Abstract
Neurogenesis continues in the mammalian subventricular zone (SVZ) throughout life. However, the signaling and cell–cell interactions required for adult SVZ neurogenesis are not known. In vivo, migratory neuroblasts (type A cells) and putative precursors (type C cells) are in intimate contact with astrocytes (type B cells). Type B cells also contact each other. We reconstituted SVZ cell–cell interactions in a culture system free of serum or exogenous growth factors. Culturing dissociated postnatal or adult SVZ cells on astrocyte monolayers—but not other substrates—supported extensive neurogenesis similar to that observed in vivo. SVZ precursors proliferated rapidly on astrocytes to form colonies containing up to 100 type A neuroblasts. By fractionating the SVZ cell dissociates with differential adhesion to immobilized polylysine, we show that neuronal colony-forming precursors were concentrated in a fraction enriched for type B and C cells. Pure type A cells could migrate in chains but did not give rise to neuronal colonies. Because astrocyte-conditioned medium alone was not sufficient to support SVZ neurogenesis, direct cell–cell contact between astrocytes and SVZ neuronal precursors may be necessary for the production of type A cells.
Throughout life, the subventricular zone (SVZ) retains a neurogenic population of cells (reviewed in refs. 1–5). Cells born in the neonatal (6) and adult rodent (7) SVZ migrate along a restricted pathway called the rostral migratory stream to the olfactory bulb where they differentiate into interneurons. The SVZ of adult mice lies directly under the ependyma of the lateral wall of the lateral ventricle (8).
Three SVZ cell types have been identified based on their ultrastructural and immunocytochemical characteristics (2, 7, 9): (i) type A cells are migratory neuroblasts; (ii) type B cells are astrocytes; and (iii) type C cells are putative precursors. The spatial relationship between these cells is depicted in Fig. 1 Inset. Both type A and type C cells are apposed to the type B astrocytes. Type B cells also have intercellular contacts with each other. Type A cells migrate in chains through glial tubes composed of type B cells, and type C cells are found as small clusters along these chains of migrating neuroblasts. Type A cells are immunopositive for neuronal markers including neuron-specific tubulin (Tuj1) and the polysialylated form of the neural cell-adhesion molecule (PSA-NCAM). Type B cells contain glial-fibrillary acidic protein (GFAP), a marker of astrocytes. Type C cells are ultrastructurally immature and do not stain for either neuronal or glial markers.
Figure 1.
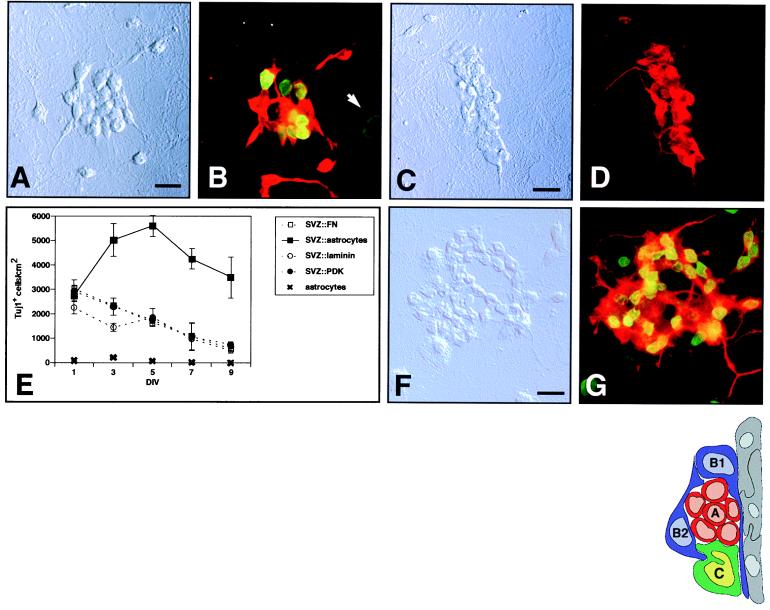
Astrocyte monolayers support the proliferation and differentiation of SVZ cells. (A and C) Differential interference contrast (DIC) images of typical colonies from postnatal SVZ precursors. (B and D) Epifluorescent images of the postnatal SVZ precursors depicted in A and C, respectively. (B) Colonies are immunopositive for the neuronal marker, neuron-specific tubulin (Tuj1; red), and have incorporated BrdUrd (green) between 4 and 5 days in vitro (DIV). (D) Colonies are also immunopositive for neuronal marker MAP-2. (E) Time course of SVZ neurogenesis. Tuj1+ cell density in coculture vs. culture on different substrates is reported at 2-day intervals. Error bars = SEM of triplicate cultures. Astrocyte monolayers alone did not produce Tuj1+ cells at any time point. (F) DIC image of a neuronal colony from the adult SVZ. (G) Respective epifluorescence showing BrdUrd incorporation (yellow-green) and Tuj1 immunoreactivity (red). All above cultures were photographed at 5 DIV. (Bars = 10 μm.) (Inset) Schematic of SVZ cellular architecture as seen in a coronal section. The ventricular cavity would be to the right of the ependymal cell layer (gray). Type A migratory neuroblasts (red) migrate through glial tubes formed by type B cells (blue). The direction of migration would be perpendicular to the plane of the page. Type C cells (green) also are in intimate contact with type B astrocytes. Type A, B2, and C are mitotic.
