Abstract
Studying the influence of chemokine receptors (CCRs) on monocyte fate may reveal information about which subpopulations of monocytes convert to dendritic cells (DCs) and the migration pathways that they use. First, we examined whether prominent CCRs on different monocyte subsets, CCR2 or CX3CR1, mediated migration events upstream of the accumulation of monocyte-derived DCs in lymph nodes (LNs). Monocytes were labeled and traced by uptake of latex microspheres in skin. Unexpectedly, neither CCR2 nor CX3CR1 were required. However, absence of CCR2 led to an increased labeling of the minor Gr-1int monocyte population, and the number of latex+ DCs that emigrated to LNs was correspondingly increased. Characterization of Gr-1int monocytes revealed that they selectively expressed CCR7 and CCR8 mRNA in blood. CCR7 and CCR8 pathways were used by monocyte-derived DCs during mobilization from skin to LNs. The role of CCR8 in emigration from tissues also applied to human monocyte-derived cells in a model of transendothelial trafficking. Collectively, the data suggest that Gr-1int monocytes may be most disposed to become a lymphatic-migrating DCs. When these monocyte-derived DCs exit skin to emigrate to LNs, they use not only CCR7 but also CCR8, which was not previously recognized to participate in migration to LNs.
Keywords: chemotaxis, endothelium, inflammation, lymphatic system, macrophage
Introduction
Monocytes differentiate into either macrophages or migratory LN-homing DCs in vivo (1). Little is known about the molecular events that guide the “decision” of a monocyte to become a macrophage or a migratory DC. Likewise, we know very little about the molecules that mediate the migration of monocyte-derived DCs from blood, through tissue, to LNs. Different chemokine receptor (CCR) profiles are known to characterize different subsets of monocytes; tracing the roles and expression of CCRs can lend information about the type of monocyte that becomes a DC versus a macrophage.
In both mouse and human, there are two major subsets of monocytes. They are distinguished in part by CCR expression patterns (2–4), and in mouse by differential levels of cell surface Gr-1 (4). “Classical” monocytes in both species are CCR2+, whereas another population of monocytes in both species expresses CCR2 only at low levels and characteristically show higher levels of surface CX3CR1 than the other monocytes (3, 4). A consensus does not exist on a possible lineage relationship between these subsets, but some evidence suggests that CCR2+ monocytes are the precursors for CCR2− monocytes (5), and that these two phenotypes are separated by a third “transitional” subset (5). The transitional monocyte subset has not been well studied in mice, but some analysis of this population has been done using human monocytes (3, 6). They are CD64+CD14+CD16+ and express CCR2. Interestingly, they tend to express the whole range of CCRs that otherwise distinguish the two major subsets, in addition to unique expression of other CCRs (3). Although very low in frequency in blood, their wider range of CCR expression may make them the most flexible population of monocytes for recruitment to different chemokine signals.
In this paper, we characterized the roles of several CCRs in mediating migration events that were especially relevant to the appearance of monocyte-derived DCs in LNs via lymphatics. CCR2 is a key mediator of monocyte migration to peritoneal cavity (7, 8) and into LNs via high endothelial venule (9) during robust inflammatory reactions evoked by pathogen-derived components, but such components can prevent the differentiation of monocytes into lymph-homing DCs (10). The trafficking of some APCs also relies on CCR2 (11–14), although it is not clear that these APCs derive from monocytes or another CCR2+ precursor. Our data indicate that CCR2 is unexpectedly not essential for any of the migratory events that lead to the accumulation of monocyte-derived DCs in LNs from skin, as assessed in CCR2−/− mice, under the relatively mild inflammatory conditions studied. CX3CR1, studied using CX3CR1gfp knockin mice (4, 15), was also dispensable for the accumulation of monocytic DC precursors in skin or later in LNs. Analysis in these knockout/knockin mouse strains pointed to “transition state” (Gr-1int) monocytes as likely being the population monocytes that most readily convert to lymph-homing DCs. In blood, Gr-1int monocytes were already oriented for differentiation to DCs, as they selectively expressed mRNA for CCR7 and CCR8, two CCRs that we show are required for emigration of monocyte-derived DCs from skin to LNs. Thus, this work unravels new aspects of the migratory pathways used by monocyte-derived DCs during recruitment from blood into skin and subsequent mobilization to LNs.
Materials and Methods
Mice.
All procedures described herein involving mice were approved by the Institutional Animal Care and Use Committee at Mt. Sinai School of Medicine. CCR8−/− mice (16) were backcrossed 10 times onto the C57BL/6 strain using breeders from Charles River Laboratories. Analyses were conducted in these or C57BL/6 WT mice (Charles River Laboratories or Jackson ImmunoResearch Laboratories), CX3CR1gfp/+ (15), CX3CR1gfp/gfp (15), CCR2−/− (7, 8), or plt/plt mice (17, 18) all on a C57BL/6 background.
Quantification of Monocyte-derived DC Migration to LNs.
FITC-labeled polystyrene microspheres (1-μm diameter; Polysciences) or 1-μm red fluorescent microspheres (T-8883; Molecular Probes) were diluted to 0.1% (wt/vol), and 10 μl was injected into the shaved back skin overlying the region of the brachial LN of anesthetized CCR8+/+ or CCR8−/− C57BL/6 mice. Two injections were made on each side. 3 d later, brachial LNs were pooled from each injected mouse and digested with collagenase D free of tryptic activity (Boehringer) for 30 min. Single cell suspensions were generated by pressing LN cells through a 70-μM cell strainer and were stained with conjugated mAbs from BD Biosciences. For each mouse, the whole LN preparation was analyzed by flow cytometry, and the number of cells bearing two or more fluorescent particles was quantified. Cells bearing only one microsphere/cell are not clearly of monocyte origin, in contrast with the more phagocytic cells, and their accumulation in LNs does not correlate with that of monocyte-derived cells (1).
Dermal Cell Suspensions.
Areas of the dermis where beads were injected were visible due to the focal location of the bright fluorescent beads in the skin. These areas were excised with dissecting scissors and digested with 1.8 mg/ml of LPS-free Blendzyme Liberase III obtained from Roche diluted in RPMI 1640 for 30 min. EDTA was added, and cells were passed through a 70-μm strainer before flow cytometric staining.
Immunostaining.
Frozen section of WT skin and LNs were fixed in acetone, stained with rat anti-CCL1 mAb (R&D Systems), and detected with Cy3-conjugated anti–rat IgG (Jackson ImmunoResearch Laboratories). A polyclonal rabbit anti–LYVE-1 Ab (Upstate Biotechnology) was used to identify lymphatic vessels detected with FITC-conjugated anti–rabbit IgG (Jackson ImmunoResearch Laboratories).
Mouse Blood Monocytes.
Whole blood was subjected to red cell lysis using Pharmlyse (BD Biosciences) and washed twice in DMEM containing 5 mM EDTA and 0.5% BSA. Cells were incubated in HBSS containing 0.02% NaN3 and 2 mM EDTA, 2% FBS, 1% normal mouse serum, and 20 μg/ml CD16/CD32 Fc receptor–blocking mAb (BD Biosciences). Four-color staining for flow cytometric analysis was conducted using combinations of the following mAbs. All mAbs were from BD Biosciences, except where otherwise indicated: F4/80 (Serotec), CD115 (eBioscience), Gr-1, IgG2a isotype control, mouse IgG1, rat IgG2a, or hamster IgG. Clodronate was a gift from Roche and was incorporated into liposomes as described previously (19). In some mice, blood monocytes were eliminated with these liposomes by i.v. injection of 0.2 ml into the lateral tail vein (5). Control liposomes that incorporated PBS instead of clodronate were used, but without affecting total monocytes or subpopulations.
Real-Time PCR.
