLA-DR− and CD3+ or HLA-DR+ populations were then cocultured with PM1 cells, and p24 antigen production was assessed on day 7. As shown in Fig. 5 C, HLA-DR+ cells accounted for as much as 90% of HIV-1 dissemination, whereas CD3+ cells contributed for only 30%. Together, these results suggest that HIV-1 dissemination by migratory cells is largely mediated by a CD3−HLA-DR+ cell subset. However, DC-T cell conjugates will also be represented in the single positive fractions and may contribute to the overall signal. This might be especially true for the CD3+ population.
Visualizing HIV-1 Associated with CD4+ T Cells and iMDDCs.
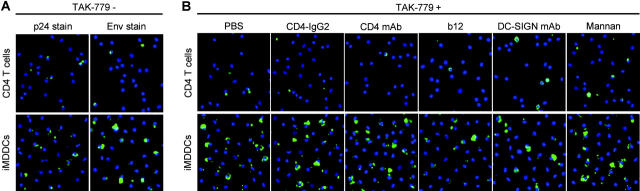
To determine efficiency and specificity of HIV-1 capture by the two migratory cell populations (HLA-DR+, CD3+; see Fig. 5 C) in more detail, further investigations were performed using purified CD4+ T cells and iMDDCs pulsed with AT-2–inactivated HIV-1ADA (R5), and virus was detected by p24 antigen and Env staining. AT-2 virus capture by DCs has been shown to reflect the capture of live virus (15), providing a safe and reliable measure of DC-virus interplay. As shown in Fig. 6 A, in the absence of TAK-779 HIV-1 captured by iMDDCs was far more efficient than that observed for CD4+ T cells as assessed by both anti-p24 mAb and biotinylated CD4-IgG2 (Env gp120) stains. In the presence of 1 μM TAK-779 (Fig. 6 B), no significant reduction of bound HIV-1 was observed in iMDDCs and CD4+ T cells (compared with PBS control), indicating that TAK-779 does not block HIV-1 uptake. But the amount of cell-associated virus captured by CD4+ T cells was decreased in the presence of CD4-IgG2 (20 μg/ml), CD4 mAb RPA-T4 (20 μg/ml), and b12 (20 μg/ml) but not in the presence of DC-SIGN mAb (20 μg/ml) or mannan (200 μg/ml) in CD4+ T cells (Fig. 6 B). In contrast, for iMDDCs no significant reduction in HIV-1 uptake was detected in the presence of any of the inhibitors (CD4-IgG2, CD4 mAb, b12, DC-SIGN mAb, and mannan) in combination with TAK-779 (Fig. 6 B).
Figure 6.
Visualizing HIV-1 captured by CD4+ T cells or iMDDCs. iMDDCs or CD4+ T cells were incubated for 1 h at 37°C with (A) PBS or (B) TAK-779 combined with a second inhibitor as listed. After exposure to AT-2 HIV-1ADA for 2 h at 37°C, cells were washed, adhered to alcian blue–pretreated slides, and monitored for the presence of (A) Env versus p24 gag protein using the biotinylated CD4-IgG2 and HRP-conjugated streptavidin versus an anti-p24 mAb combined with a HRP-conjugated GAM Ab or (B) Env using the biotinylated CD4-IgG2 and HRP-conjugated steptavidin. Signal amplification was achieved by using the TSA kit. Nuclei were stained with DAPI. Representative results out of three independent experiments are shown.
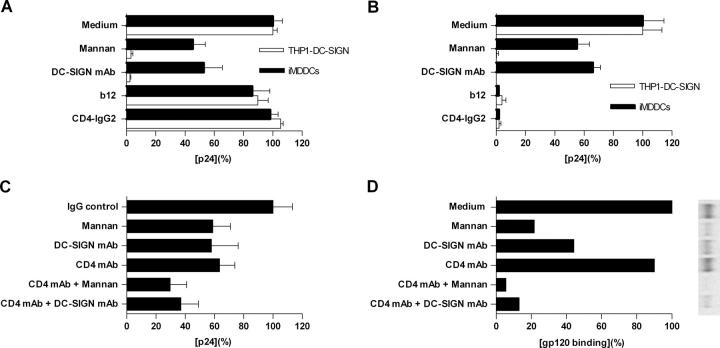
mAb b12 and CD4-IgG2 Render Bound Virus Noninfectious.
