Abstract
Signaling lymphocyte activation molecule (SLAM), a glycoprotein expressed on activated lymphocytes and antigen-presenting cells, has been shown to be a coregulator of antigen-driven T cell responses and is one of the two receptors for measles virus. Here we show that T cell receptor–induced interleukin (IL)-4 secretion by SLAM−/− CD4+ cells is down-regulated, whereas interferon γ production by CD4+ T cells is only slightly up-regulated. Although SLAM controls production of IL-12, tumor necrosis factor, and nitric oxide in response to lipopolysaccharide (LPS) by macrophages, SLAM does not regulate phagocytosis and responses to peptidoglycan or CpG. Thus, SLAM acts as a coreceptor that regulates signals transduced by the major LPS receptor Toll-like receptor 4 on the surface of mouse macrophages. A defective macrophage function resulted in an inability of SLAM−/− C57Bl/6 mice to remove the parasite Leishmania major. We conclude that the coreceptor SLAM plays a central role at the interface of acquired and innate immune responses.
Keywords: SLAM, macrophage, T cell, L. major
Introduction
The self-ligand glycoprotein, signaling lymphocyte activation molecule (SLAM or CD150), is not only found on the surface of activated and memory T cells, but also on the surface of activated B cells, dendritic cells, and macrophages (1). SLAM is thought to play an important role in adhesion between T cells and APCs and has been shown to act as a coreceptor in TCR-dependent responses (1, 2). SLAM, together with CD46, is one of the two receptors for measles virus (3).
In T cells, SLAM-initiated signal transduction is in part controlled by SLAM-associated protein (SAP or SH2D1A; reference 4). SAP is a single SH2 domain protein adaptor that binds to specific tyrosine motifs on the cytoplasmic tail of SLAM and five SLAM-related receptors (CD84, CD229, CD244, NTB-A, and CS1; reference 2). As an adaptor, SAP recruits Fyn, which in turn induces tyrosine phosphorylation of the SLAM receptors and subsequently recruits a set of signaling molecules that includes SHP, Shc, Dok1/2, and RasGap (5–7). EAT-2, which is structurally related to SAP, may have a similar function in APCs (2).
An important function in immunobiology was attributed to SLAM and related receptors because mutations in SAP cause a severe immunodeficiency termed X-linked lymphoproliferative syndrome (2, 4, 8). SAP mutations in X-linked lymphoproliferative syndrome result in three major disease manifestations: fulminant infectious mononucleosis, B cell lymphomas, and dys-gammaglobulinemia (2). TCR-induced IL-4 production is impaired in SAP−/− T cells (9, 10), as judged by in vivo and in vitro experiments. SAP−/− mice are also hyperresponsive to infection with the lymphocytic choriomeningitis virus with increased numbers of IFN-γ–producing cells observed in the spleen and liver (9, 10).
Because SAP partakes in signal transduction initiated by six SLAM family members in T cells, we hypothesize that the diverse outcomes of a SAP mutation are the consequences of a combination of contributions of SLAM-like receptors. To dissect the individual contribution of SLAM signaling to immune responses, we generated a mouse that is defective in SLAM. Here we show that SLAM, like SAP (9, 10), is involved in the regulation of Th2 development. In contrast to the SAP−/− C57BL/6 mice, SLAM−/− C57BL/6 mice also have impaired macrophage functions and consequently a defective immune response to infection with the parasite Leishmania major.
Materials and Methods
Generation of SLAM-deficient Mice.
A targeting construct was constructed from a 129/Sv mouse pBAC clone (11) and was placed into the plasmid vector pPNT, which includes a thymidine kinase gene expressed from the PGK promoter (PGK-tk) for positive selection. The second and third exons of the SLAM gene, IgV and IgC, encoding the complete ectodomain of SLAM, were replaced with the neomycin-resistance gene (Neo). An 8.2- and a 3.8-kb genomic DNA fragment flank the neomycin-resistance gene.
The targeting vector was linearized at a unique NotI site and electroporated into J1 embryonic stem (ES) cells (9). G418 and FIAU-resistant ES cell colonies were screened using Southern blot analysis. KpnI digestion of genomic DNA generated a 13-kb band from the endogenous WT SLAM allele, whereas the correctly targeted SLAM allele generated an additional 8.8-kb band (see Figs. 1, A and B). The single integration site was confirmed with the internal 5′ probe upon SacI digestion of the DNA (unpublished data). A SLAM+/− ES cell clone was injected into C57BL/6 and BALB/c blastocysts. Chimeric mice were bred into the appropriate background. F1 mice with germline transmission of SLAM+/− F1 mice (129/Sv × BALB/c or 129/Sv × C57Bl/6) were bred to homozygocity. SLAM−/− mice were backcrossed with WT BALB/c or C57Bl/6 mice six times and were kept under specific pathogen-free conditions.
Figure 1.
Targeted disruption of the mouse SLAM gene. (A) The targeting vector. Configuration of the WT SLAM genomic locus, the targeting vector, and the chromosomal locus after homologous recombination with the targeting vector is shown. The second and third exons of the SLAM gene, IgV and IgC, encoding the complete ectodomain of SLAM, were replaced with the neomycin resistance gene (Neo). An 8.2- and a 3.8-kb genomic DNA fragment flank the neomycin resistance gene. The locations of the two probes used in the Southern blotting analysis are shown. SP, signal peptide; TM, transmembrane region; CP1, CP2, and CP3, cytoplasmic domains; S, SacII; K, KpnI. (B) Southern blot analysis. Screening for homologous recombination events by Southern blotting of tail DNA samples used the 3′ probe in conjunction with a KpnI (K) digest. A 13-kb band is detected in WT and heterozygote mice. An 8.8-kb band, which results from a new KpnI site in the targeting vector, is detected in heterozygotes (+/−) and homozygotes (−/−). (C) RT-PCR analysis. RT-PCR products were generated with RNA from the thymus or spleen of SLAM+/+ or SLAM−/− mice. Although an SP plus CP3 primer pair detects a 1.1-kb RT-PCR product in SLAM+/+ thymocytes (T) or splenocytes (S), no RT-PCR product is detected in the thymus or spleen of SLAM−/− mice. (D) Absence of SLAM surface expression in SLAM-deficient thymocytes. SLAM−/− or SLAM+/+ thymocytes were stained with a monoclonal rat anti–mouse SLAM antibody (9D1) and analyzed by flow cytometry.
RT-PCR.
RT-PCR was performed as previously described (11) using a 5′ UT forward primer encoding the signal peptide in the first exon, 5′-GATCCCAAAGGATCCCTTTCCTGG-3′, and a reverse primer derived from the last exon CP3, 5′-GGTCCAGTTCAGTGTGGATTTTGG-3′, defining a 1.1-kb product containing full-length SLAM cDNA (see Fig. 1 C).