Monocytes stained for F4/80, CD115, and Gr-1 were subjected to flow cytometric cell sorting to separate Gr-1hi, Gr-1int, and Gr-1lo populations, all F4/80+CD115+. RNA was extracted, followed by DNase treatment. cDNA was prepared from these samples using random primers (Invitrogen) and Sensiscript RT kit (QIAGEN). These cDNA were used for real-time PCR to quantify ubiquitin, sense primer 5′-TGGCTATTAATTATTCGGTCTGCAT-3′, antisense primer 5′-GCAAGTGGCTAGAGTGCAGAGTAA-3′; CCR2, sense primer 5′-GTTACCTCAGTTCATCCA-3′, antisense primer 5′-CAAGGCTCACCATCATCGTAGTC-3′; CX3CR1, sense primer 5′-TGTCCACCTCCTTCCCTGAA-3′, antisense primer 5′-TCGCCCAAATAACAGGCC-3′; CCR7, sense primer 5′-CACGCTGAGATGCTCACTGG-3′, antisense primer 5′-CCATCTGGGCCACTTGGA-3′; and CCR8 expression, sense primer 5′-TGACCGACTACTACCCTGATTTCTT-3′, antisense primer 5′-GCTGCCCCTGAGGAGGAA-3′. The cDNA was amplified in the presence of QuantiTech SYBR green PCR master mix oligonucleotides and HotStarTaq DNA Polymerase (QIAGEN) in a Roche Lightcycler. The relative expression of each CCR was calculated against the relative abundance of cDNA encoding for ubiquitin. Using this method, relative differences in copy number for a given cDNA can be discerned, but comparisons between different cDNAs (i.e., relative expression of CCR2 vs. CCR8) are not valid.
In Vitro Culture of Monocytes Retrieved from Peritoneal Lavage (10).
Mice received 1 ml of 4% thioglycollate i.p., and 0.5 ml of FITC-labeled microspheres (0.02% wt/vol; Polysciences) 18 h later. Cells retrieved from peritoneal lavage were sorted using the same protocol as for blood monocytes and cultured for 2 d in growth medium supplemented with conditioned medium from GM-CSF–producing J588L cells. Additions to the growth medium in some samples included neutralizing goat anti–mouse CCL1 polyclonal Ab (R&D Systems) or goat anti–mouse CCL19 polyclonal Ab (R&D Systems) at 5 or 10 μg/ml.
Allostimulation.
For allostimulation assays, splenic BALB/c T cells were isolated by negative selection to remove MHC II positive cells using Dynal magnetic beads and removal of additional macrophages by adherence. Graded doses of test antigen-presenting cells were cultured with 105 T cells per microtiter well. 4 d later, 4 μCi/ml [3H]thymidine was added. Cells were harvested, and incorporated [3H]thymidine was quantified 15 h after addition of this tracer.
Human Monocyte Cultures and Reverse Transmigration Assay.
Reverse-transmigrated and monocyte-derived cells were studied using previously described methods (20, 21). Second passage human umbilical vein endothelial cells were cultured on bovine type I collagen gels. Blood was drawn according to guidelines approved by the Internal Review Board of Mt. Sinai School of Medicine. PBMCs were applied to the endothelium in some experiments in the presence of anti-CCR8 mAb or IgG1 isotype control mAb obtained from R&D Systems, incubated for 1.5 h, washed thoroughly in medium to remove nonmigrated cells from above the endothelium, and continued in culture for 48 h. Monocytes migrated across the endothelium during the 1.5-h incubation (22). Neutralizing or control mAbs were included in the medium (5 μg/ml each) and were applied to the cultures after the wash at 1.5 h. Neutralizing mouse anti–human CCR8 mAbs 3B10, 2D10, and 5B11 were generated as described previously (23, 24).
For quantification of reverse transmigration, samples were fixed at 1.5 h or at 48 h. Differential interference contrast was used to quantify the number of monocyte-derived cells that were beneath the endothelium in at least five high-power fields per sample. Each experiment included three to six replicates per parameter tested. Percent of reverse transmigrated cells was calculated as the percent decrease in the number of monocyte-derived cells beneath the endothelial monolayer at 48 h relative to the number present at 1.5 h. Preparation of CD16− monocytes and their culture in TGFβ1 to induce CD16+ cells was conducted as described previously (25).
Immunoblots, PCR, and Flow Cytometry to Detect Human CCR8.
For flow cytometry, monocyte-derived cells were stained with anti–human CCR8 mAb 3B10 (23, 24), detected with biotinylated anti–mouse Ab (Jackson ImmunoResearch Laboratories), followed by streptavidin allophycocyanin (Caltag). For immunoblots, plasma membrane-enriched microsomes from 1–3 × 106 purified cells were prepared, subjected to SDS-PAGE, and transferred to nitrocellulose membranes. After blocking with 5% milk, the membrane was incubated overnight with 2 μg/ml anti-CCR8 mAbs 2D10 or 3B10, or anti–β-actin mAb (Santa Cruz Biotechnology, Inc.) and detected with horseradish peroxidase–conjugated anti–mouse or anti–rat IgG, respectively, and ECL substrate (Amersham Biosciences).
Cultured human skin explants served as a source of human skin DCs and their CD14+ precursors (26). Emigrated cells collected 2 d after onset of explant culture were incubated with anti-CD14 magnetic microbeads (Miltenyi Biotec) to positively select CD14+ emigres. CD14− DCs were selected and purified from skin T cells using anti–HLA-DR mAb-coupled magnetic beads (Dynal). For PCR, synthesis of cDNA from purified monocyte mRNA was performed using random primers. Amplification was for 30 cycles with annealing temperature of 54°C for 45 s and extension at 72°C for 45 s. Primers to amplify human CCR8 cDNA were 5′-TGGCCCTGTCTGACCTGCTTT-3′ and 5′-GGCATAAGTCAGCTGTTGGCT-3′, which amplified a 613-bp product. Amplification of GAPDH used primers 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′, which amplifies a 412-bp product.
Statistics.
Statistical analysis was conducted using JMP software and Prizm software. Statistical tests were performed using All Pairs Tukey-Kramer analysis and/or two-tailed Student's t test.
Online Supplemental Material.
Fig. S1 illustrates that depletion of peripheral blood monocytes eliminates the appearance of latex (LX)-bearing cells in the draining lymph node. Fig. S2 depicts the use of CD115 and F4/80 co-staining to identify mouse blood monocytes, and their further division into three subsets distinguished by differing levels of Gr-1. Fig. S3 shows PCR products for CCR8 and GAPDH in human blood monocyte populations before and after coculture with endothelium. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20032152/DC1.
Results
Tracing Monocytes That Convert to Phagocytic Lymph-homing DCs Using CX3CR1 Knockin Mice.
An excellent means to track monocyte subsets is through the use of mice bearing a GFP reporter knocked-in to the CX3CR1 locus (4, 9, 15). Gr-1hi monocytes express lower levels of GFP (GFPmed) than Gr-1lo monocytes (GFPhi; reference 4), corresponding to the somewhat higher levels of CX3CR1 in this monocyte population. CX3CR1gfp mouse blood also contains a small Gr-1int population of monocytes (∼10% of total monocytes), as anticipated from previous work (5). The mean fluorescence of GFP in this population was intermediate between the Gr-1hi and Gr-1lo populations (Fig. 1, A and B).
Figure 1.
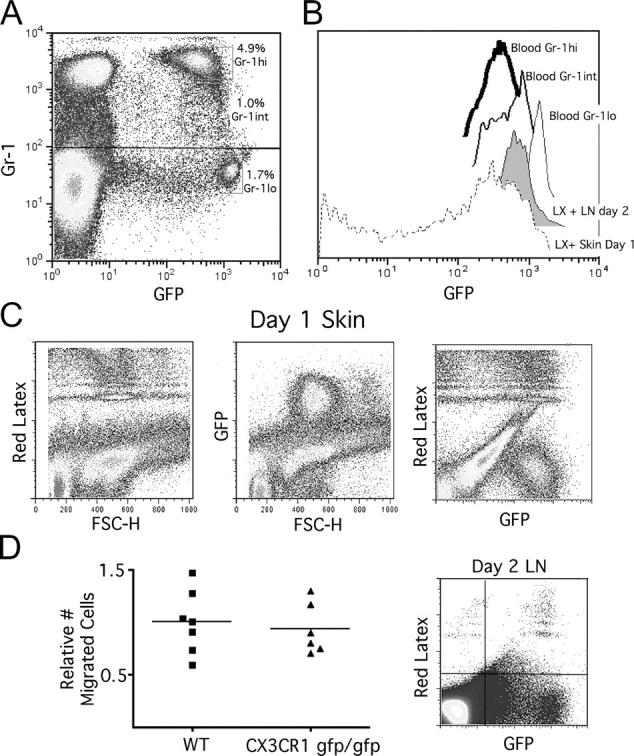
Analysis of monocyte trafficking and microsphere transport to LNs in CX3CR1gfp/gfp mice. (A) Monocyte subsets were identified in blood of CX3CR1gfp/gfp mice as GFP+ cells with differing levels of Gr-1, revealing the presence of a small Gr-1int subpopulation in addition to previously described Gr-1hi and Gr-1lo populations. (B) GFP fluorescent intensities of these subsets, stable and noninterconverting for at least 1 d (4), were compared with the fluorescent intensities of cells that engulfed red fluorescent microspheres deposited in skin, examined 14 h after injection, and in cells that emigrated from skin to LNs by day 2. The same settings in flow cytometry were used to acquire all of these data, making them directly comparable. (C) Profiles of the cells in skin at the site of microsphere injection at 14 h are shown. (D) Quantification and profiles of LX-bearing cells that migrated to LNs were analyzed at day 2. To combine data from different experiments, the mean number of migrated cells in WT mice was set equal to 1.0 for each experiment, and relative values for all WT and knock-out individuals in that experiment were calculated. Six mice were studied for each part of the figure over the course of two experiments with three mice in each group.