To test the hypothesis that enough Env-bound b12 or CD4-IgG2 remains associated with the virus taken up by migratory cells for the virus to be neutralized, iMDDCs and DC-SIGN–expressing THP1 cells were used to study HIV-1 capture and transfer. As shown in Fig. 7 A, b12 (20 μg/ml) showed only a marginal effect, and CD4-IgG2 (20 μg/ml) had no effect on HIV-1BaL capture by iMDDCs and DC-SIGN–expressing THP1 cells. Although DC-SIGN mAb (20 μg/ml) and mannan (200 μg/ml) partially blocked HIV-1 uptake by iMDDCs (∼55% by mannan and ∼45% by DC-SIGN mAb), they completely blocked HIV-1 capture by DC-SIGN–expressing THP1 cells, supporting the involvement of attachment receptors other than MCLRs for HIV-1 capture by iMDDCs. Similar observations were made in the HIV-1 capture and transfer experiment (Fig. 7 B). DC-SIGN mAb and mannan completely blocked HIV-1BaL transfer from DC-SIGN–expressing THP1 cells to target cells (>95%) but only partially blocked transfer of HIV-1 BaL from iMDDCs to indicator cells (∼35 and ∼45%, respectively), again suggesting the involvement of other receptors in HIV-1 capture than those blocked by DC-SIGN mAb or mannan. Of interest, b12 and CD4-IgG2, which had no effect on HIV-1 association with iMDDCs and DC-SIGN–expressing THP1 cells, completely inhibited the transmission of infectious virus from iMDDCs and DC-SIGN–expressing THP1 cells to target cells (>95%). Thus, although virus neutralized by either b12 or CD4-IgG2 still binds to iMDDCs/DC-SIGN–expressing THP1 cells, such neutralized virus does not infect T cells in trans.
Figure 7.
mAb b12 and CD4-IgG2 render bound virus noninfectious. iMDDCs and DC-SIGN-expressing THP1 cells were incubated with indicated inhibitors for 1 h at 37°C before exposure to HIV-1BaL for 2 h at 37°C. Cells were extensively washed and either lysed for capture assay (A) or cocultured with PM1 cells (B and C) for transfer assay. (A) Virus captured by iMDDCs or THP1-DC-SIGN in the absence of inhibitors was defined as 100% and was 1.76 ng/ml (THP1-DC-SIGN) and 1.58 ng/ml (iMDDCs). (B) Virus transfer from iMDDCs or THP1–DC-SIGN cells to PM1 cells in the absence of inhibitors was defined as 100% and was 65.8 ng/ml (THP1–DC-SIGN) and 59.4 ng/ml (iMDDCs). (C) Virus transfer from iMDDCs to PM1 cells in the absence of inhibitors was defined as 100% and was 83.6 ng/ml. The data shown are representative of at least two independent experiments yielding similar results with each p24 shown as the mean (± SD) of triplicate determinations. (D) Radiolabeled BaL gp120 binds to iMDDCs in the presence or absence of inhibitors. Autoradiographed gels were analyzed and quantitated using the PhosphorImager. One representative experiment out of three is shown. The value (band intensity) in the absence of inhibitors was arbitrarily set to 100%.
MCLRs and CD4 Are the Principal Receptors for gp120, But Other Mechanisms May Account for Virus Capture and Transfer.
Based on the observation that CD4 mAb in combination with either DC-SIGN mAb (20 μg/ml) or mannan (200 μg/ml) only partially inhibited HIV-1 uptake by iMDDCs and transfer to target cells (60–70%; Fig. 7 C), we examined whether there are additional receptors involved for HIV-1 binding to iMDDCs. To do so, gp120 binding to iMDDCs in the presence or absence of specific inhibitors was performed. As shown in Fig. 7 D, mannan (200 μg/ml) and DC-SIGN mAb (20 μg/ml) alone inhibited BaL gp120 binding to iMDDCs (∼80 and ∼55%, respectively). CD4 mAb (20 μg/ml) by itself had a marginal inhibitory effect on BaL gp120 binding to iMDDCs (∼10%). In contrast, the combination of CD4 mAb with mannan (>95%) or DC-SIGN mAb (∼85%) almost completely blocked BaL gp120 binding to iMDDCs. Together these data suggest that BaL gp120 binding to iMDDCs mainly involves MCLRs and CD4. The inefficient blockade of virus transfer by CD4 mAb and mannan in combination (Fig. 7 C) suggests that other mechanisms may account for virus transfer by iMDDCs.