Mice.
SAP−/− C57BL/6 mice (9), SLAM−/− BALB/c and SLAM−/− C57BL/6 mice, and TCR transgenic DO11.10 BALB/c mice (12) were kept under specific pathogen-free conditions. SAP−/− × DO11.10 BALB/c mice and SLAM−/− × DO11.10 BALB/c mice were generated by crossing mice that were homozygous for each mutation until double homozygocity was reached. This was judged by the appropriate PCR reactions (9 and this paper) or by staining with monoclonal antibody KJ-126 that detects the αβ TCR, which is expressed on the surface of the OVA-specific CD4+ cells of DO11.10 (12).
Flow Cytometry.
Surface staining analysis was performed as previously described (13, 14). In brief, cells were suspended at 5 × 106 cells/ml in PBS. Anti-mSLAM (9D1) was used at 10 μg/ml and detected with 10 μg/ml biotin-conjugated goat polyclonal anti–rat Ig (BD Biosciences), followed by 1 μg/ml streptavidin-conjugated Red 670 (Invitrogen).
For cytoplasmic staining of IFN-γ production, splenic T cells were resuspended at 106/ml and stimulated with 5 μg/ml plate-bound anti-CD3ɛ for 3 d. 2 h before harvesting, 1 μg/ml Brefeldin A was added to the cultures. Cells were stained with FITC-conjugated anti-CD4 or biotin-conjugated anti-CD8 (BD Biosciences) for 30 min at 4°C in a balanced salt solution supplemented with1% FCS. Cells were then washed twice in buffered saline before fixation in buffered saline/4% paraformaldehyde for 10 min at room temperature. After fixation, cells were washed twice with balanced salt solution/1% FCS and resuspended in permeabilization buffer (PBS, 0.1% saponin, and 1% FCS) containing anti–IFN-γ monoclonal antibody (BD Biosciences) for 30 min. The percentage of cells expressing cytoplasmic IFN-γ was determined by flow cytometry.
ELISA Assays for Cytokine Secretion.
All cytokine ELISA kits were obtained from BD Biosciences. Tissue culture supernatants were stored at −80°C until analysis. ELISA assays were performed according to the manufacturer's instructions. Standard curves were generated using known amounts of purified murine rIL-4, rIL-6, rIL-12p40, rIL-12p70, or TNF-α (BD Biosciences).
In Vitro T Cell Assays.
CD4+ cells were purified using negative selection columns (9) cultured with 5 μg/ml anti-CD3ɛ antibody (2C11)–coated plates and 5 μg/ml soluble anti-CD28 or with a combination of 10 ng/ml PMA and 1 μM ionomycin in the presence of 10 ng/ml recombinant IL-2 (BD Biosciences) for 72 h. Supernatants were analyzed for IL-4 or IFN-γ by ELISA as previously described (9). IFN-γ production was determined by cytoplasmic staining and by flow cytometry as previously described (9).
In Th1- or Th2-polarized experiments, exogenous cytokines or anti-cytokine antibodies were added to the anti-CD3 and anti-CD28 antibody, as indicated. Th2 cell differentiation was promoted in the presence of 100 ng/ml IL-4 and 10 μg/ml anti–IL-12 antibody, and Th1 differentiation was promoted by 10 μg/ml anti–IL-4 antibody and 1 ng/ml IL-12 (all from BD Biosciences). After the cells were cultured for 4 d, they were washed and resuspended at 106 cells/ml. The secondary culture used only plate-bound anti-CD3 and lasted for 24 h. As described above, 10 ng/ml IL-2 (BD Biosciences) was added to all cultures (9).
For IL-4 and IFN-γ secretion by CD4+ T cells isolated from SLAM−/− × DO11.10 or DO11.10 mice, the percentage of KJ-126+ CD4+ cells from each mouse was determined by flow cytometry using KJ-126 FITC (Caltag) and CD4-PE (BD Biosciences). Equal numbers of KJ-126+ CD4+ cells were plated in complete media at 2 × 105 cells/well in a 24-well plate for 3 d in the presence of 2 × 106 APCs and 1.0 μg of an OVA peptide, OVA 323–339. Surface staining analysis was performed with anti–mouse SLAM (9D1) as previously described (13). Secondary stimulations were performed for 24 h under the same conditions. IL-4 and IFN-γ secretion was determined at day 3 of the primary stimulation and 24 h after the secondary stimulation by ELISA.
Cytokine Production by In Vitro–stimulated Macrophages.
Resting or thioglycollate-elicited macrophages were obtained by peritoneal lavage as previously described (13). Macrophages were stimulated in a medium containing 2 ng/ml LPS, 200 U/ml IFN-γ, 2 ng/ml LPS plus 200 U/ml IFN-γ, and 10 μM CpG or 25 μg/ml peptidoglycan.
In other experiments, 2 × 106 macrophages were activated with increasing amounts of LPS (1, 10, or 100 ng/ml). After the cells were incubated for 24 h, supernatants were assayed for IL-12p70, IL-12p40, TFN-α, or IL-6 by ELISA (BD Biosciences).
For antibody stimulation of macrophages, cells were cultured for 24 h with rat anti–mouse SLAM (9D1) at concentrations ranging between 0 to 10 μg/ml in the presence of blocking Fc fragments (13). To each well, a rat anti–mouse IgG1 isotype control antibody was added (BD Biosciences) in a concentration range from 10 to 0 μg/ml resulting in a final concentration of 10 μg/ml Ig at each experimental point.
Nitric oxide (NO) production was quantified by the accumulation of nitrite (as a stable end product) in the supernatants by the standard Griess reaction as previously described (15). In brief, 50 μl of the supernatants was removed from 24-well plates and incubated with an equal volume of Griess reagent (1% sulfanilamide/0.1% naphthalene diamine dihydrocloride/2.5% H3PO4) at room temperature for 10 min. The absorbance at 570 nm was determined with a microplate reader. Conversion of absorbance to micromolar concentration of NO was deduced from a standard curve using a known concentration of NaNO2 diluted in DMEM medium. All determinations were performed in quadruplicate and expressed as micromolar concentration of NO.
Antigen Presentation by Macrophages.
To quantify the in vitro proliferative response to OVA peptide, thioglycollate-elicited macrophages (2 × 105/well) from SLAM+/+ or SLAM−/− mice were cocultured with KJ-126+ CD4+ splenic T cells from DO11.10 mice (105/well) in DMEM medium supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS for 3 d. OVA peptide was added at the indicated concentrations (from 0.01 to 1.0 μg/ml). The cells were cultured for 72 h and [3H]TdR (1 μCi /well) was added for the last 18 h of the assay. The cells were harvested onto filters using an automated cell harvester.