To trace monocytes into skin and later into LNs, red fluorescent microspheres were injected i.c. into the shaved back skin of mice (1). 14 h after bead injection into the skin of CX3CR1gfp/+ or CX3CR1-deficient (gfp/gfp) mice, the vast majority of phagocytic, bead-bearing monocytes had a uniform size and GFP intensity that overlapped with Gr-1hi and Gr-1int monocytes (Fig. 1, B and C). Very few, if any GFPhi Gr-1lo monocytes were apparent, consistent with the inability of this subset to respond to inflammatory chemokines (4). A minority (25%) of bead-bearing cells were GFP− (Fig. 1 C). These may be derived from resident macrophages, which are generally GFP− in CX3CR1 knockin mice (15), or they may be other types of phagocytes like neutrophils, or even dying cells that have leaked GFP. Overall, these data indicate that tracer beads are engulfed by monocytes of two phenotypes, Gr-1hi and Gr-1int, and indicate that monocyte-derived DCs that traffic through skin before mobilizing to LN do not derive from CCR2− Gr-1lo monocytes under the conditions examined.
In the draining LN, analyzed at day 2 when most LX+ cells appear in LNs, there were no differences in the number of bead-bearing monocyte-derived DCs in CX3CR1+/+ or CX3CR1-deficient (gfp/gfp) mice (Fig. 1 D), indicating that CX3CR1 is not essential for any step in the migration of monocytes that become LN DCs. The majority of bead+ cells in the LN were GFP+. In the LNs, LX+ cells were largely Gr-1lo, but most expressed the same level of GFP as Gr-1int monocytes (Fig. 1, B and D). The half-life of GFP in the positive cells is relatively long (15) and, therefore, can serve for some time as a marker for cell lineage.
A few GFP− LX+ cells were also present in the LNs (Fig. 1 D, right). These may be derived from GFP− precursors, such as tissue macrophages, that engulfed beads in the skin, but these were more likely derive from cells whose integrity has become compromised during LN processing because these cells had lower FSC-H than the GFP+ cells and any of the original LX-bearing cells in the skin. Furthermore, i.v. injection of clodronate liposomes was used to deplete blood monocytes (5) without affecting skin macrophages (unpublished data). This procedure resulted in an 87% average reduction in the number of bead+ monocyte-derived DCs in the LNs (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20032152/DC1) and correspondingly markedly diminished uptake of microspheres in the skin. This result confirms that macrophages, or any other tissue phagocyte, contribute marginally to the microsphere-bearing cell pool in the LNs. Rather, monocytes that derive either from Gr-1hi or Gr-1int subsets, but apparently not from the Gr-1lo subset, are the major precursor. The GFP intensity of LN LX+ cells most closely overlapped with the intensity of the Gr-1int population.
CCR2 Is Not Required for Trafficking Events That Precede the Appearance of Phagocytic DCs in the LN: Quantitative Correlation between the Number of Skin Gr-1int LX+ Monocytes and the Number of LX+ Cells That Home to LNs.
CCR2 is a major CCR for inflammatory monocyte recruitment to inflamed sites (7, 8, 27). Its expression particularly characterizes Gr-1hi monocytes (4) that were abundantly recruited to engulf LX microspheres. Thus, we tested whether the absence of CCR2 affected migration of monocytes into skin that would later differentiate into phagocytic, LN-homing DCs. First, we compared blood monocytes between CCR2+/+ and CCR2−/− mice. To examine monocytes in blood, we stained mouse leukocytes to identify M-CSF receptor (CD115)+ F4/80+ monocytes with expected FSC-H/SSC-H properties (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20032152/DC1) and identified the three subsets with differential levels of Gr-1. This cell surface phenotype was appropriate for defining monocytes because this population was selectively reduced in monocyte-deficient op/op mouse blood (unpublished data). CCR2−/− mice showed a consistent and marked reduction in the frequency of Gr-1hi blood monocytes, accompanied by a more pronounced Gr-1int monocyte population (Fig. 2 A). This was not likely due to impaired development of Gr-1hi monocytes in these mice because Gr-1hi monocytes appeared abundantly and initially were the most numerous monocytes in CCR2−/− blood after clodronate liposome treatment to transiently eliminate blood monocytes (unpublished data), just as observed in WT monocytes (5).
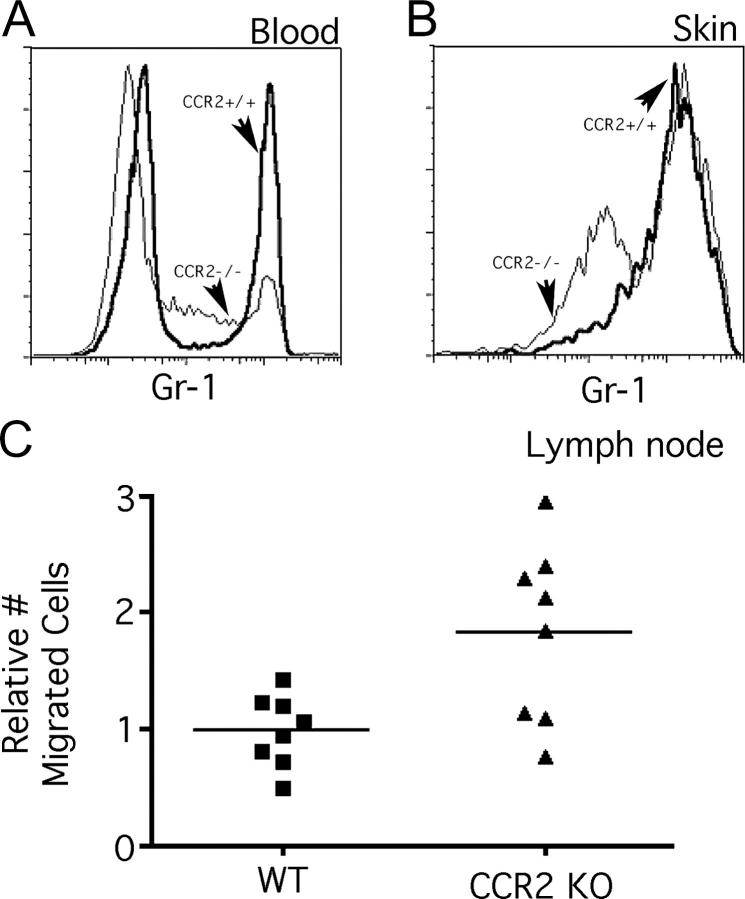
Figure 2.
Analysis of monocyte trafficking and microsphere transport to LNs in CCR2−/− mice. (A) The relative distribution of the various blood monocyte subsets was compared between CCR2+/+ and CCR2−/− mice. (B) The relative proportions of Gr-1hi and Gr-1int phagocytes bearing LX were also compared in the skin, 14 h after injection of green fluorescent microspheres. (A and B) Bold line profiles identify CCR2+/+ monocytes. and thin lines identify CCR2−/− monocytes. (C) The number of DCs bearing two or more LX particles in the draining LNs was quantified 2 d later. The increased number of LX+ cells in CCR2−/− LNs was statistically significant: P < 0.04.