Discussion
In vivo studies from the SIV-infected macaque model and human epidemiologic evidence together suggest that during sexual transmission HIV-1 enters the mucosa of the cervix and vagina (2). To enter coreceptor-transfected cells, HIV-1 has been reported to utilize more than a dozen seven-transmembrane receptors (30), including CCR5, CCR3, CCR2b, and CXCR4, which are expressed in the human cervix and vaginal mucosa (6, 31, 32). By conducting coreceptor-targeted blocking experiments, our results indicate that the only relevant coreceptors for HIV-1 in cervix are CCR5 and CXCR4, suggesting that CCR5 and CXCR4 are the predominant coreceptors for HIV-1 infection of cervix in vivo. These results are consistent with what was found in studies of primary human and macaque lymphocytes (33). The R5X4 isolate 2076, which uses both CCR5 and CXCR4 (23), was not inhibited by either the CCR5 inhibitor TAK-779 or the CXCR4 inhibitor AMD3100, whereas inhibition was complete when both inhibitors were present. Thus, R5X4 isolates may enter the cervix via both coreceptors, CCR5 and CXCR4. Given the broad repertoire of viral isolates present in infectious semen (5), these findings provide information that both CCR5 and CXCR4 should be considered when developing topical microbicides.
The distribution of DCs in vagina and ectocervix may allow them to be one of the first cell types to contact HIV-1. DCs express CD4, DC-SIGN, and MCLRs that may facilitate capture of HIV-1. It has been documented that different subsets of DCs express unique members of the MCLR family. For instance, DCs in vaginal epithelium express langerin (CD207) but not DC-SIGN and MR, whereas DCs in the lamina propria express DC-SIGN and MR but not langerin (9, 24). By using an ex vivo cervical explant model in which the cells represent the resident immune population in terms of their phenotype and anatomical location, we demonstrate that blockade of the primary receptors for HIV-1 infection, CD4 or CCR5 or/and CXCR4, is sufficient to inhibit localized HIV-1 infection of human cervical tissue. Interestingly, GalCer or MR appeared to play no part in localized infection of cervical tissue and dissemination of virus to target cells in our system (25, 26, 34). In the present study, MR was found to be expressed on DC subset of cells emigrated from cervical tissues. MR mAb (clone19.2; BD Biosciences) was unable to block HIV-1 take-up by migratory cells, suggesting that HIV-1 could bind to CD4, other MCLRs, and/or other MR (not recognized by the mAb). However, it is possible that the partial blocking activities of the MR (and other) mAb (25) may have contributed to the incomplete inhibition we observed. Indeed, further studies indicate that inhibition of HIV-1 uptake and subsequent transmission to T lymphocytes by migratory cells required blockade of both CD4 and MCLRs. The observation that mannan had no effect on HIV-1 infection within tissues across a range of viral titers (5 × 103–5 × 105 TCID50) suggests that MCLRs may have little impact on localized infection within cervical tissue. In contrast, MCLR-dependent uptake and dissemination of virus by migratory cells occurs even in tissue explants that remain p24 negative after in vitro exposure to HIV-1 (unpublished data). This suggests that dissemination of virus by migratory cells may be more efficient than establishment of localized infection.
Although DC-SIGN seems to be responsible for a considerable fraction of HIV-1 uptake by MCLRs on cervical cells, additional receptors are also likely to contribute as has been reported for other DC subsets (24). Flow cytometric analyses of cells emigrating from tissue explants reveal two major populations, CD3+HLA-DR− and CD3−HLA-DR+. Further experiments demonstrated that migratory DCs were large and irregular in shape and express high level of HLA-DR; these DCs also express MR, DC-SIGN, and the mature DCs marker CD83. The number of langerin-positive cells was very low (<1%). Although DCs were consistently observed within migratory population, the number of DC-SIGN+ cells appeared more variable (5–25% from donor to donor, n = 7; unpublished data). The relatively low expression of DC-SIGN on migratory cells may be due to its down-regulation after DC migration as suggested by findings in skin and tonsil models (24). It is not clear if there is interindividual heterogeneity regarding DC-SIGN expression. Recent studies indicate that inflammatory diseases and acute HIV-1 infection may increase DC-SIGN–positive DC populations (35, 36), implicating the possibility of DC-SIGN heterogeneity. More individuals need to be investigated to address this issue.