Phagocytosis of Escherichia coli F18 by Macrophages.
After macrophages were plated at 106 cells/ml E. coli, F18 bacteria were added to the culture media at a concentration of 107 bacteria/ml. After 1 h incubation at 37°C, the cells were washed three times with PBS and lysed using 1% Triton in H2O for 10 min at 4°C. Dilutions of the lysate were streaked onto LB plates and incubated for 24 h. Phagocytosis rates were obtained by counting colonies after 24 h (16, 17).
Infection with L. major.
Mice were infected subcutaneously into one hind footpad with 2 × 106 L. major stationary phase promastigotes in a final volume of 50 μl as previously described (18). The development of lesions was monitored with a vernier caliper, measuring increase in footpad thickness compared with the uninfected footpad. To determine parasite loads, infected mice were killed and their footpads were collected and homogenized. The number of amastigotes in each footpad was counted with a Nuebaer hemocytometer. Data are expressed as mean ± SEM number of amastigotes per footpad. Leishmaniacidal activity was determined as previously described (18–20).
T Cell Assays after Infection with L. major.
The draining inguinal lymph nodes from mice infected with L. major were removed aseptically and cell suspensions were prepared, as previously described (18–20). 100 μl aliquots containing 3 × 105 lymph node cells in complete RPMI medium were added in triplicate to the wells of 96-well flat-bottomed tissue culture plates containing 20 μg/ml soluble L. major antigen per well in different concentrations (18–20). After incubation at 37°C in 5% CO2 for 60 h, 100 μl of the culture supernatant was removed from each well and stored at −70°C for cytokine assays and replaced with 100 μl complete medium containing the appropriate μCi of tritiated thymidine. After an additional 12 h of incubation at 37°C, it was harvested onto filter paper with a cell harvester. Thymidine uptake was measured by liquid scintillation using a β counter with 1 ml Optiscint added to the filter discs in vials and counted for 5 min each. The stimulation index values at the end of the L. major infection were calculated as the quotient of antigen stimulated/unstimulated cells.
Online Supplemental Material.
Results of experiments, which complement the data presented in the paper, are shown in Figs. S1–S6. In Fig. S1, IL-4 and IFN-γ secretion by resting and anti-CD3/CD28 activated SLAM−/− C57BL/6 and SLAM−/− BALB/c T cells. In Fig. S2, IL-4 and IFN-γ secretion by resting and anti-CD3/CD28 activated CD4+ cells purified from the spleens of SLAM−/− BALB/c mice. Secretion of IL-4 and IFN-γ by SLAM−/− C57BL/6 and WT C57BL/6 CD4+ cells cultured under Th1- and Th2-polarizing conditions is shown in Fig. S3. In Fig. S4, secretion of IL-4 and IFN-γ by SLAM−/− BALB/c and WT BALB/c CD4+ cells cultured under Th1- and Th2-polarizing conditions is shown. In Fig. S5, T cell receptor–mediated Ca2+ entry in CD4+ T cells of WT SLAM−/− and SAP−/− C57BL/6 mice is identical. Altered IL-12, TNF-α, IL-6, and NO production by peritoneal macrophages from SLAM−/− BALB/c mice is shown in Fig. S6. Figs. S1–S6 are available at http://www.jem.org/cgi/content/full/jem.20031835/DC1.
Results
Deviation of Cytokine Production by SLAM−/− CD4+ and CD8+ T Cells.
A mouse with a targeted disruption of the second and third exon of the SLAM gene was generated by homologous recombination in ES cells (Fig. 1, A and B). SLAM-deficient mice were fertile, morphologically indistinguishable from WT littermates, and no defects in their T, B, or NK cell development were detected by cell surface marker analyses. No SLAM mRNA was detected in mice that were homozygous for the gene defect (Fig. 1 C) and SLAM-deficient thymocytes, which are the highest SLAM expressors in WT mice, do not stain with the anti–mouse SLAM antibody 9D1 (Fig. 1 D).
To determine whether SLAM−/− CD4+ T cells deviated in their cytokine production along the lines of SAP−/− T cells, several in vitro assays were performed. First, total T cells purified from the spleen of SLAM−/− C57BL/6 and WT C57BL/6 mice were triggered with a combination of anti-CD3 and anti-CD28. Production of IFN-γ was then measured by cytoplasmic staining and cytofluorimetric analyses using anti-CD8 and anti-CD4 antibodies. Although SLAM−/− CD8+ T cells produce more IFN-γ than WT C57BL/6 CD8+ T cells, this difference is less dramatic in CD4+ cells (unpublished data). A modest increased IFN-γ secretion by T cells was found by ELISA, regardless of the genetic background (Fig. S1, B and D, which is available at http://www.jem.org/cgi/content/full/jem.20031835/DC1). Stimulation of total T cells from either SLAM−/− C57BL/6 or SLAM−/− BALB/c mice with anti-CD3 alone or with a combination of anti-CD3 and anti-CD28, also resulted in a reduced production of IL-4, presumably by CD4+ cells (Fig. S1, A and C).
Indeed, purified CD4+ cells isolated from SLAM−/− C57BL/6 mice activated with a combination of anti-CD3 and anti-CD28 secreted reduced amounts of IL-4 in a primary stimulation compared to WT C57BL/6 CD4+ cells (Fig. 2 A). Using the same stimulation conditions, we consistently observed a slight increase in IFN-γ secretion by SLAM−/− C57BL/6 CD4+ cells versus WT C57BL/6 CD4+ cells (Fig. 2 B). Upon primary or secondary stimulation with anti-CD3 and anti-CD28, CD4+ cells from purified SLAM−/− BALB/c mice also produced less IL-4 than their WT counterparts (Fig. S2, which is available at http://www.jem.org/cgi/content/full/jem.20031835/DC1). However, when polarizing Th2 conditions were used, SLAM−/− C57BL/6 and WT C57BL/6 CD4+ cells secreted comparable amounts of IL-4 (Fig. S3, which is available at http://www.jem.org/cgi/content/full/jem.20031835/DC1). Using polarizing Th2 conditions in the second stimulation, SLAM−/− BALB/c and WT BALB/c CD4+ cells also secreted comparable amounts of IL-4 (Fig. S4, which is available at http://www.jem.org/cgi/content/full/jem.20031835/DC1). The IFN-γ production remained slightly higher when the cells were cultured under Th1-polarizing conditions (Figs. S3 and S4). Taken together, these results were indicative of an impaired production of IL-4 by CD4+ cells purified from SLAM−/− C57BL/6 and SLAM−/− BALB/c mice in primary and secondary stimulations. However, IL-4 secretion by purified CD4+ cells readily reaches a WT level in the presence of IL-4. These SLAM−/− C57BL/6 and SLAM−/− BALB/c CD4+ cells also consistently produce more IFN-γ than WT cells. As in SAP−/− mice, the increase in IFN-γ production by SLAM−/− CD8+ cells appears more robust than that of SLAM−/− CD4+ cells.