Next, we examined the profile of skin-infiltrating phagocytes in CCR2+/+ and CCR2−/− mice. We focused on the period just after the plateau in their recruitment (12–14 h), when the cells would be expected to most closely resemble their blood counterparts. As expected from the fact that bead-bearing cells were mostly GFP+ in CX3CR1gfp mice, the overwhelming majority of the phagocytes that engulfed LX were F4/80+ (unpublished data). They were also largely comprised of Gr-1hi cells (Fig. 2 B), consistent with observations of monocyte recruitment to sites of inflammation (4, 5). Gr-1int monocytes bearing beads were also observed (Fig. 2 B), but Gr-1lo monocytes were absent. These data agree with the profile of recruited monocytes in CX3CR1gfp/gfp mice (Fig. 1 B). Gr-1hi cells were also observed in CCR2−/− mice (Fig. 2 B), despite the fact that attenuated numbers of Gr-1hi monocytes in blood persisted even after injection of the LX beads in skin (not depicted). In CCR2−/− mice, as in CCR2+/+ mice, LX-bearing cells also included Gr-1int monocytes (Fig. 2 B). These were relatively few in CCR2+/+ mice, but were two- to threefold enriched in CCR2−/− mice (Fig. 2 B). The total yield of LX-bearing skin cells was similar between CCR2+/+ and CCR2−/− preparations.
Our next step entailed quantifying the accumulation of LX-bearing, monocyte-derived DCs in the LNs. The number of these DCs was not reduced, but was unexpectedly nearly doubled in CCR2−/− mice (Fig. 2 C). This increase in the number of migratory LX-bearing DCs in the LN closely correlated with the increase in Gr-1int F4/80+ cells in the skin, raising the possibility that Gr-1int monocytes may be the more efficient precursors for DCs, compared with Gr-1hi phagocytes. These data, together with those from CX3CR1gfp/gfp mice, turned our attention to Gr-1int monocytes.
Gr-1int Blood Monocytes Possess Allostimulatory Capacity, Along with Gr-1lo Monocytes, and Selectively Express mRNA for CCR7 and CCR8.
In profiling blood monocytes, we first noted that both Gr-1int and Gr-1lo monocytes possessed higher costimulatory activity (Fig. 3 A) than Gr-1hi blood monocytes. These data suggest that Gr-1int and Gr-1lo monocytes resemble populations of human CD16+ monocytes in their increased expression of costimulatory activity after isolation (6, 28), relative to more classical monocytes.
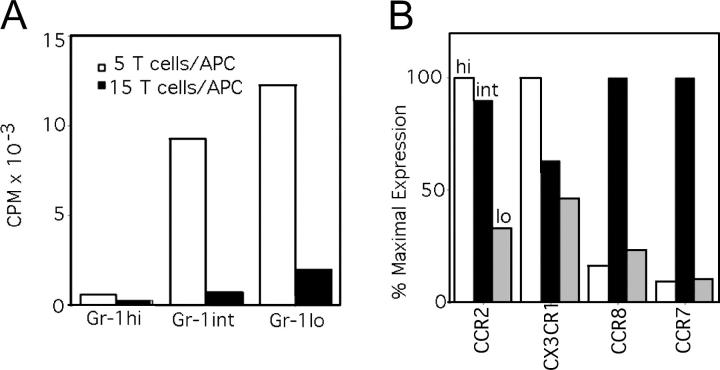
Figure 3.
Analysis of monocyte subsets in blood: expression of costimulatory activity and CCR mRNA. (A) Sorted Gr-1hi, Gr-1int, and Gr-1lo subsets were irradiated and used as APCs in an MLR with BALB/c T cells. This experiment is representative of two conducted experiments, both showing similar results. (B) Real-time PCR for CCR2, CX3CR1, CCR7, and CCR8 mRNA was conducted. Data are plotted as the percentage of expression relative to the subset with the highest expression of a particular CCR. These data were repeated in a similar pattern in three independent sorting experiments.
We analyzed mRNA from monocyte subsets for expression of CCRs. We stained F4/80+ CD115+ cells in WT blood and sorted these cells into three populations: Gr-1hi, Gr-1int, and Gr-1lo monocytes (Fig. S2). In addition to conducting real-time PCR analysis for CCR2 and CX3CR1, we examined mRNA for the CCRs CCR7, a major mediator of DC migration via lymphatics, and CCR8, as we became interested in CCR8 after finding that human CD16+ monocytes expressed mRNA for CCR8 more significantly than did CD16− monocytes (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20032152/DC1). As expected, CCR2 mRNA was expressed in both Gr-1hi and Gr-1int monocytes, but reduced in Gr-1lo monocytes (Fig. 3 B). CX3CR1 mRNA was found in all populations (Fig. 3 B), also as expected, although sorted Gr-1lo monocytes did not show the highest levels of CX3CR1 mRNA (Fig. 3 B), which apparently differs from protein levels (4). Strikingly, CCR7 and CCR8 mRNA were selectively expressed by Gr-1int monocytes (Fig. 3 B).
CCR8 Regulates Emigration of Monocyte-derived DCs to LNs without Acting Redundantly with a Role for CCR7 Ligands.
Given these findings, we set out to determine whether selective expression of CCR7 or CCR8 mRNA by this population might relate to a role for these receptors in migratory events that lead to the accumulation of monocyte-derived DCs in LNs. Although CCR7 is known to be a major regulator of DC migration, the role of this receptor or its ligands in the migration of monocyte-derived, phagocytic DCs is unknown. Thus, we tested whether the CCR7 or CCR8 pathways may be involved in the migration of monocyte-derived DCs to LNs. Analysis of migration to LNs in CCR8−/− mice revealed a 50% mean inhibition in the accumulation of LX+ cells in LNs (Fig. 4 A). In contrast with the findings in CCR2−/− mice (Fig. 2), CCR8−/− mouse skin showed no alterations in phenotype of phagocytic cells, phagocyte yield, or distribution between Gr-1hi and Gr-1int subsets (Fig. 4 A, inset) at any time point, indicating normal recruitment of monocytes in CCR8−/− mice.
Figure 4.
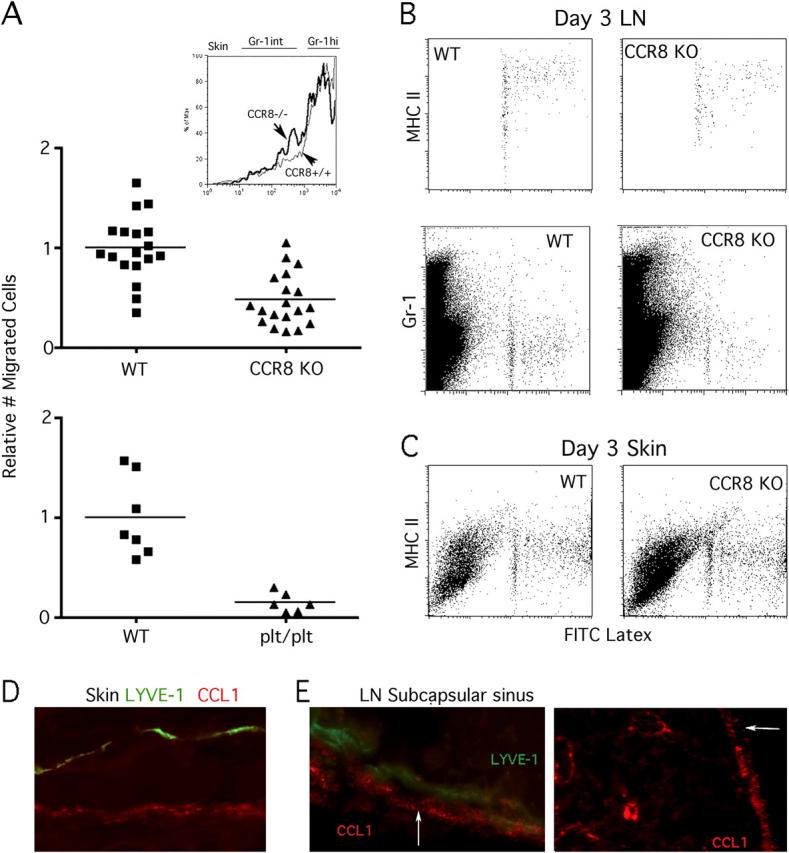
Analysis of cell-mediated microsphere transport to LNs in CCR8−/− and plt/plt mice. (A) Green fluorescent LX microspheres were injected into the skin of CCR8-deficient mice and plt/plt mice that were compared with age- and sex-matched WT C57BL/6 counterparts. The number of DCs bearing two or more LX particles in the draining LNs was quantified 3 d later. To combine data from different experiments, the mean number of migrated cells in WT mice was set equal to 1.0 for each experiment, and relative values for all WT and knock-out individuals in that experiment were calculated. Inset shows Gr-1 staining intensity among gated bead+ cells from CCR8−/− (bold line) and CCR8+/+ mice (thin line) in the skin 14 h after bead injection. (B and C) Plots show MHC II (I-Ab) and Gr-1 levels in LNs and skin of WT and CCR8−/− mice. LN plots (B) are quantitative comparisons, as they depict the entire population of LX-bearing cells recovered from pooled brachial LNs from individual mice. Skin plots (C) are not quantitative comparisons. Where available, plots depict all acquired events, although in some experiments (for MHC II staining in LN), LX+ cells were gated during acquisition to reduce file size. (D) High power magnification of WT skin section stained for LYVE-1 (green) and CCL1 (red). (E) LYVE-1 (green) and CCL1 (red) in LN subcapsular sinus (left, high power magnification). CCL1 staining in LN at low power (right). Outer subcapsule is indicated by an arrow.