The type B cells can be classified further: B1 cells contact the ependyma and B2 cells do not. Type A, B2, and C cells are mitotic. Based on their immature characteristics, mitotic activity, and juxtaposition to the chains of type A cells, the type C cells have been proposed to be the immediate precursors of the migratory type A neuroblasts (9).
How SVZ neurogenesis is maintained in the adult brain is not well understood. One idea is that neuronal precursors can proliferate only in a restricted microenvironment provided by specific cell types and their particular arrangement. Astrocytes produce a variety of intercellular signals, both soluble and membrane associated, that influence the development of the central nervous system (10). Because astrocytes are in intimate contact with all of the SVZ cell types in vivo, we explored the possibility that astrocytes provide a neurogenic microenvironment for SVZ cells.
MATERIALS AND METHODS
SVZ Cell Dissociation and Fractionation.
SVZ was dissected from coronal slices of P6-P10 or adult CD-1 mouse brains (Charles River Breeding Laboratories) and dissociated with papain as described (7, 8); 30 pups were used for each fractionation.
For fractionation, 10-cm tissue-culture dishes (Corning) were treated with 0.1 mg/ml poly-d-lysine (PDK; Sigma) for 30 min. SVZ cells were plated at 2,000–4,000 cells per cm2 in DMEM with 10% (vol/vol) FCS. After 16–20 h of incubation at 37°C in 5.7% CO2 (used for all cultures), the medium was gently aspirated and replaced with 10 ml of the serum-free NB/B27 (GIBCO). By using a pipette, the NB/B27 was streamed over the surface of the plate four times at a rate of ≈2.5 ml/s. The medium containing released cells was collected (fraction 1), and 10 ml of NB/B27 was then streamed with a pipette over the plate 10 times at ≈5 ml/s and collected (fraction 2). The third collection was performed with 10 ml of PBS streamed with a pipette over the surface 20 times at ≈5 ml/s (fraction 3). The plate was then incubated with 1 ml of 0.25% trypsin/1 mM EDTA (GIBCO) for 5 min at 37°C. The trypsin-treated cells were then collected in 10 ml of NB/B27 (fraction 4) containing 1% ovomucoid albumin (Worthington) and 0.1 mg/ml DNase I (Worthington). The cell pellet of fraction 1 was treated with trypsin like fraction 4. All fractions were washed three times by centrifugation and resuspension in NB/B27. PKH26 dye labeling was performed as described (11).
Cell Culture.
Type I astrocytes were generated as described (12). Briefly, cortices or SVZ from P2-P4 mouse pups were dissected from coronal slices; the meninges were removed; and samples were incubated with 0.25% trypsin/0.5 mM EDTA. Explants were then triturated, and cells were plated into T-75 flasks in DMEM with 10% (vol/vol) FCS. At confluence, the flasks were shaken at 300 rpm for 16 h to remove neurons and oligodendrocytes. When we used this method, >95% of cells were GFAP+.
LabTek (Nunc) 16-well glass culture slides were treated with 0.5 mg/ml PDK of >300,000 molecular weight (Sigma). Fibronectin and laminin substrates (Boehringer) were used at 2 and 5 μg/cm2 respectively. To prepare astrocyte or 3T3 cell monolayers, cells were plated at 50,000 per cm2 in DMEM/10% (vol/vol) FCS. At confluence, monolayers were rinsed with four changes of NB/B27. During culture, the NB/B27 medium was half-changed every 4 days.