Although simultaneous blockade of CD4 and DC-SIGN did not completely suppress HIV-1 transmission from migratory cells to T cells, direct targeting of HIV-1 by the neutralizing mAb b12 and sCD4 fusion protein CD4-IgG2 was sufficient to inhibit both localized infection and dissemination pathways. Using iMDDCs and DC-SIGN–expressing THP1 cells, it has been demonstrated that virus neutralized by either b12 or CD4-IgG2 still binds to iMDDCs and DC-SIGN, but the bound virus remains noninfectious. These in vitro observations are supported by the demonstration that vaginal application of b12 but not the CCR5 inhibitor CMPD167 can prevent SHIV-162P4 transmission to macaques (37, 38). Of interest, HIV-1 uptake appears more complex in iMDDCs as blockade of both CD4 and MCLRs was unable to completely inhibit HIV-1 uptake by iMDDCs and subsequent transfer to T cells, whereas gp120 binding assays indicate that MCLRs and CD4 are the main receptors for gp120 on iMDDCs. Our findings suggest the existence of additional pathways for HIV-1 virus capture/transfer by iMDDCs. Although it is accepted that MDDCs can capture HIV via DC-SIGN, conflicting data have been reported regarding the proportional contribution of DC-SIGN to HIV-1 uptake by MDDCs (9, 13, 14, 17, 18, 39–41). This inconsistency may be attributed to difference in viral strain, inhibitor used, MDDCs preparation, and methodology.
These findings have particular significance for the design of potential topical microbicides for the prevention of HIV-1 infection of women (1, 42). Topically applied compounds will form a diffusion gradient across mucosal tissue dependent on their permeability characteristics. Agents targeted against HIV-1 itself, such as b12 mAb and CD4-IgG2, will be active within the vaginal or cervical mucosa but will need to be maintained at sufficiently high levels to neutralize incoming virus before uptake and dissemination by migratory cells (37). In contrast to b12 mAb and CD4-IgG2, many fusion and attachment inhibitors, including coreceptor inhibitors, need to reach target cells within genital mucosa before or concomitant with viral exposure (38). However, uptake of HIV-1 by migratory cells may transport virus away from localized inhibitory concentrations of topically applied agents rendering them ineffective.
These observations suggest that strategies aimed at blockade of HIV-1 uptake by cells within genital mucosa should target both localized infection and dissemination pathways and provide a frame of reference for future in vitro evaluation of microbicide candidates. Our identification of the predominant receptors involved in HIV-1 infection and dissemination within human cervical tissue suggests that targeted blockade of attachment and fusion receptors may protect against HIV-1 transmission. These findings may provide important direction for the successful development of effective HIV-1 prevention strategies.
Acknowledgments
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: human IL-2 from Dr. Maurice Gately, Hoffmann-La Roche Inc.; vTF7-3 from Dr. Tom Fuerst and Dr. Bernard Moss; HIVIG from NABI and National Heart, Lung, and Blood Institute; HIV-1 p24 mAb (183-H12-5C) from Dr. Bruce Chesebro and Kathy Wehrly.
We thank Faith Johnson and Carrie Victor-Smith for coordination and collection of tissue samples and the Obstetrics and Gynecology Department and Pathology Department of St. George's Hospital Medical School for their assistance in obtaining cervical tissue. We thank Drs. Zhiming Huo and Paul McKay for helpful discussion on flow cytometry.
This work was supported by Medical Research Council grant (G9828308) and the US National Institute of Health (AI52048). J.P. Moore is a Stavros S. Niarchos Scholar. M. Pope is an Elizabeth Glaser Scientist, and this work was supported by National Institutes of Health grants R01 AI40877 and R21 AI52060.
Abbreviations used in the paper: Env, envelope glycoprotein; DC-SIGN, DC-specific intercellular adhesion molecule–grabbing integrin; GalCer, galactosyl ceramide; GAM, goat anti–mouse; iMDDC, immature MDDC; MCLR, mannose-binding C-type lectin receptor; MDDC, monocyte-derived DC; MR, mannose receptor.