Figure 2.

Deviation of cytokine production by CD4+ and CD8+ SLAM−/− T cells. (A) IL-4 secretion by CD4+ cells purified from SLAM−/− or SLAM+/+ C57BL/6 mice. SLAM−/− or SLAM+/+ C57BL/6 CD4+ cells were purified using negative selection columns. In the primary stimulation (−1°−) CD4+ cells were stimulated with 5 μg/ml anti-CD3ɛ antibody (2C11)–coated plates and 5 μg/ml soluble anti-CD28, or with a combination of 10 ng/ml PMA and 1 μM ionomycin in the presence of 10 ng/ml recombinant IL-2 (BD Biosciences; P/I) for 72 h. IL-4 in the cell culture supernatants was determined by ELISA. (B) IFN-γ secretion by CD4+ cells purified from SLAM−/− or SLAM+/+ C57BL/6 mice. SLAM−/− or SLAM+/+ C57BL/6 CD4+ cells were purified using negative selection columns. CD4+ cells were stimulated as described in A and IFN-γ in the cell culture supernatants was determined by ELISA. (C) Impaired IL-4 secretion by SLAM−/− × DO11.10 CD4+ and SAP−/− × DO11.10 CD4+ cells upon stimulation with APCs and OVA peptide. IL-4 secretion after primary (−1°−) and secondary (−2°−) stimulation of CD4+ T cells isolated from SLAM−/− × DO11.10, SAP−/− × DO11.10, or DO11.10 TCR transgenic mice were determined. Equal numbers of purified KJ-126+/CD4+ T cells isolated from SLAM−/− × DO11.10, SAP−/− × DO11.10, or DO11.10 TCR transgenic BALB/c mice (2 × 105 cells/well) were stimulated for 3 d with APCs (2 × 106) pulsed with 1.0 μ of the OVA peptide OVA 323–339. Secondary stimulations were performed for 24 h under the same conditions. IL-4 secretion was determined at day 3 of the primary stimulation and 24 h after the secondary stimulation by ELISA. (D) IFN-γ secretion by SLAM−/− × DO11.10 CD4+ and SAP−/− × DO11.10 CD4+ cells upon stimulation with APCs and OVA peptide. Cells described in C were analyzed for IFN-γ by ELISA after primary (−1°−) and secondary (−2°−) stimulation. (E) Transcription of IL-4 and IFN-γ in NKT cells after anti-CD3 administration. RT-PCR products of IL-4, IFN-γ, and GAPDH were generated with spleen RNA samples isolated from anti-CD3 or PBS-injected mice. SLAM−/− C67BL/6 and WT C67BL/6 littermates as well as SLAM−/− BALB/c and WT BALB/c were used. The RT-PCR products were analyzed on a 1% agarose gel and visualized by UV light.
Because the results obtained with SLAM−/− mice indicated impaired development of Th2 cells and were similar to those previously observed with SAP−/− CD4+ cells (9, 10), we compared the antigen responses of SLAM−/− CD4+ cells and SAP−/− CD4+ cells. Reduced levels of IL-4 secretion were detected after triggering of both SLAM−/− DO11.10 CD4+ cells (KJ-126+ cells) or SAP−/− DO11.10 CD4+ cells (KJ-126+ cells) with OVA peptide–pulsed APCs (Fig. 2 C). The defect in IL-4 secretion by SLAM−/− KJ-126+/CD4+ cells was less robust than that of SAP−/− KJ-126+/CD4+ cells because after a secondary stimulation with antigen-pulsed APCs, WT levels of IL-4 secretion were detected (Fig. 2 C). IFN-γ secretion by either SLAM−/− or SAP−/− DO11.10 CD4+ cells was similar to that by WT BALB/c DO11.10 CD4+ cells (Fig. 2 D).
It is well established that shortly after injection of WT mice with anti-CD3, large amounts of IL-4, IFN-γ, and other key cytokines are produced immediately by NKT cells (21, 22). To test whether IL-4 gene activation was defective in SLAM−/− NKT cells, SLAM−/− BALB/c and SLAM−/− C57BL/6 mice were injected intravenously with anti-CD3 and cytokine mRNA levels were analyzed 90 min after injection by RT-PCR. As shown in Fig. 2 E, IL-4 and IFN-γ mRNA levels in SLAM-deficient NKT cells were indistinguishable from those of the corresponding WT mice, regardless of the strain background.
Taken together, the data indicate that the coreceptor SLAM positively regulates TCR-induced IL-4 production by naive and activated CD4+ T cells, but not by NKT cells. By contrast, SLAM signals negatively interfere with TCR-induced IFN-γ secretion by CD8+ and to a lesser extent by CD4+ cells.
The SLAM-induced signals that are involved in coactivation of the IL-4 gene are as yet unknown. Signal transduction by SLAM is in part governed by SAP, which is an adaptor that links the src kinase FynT to the SLAM receptor. SAP appears to bind to the SH3 domain of FynT and it therefore directly couples FynT to SLAM (6, 7). Thus, triggering of the SLAM receptor by anti-SLAM induces SAP-dependent tyrosine phosphorylation of a number of targets, including SHIP, Dok1, Dok2, and RasGAP (5). The SLAM/SAP signal transduction pathway that interferes with TCR-induced activation of the IL-4 gene in CD4+ cells does not involve Ca2+-dependent mechanisms, as judged by single cell Ca2+ influx experiments (Fig. S5, which is available at http://www.jem.org/cgi/content/full/jem.20031835/DC1).
Impaired Responses to LPS by SLAM−/− Macrophages.
Because SLAM is also expressed on activated macrophages (13, 23), we examined whether SLAM controls macrophage functions. To this end, purified resting peritoneal macrophages were stimulated with LPS, IFN-γ, or with a combination of LPS and IFN-γ. As shown in Fig. 3, A and B, the levels of secreted TNF-α and IL-12 by activated SLAM−/− C57BL/6 macrophages are reduced when compared with those of WT mice. Similarly, NO production was impaired in LPS/IFN-γ–stimulated SLAM−/− C57BL/6 macrophages (Fig. 3 C). By contrast, LPS-stimulated SLAM−/− macrophages secrete IL-6 at levels that are higher than WT levels (Fig. 3 D).
Figure 3.