For migration assays, fraction 1 cells were reaggregated by incubating cells at 750,000 cells per cm2 in untreated glass LabTek wells in NB/B27 or DMEM/10% (vol/vol) FCS. Aggregates of type A cells were collected gently with a pipette, transferred to a microfuge tube, and washed three times before they were embedded in Matrigel (Collaborative Biomedical Products, Bedford, MA) and culture (13).
For astrocyte-medium-conditioning experiments, cleaned 7-mm round glass coverslips coated with astrocyte monolayers, PDK, fibronectin, or laminin were placed into 35-mm culture dishes containing astrocyte monolayers in 1 ml of serum-free medium. Dissociated SVZ cells were then added to the entire 35-mm dish, and cells were allowed to settle onto the coverslips. In other experiments, medium conditioned for 2–5 days was concentrated on Centiplus 3 units (Amicon). Astrocyte membranes were isolated as described (14). Astrocyte monolayers were killed by either heating to 55°C for 30 min or treatment with 70% (vol/vol) ethanol for 30 min followed by extensive washing.
Cells were counted at ×200 with a computer-assisted mapping system (15). All data shown are representative of at least three different experiments.
Immunocytochemistry.
All cultures were fixed with phosphate-buffered 3% (vol/vol) paraformaldehyde. Antibodies were diluted in PBS/0.1% Triton X-100/10% (vol/vol) horse serum and incubated at room temperature for 2 h. Antibody dilutions were 1:1,000 for Tuj1 (Berkeley Antibody Company); 1:100 for sheep anti-BrdUrd (Fitzgerald); 1:500 for anti-MAP-2 (Sigma); 1:1,000 for anti-menB (recognizes PSA-NCAM; gift of G. Rougon, Universite Aix-Marseille II, Marseille, France); 1:500 for rabbit anti-GFAP (Sigma); and 1:500 for anti-S100 (Sigma). Secondary antibodies were from Jackson ImmunoResearch. Controls in which primary antibodies were omitted or replaced with irrelevant IgG resulted in no detectable staining for both fluorescent and diaminobenzidine protocols.
Cultures were counterstained with Hoechst 33258 (Molecular Probes).
RESULTS
Astrocyte Monolayers Support Neurogenesis of Dissociated Postnatal and Adult SVZ Cells.
Confluent astrocyte monolayers were prepared from neonatal brains (12). Onto these monolayers, we plated dissociated SVZ cells from postnatal day-6 to day-10 mice at 5,000 cells per cm2 in serum-free medium. After 1 DIV, single, well isolated SVZ cells were observed on top of the astrocytes by phase-contrast microscopy. By 3 DIV, clusters of three to five cells having a rounded, phase-bright morphology appeared in the astrocyte coculture, and these clusters continued to form larger colonies (Fig. 1A). SVZ cells plated onto PDK, laminin, or fibronectin substrates remained as single cells and did not form colonies at any time point.
Colonies that formed on astrocytes were immunopositive for both a neuron-specific β-tubulin identified by monoclonal antibody Tuj1 (Fig. 1B), and the neuron-specific microtubule-associated protein MAP2 (Fig. 1 C and D). Furthermore, SVZ-derived colonies were immunopositive for PSA-NCAM (data not shown). The presence of the above markers and the small (<10-μm diameter) rounded somas with one or two processes identified the cells in the colonies as SVZ type A neuroblasts. To confirm that these neuronal colonies arise by proliferation, a nucleotide analog, BrdUrd, was added to cocultures at 4 DIV. Within 24 h, about half of the Tuj1+ cells were labeled (Fig. 1B).
The supporting astrocyte monolayers were initially prepared from SVZ cell dissociates. However, SVZ astrocyte preparations alone gave rise to a small number of neuronal colonies, suggesting that neuronal precursors were retained in these astrocyte monolayers. It is possible that SVZ astrocytes themselves are neuronal precursors. We therefore used cortically derived astrocytes (12), which were morphologically and immunocytochemically (GFAP+ and S100+) similar to SVZ type B cells. Cortical astrocytes alone never gave rise to neuronal colonies but supported SVZ neurogenesis. To confirm that the colonies arose from SVZ-derived cells, we used transgenic SVZ cells that constitutively express β-galactosidase (16). All Tuj1+ colonies on nontransgenic astrocyte monolayers were X-Gal+ (data not shown).