Accumulation of LX+ DCs in LNs was reduced by 90% (Fig. 4 A) in plt/plt mice that lack several genes encoding CCR7 ligands (29, 30) and that show greatly diminished Langerhans cell recruitment to LNs (17). Thus, a role for CCR8 in mediating accumulation of monocyte-derived DCs in LNs does not replace a need for CCR7 ligands in emigration, arguing that the two molecules function at different points in a common pathway downstream of recruitment into skin. Although there were fewer phagocytic DCs in the LNs of CCR8-deficient mice, the phenotype of the emigrated cells that were there was similar to WT mice. For example, these cells expressed very high levels of MHC II (Fig. 4 B, top). We also noted that the LX+ cells of WT mice were Gr-1lo in the LNs. In contrast, for up to 3 d in skin there were no MHC IIhi or Gr-1lo LX+ cells present, only MHC IIint cells (Fig. 4 C and not depicted). This pattern was also true in plt/plt mice (unpublished data), suggesting against our expectations that failure to emigrate to LNs does not lead to a build-up of mature LX+ DCs in the skin. Thus, maturation may normally continue or complete as the cells are en route to the LNs.
The expression of CCL1, the ligand for CCR8, in skin (Fig. 4 D) revealed no apparent staining of LYVE-1+ lymphatic capillaries (31), but other vessels, likely blood vessels, were positive as expected (32). In LNs, CCL1 was expressed in the subcapsule of the LNs (Fig. 4 E) and was also observed in paracortical vessels that are likely high endothelial venules (Fig. 4 E, right). These data raise the possibility that CCL1/CCR8 may function downstream of entry into lymphatics by regulating entry in the subcapsular sinus of the LNs.
Gr-1int Monocytes More Readily Differentiate into Potent APCs Than Gr-1hi Monocytes: Role for CCR8.
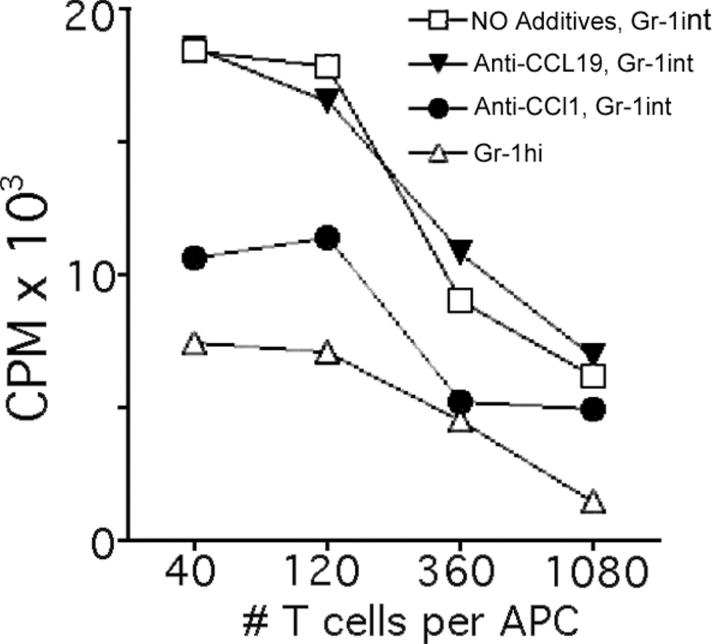
We focused further on whether Gr-1int monocytes that were recruited to tissues might be more efficient precursors for DCs, compared with Gr-1hi monocytes. Gr-1int and Gr-1hi monocytes are similarly recruited to the inflamed peritoneum (5), as we observed in skin. Thus, we took advantage of the ease of retrieving these cells from the peritoneum for experiments that require greater cell yields. Sorted Gr-1int and Gr-1hi monocytes that had recently migrated into the peritoneal cavity after injection of thioglycollate were collected and cultured in GM-CSF for 2 d, which led some monocytes to acquire characteristics of DCs (17). Next, allostimulatory activity was assessed. In some samples, neutralizing mAbs to CCL1, the ligand of CCR8, or mAb to CCL19, a ligand of CCR7, was included during the 2-d GM-CSF treatment, but not added during the T cell stimulation period. Differentiation was analyzed by allostimulatory activity because marker stainings were unreliable after the initial three-color sorting of the two populations. Gr-1int monocytes developed a much more robust allostimulatory capacity than Gr-1hi monocytes (Fig. 5). The capacity to stimulate T cell proliferation by Gr-1int monocyte-derived cells was partially blocked by anti-CCL1, but not affected by anti-CCL19, which served as a control (Fig. 5). Thus, these data support the earlier indications that Gr-1int monocytes more efficiently become DCs and suggest that signals via CCL1/CCR8 participate in this process.
Figure 5.
Allostimulatory capacity of monocyte subsets sorted from the periphery and effect of an antagonist to the CCR8 ligand CCL1. (A) Gr-1hi and Gr-1int recruited monocytes from the peritoneal lavage of C57BL/6 CCR8+/+ mice were sorted to purity by flow cytometry using mAbs to F4/80 and Gr-1. The cells were cultured separately in GM-CSF for 2 d in the absence of added mAb, or in the presence of 5 μg/ml of neutralizing mAbs to chemokines CCL1 (TCA-3) or CCL19. These cells were washed to remove residual antibodies and cultured with BALB/c T cells for evaluation of their potential to support allogeneic T cell proliferation in a mixed lymphocyte reaction. These data depict one out of two experiments conducted with similar results.
CCR8 Mediates Reverse Transmigration of Human Monocyte-derived DCs.
A role for CCR8 in emigration of monocyte-derived DCs to LNs has not been anticipated in the literature because little is known about the role of this CCR on phagocytes. Thus, we wondered whether CCR8 may also be relevant to the migration of human monocyte-derived cells in the reverse transmigration model (21, 22) that we have proposed involves analogous steps in migration as observed for monocyte-derived DCs in vivo.
CCR8 mRNA has been detected in freshly isolated, total human monocytes (33). When we tested for CCR8 mRNA in monocyte subsets, it was apparent that higher levels of CCR8 mRNA were found in bulk CD16+ monocytes, relative to CD16− monocytes (Fig. S3). Surface protein levels were very low, even on CD16+ monocytes (unpublished data). During culture with endothelial cells grown on collagen matrices, CCR8 mRNA and protein expression was induced in all monocyte-derived cells, whether or not they joined the reverse-transmigrating population or remained in the subendothelium (Fig. 6 A and not depicted). An additional analysis of the expression of CCR8 by HLA-DR+ cells that migrated from human skin explants revealed CCR8 protein associated with the CD14+ dermal precursors of DCs (26, 34), but not CD14− emigrated Langerhans cells (Fig. 6 B). In agreement with the implication that CCR8 may selectively regulate trafficking of monocyte-derived DCs, and not affect Langerhans cells, is the finding that, in CCR8−/− mice, Langerhans cell trafficking to LNs in response to contact sensitizers is normal (unpublished data).
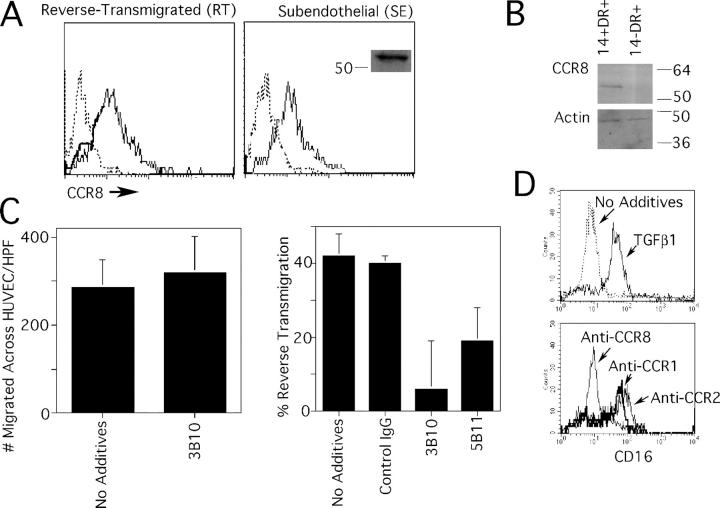
Figure 6.