Altered IL-12, TNF-α, IL-6, and NO production by peritoneal macrophages from SLAM−/− C57BL/6 mice. (A) IL-12 production by SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 peritoneal macrophages. Resting peritoneal macrophages from 12 SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 mice were stimulated with 200 U/ml IFN-γ, 2 ng/ml LPS, or with IFN-γ plus LPS for 24 h. IL-12p70 was determined by ELISA as described in Materials and Methods. M, cells incubated in medium only. (B) TNF-α production by SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 peritoneal macrophages. Resting peritoneal macrophages from SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 mice were stimulated as described in A. TNF-α was determined by ELISA in the same culture supernatants as described in Materials and Methods. M, cells incubated in medium only. (C) IL-6 production by SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 peritoneal macrophages. Resting peritoneal macrophages from SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 mice were stimulated as described in A. IL-6 was determined by ELISA in the same culture supernatants as described in Materials and Methods. M, cells incubated in medium only. (D) NO production by SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 peritoneal macrophages. Resting peritoneal macrophages from SLAM−/− C57BL/6 or WT SLAM+/+ C57BL/6 mice were stimulated as described in A. NO was determined in the same culture supernatants as described in Materials and Methods. M, cells incubated in medium only. (E) IL-12p40 production is impaired in thioglycollate-induced peritoneal macrophages from SLAM−/− C57BL/6 mice, but not from SAP−/− C57BL/6 mice. Thioglycollate-elicited macrophages were isolated from the peritoneal cavity of SLAM−/−, SAP−/−, or WT SLAM+/+ C57BL/6 mice. 2 × 106 macrophages were activated with increasing amounts of LPS (at 1, 10, or 100 ng/ml). Supernatants were collected after 24 h and IL-12p40 was determined by ELISA. (F) IL-12p40 production by macrophages in response to CpG and PGN. Thioglycollate-elicited peritoneal SLAM−/− or SLAM+/+ BALB/c macrophages (106 per well) were treated with 10 μM CpG or 25 μg/ml peptidoglycan for 24 h. Supernatants were removed and IL-12p40 levels were determined by ELISA. (G) TNF-α production by macrophages in response to CpG and PGN. Thioglycollate-elicited peritoneal SLAM−/− or SLAM+/+ BALB/c macrophages (106 per well) were treated with 10 μM CpG or 25 μg/ml peptidoglycan for 24 h. Supernatants were used to determine IL-12p40 levels by ELISA. (H) Antigen presentation by macrophages. 2 × 105 thioglycollate-elicited peritoneal SLAM−/− or SLAM+/+ BALB/c macrophages were pulsed with 0.01–1 μg/ml of the OVA peptide OVA 323–339. Next, antigen-pulsed macrophages were incubated for 3 d with 105 KJ126+ CD4+ T cells isolated from DO11.10 TCR transgenic mice. To determine DNA synthesis, cultures were pulsed with [3H]thymidine in the final 18 h. (I) Phagocytosis of F18 E. coli by macrophages. Thioglycollate-elicited peritoneal SLAM−/− or SLAM+/+ BALB/c macrophages were incubated with E. coli F18 bacteria. After 1 h, cells were lysed and phagocytosis rates were obtained by counting colonies after 24 h of incubation. CFU, colony-forming units.
Using purified resting peritoneal macrophages from SLAM−/− BALB/c mice, the same results were obtained (Fig. S6, which is available at http://www.jem.org/cgi/content/full/jem.20031835/DC1). Importantly, when macrophages from the peritoneal cavity of thioglycollate-treated SLAM−/− BALB/c mice were stimulated with LPS, a low level of IL-12 secretion was again detected (Fig. 3 E). This result indicates that the impaired cytokine production is not dependent upon the method of isolation of the macrophages. As expected from the absence of SAP in macrophages (4, 8), SAP−/− BALB/c (Fig. 3 E) macrophages did not display a defect in IL-12 production.
The possibility that SLAM−/− peritoneal cavity macrophages lack one of the members of the known LPS receptor complex, i.e., Toll-like receptor (TLR)-4/MD-2 and CD14, or the IFN-γ receptor, was excluded by FACS® analyses (unpublished data). Importantly, when SLAM−/− macrophages were stimulated with CpG or peptidoglycan, TNF secretion was comparable to that of WT macrophages (Fig. 3 F). Thus, TLR-2 and TLR-9 receptor–initiated signal transduction is intact in SLAM−/− macrophages. The collective results obtained were consistent regardless of whether the macrophages were isolated from SLAM−/− C57Bl/6 or SLAM−/− BALB/c mice and of the method of preparation of peritoneal macrophages.
Two sets of experiments showed that there is no global defect in the SLAM−/− macrophages. First, SLAM−/− and WT BALB/c peritoneal macrophages, pulsed with OVA peptide, were used to induce proliferation of KJ-126+ CD4+ T cells derived from the DO11.10 mouse, which is specific for H-2d class II and OVA peptide. SLAM−/− BALB/c and WT BALB/c peritoneal macrophages were equally efficient in stimulating DNA synthesis of DO11.10 CD4+ cells (Fig. 3 H). Second, phagocytosis of E. coli F18 bacteria by SLAM−/− BALB/c macrophages was intact (Fig. 3 I). Together, these analyses demonstrate that SLAM specifically regulates the production of IL-12, TNF-α, NO, and IL-6 upon triggering of TLR-4 by LPS.
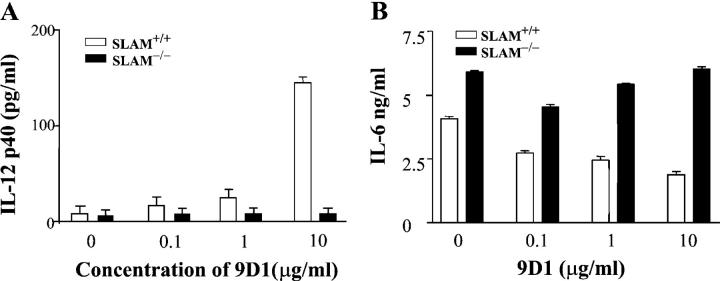
To test whether SLAM functions as a coreceptor on the surface of WT macrophages, the anti–mouse SLAM monoclonal antibody 9D1 (13) was used. As shown in Fig. 4 A, 9D1 induces IL-12 secretion by WT macrophages, whereas incubation with increasing concentrations of 9D1 negatively interferes with IL-6 production by WT macrophages (Fig. 4 B). Importantly, SLAM−/− BALB/c macrophages served as negative controls, thus excluding the possibility that anti-SLAM affected IL-6 and IL-12 production by nonspecific Fc receptor binding. Furthermore, an isotype control antibody was included in these experiments.
Figure 4.