We performed experiments to determine the time course of neuronal production on astrocyte monolayers, PDK, laminin, and fibronectin substrates (Fig. 1E). There was no difference of SVZ-cell plating efficiency among the different conditions. The density of Tuj1+ cells in astrocyte coculture increased rapidly, reaching a maximum at 5 DIV (Fig. 1E). On the other culture substrates tested, the density of Tuj1+ cells decreased linearly. This decline was attributed to Tuj1+ cell death, as Tuj1+ cell debris increased with time.
Astrocyte monolayers also supported neurogenesis of adult-derived SVZ cells. SVZ cells dissociated from 3-month-old CD-1 mice were plated onto astrocyte monolayers or PDK substrate. Neuronal colonies identical in appearance to those derived from postnatal cells formed in astrocyte coculture (Fig. 1F); none were observed on the PDK substrate. Staining for Tuj1 and BrdUrd incorporation between 4 and 5 DIV confirmed the neuronal nature and proliferative origin of these adult-derived colonies (Fig. 1G).
Culture medium continuously conditioned by astrocytes or a 20-fold concentration of this conditioned medium did not support SVZ neurogenesis on PDK, laminin, fibronectin, membranes isolated from cultured astrocytes, or killed astrocyte monolayers (see Materials and Methods). Hence, it is likely that direct cell contact between SVZ precursors and live astrocytes is necessary for neurogenesis. Monolayers of 3T3 fibroblasts did not support SVZ neurogenesis (data not shown), indicating that the neurogenic cell–cell interaction is specific to astrocytes.
SVZ Cell Types Can Be Fractionated by Differential Adhesion to PDK-Treated Plastic Substrates.
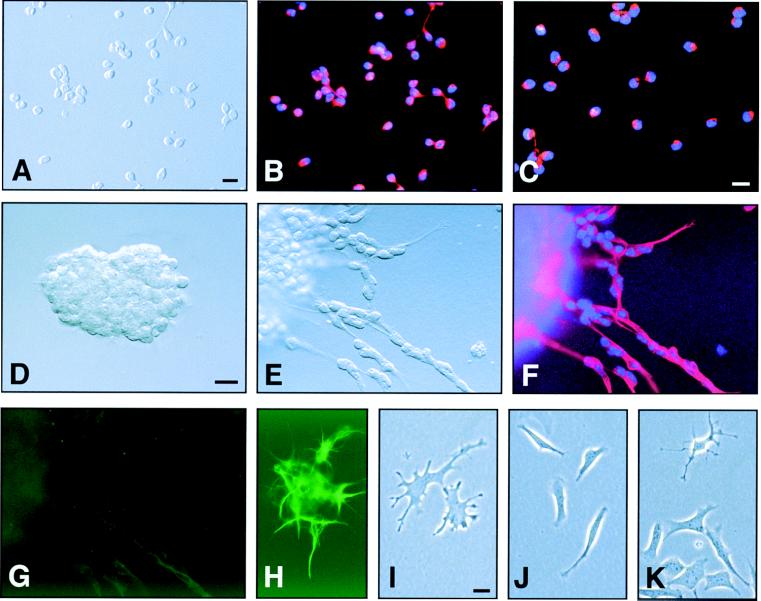
SVZ dissociates were preplated onto PDK-treated plastic culture dishes for 16–20 h, separating SVZ cells into two populations: flat, phase-dark, tightly adherent cells and rounded, phase-bright, loosely attached cells. The loosely attached cells were first removed from the preplate culture by serial rinses of culture medium. The first fraction of cells (fraction 1) was essentially homogeneous as assessed by both morphology (Fig. 2A) and immunocytochemistry (Fig. 2 B and C). Fraction 1 reproducibly consisted of Tuj1+, PSA-NCAM+ cells with less than 1% contamination by GFAP+ glial cells (see Fig. 3 Inset); hence, we designated fraction 1 the type A cell fraction. Type A cell identity was confirmed further in a chain-migration assay. After 6 h in vitro, aggregated type A cells (Fig. 2D) began forming chains and migrated away from the aggregate (Fig. 2E). The migratory A cells were homogeneously Tuj1+ (Fig. 2F) and GFAP− (Fig. 2G). These results confirm a previous report that SVZ cells can migrate in chains in the absence of glia (13).
Figure 2.