References
- 1.Shattock, R.J., and J.P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25–34. [DOI] [PubMed] [Google Scholar]
- 2.Miller, C.J., and R.J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59–67. [DOI] [PubMed] [Google Scholar]
- 3.Greenhead, P., P. Hayes, P.S. Watts, K.G. Laing, G.E. Griffin, and R.J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, K.B., B.K. Patterson, G.J. Naus, D.V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475–479. [DOI] [PubMed] [Google Scholar]
- 5.Coombs, R.W., P.S. Reichelderfer, and A.L. Landay. 2003. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 17:455–480. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, L., T. He, A. Talal, G. Wang, S.S. Frankel, and D.D. Ho. 1998. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J. Virol. 72:5035–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agace, W.W., A. Amara, A.I. Roberts, J.L. Pablos, S. Thelen, M. Uguccioni, X.Y. Li, J. Marsal, F. Arenzana-Seisdedos, T. Delaunay, et al. 2000. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr. Biol. 10:325–328. [DOI] [PubMed] [Google Scholar]
- 8.Pohlmann, S., F. Baribaud, and R.W. Doms. 2001. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 22:643–646. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek, T.B., D.S. Kwon, R. Torensma, S.J. van Vliet, G.C. van Duijnhoven, J. Middel, I.L. Cornelissen, H.S. Nottet, V.N. KewalRamani, D.R. Littman, C.G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 100:587-597. [DOI] [PubMed] [Google Scholar]
- 10.Frank, I., and M. Pope. 2002. The enigma of dendritic cell-immunodeficiency virus interplay. Curr. Mol. Med. 2:229–248. [DOI] [PubMed] [Google Scholar]
- 11.Van Kooyk, Y., and T.B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697–709. [DOI] [PubMed] [Google Scholar]
- 12.Jameson, B., F. Baribaud, S. Pohlmann, D. Ghavimi, F. Mortari, R.W. Doms, and A. Iwasaki. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turville, S.G., J.J. Santos, I. Frank, P.U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, et al. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 103:2170–2179 [DOI] [PubMed] [Google Scholar]
- 14.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865–12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J.D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allaway, G.P., K.L. Davis-Bruno, G.A. Beaudry, E.B. Garcia, E.L. Wong, A.M. Ryder, K.W. Hasel, M.C. Gauduin, R.A. Koup, J.S. McDougal, et al. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retroviruses. 11:533–539. [DOI] [PubMed] [Google Scholar]
- 17.Wu, L., T.D. Martin, R. Vazeux, D. Unutmaz, and V.N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R.W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, Q., J.O. Trent, G.D. Tomaras, Z. Wang, J.L. Murray, S.M. Conolly, J.M. Navenot, A.P. Barry, M.L. Greenberg, and S.C. Peiper. 2000. Identification of Env determinants in V3 that influence the molecular anatomy of CCR5 utilization. J. Mol. Biol. 302:359–375. [DOI] [PubMed] [Google Scholar]
- 20.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, et al. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA. 96:5698–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simmons, G., P.R. Clapham, L. Picard, R.E. Offord, M.M. Rosenkilde, T.W. Schwartz, R. Buser, T.N. Wells, and A.E. Proudfoot. 1997. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 276:276–279. [DOI] [PubMed] [Google Scholar]
- 22.Hatse, S., K. Princen, G. Bridger, E. De Clercq, and D. Schols. 2002. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 527:255–262. [DOI] [PubMed] [Google Scholar]
- 23.Simmons, G., J.D. Reeves, A. McKnight, N. Dejucq, S. Hibbitts, C.A. Power, E. Aarons, D. Schols, E. De Clercq, A.E. Proudfoot, et al. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72:8453–8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turville, S.G., P.U. Cameron, A. Handley, G. Lin, S. Pohlmann, R.W. Doms, and A.L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975–983. [DOI] [PubMed] [Google Scholar]