Monoclonal anti-SLAM antibody regulates IL-6 and IL-12 production by WT macrophages. IL-12 and IL-6 production by SLAM−/− or SLAM+/+ C57BL/6 thioglycollate-elicited peritoneal macrophages was determined in response to the anti-SLAM antibody 9D1. 106 peritoneal macrophages from SLAM−/− or SLAM+/+ C57BL/6 mice were treated for 24 h with 9D1 at the concentrations indicated. To each well, a rat anti–mouse IgG1 isotype control antibody was added (BD Biosciences) in a concentration range from 10 to 0 μg/ml, resulting in a final concentration of 10 μg/ml Ig in each experimental point. Supernatants were collected and analyzed for IL-12 p40 (A) or IL-6 (B) by ELISA.
Results of the antibody experiments support the notion that SLAM confers a positive signal to LPS-induced IL-12 production by macrophages. The antibody data combined with the SLAM−/− macrophage studies also support the hypothesis that SLAM negatively controls IL-6 production. Together, the in vitro and in vivo data demonstrate that in both SLAM−/− C57BL/6 and SLAM−/− BALB/c mice, macrophage functions related to SLAM signaling are impaired.
SLAM−/− C57Bl/6 Mice Are Susceptible to Infection with L. major.
To determine the in vivo effect of the absence of the coreceptor SLAM, SLAM−/− BALB/c mice were infected with L. major. Initial foodpad lesions in BALB/c mice in response to the parasite L. major result in part from a rapid response by CD4+ T and NKT cells producing large amounts of IL-4 early after infection (24). In spite of the reduced in vitro IL-4 production by SLAM−/− T cells (Fig. S2), SLAM−/− BALB/c mice responded to the parasite in the same manner as WT BALB/c mice (Fig. 5 A). Thus, a minimal in vivo IL-4 production during the initial phase of infection of SLAM−/− BALB/c mice with L. major appears to be sufficient for the typical BALB/c response. The WT level of IL-4 production by the SLAM−/− NKT cells (Fig. 2 E), is likely to be a source of this key cytokine. By contrast, the size of the footpad lesions in the SAP−/− BALB/c mice was significantly smaller than that of the WT BALB/c mice throughout the course of infection because of the low level of IL-4 secretion by all CD4+ T cells, including NKT cells (9).
Figure 5.

SLAM−/− C57BL/6 mice are not resistant to infection with L. major. (A) Response to L. major infection by SLAM−/− BALB /c mice. SLAM−/− or SLAM+/+ BALB/c mice were infected in one footpad with 106 L. major stationary phase promastigotes. The size of footpad lesions of 10 infected mice of each strain was measured until 9 wk after infection and the change in footpad depth was plotted against time (in weeks). (B) Response to L. major infection by SLAM−/− C57BL/6 mice. SLAM−/− or SLAM+/+ C57BL/6 mice were infected with L. major as described in A. 30 mice were used in 3 experiments. A representative independent experiment with 12 mice is shown. ▴, SLAM−/− mice; ▪, SLAM+/+ mice. (C) Response to L. major infection by SAP−/− C57BL/6 mice. 10 SAP−/− or WT (SAP+/+) C57Bl/6 mice were infected with L. major as described in A. ▴, SAP−/− mice; ▪, WT SAP+/+ mice.
C57Bl/6 mice respond differently to infection with the parasite L. major than BALB/c mice, as C57Bl/6 mice eventually remove the parasite by mechanisms that involve Th1 cells as well as activated macrophages (24). As judged by measuring the size of footpad lesions, SLAM−/− C57BL/6 mice did not efficiently heal the infection with L. major (Fig. 5 B). This impaired ability to clear the parasite was confirmed by increased parasite titers in infected SLAM−/− C57BL/6 mice (log parasite titer, 4.16 ± 1.03), compared with WT C57BL/6 mice (log parasite titer, 1.82 ± 0.72; P < 0.005) at the end of the experiment.
The inability of SLAM−/− C57BL/6 mice to heal the L. major lesions is consistent with defective IL-12 and TNF-α production by SLAM−/− macrophages because secretion of IL-12 and TNF-α is known to be required for sustaining the “healing phenotype” in C57BL/6 mice (24–26). Moreover, the in vitro leishmaniacidal activity of SLAM−/− peritoneal macrophages was markedly reduced. SLAM−/− C57BL/6 macrophages were less efficient in killing parasites in vitro (73 ± 8% infectivity) than WT macrophages (37 ± 12%). Because our in vitro studies showed impaired NO production (Fig. 3), and as NO plays a major role in the removal of L. major by macrophages (26), the inability of SLAM−/− C57BL/6 mice to combat L. major infection provides further evidence for a macrophage defect.
Based upon our in vitro CD4+ T cell analyses (Fig. 2), the abnormal response to L. major by SLAM−/− C57BL/6 mice was unlikely to have been caused by a defective Th1 response. Indeed, when SLAM−/− and WT C57BL/6 T cells isolated from the lymph nodes of infected animals were examined at the end of the experiment, both mutant and WT CD4 cells secreted comparable amounts of IFN-γ (SLAM−/−, 6,905 ± 1,426 pg/ml; WT, 9,213 ± 120 pg/ml). As expected for C57BL/6 mice (24–26), at the end of the infection only very low levels of IL-4 (<20 pg/ml) were secreted either by mutant or by WT T cells in response to L. major antigen. DNA synthesis of these SLAM−/− T cells was similar to that of WT C57BL/6 T cells. The stimulation index was 6.14 ± 1.41 for SLAM−/− T cells (n = 12) and 7.2 ± 4.87 for WT T cells (n = 12). Thus, upon infection of C57BL/6 mice with L. major, the relevant macrophage responses, but not the T cell responses, were affected by the SLAM mutation. This conclusion was indirectly supported by analysis of an L. major infection of SAP−/− C57BL/6 mice, which have a T cell defect similar to that of the SLAM−/− C57BL/6 mice (Fig. 2 D; references 9 and 10) The SAP−/− C57BL/6 mice were able to remove the parasite and heal the footpad lesions with the same kinetics as WT C57BL/6 mice (Fig. 5 C). Taken together, the data strongly support the notion that the abnormalities observed in macrophages, but not those found in T cells, account for the impaired responses by the SLAM−/− C57BL/6 mice to infection with L. major.
Discussion
These analyses of SLAM−/− T cells demonstrate that SLAM-induced signal transduction differentially modulates TCR-induced IFN-γ and IL-4 production. SLAM negatively interferes with IFN-γ production in CD8+ T cells and to a lesser extent in CD4+ T cells. By contrast, SLAM augments IL-4 secretion induced by engagement of TCR on the surface of CD4+ T cells. As the absence of SAP influences TCR responses in a very similar manner, it appears that SLAM and SAP cooperate toward the same sets of signals. Thus, the SLAM/SAP receptor–adaptor complex is one of the few cell surface–signaling units that contributes to cell fate during terminal differentiation into Th1 and Th2 cells. Because the adaptor SAP operates as a control element of signal transduction pathways of at least six members of the SLAM family of T cell surface receptors, the less stringent control of IL-4 production in SLAM−/− mice compared with SAP−/− mice can be explained by overlapping functions of the other receptors (2).