Fractionation of SVZ cells by differential adhesion yields populations of cells with distinct characteristics. (A–C) Fraction 1, type A cells. See Results and Fig. 3 for details. (A) DIC image of isolated type A cells. (B) Epifluorescent image showing Tuj1 staining (red). (C) Purified type A cells are PSA-NCAM+ (red) and can migrate in chains. (D) Aggregate of type A cells immediately after they were embedded in Matrigel. (E) Chain migration from aggregates after 6 h in culture. The culture in E was double-stained for Tuj1 (F) and GFAP (G). (H) A positive control for GFAP staining (green) in Matrigel culture. (I–K) Fraction 4, B/C cells. Most of the adherent cells had a flattened, spread, phase-dark appearance, and ≈30% of these cells were GFAP+ (see Fig. 3 Inset). Nuclei are counterstained with Hoechst 33258 (blue). (Bar = 10 μm.)
Figure 3.
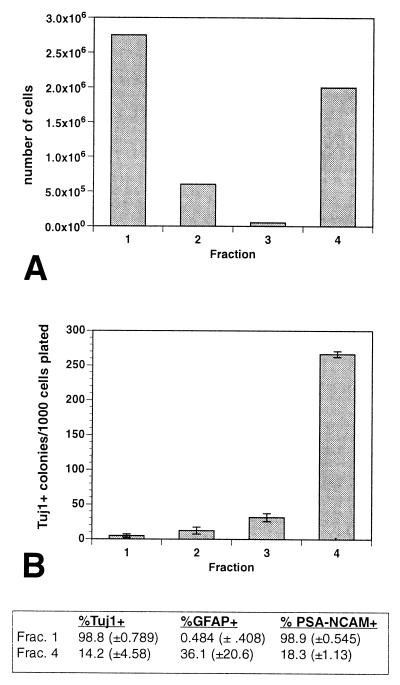
The four fractions of SVZ cells have different yields and abilities to generate Tuj1+ colonies. (A) Fractionation yield. Fraction 4 was collected after treatment with trypsin. (B) Equal numbers of cells from the different fractions in A were plated onto astrocyte monolayers. Cultures were fixed at 5 DIV. Clusters of more than four Tuj1+ cells were counted as colonies. Error bars = SEM of triplicate cultures. (Inset) Immunocytochemistry of fraction 1 (type A cells) and fraction 4 (type B/C cells) purity. For every SVZ fractionation, an aliquot of each fraction was plated for immunostaining for type A markers (Tuj1 and PSA-NCAM) and a type B marker (GFAP). Between 500 and 1,000 cells were counted in each fraction. Standard deviation is indicated in parentheses. Note that in fraction 4, almost half of the cells were immunonegative for all three markers; these are putative type C cells. There is no marker specific for type C cells at present.
Fraction 1 contained the greatest number of cells (Fig. 3A). Fractions 2 and 3 were collected with continued and more vigorous rinsing of the preplate. Fraction 4 was collected after a brief treatment of trypsin. The yield of fraction 4 was much greater than that of fraction 3, suggesting that the last collection was selective for those cells tightly adherent to the preplate. Fraction 4 cells were mostly flat and phase-dark (Fig. 2 I–K), and most were immunonegative for Tuj1 and PSA-NCAM (Fig. 3 Inset). About one-third of these cells expressed GFAP. Because fraction 4 was enriched for cells whose morphological and immunocytochemical profile resembled type B (astrocytes) and type C (immature cells) cells, we designated this fraction the B/C cell fraction.
Each fraction was tested for Tuj1+ colony formation in astrocyte coculture (Fig. 3B). The neuronal colony-forming precursors were found mostly in fraction 4. About 25% of the plated fraction 4 cells formed neuronal colonies. In other experiments in which the SVZ-derived cells first were labeled with lipophilic fluorescent dye PKH26, many fraction 4 cells were observed to attain a flat, astrocyte morphology. Thus, fraction 4 cells give rise to both glia and neurons. The small number of cells in fractions 2 and 3 had cellular compositions and colony-forming potentials intermediate to those of fractions 1 and 4; therefore, these fractions were not studied further.
Type A Cells Do Not Proliferate to Form Colonies in Astrocyte Coculture.