Expression of CCR8 by human monocyte-derived cells and skin DCs, and its role in reverse transmigration. Monocytes were cocultured with endothelial cells grown on a type I collagen gel for 48 h, permitting the separation of the population into reverse-transmigrating DCs (RT) or subendothelial macrophages (SE). (A) Anti-CCR8 mAb 3B10 (thin solid line) reacted with both RT and SE monocyte-derived cells. Isotype-matched mAb was used to establish the baseline for negative staining (dotted line). Anti-CCR8 mAb 3B10 specifically recognized an ∼54 kD band by immunoblot, shown only for SE monocyte-derived cells (inset). (B) CD14+ DC precursors from human skin, but not CD14− DCs, were positive for CCR8 by immunoblot. Blotting for β-actin was conducted as a loading control. (C) Inclusion of neutralizing anti-CCR8 mAb 3B10 during the assay when monocytes traverse endothelium in the apical-to-basal direction had no effect (left), but 3B10 anti-CCR8 mAb and anti-CCR8 mAb 5B11 significantly (P < 0.005) inhibited reverse transmigration in more than five independent experiments. (D) CD16− monocytes were selected by magnetic depletion to remove CD16+ monocytes and cultured overnight in the presence or absence of TGFβ1 to induce CD16 (top). In some samples, 20 ng/ml TGFβ1 was added together with neutralizing mAbs to CCR1, CCR2, or CCR8. Expression of CD16 was monitored by flow cytometry. This result is representative of five experiments.
When we tested the effect of neutralizing CCR8 activity, we observed that the initial migration of monocytes across endothelium to enter the collagen gels was not blocked by anti-CCR8 mAb (Fig. 6 C, left), suggesting that CCR8 did not participate in recruitment of monocytes. In contrast, the reverse transmigration of monocyte-derived cells that accompanies DC differentiation was significantly blocked by anti–human CCR8 mAbs (Fig. 6 C, right). Thus, CCR8 is not required for recruitment of monocytes, but participates importantly in the migratory clearance of monocyte-derived DCs from tissues in humans, as it does in mice.
We examined how neutralizing CCR8 may affect monocytes in the absence of endothelium. Anti-CCR8 mAb interfered with conversion of monocytes from CD16− monocytes to CD16+ monocytes. Specifically, CD16 can be induced on monocytes by treatment with TGFβ1. This induction was totally blocked by anti-CCR8 mAb, but not by mAb to CCR1 or CCR2, as controls (Fig. 6 D). The effect of anti-CCR8 in preventing CD16 expression was not related to loss or down-regulation of CD16 after induction because anti-CCR8 mAb did not change surface levels of CD16 once induced (unpublished data). These data point to a role for CCR8 in the transition between human monocyte subsets.
Discussion
Monocyte Subsets: Differentiation and Trafficking.
Two discrete monocyte subsets have been described in both humans and mice (4). The difference in CCRs expressed in these populations is one feature that suggests the subsets may have different homing properties and likely distinct functions. Often overlooked in the classification is the presence of a third subset, relatively well characterized as CD64+CD14+CD16+ cells among human monocytes (6, 35), and only recently recognized in mouse monocytes as Ly-6C Gr-1int cells (5).
Herein, we traced monocyte subsets and their usage of CCRs in a model that involves minimal ex vivo culture or manipulations such as adoptive transfer. In the assay used, most monocytes differentiate into tissue macrophages, whereas others simultaneously become DCs that home to the T cell zone of draining LNs (1). Monocyte-derived cells are traced through their acquisition of fluorescent particles (1), and if these particles are coupled to protein antigens, LN particle-bearing cells present the antigen to T cells (10). First, we asked whether either of the chemokine pathways that distinguish the two major monocyte subsets from each other (CCR2 and CX3CR1) are required to attract monocytes to the site of particle deposition, in turn affecting the appearance of monocyte-derived DCs in LNs.
Earlier work suggested that human CD16+ monocytes preferentially differentiate into migratory DCs. The equivalents of these human CD16+ monocytes are thought to be the CCR2− CX3CR1+Gr-1lo monocytes (4). Contrary to our expectations, Gr-1lo monocytes (4) did not become particle-bearing LN DCs in the assay. Indeed, no Gr-1lo (GFPhi in CX3CR1gfp mice) monocytes were recruited to the skin site. Instead, most particles were acquired by Gr-1hi and Gr-1int monocytes, populations known to migrate to sites of inflammation (4, 5). These subsets express CCR2 (4) and use CCR2 to emigrate to sites of inflammation (7–9) and lung infection (13, 36), for example. However, these monocyte subsets were unexpectedly still capable of migration to the skin site of microsphere injection in the absence of CCR2, suggesting usage of other CCRs and possible redundancy among them in this particular context. This result differs from other work in which migration of antigen-bearing APCs to the LNs after intramuscular immunization was remarkably reduced in CCR2−/− mice (11). Differences may be technical because we did not purify different LN populations before analysis, or the major difference could be in the model studied. The present model is minimally inflammatory and, therefore, may involve different CCRs during monocyte recruitment. Moreover, the assay used herein explicitly focuses on monocyte-derived DCs, which appear to migrate more readily to LNs in the absence rather than the presence of microbial stimuli (10), giving rise to the possibility that the cells we trace participate in a more regulatory role in immunity. In an earlier paper (11), it is possible that the migrating population did not derive from monocytes, but could rather arise from other DC precursors that use CCR2 in steps upstream of emigration to LNs, including Langerhans cells and their precursors (12, 14).
During our study of different knockout and knockin strains of mice, several points suggested that, between recruited Gr-1hi and Gr-1int monocytes, the latter may be more likely to give rise to LN DCs. First, shifts in the proportion of Gr-1int monocytes in the skin correlated with the magnitude of LX+ cells in LNs. That is, Gr-1int monocytes were more numerous in the skin of CCR2−/− mice, and the number of LX bead+ monocyte-derived DCs was surprisingly and correspondingly increased in the LN of CCR2−/− mice. Indeed, the fraction of Gr-1int monocytes in the total LX+ monocyte pool agrees with the relative fraction of monocytes earlier predicted to become DCs (1). Second, the GFP intensity of LN DCs bearing microspheres overlapped closely with Gr-1int monocytes in CX3CR1gfp/gfp mice. Furthermore, expression of CCR7 and CCR8 mRNA particularly characterized Gr-1int monocytes, and we observed that CCR7 and CCR8 regulates accumulation of LX-bearing DCs in the LNs. Finally, in culture, Gr-1int but not Gr-1hi monocytes more readily acquired APC function during the same interval of time that the migration assay from skin to LN takes places (2-d migration and 2-d culture in GM-CSF). Therefore, it appears that Gr-1int monocytes are the best candidates among monocytes to give rise to lymphatic trafficking DCs.
An argument can be made that Gr-1hi monocytes are the precursors for Gr-1int monocytes, which in turn are the precursors for Gr-1lo monocytes (5). If so, what concern should there be over tracing the fate of Gr-1hi versus Gr-1int monocytes because Gr-1hi monocytes may eventually trickle down the lineage and differentiate into and along the same paths as Gr-1int monocytes? Once a monocyte leaves the bloodstream, the cues that it receives from the endothelium through which it traverses, from the matrix through which it migrates, and from the cells and molecules in environment, strongly influence its maturation. Even if a relatively immature monocyte (Gr-1hi) will become a Gr-1int monocyte in the blood, how far that monocyte has differentiated in the blood by the time of diapedesis may affect how it responds to the environment and whether it is diverted to a macrophage or DC. At certain points in differentiation, a monocyte may be more susceptible to receive cues that will direct a migratory DC phenotype. It is interesting in that regard to consider our finding that Gr-1int monocytes, in contrast with Gr-1hi and Gr-1lo monocytes, selectively expressed mRNA for CCR7 and CCR8. This result shows that Gr-1int monocytes are more than a cell type midway between the two other subsets, but indeed express distinctive identifiers. The presence of these CCR mRNAs may signify, along with possibly other unknown characteristics, a transient readiness or preparation to receive particular signals for differentiation or migration.