The notion that SLAM is a coreceptor in TCR-driven functions was originally developed in studies with anti-SLAM antibodies. As stimulation of TCR in the presence of anti-SLAM antibody results in increased IFN-γ production (1, 2), both antibody treatment and removal of the SLAM receptor appear to eliminate the negative costimulatory signal. However, the present data also show that TCR-induced IL-4 secretion by SLAM-deficient T cells is impaired, whereas treatment with anti-SLAM has no effect on IL-4 gene activation (1, 2). It is conceivable that anti-SLAM interrupts the interaction of SLAM with its ligand SLAM on a second cell, i.e., APC. It is also conceivable that anti-SLAM causes clustering of SLAM molecules on a T cell, which then brings SLAM clusters in proximity of the TCR. In general, differences between antibody effects and findings in knockout mice have been reported. For example, in the case of the cell surface receptor CD2, anti-CD2 has an effect on T cell functions, but disruption of the CD2 gene does not have functional consequences for the T cell (27). Furthermore, anti–CTLA-4 had been thought at first to have costimulatory properties until further studies and results with the CTLA-4−/− T cells showed that the antibody inhibited a negative signal (28, 29). In addition, triggering a receptor with antibodies can also give different outcomes than the use of a soluble form of ligand, as shown in studies of SLAM. Triggering of human SLAM either by its ligand (i.e., Fc-SLAM) or by anti-SLAM antibody has different outcomes. Punnonen et al. (30) show that a soluble human SLAM Ig protein induces responses in T and B cells that differ dramatically from the responses induced by anti–human SLAM monoclonal antibodies.
The signal transduction events that are initiated by engagement of SLAM use a pathway that leads to activation of the Ser/Thr kinase Akt (23). Furthermore, signal transduction by SLAM is in part governed by SAP, which is an adaptor that links the src kinase FynT to the SLAM receptor. SAP appears to bind to the SH3 domain of FynT and it therefore directly couples and activates FynT to SLAM (5–7, 31). In addition, overexpression experiments and analysis of Fyn−/− T cells clearly show that SLAM can be phosphorylated by other src kinases in a SAP-dependent manner (31). But how SLAM-induced or SAP-controlled signaling networks are involved in activation of the IL-4 gene is unknown. Because the SLAM- and SAP-controlled signal transduction pathways do not involve Ca2+-dependent mechanisms (Fig. S5), a further dissection of the molecular underpinnings of the role of SLAM or SAP in governing TCR-induced IL-4 production is required.
The role of SLAM as a TLR-4 coreceptor on the surface of macrophages was established by experiments with LPS and SLAM−/− BALB/c and SLAM−/− C57BL/6 macrophages as well as by the use of an anti-SLAM monoclonal antibody. In response to antibody, IL-12 secretion by purified macrophages is induced and IL-6 production is impaired. In the SLAM−/− macrophage, LPS induced IL-12 and TNF secretion is impaired and SLAM−/− macrophages overproduce IL-6 even in the absence of any extraneous stimuli. The two types of responses suggest that SLAM signaling negatively influences IL-6 production and augments IL-12 secretion. Moreover, both SLAM−/− BALB/c and SLAM−/− C57BL/6 macrophages produce reduced amounts of NO upon stimulation with LPS.
The inefficiency of SLAM−/− C57BL/6 macrophages in killing L. major is consistent with both an impaired NO production and reduced levels of IL-12 and TNF production. The signaling pathways involved in the coreceptor function of SLAM with respect to production of IL-12, TNF-α, and NO in response to LPS is currently being explored.
Infection of SLAM−/− BALB/c mice with L. major followed the typical BALB/c response, i.e., uncontrolled increase of footpad swelling. The WT level of IL-4 production by the SLAM−/− NKT cells and the residual in vivo IL-4 production are undoubtedly factors in the early responses to the parasite (24–26). We speculate that a contribution of the macrophage defect of SLAM−/− BALB/c mice may also contribute to the sustained response. By contrast, as previously reported (9), the size of the footpad lesions in the SAP−/− BALB/c mice was significantly smaller than that of the WT BALB/c mice throughout the course of infection because of the low level of IL-4 secretion by all CD4+ T cells and the absence of a macrophage defect.
The use of SLAM as one of the two receptors for measles virus suggests that SLAM functions are required for both innate and acquired immune responses. Although engagement of the measles virus receptors CD46 and SLAM on dendritic cells or macrophages has been linked to regulation of IL-12 production, the role of IL-12 in the immunosuppression is not understood. As measles virus–induced immune suppression has a strong T cell component as well (32–34), further studies to elucidate the molecular mechanisms of SLAM signaling in T cells and APCs are needed. In that regard, the availability of well-defined transgenic mouse strains that correctly express human SLAM on a SLAM−/− background will be invaluable for the study of aberrant immune responses to measles virus. Thus, the availability of SLAM-deficient mice will allow for studies that provide a better understanding of the role of innate and adaptive immune responses to both parasites and viruses.
Acknowledgments
We are grateful to Lina Du and John Burgess for their technical assistance in generating the SLAM−/− mice, and Drs. Pablo Engel, Stephen Laroux, David Perkins, Massimo Morra, and Hongbin Ji for critical review of the manuscript.
D. Howie was supported by a postdoctoral fellowship from the Leukemia and Lymphoma Society of America and S. Feske was supported by a fellowship from the Cancer Research Institute. This work is supported by a grant from the National Institutes of Health to C. Terhorst (AI-015066).
The online version of this article contains supplemental material.
A. Satoskar's present address is Department of Microbiology, Ohio State University, 484 West 12th Ave., Columbus, OH 43210.
Abbreviations used in this paper: ES, embryonic stem; NO, nitric oxide; SAP, signaling lymphocyte activation molecule–associated protein; SLAM, signaling lymphocyte activation molecule; TLR, Toll-like receptor.