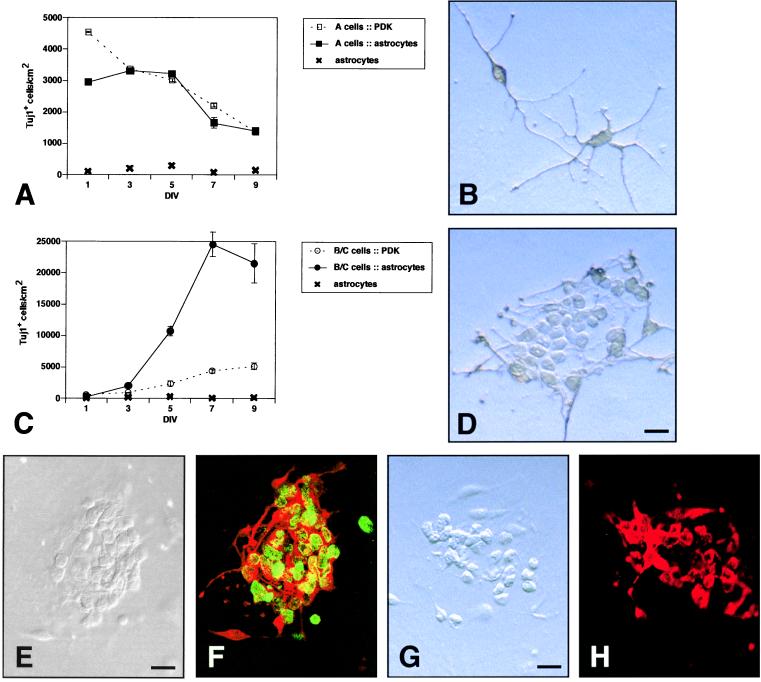
In vivo (17) and in vitro (18) studies have shown that type A cells undergo mitosis, but whether these cells have the potential to proliferate extensively is not known. Previous studies in vivo (7, 9, 18) left open the possibility that type A cells divide symmetrically to provide a continuous supply of themselves. To investigate whether coculture with astrocytes can stimulate type A cells to produce greater numbers of themselves, we plated purified type A cells at 5,000 cells per cm2 onto astrocyte monolayers or PDK substrate. On both astrocytes and PDK, the density of Tuj1+ cells decreased at similar rates (Fig. 4A), showing that astrocyte coculture does not stimulate type A cell neurogenesis nor enhance type A cell survival. On astrocytes, type A cells did not form colonies but instead remained as single cells and extended neurites (Fig. 4B).
Figure 4.
Fraction 1 (type A cells) and fraction 4 (type B/C cells) in astrocyte coculture. (A) Time course of a cell–astrocyte coculture vs. culture on PDK. Error bars = SEM of triplicate cultures. (B) Tuj1+ cells stained with diaminobenzidine in a cell–astrocyte coculture at 5 DIV. (C) Time course of B/C cell–astrocyte coculture vs. culture on PDK. (D) Typical colony of Tuj1+ cells stained with diaminobenzidine in astrocyte coculture at 5 DIV. (E and F) Cocultures were exposed to BrdUrd from 2 to 4 DIV. (E) DIC image of a neuronal colony. (F) Same colony double-stained for Tuj1 (red) and BrdUrd (green). DIC (G) and epifluorescent (H) images of a neuronal colony stained for PSA-NCAM. (Bars = 10 μm.)
The Type B/C Cell Fraction Contains Precursors Capable of Proliferating to Form Colonies of Type A Cells.
It has been proposed that the type C cells are the immediate precursors of type A cells (9) and that type B cells are the precursors of either type A or C cells (2). We plated B/C cells at 5,000 cells per cm2 onto either astrocyte monolayers or PDK substrate. At 1 DIV, there were fewer than 600 Tuj1+ cells per cm2 in both culture conditions (Fig. 4C). By 3 DIV, phase-bright colonies of fewer than eight rounded cells were observed by phase-contrast microscopy in the astrocyte cocultures, and these colonies continued to grow (Fig. 4D, 5 DIV). The cells proliferated until reaching a plateau of 23,000 Tuj1+ cells per cm2 at 9 DIV (Fig. 4C), a nearly 40-fold increase in neuronal cell number. To confirm that most Tuj1+ cells counted in the B/C–astrocyte coculture arose from proliferation, we labeled dividing cells with BrdUrd between 2 and 4 DIV. Double-staining for Tuj1 and BrdUrd at 4 DIV indicated that 87% (SEM = 4%; n = 3) were double-labeled (Fig. 4 E and F). Thus, most Tuj1+ cells counted in this neurogenesis assay were born in culture. Colonies at 5 DIV were also immunopositive for another marker of type A cells, PSA-NCAM (Fig. 4 G and H).