Role of CCR8 in Monocyte-derived DC Differentiation and Migration to the LNs.
Little is known about CCR8, the CCR for CCL1 (TCA-3 in mouse, I-309 in human; reference 37), in the biology of DCs. It is well known to be expressed by eosinophils and particular populations of T cells, including Th2 cells, regulatory T cells, and skin-homing T cells (38–40). CCR8 is also expressed by monocytes (33), NK cells (41), and nonhematopoietic cells including endothelial cells (23) and smooth muscle cells (24). Phagocytically active macrophages in the CNS up-regulate CCR8 (42), and the production of CCL1 in the early stage of inflammation in experimental allergic encephalitis is a pivotal mediator in the magnitude of inflammation observed (43). CCR8−/− mice show delayed inflammation in this model (43). Studies in CCR8−/− mice reveal that it may mediate Th2 responses (16), whereas others report no obvious defect in immunologic priming to soluble antigens administered in potent adjuvant solutions (44, 45). Our studies, as well as aforementioned others, suggest that the immune response to particulates may be more affected than to soluble antigens because monocyte-derived DCs are probably APCs for the presentation of particulate, but not soluble antigen (1, 10). Alternately, because we observe only a partial block in migration of monocyte-derived DCs in the absence of CCR8, a major effect on immunity may not be observed even when presentation is restricted to monocyte-derived DCs.
Antigen-presenting DCs have not been characterized with regard to CCR8 expression, but recent evidence suggests that they induce CCL1 mRNA in response to microbial inflammatory stimuli such as schistosomal egg infection (46). In another work, human monocyte-derived DCs, but not macrophages, expressed mRNA for CCL1 (35) and, interestingly, the most mature CD16+ monocyte subset (CCR2−) was the only of the three human monocyte subsets that also expressed CCL1 (35). In these past studies, functions for CCL1 and CCR8 were completely unknown. Our data begin to shed some light on roles for CCR8 in regulating monocytes, macrophages, and DC biology. Finding of a novel role for CCR8 in affecting access of monocyte-derived DCs to LNs provides new information that might help to explain why some micro-organisms target the function of CCR8 or CCL1 (47–49).
We observed fewer LX+ monocyte-derived DCs in the LNs of CCR8−/− mice compared with WT mice. CCR8 was not required for monocyte recruitment to skin because the number and phenotype (Gr-1hi and Gr-1int) of phagocytic monocytes in the skin were similar, suggesting that it more likely affects mobilization from skin to LNs. However, CCL1/CCR8 probably does not function to attract phagocytic DCs to lymphatics because CCR7 ligands fulfill this role and because CCL1 was not obviously expressed by lymphatic vessels, consistent with data from human skin (32). Instead, CCL1 was observed in the LN subcapsule. It is not known whether emigration of cells into the LN via afferent lymphatics requires chemokine signals to specifically enter the subcapsular sinus. If so, CCL1 is positioned to play a role at this point of entry, thereby possibly explaining the reduced numbers of LX+ cells in CCR8−/− mice. In vitro differentiation studies in both human and mouse cultures additionally, or alternatively, suggest that CCR8 and its ligand CCL1 may participate in differentiation of monocytes to DCs. CCR8 may even participate in the transition of monocytes from one subset to another. However, full differentiation to mature DCs may not be a prerequisite for migration into LNs via lymphatics, as our data indicate that some stages of maturation to DCs occur while the cells are in transit to the LN or after their arrival.
In some settings, CCL1 and CCR8 regulate survival (50–52), raising the possibility that differential survival, not migration, accounts for the decreased the number of phagocytically labeled DCs in CCR8−/− LNs. However, this possibility is inconsistent with our studies using human monocytes in a model of transendothelial trafficking, where we can evaluate both the migratory and sessile fraction of cells quantitatively. In this model, fewer reverse-transmigrating cells corresponded with increased numbers of monocyte-derived cells in the subendothelium. Furthermore, in the LX bead assay, death of phagocytes in the LN results in passage of the microspheres to other cells (unpublished data), so that the original number of migrated cells can be traced for up to 10 d, even after apoptosis of input DCs.
In summary, this work begins to unravel the role of CCRs in the migration of monocytes that ultimately develop into lymphatic trafficking DCs. The findings confirm some expected results, such as the role of the CCR7 pathway in migration of monocyte-derived DCs to LNs; bring us some surprises like the lack of a role for CCR2 and CX3CR1 in the generation of these migratory LN DCs; and reveal a novel role for the CCR CCR8 in migration of monocyte-derived DCs via lymphatics.
Acknowledgments
This work is dedicated to the memory of Dr. Peter C. Harpel, whose enthusiasm for CCR8 helped initiate and shape this project. We are grateful to the New York Firefighter's Skin Bank for the preparation and provision of human skin explants. Clodronate was a gift from Roche Diagnostics GmbH.
This work was supported in part by grants from the National Institutes of Health, an Investigator Award from the Cancer Research Institute (to G.J. Randolph), and funds from the Irene Diamond Foundation (to S. Lira). S. Lira is an Irene Diamond Associate Professor of Immunology. Fellowship support included funds from the German Research Foundation (to F. Tacke), the European Molecular Biology Organization (to V. Angeli), and the National Institutes of Health (to E. Edwards).
The authors have no conflicting financial interests.
Abbreviations used in this paper: CCR, chemokine receptor; LX, latex.
References
- 1.Randolph, G.J., K. Inaba, D.F. Robbiani, R.M. Steinman, and W.A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 11:753–761. [DOI] [PubMed] [Google Scholar]
- 2.Passlick, B., D. Flieger, and H.W. Ziegler-Heitbrock. 1989. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 74:2527–2534. [PubMed] [Google Scholar]
- 3.Ancuta, P., R. Rao, A. Moses, A. Mehle, S.K. Shaw, F.W. Luscinskas, and D. Gabuzda. 2003. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J. Exp. Med. 197:1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geissmann, F., S. Jung, and D.R. Littman. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 19:71–82. [DOI] [PubMed] [Google Scholar]
- 5.Sunderkotter, C., T. Nikolic, M.J. Dillon, N. Van Rooijen, M. Stehling, D.A. Drevets, and P.J. Leenen. 2004. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172:4410–4417. [DOI] [PubMed] [Google Scholar]
- 6.Grage-Griebenow, E., H.D. Flad, and M. Ernst. 2001. Heterogeneity of human peripheral blood monocyte subsets. J. Leukoc. Biol. 69:11–20. [PubMed] [Google Scholar]
- 7.Boring, L., J. Gosling, S.W. Chensue, S.L. Kunkel, R.V. Farese Jr., H.E. Broxmeyer, and I.F. Charo. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuziel, W.A., S.J. Morgan, T.C. Dawson, S. Griffin, O. Smithies, K. Ley, and N. Maeda. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA. 94:12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palframan, R.T., S. Jung, G. Cheng, W. Weninger, Y. Luo, M. Dorf, D.R. Littman, B.J. Rollins, H. Zweerink, A. Rot, and U.H. von Andrian. 2001. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194:1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotta, G., E.W. Edwards, S. Sangaletti, C. Bennett, S. Ronzoni, M.P. Colombo, R.M. Steinman, G.J. Randolph, and M. Rescigno. 2003. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J. Exp. Med. 198:1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters, W., M. Dupuis, and I.F. Charo. 2000. A mechanism for the impaired IFN-gamma production in C-C chemokine receptor 2 (CCR2) knockout mice: role of CCR2 in linking the innate and adaptive immune responses. J. Immunol. 165:7072–7077. [DOI] [PubMed] [Google Scholar]
- 12.Sato, N., S.K. Ahuja, M. Quinones, V. Kostecki, R.L. Reddick, P.C. Melby, W.A. Kuziel, and S.S. Ahuja. 2000. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major–resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynor, T.R., A.C. Herring, M.E. Dorf, W.A. Kuziel, G.B. Toews, and G.B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168:4659–4666. [DOI] [PubMed] [Google Scholar]