References
- 1.Cocks, B.G., C.C. Chang, J.M. Carballido, H. Yssel, J.E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature. 376:260–263. [DOI] [PubMed] [Google Scholar]
- 2.Engel, P., M.J. Eck, and C. Terhorst. 2003. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 3:813–821. [DOI] [PubMed] [Google Scholar]
- 3.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 406:893–897. [DOI] [PubMed] [Google Scholar]
- 4.Sayos, J., C. Wu, M. Morra, N. Wang, X. Zhang, D. Allen, S. van Schaik, L. Notarangelo, R. Geha, M.G. Roncarolo, et al. 1998. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 395:462–469. [DOI] [PubMed] [Google Scholar]
- 5.Latour, S., G. Gish, C.D. Helgason, R.K. Humphries, T. Pawson, and A. Veillette. 2001. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat. Immunol. 2:681–690. [DOI] [PubMed] [Google Scholar]
- 6.Chan, B., A. Lanyi, H.K. Song, J. Griesbach, M. Simarro-Grande, F. Poy, D. Howie, J. Sumegi, C. Terhorst, and M.J. Eck. 2003. SAP couples Fyn to SLAM immune receptors. Nat. Cell Biol. 5:155–160. [DOI] [PubMed] [Google Scholar]
- 7.Latour, S., R. Roncagalli, R. Chen, M. Bakinowski, X. Shi, P. Schwartzberg, D. Davidson, and A. Veillette. 2003. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signaling in immune regulation. Nat. Cell Biol. 5:149–154. [DOI] [PubMed] [Google Scholar]
- 8.Coffey, A.J., R.A. Brooksbank, O. Brandau, T. Oohashi, G.R. Howell, J.M. Bye, A.P. Cahn, J. Durham, P. Heath, P. Wray, et al. 1998. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20:129–135. [DOI] [PubMed] [Google Scholar]
- 9.Wu, C., K.B. Nguyen, G.C. Pien, N. Wang, C. Gallo, D. Howie, M.R. Sosa, M.J. Edwards, P. Borrow, A.R. Satoskar, et al. 2001. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat. Immunol. 2:410–414. [DOI] [PubMed] [Google Scholar]
- 10.Czar, M., E.N. Kersh, L.A. Mijares, G. Lanier, J. Lewis, G. Yap, A. Chen, A. Sher, C.S. Duckett, R. Ahmed, et al. 2001. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DHP/SAP. Proc. Natl. Acad. Sci. USA. 98:7449–7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, N., M. Morra, C. Wu, C. Gullo, D. Howie, T. Coyle, P. Engel, and C. Terhorst. 2001. CD150 is a member of a family of genes that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics. 53:382–394. [DOI] [PubMed] [Google Scholar]
- 12.Murphy, K., A. Heimberger, and D. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science. 250:1720–1723. [DOI] [PubMed] [Google Scholar]
- 13.Howie, D., S. Okamoto, S. Rietdiik, K. Clarke, N. Wang, C. Gullo, J. Bruggeman, S. Manning, A. Coyle, E. Greedfield, et al. 2002. The role of SAP in murine CD150 (SLAM)-mediated T-cell proliferation and interferon γ production. Blood. 100:2899–2907. [DOI] [PubMed] [Google Scholar]
- 14.Howie, D., M. Simarro, J. Sayos, M. Gurirado, J. Sancho, and C. Terhorst. 2002. Molecular dissection of the signaling and co-stimulatory function of CD150 (SLAM) and its interaction with the XLP gene product SAP. Blood. 99:957–965. [DOI] [PubMed] [Google Scholar]
- 15.Aramaki, Y., H. Arima, T. Hara, and S. Tsuchiya. 1996. Liposomal induction of a heat-stable macrophage priming factor to induce nitric oxide in response to LPS. Pharm. Res. 13:1389–1392. [DOI] [PubMed] [Google Scholar]
- 16.Peiser, L., P. Gough, T. Kodama, and S. Gordon. 2000. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect. Immun. 68:1953–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsukawa, A., C. Hogaboam, N. Lukacs, P. Lincoln, H. Evanoff, and S. Kunkel. 2000. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J. Immunol. 164:5362–5368. [DOI] [PubMed] [Google Scholar]
- 18.Satoskar, A., M. Stamm, X. Zhang, M. Okano, C. Terhorst, T. David, and B. Wang. 1999. Mice lacking NK cells develop an efficient Th1 response and control cutaneous Leishmania major infection. J. Immunol. 162:6747–6754. [PubMed] [Google Scholar]
- 19.Satoskar, A., M. Bozza, M. Rodriguez, G. Lin, and T. Daivd. 2001. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoskar, A., H. Bluethmann, and J. Alexander. 1995. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect. Immun. 63:4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimoto, T., and W. Paul. 1994. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimoto, T., A. Bendelac, J. Hu-Li, and W. Paul. 1995. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc. Natl. Acad. Sci. USA. 92:11931–11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro, A., T. Hauser, B. Cocks, J. Abrams, S. Zurawski, T. Churakova, F. Zonin, D. Robinson, S. Tangye, G. Aversa, et al. 1999. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): differential expression and responsiveness in Th1 and Th2 cells. J. Immunol. 163:5860–5870. [PubMed] [Google Scholar]
- 24.Sacks, D., and N. Nobern-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845–858. [DOI] [PubMed] [Google Scholar]
- 25.Park, A.Y., B.D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896–902. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan and H. Korner. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J Immunol. 166:4012–4019. [DOI] [PubMed] [Google Scholar]
- 27.Killeen, N., S.G. Stuart, and R. Littman. 1992. Development and function of T cells in mice with a disrupted CD2 gene. EMBO J. 1:4329–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tivol, E.A., F. Borriello, A.N. Schweitzer, W.P. Lynch, J.A. Bluestone, and A.H. Sharpe. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 5:541–547. [DOI] [PubMed] [Google Scholar]
- 29.Walunas, T.L., D.J. Lenschow, C.Y. Bakker, P.S. Linsley, G.J. Freeman, J.M. Green, C.B. Thompson, and J.A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1:405–413. [DOI] [PubMed] [Google Scholar]
- 30.Punnonen, J., B. Cocks, J. Carballido, B. Bennett, D. Peterson, G. Aversa, and J.E. de Vries. 1997. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J. Exp. Med. 185:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simarro, M., A. Lanyi, D. Howie, F. Poy, J. Bruggeman, J. Sumegi, M. Eck, and C. Terhorst. 2004. SAP controls FynT kinase activity and is required for phosphorylation of SLAM-related receptors. Int. Immunol. In press. [DOI] [PubMed] [Google Scholar]
- 32.Avota, E., A. Avots, S. Niewiesk, L.P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disrupton of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725–731. [DOI] [PubMed] [Google Scholar]
- 33.Karp, C., M. Wysocka, L.M. Wahl, J.M. Ahearn, P.J. Cuomo, B. Sherry, G. Trinchieri, and D.E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 273:228–231. [DOI] [PubMed] [Google Scholar]
- 34.Roscic-Mrkic, B., R.A. Schwendener, B. Odermatt, A. Zuniga, J. Pavlovic, M.A. Billeter, and R. Cattaneo. 2001. Role of macrophages in measles virus infection of genetically modified mice. J. Virol. 75:3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]