There was a modest increase in Tuj1+ cell number when fraction 4 B/C cells were cultured on PDK (Fig. 4C). However, we noticed that in these cultures, some SVZ astrocytes had proliferated, and the Tuj1+ colonies were always on top of the flat SVZ-derived astrocytes. We thus infer that astrocytes from fraction 4 proliferated on the PDK substrate and served as a substrate for the neuronal precursors, inducing the formation of a few type A cell colonies.
DISCUSSION
We show that, in vitro, contact with astrocytes supports the proliferation of SVZ neuronal precursors and differentiation into type A neuroblasts. Although type A cells divide (7, 17, 18), we provide evidence that SVZ neurogenesis is not solely due to symmetric division of type A cells. Instead, a cell population enriched for type B and C cells gave rise to large colonies of newly born type A cells. Astrocytes did not enhance the survival of type A cells; thus, the large numbers of these cells produced in the cocultures of type B/C cells with astrocytes cannot be attributed to the enhanced survival of the newly born neuroblasts. Thus, we propose that astrocytes support the proliferation of precursors in the B/C cell fraction and their subsequent differentiation into type A cells.
In our cultures, direct contact between astrocytes and precursors in the B/C cell fraction may be necessary for the neurogenesis. Astrocytes did not seem to produce robust soluble factors sufficient for stimulating production of type A cells. Medium continuously conditioned by astrocytes, or a 20-fold concentrate of this medium, did not support neurogenesis of SVZ cells cultured on standard substrates. Soluble factors produced by astrocytes may be necessary for SVZ neurogenesis but not sufficient. Our in vitro experiments suggest that the intimate contact in vivo among type B cells (SVZ astrocytes) and/or type B with type C cells is critical for maintaining the extensive neurogenesis of this region.
Direct contact with astrocytes also regulates neurogenesis of other precursors in vitro. Olfactory receptor precursors cultured on cortical astrocytes proliferate extensively and differentiate into mature olfactory receptor neurons (19, 20). Cortical neuron precursors from the embryo are maintained in division by contact with astrocytes (14). Although astrocytes are not present in either the olfactory epithelium where olfactory precursors reside or the developing ventricular zone, contact with astrocytes stimulates neurogenesis of precursors from these germinal zones. In contrast, neuronal precursors from the external germinal layer of the developing cerebellum cease neurogenesis when in contact with astrocytes (21). Hence, different precursors respond differently to the astrocyte microenvironment. Other examples of direct cell–cell contact regulating precursor cell biology also exist in hematopoiesis (reviewed in ref. 22) and Caenorhabditis elegans germ-line precursor development (23).
Astrocytes may present a neurogenic microenvironment at their cell surface with a combination of growth factors, extracellular matrix, and membrane-bound molecules. It is possible that the extracellular milieu at the interface between astrocytes and precursors concentrates and/or stabilizes soluble factors. Growth factors are often bound to extracellular matrix or membrane-bound molecules (reviewed in ref. 24). Temple et al. (14) found that embryonic cortical precursors continue to divide in conditioned medium supplemented with membranes prepared from cultured astrocytes or glial cells. We attempted similar experiments in which we cultured SVZ precursors in conditioned medium on astrocyte membrane preparations or killed astrocyte monolayers; however, such cultures did not support the in vitro neurogenesis. SVZ neurogenesis may require cell–cell interaction with live astrocytes. An intriguing possibility is that contact with SVZ precursors induces astrocytes to generate a neurogenic microenvironment at their cell surface.
Adult-derived central nervous system precursors have been studied extensively in vitro with exogenous soluble growth factors (reviewed in ref. 25). High concentrations of either epidermal growth factor (26, 27) or basic fibroblast growth factor (28–30) have been used to propagate SVZ cells in vitro. On mitogen withdrawal, these amplified cells cease proliferating and differentiate into mixed cultures of neurons, astrocytes, and oligodendrocytes. For epidermal growth factor-amplified cells, insulin-like growth factor I enhances neuronal differentiation (31), and platelet-derived growth factor directs neuronal differentiation of SVZ cells propagated with basic fibroblast growth factor (30). It will be important to determine whether astrocyte production of these and other soluble factors promote neurogenesis in the SVZ. In addition, we must consider that, although soluble growth factors are likely to participate in SVZ neurogenesis, signals arising from growth factor receptors can be modulated by integrins and other membrane-bound molecules (reviewed in refs. 32 and 33).