- 25.Turville, S.G., J. Arthos, K.M. Donald, G. Lynch, H. Naif, G. Clark, D. Hart, and A.L. Cunningham. 2001. HIV gp120 receptors on human dendritic cells. Blood. 98:2482–2488. [DOI] [PubMed] [Google Scholar]
- 26.Meng, G., X. Wei, X. Wu, M.T. Sellers, J.M. Decker, Z. Moldoveanu, J.M. Orenstein, M.F. Graham, J.C. Kappes, J. Mestecky, et al. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8:150–156. [DOI] [PubMed] [Google Scholar]
- 27.Burton, D.R., J. Pyati, R. Koduri, S.J. Sharp, G.B. Thornton, P.W. Parren, L.S. Sawyer, R.M. Hendry, N. Dunlop, P.L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 266:1024–1027. [DOI] [PubMed] [Google Scholar]
- 28.Pope, M., M.G. Betjes, N. Romani, H. Hirmand, P.U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R.M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 78:389–398. [DOI] [PubMed] [Google Scholar]
- 29.Pope, M., D. Elmore, D. Ho, and P. Marx. 1997. Dendrite cell-T cell mixtures, isolated from the skin and mucosae of macaques, support the replication of SIV. AIDS Res. Hum. Retroviruses. 13:819–827. [DOI] [PubMed] [Google Scholar]
- 30.Clapham, P.R., and A. McKnight. 2002. Cell surface receptors, virus entry and tropism of primate lentiviruses. J. Gen. Virol. 83:1809–1829. [DOI] [PubMed] [Google Scholar]
- 31.Patterson, B.K., A. Landay, J. Andersson, C. Brown, H. Behbahani, D. Jiyamapa, Z. Burki, D. Stanislawski, M.A. Czerniewski, and P. Garcia. 1998. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am. J. Pathol. 153:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hladik, F., G. Lentz, R.E. Akridge, G. Peterson, H. Kelley, A. McElroy, and M.J. McElrath. 1999. Dendritic cell-T-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J. Virol. 73:5833–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, Y., B. Lou, R.B. Lal, A. Gettie, P.A. Marx, and J.P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893–6910.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen, D.G., and J.E. Hildreth. 2003. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur. J. Immunol. 33:483–493. [DOI] [PubMed] [Google Scholar]
- 35.te Velde, A.A., Y. van Kooyk, H. Braat, D.W. Hommes, T.A. Dellemijn, J.F. Slors, S.J. van Deventer, and F.A. Vyth-Dreese. 2003. Increased expression of DC-SIGN+IL-12+IL-18+ and CD83+IL-12-IL-18− dendritic cell populations in the colonic mucosa of patients with Crohn's disease. Eur. J. Immunol. 33:143–151. [DOI] [PubMed] [Google Scholar]
- 36.Lore, K., A. Sonnerborg, C. Brostrom, L.E. Goh, L. Perrin, H. McDade, H.J. Stellbrink, B. Gazzard, R. Weber, L.A. Napolitano, et al. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 16:683–692. [DOI] [PubMed] [Google Scholar]
- 37.Veazey, R.S., R.J. Shattock, M. Pope, J.C. Kirijan, J. Jones, Q. Hu, T. Ketas, P.A. Marx, P.J. Klasse, D.R. Burton, and J.P. Moore. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346. [DOI] [PubMed] [Google Scholar]
- 38.Veazey, R.S., P.J. Klasse, T.J. Ketas, J.D. Reeves, M. Piatak, Jr., K. Kunstman, S.E. Kuhmann, P.A. Marx, J.D. Lifson, J. Dufour, et al. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon, D.S., G. Gregorio, N. Bitton, W.A. Hendrickson, and D.R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans- enhancement of T cell infection. Immunity. 16:135–144. [DOI] [PubMed] [Google Scholar]
- 40.Chehimi, J., Q. Luo, L. Azzoni, L. Shawver, N. Ngoubilly, R. June, G. Jerandi, M. Farabaugh, and L.J. Montaner. 2003. HIV-1 transmission and cytokine-induced expression of DC-SIGN in human monocyte-derived macrophages. J. Leukoc. Biol. 74:757–763. [DOI] [PubMed] [Google Scholar]
- 41.Kawamura, T., F.O. Gulden, M. Sugaya, D.T. McNamara, D.L. Borris, M.M. Lederman, J.M. Orenstein, P.A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA. 100:8401–8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turpin, J.A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs. 11:1077–1097. [DOI] [PubMed] [Google Scholar]