- 14.Merad, M., M.G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I.L. Weissman, J.G. Cyster, and E.G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung, S., J. Aliberti, P. Graemmel, M.J. Sunshine, G.W. Kreutzberg, A. Sher, and D.R. Littman. 2000. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20:4106–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chensue, S.W., N.W. Lukacs, T.Y. Yang, X. Shang, K.A. Frait, S.L. Kunkel, T. Kung, M.T. Wiekowski, J.A. Hedrick, D.N. Cook, et al. 2001. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J. Exp. Med. 193:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunn, M.D., S. Kyuwa, C. Tam, T. Kakiuchi, A. Matsuzawa, L.T. Williams, and H. Nakano. 1999. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano, H., and M.D. Gunn. 2001. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 166:361–369. [DOI] [PubMed] [Google Scholar]
- 19.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 174:83–93. [DOI] [PubMed] [Google Scholar]
- 20.Randolph, G.J., S. Beaulieu, S. Lebecque, R.M. Steinman, and W.A. Muller. 1998. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 282:480–483. [DOI] [PubMed] [Google Scholar]
- 21.Randolph, G.J., T. Luther, S. Albrecht, V. Magdolen, and W.A. Muller.1998. Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood. 92:4167–4177. [PubMed] [Google Scholar]
- 22.Randolph, G.J., S. Beaulieu, M. Pope, I. Sugawara, L. Hoffman, R.M. Steinman, and W.A. Muller. 1998. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. USA. 95:6924–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque, N.S., J.T. Fallon, M.B. Taubman, and P.C. Harpel. 2001. The chemokine receptor CCR8 mediates human endothelial cell chemotaxis induced by I-309 and Kaposi sarcoma herpesvirus-encoded vMIP-I and by lipoprotein(a)-stimulated endothelial cell conditioned medium. Blood. 97:39–45. [DOI] [PubMed] [Google Scholar]
- 24.Haque, N.S., J.T. Fallon, J.J. Pan, M.B. Taubman, and P.C. Harpel. 2004. Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion. Blood. 103:1296–1304. [DOI] [PubMed] [Google Scholar]
- 25.Randolph, G.J., G. Sanchez-Schmitz, R.M. Liebman, and K. Schakel. 2002. The CD16+ (FcγRIII+) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J. Exp. Med. 196:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larregina, A.T., A.E. Morelli, L.A. Spencer, A.J. Logar, S.C. Watkins, A.W. Thomson, and L.D. Falo Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151–1158. [DOI] [PubMed] [Google Scholar]
- 27.Kurihara, T., G. Warr, J. Loy, and R. Bravo. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186:1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grage-Griebenow, E., R. Zawatzky, H. Kahlert, L. Brade, H. Flad, and M. Ernst. 2001. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur. J. Immunol. 31:48–56. [DOI] [PubMed] [Google Scholar]
- 29.Vassileva, G., H. Soto, A. Zlotnik, H. Nakano, T. Kakiuchi, J.A. Hedrick, and S.A. Lira. 1999. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 190:1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luther, S.A., H.L. Tang, P.L. Hyman, A.G. Farr, and J.G. Cyster. 2000. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA. 97:12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson, D.G. 2003. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc. Med. 13:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Schaerli, P., L. Ebert, K. Willimann, A. Blaser, R.S. Roos, P. Loetscher, and B. Moser. 2004. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 199:1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiffany, H.L., L.L. Lautens, J.L. Gao, J. Pease, M. Locati, C. Combadiere, W. Modi, T.I. Bonner, and P.M. Murphy. 1997. Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J. Exp. Med. 186:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larregina, A.T., A.E. Morelli, L.A. Spencer, A.J. Logar, S.C. Watkins, A.W. Thomson, and L.D. Falo Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151–1158. [DOI] [PubMed] [Google Scholar]
- 35.Ancuta, P., L. Weiss, and N. Haeffner-Cavaillon. 2000. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur. J. Immunol. 30:1872–1883. [DOI] [PubMed] [Google Scholar]
- 36.Peters, W., J.G. Cyster, M. Mack, D. Schlondorff, A.J. Wolf, J.D. Ernst, and I.F. Charo. 2004. CCR2-dependent trafficking of F4/80dim macrophages and CD11cdim/intermediate dendritic cells is crucial for T cell recruitment to lungs infected with Mycobacterium tuberculosis. J. Immunol. 172:7647–7653. [DOI] [PubMed] [Google Scholar]
- 37.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity. 12:121–127. [DOI] [PubMed] [Google Scholar]
- 38.Campbell, J.D., and K.T. HayGlass. 2000. T cell chemokine receptor expression in human Th1- and Th2-associated diseases. Arch. Immunol. Ther. Exp. (Warsz) 48:451–456. [PubMed] [Google Scholar]
- 39.Iellem, A., M. Mariani, R. Lang, H. Recalde, P. Panina-Bordignon, F. Sinigaglia, and D. D'Ambrosio. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colantonio, L., A. Iellem, F. Sinigaglia, and D. D'Ambrosio. 2002. Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1/I-309. Eur. J. Immunol. 32:3506–3514. [DOI] [PubMed] [Google Scholar]
- 41.Inngjerdingen, M., B. Damaj, and A.A. Maghazachi. 2000. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J. Immunol. 164:4048–4054. [DOI] [PubMed] [Google Scholar]
- 42.Trebst, C., S.M. Staugaitis, P. Kivisakk, D. Mahad, M.K. Cathcart, B. Tucky, T. Wei, M.R. Sandhya Rani, R. Horuk, K.D. Aldape, et al. 2003. CC chemokine receptor 8 (CCR8) in the central nervous system is associated with phagocytic macrophages. Am. J. Pathol. 162:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy, C.A., R.M. Hoek, M.T. Wiekowski, S.A. Lira, and J.D. Sedgwick. 2002. Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J. Immunol. 169:7054–7062. [DOI] [PubMed] [Google Scholar]
- 44.Chung, C.D., F. Kuo, J. Kumer, A.S. Motani, C.E. Lawrence, W.R. Henderson Jr., and C. Venkataraman. 2003. CCR8 is not essential for the development of inflammation in a mouse model of allergic airway disease. J. Immunol. 170:581–587. [DOI] [PubMed] [Google Scholar]
- 45.Goya, I., R. Villares, A. Zaballos, J. Gutierrez, L. Kremer, J.A. Gonzalo, R. Varona, L. Carramolino, A. Serrano, P. Pallares, et al. 2003. Absence of CCR8 does not impair the response to ovalbumin-induced allergic airway disease. J. Immunol. 170:2138–2146. [DOI] [PubMed] [Google Scholar]
- 46.Trottein, F., N. Pavelka, C. Vizzardelli, V. Angeli, C.S. Zouain, M. Pelizzola, M. Capozzoli, M. Urbano, M. Capron, F. Belardelli, et al. 2004. A type I IFN-dependent pathway induced by Schistosoma mansoni eggs in mouse myeloid dendritic cells generates an inflammatory signature. J. Immunol. 172:3011–3017. [DOI] [PubMed] [Google Scholar]
- 47.Luttichau, H.R., J. Stine, T.P. Boesen, A.H. Johnsen, D. Chantry, J. Gerstoft, and T.W. Schwartz. 2000. A highly selective CC chemokine receptor (CCR)8 antagonist encoded by the poxvirus molluscum contagiosum. J. Exp. Med. 191:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holst, P.J., H.R. Luttichau, T.W. Schwartz, and M.M. Rosenkilde. 2003. Virally encoded chemokines and chemokine receptors in the role of viral infections. Contrib. Microbiol. 10:232–252. [DOI] [PubMed] [Google Scholar]
- 49.Najarro, P., H.J. Lee, J. Fox, J. Pease, and G.L. Smith. 2003. Yaba-like disease virus protein 7L is a cell-surface receptor for chemokine CCL1. J. Gen. Virol. 84:3325–3336. [DOI] [PubMed] [Google Scholar]
- 50.Ruckes, T., D. Saul, J. Van Snick, O. Hermine, and R. Grassmann. 2001. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood. 98:1150–1159. [DOI] [PubMed] [Google Scholar]
- 51.Louahed, J., S. Struyf, J.B. Demoulin, M. Parmentier, J. Van Snick, J. Van Damme, and J.C. Renauld. 2003. CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/ CCL1 and vMIP-I. Eur. J. Immunol. 33:494–501. [DOI] [PubMed] [Google Scholar]
- 52.Spinetti, G., G. Bernardini, G. Camarda, A. Mangoni, A. Santoni, M.C. Capogrossi, and M. Napolitano. 2003. The chemokine receptor CCR8 mediates rescue from dexamethasone-induced apoptosis via an ERK-dependent pathway. J. Leukoc. Biol. 73:201–207. [DOI] [PubMed] [Google Scholar]