SVZ precursors cultured on astrocytes undergo extensive neurogenesis, generating an in vitro model of the adult SVZ. Our model is not unlike the cultures used to study blood-cell development in which precursors cultured on bone-marrow stromal cells undergo hematopoiesis. Our colony-forming assay can be exploited in the same way that hematopoiesis models have been used to discover the signals that direct proliferation and differentiation. For potential therapeutic applications of SVZ cells, it will be necessary to consider the cell–cell interactions both in culture and at the transplant site. A greater understanding of the interaction between precursors and astrocytes may lead to an advancement in the therapeutic potential of SVZ cells and other neural precursors.
Acknowledgments
We thank G. Rougon for the anti-menB antibody and H. Wichterle for comments on the manuscript. This work was supported by National Institutes of Health Grant NS28478 (to A.A.B). D.A.L. was supported by National Institutes of Health Medical Scientist Training Program Grant GM07739.
ABBREVIATIONS
- SVZ
subventricular zone
- PDK
poly-d-lysine
- PSA-NCAM
polysialylated neural cell-adhesion molecule
- GFAP
glial fibrillary acidic protein
- DIV
days in vitro
- DIC
differential interference contrast
References
- 1.Alvarez-Buylla A, Lois C. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- 2.García-Verdugo J M, Doetsch F, Wichterle H, Lim D A, Alvarez-Buylla A. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.McKay R. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 4.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C G, Van der Kooy D. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 5.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 6.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 7.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 8.Doetsch F, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doetsch F, Garcia-Verdugo J M, Alvarez-Buylla A. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaheer A, Zhong W, Ue E Y, Moser D R, Lim R. Cell Mol Neurobiol. 1995;15:221–237. doi: 10.1007/BF02073330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim D A, Fishell G J, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1997;94:14832–14836. doi: 10.1073/pnas.94.26.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banker G, Goslin K. Culturing Nerve Cells. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 13.Wichterle H, Garcia-Verdugo J M, Alvarez-Buylla A. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 14.Temple S, Davis A A. Development (Cambridge, UK) 1994;120:999–1008. doi: 10.1242/dev.120.4.999. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Buylla A, Vicario D S. J Neurosci Methods. 1988;25:165–173. doi: 10.1016/0165-0270(88)90155-0. [DOI] [PubMed] [Google Scholar]
- 16.Tan S-S, Breen S. Nature (London) 1993;362:638–639. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- 17.Menezes J R L, Smith C M, Nelson K C, Luskin M B. Mol Cell Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- 18.Luskin M B, Zigova T, Soteres B J, Stewart R R. Mol Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 19.Pixley S. Neuron. 1992;8:1191–1204. doi: 10.1016/0896-6273(92)90139-5. [DOI] [PubMed] [Google Scholar]
- 20.Grill R J, Pixley S K. J Neurosci. 1997;17:3120–3127. doi: 10.1523/JNEUROSCI.17-09-03120.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao W-Q, Heintz N, Hatten M E. Neuron. 1991;6:705–715. doi: 10.1016/0896-6273(91)90168-y. [DOI] [PubMed] [Google Scholar]
- 22.Deryugina E I, Muller-Sieburg C E. Crit Rev Immunol. 1993;13:115–150. [PubMed] [Google Scholar]
- 23.Henderson S T, Gao D, Lambie E J, Kimble J. Development (Cambridge, UK) 1994;120:2913–2924. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- 24.Flaumenhaft R, Rifkin D B. Curr Opin Cell Biol. 1991;3:817–823. doi: 10.1016/0955-0674(91)90055-4. [DOI] [PubMed] [Google Scholar]
- 25.Cameron H A, Hazel T G, McKay R D G. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 26.Reynolds B, Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds B A, Weiss S. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 28.Gritti A, Parati E A, Cova L, Frolichsthal P, Galii R, Wanke E, Faravelli L, Morassutti D J, Roisen F, Nickel D D, et al. J Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer T D, Ray J, Gage F H. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 30.Johe K K, Hazel T G, Muller T, Dugich-Djordjevic M M, McKay R D G. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 31.Arsenijevic Y, Weiss S. J Neurosci. 1998;18:2118–2128. doi: 10.1523/JNEUROSCI.18-06-02118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damsky C H, Werb Z. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 33.Doherty P, Walsh F S. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]