Abstract
Human immunodeficiency virus (HIV)-1 infectivity requires actin-dependent clustering of host lipid raft–associated receptors, a process that might be linked to Rho guanosine triphosphatase (GTPase) activation. Rho GTPase activity can be negatively regulated by statins, a family of drugs used to treat hypercholesterolemia in man. Statins mediate inhibition of Rho GTPases by impeding prenylation of small G proteins through blockade of 3-hydroxy-3-methylglutaryl coenzyme A reductase. We show that statins decreased viral load and increased CD4+ cell counts in acute infection models and in chronically HIV-1–infected patients. Viral entry and exit was reduced in statin-treated cells, and inhibition was blocked by the addition of l-mevalonate or of geranylgeranylpyrophosphate, but not by cholesterol. Cell treatment with a geranylgeranyl transferase inhibitor, but not a farnesyl transferase inhibitor, specifically inhibited entry of HIV-1–pseudotyped viruses. Statins blocked Rho-A activation induced by HIV-1 binding to target cells, and expression of the dominant negative mutant RhoN19 inhibited HIV-1 envelope fusion with target cell membranes, reducing cell infection rates. We suggest that statins have direct anti–HIV-1 effects by targeting Rho.
Keywords: cholesterol, actin cytoskeleton, small GTPases, lipid rafts, prenylation
Introduction
Despite the use of available prophylactic measures, HIV-1 infection constitutes a growing pandemic, particularly in less developed countries, for which we lack adequate treatment. The most common therapeutic regime, highly active antiretroviral therapy (HAART), has improved the life quality of many HIV-1–infected individuals. Nonetheless, it is cumbersome, with serious side effects, and has resulted in the emergence of drug-resistant viruses.
One area of HIV-1 research aims to understand the interplay between virus and host cell, to block key interactions between virus and host target, and to prevent virus propagation without the inconvenience of HAART. Effort has concentrated on the HIV-1 entry and budding processes, which require the formation of large clusters between viral and host cell proteins (1). Results suggest that HIV-1 entry into and exit from the host cell require actin cytoskeleton rearrangement and adequate cholesterol levels in host and viral membranes (2–13). A means remains to be found for specific targeting of these host factors to prevent HIV-1 propagation with minimal toxicity.
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors used to treat hypercholesterolemia. HMG-CoA reductase produces mevalonic acid, a precursor for cholesterol biosynthesis and generation of isoprenoids that modify specific cell proteins posttranslationally. Rho guanosine triphosphatases (GTPases), which must be prenylated at their C terminus for function, are molecular switches that cycle between GTP-bound (active) and GDP-bound (inactive) states to control actin cytoskeleton remodeling in response to stimuli (14). By targeting HMG-CoA, statins block cholesterol biosynthesis, but also affect actin cytoskeleton rearrangement by inhibiting Rho GTPases (15).
We show that statins inhibited HIV-1 infection of SCID mice grafted with adult human PBMCs (SCID-hu-PBMC), an in vivo model of acute HIV-1 infection. Statins inhibited virus entry into and exit from target cells by targeting Rho geranylation. Strikingly, 1-mo oral statin administration reduced serum HIV-1 RNA copy number in chronically HIV-1–infected individuals not receiving HAART. Our results indicate that statins might be suitable antiretroviral drugs for more accessible AIDS treatment.
Materials and Methods
HIV-1 Infection.
Single-round infections were performed with a replication-defective pNL4-3.Luc.R-E pseudotyped with HIV-1ADA or vesicular stomatitis virus (VSV)-G envelopes (2). MT2-CCR5 cells (provided by J. Alcamí, Instituto de Salud Carlos III, Madrid, Spain) were treated with 0.4, 2, or 10 μM lovastatin (Lov) for 48 h at 37°C alone or with 200 μM l-mevalonate (Mev), 5 μM geranylgeranylpyrophosphate (GGPP), 5 μM farnesylpyrophosphate (FPP), or 5 μg/ml cholesterol (all from Sigma-Aldrich), or with 10 μM GGTI-286 or 10 μM FTI-277 (both from Calbiochem) before transduction with viral supernatants (a multiplicity of infection of 0.1) for 2 h at 37°C. Infectivity was determined after 24 h by measuring luciferase activity (2, 5). Similar experiments were performed using MT2-CCR5 cells expressing GFP-tagged wild-type Rho, wild-type Rac, or the Rho-N19 or Rac-N17 mutants (provided by F. Sánchez-Madrid, Hospital de la Princesa, Madrid, Spain). gp160-induced cell–cell fusion assays were as described previously (2).
PBMCs purified on Ficoll-Hypaque gradients (Amersham Biosciences) were activated for 2 d with 1 μg/ml PHA and 50 ng/ml IL-2, and treated for 48 h at 37°C with Lov or Lov plus Mev. Treated PBMCs were incubated with NL4-3 or BaL viral stocks (1 or 10 ng p24 antigen/106 cells) for 3 h at 37°C. Cell-free supernatants were collected daily from cultured cells (0.5 × 106/ml) and tested for p24 antigen (Beckman Coulter).
For ex vivo infection, PBMCs were purified from informed, vehicle-, or pravastatin-treated donors (40 mg/d for 14 d, oral) before and after treatment. PBMCs obtained before treatment were frozen, and pre- and posttreatment cells were infected simultaneously with two infectious doses of HIV-1 BaL stocks. After washing, cells (0.5 × 106 cells/ml) were plated with PHA and IL-2, and cultured in the absence of statins. p24 was measured at 4 and 5 d after infection (Beckman Coulter).
Murine SCID-hu-PBMC Model.
8–10-wk-old nonleaky phenotype CB.17 SCID/SCID mice were reconstituted by i.p. injection of 50 × 106 human PBMCs. 1 wk later, mice with comparable serum human immunoglobulin levels, proof of reconstitution with human cells, received 5 mg/kg Lov i.p. every 3 d, beginning 1 wk before HIV-1 NL4-3 i.p. challenge (100 TCID50/ml), until they were killed. Plasma HIV-1 RNA copy number was measured (Amplicor HIV-1 Monitor Assay; Roche Molecular Systems) 1 wk after infection. 2 wk after viral challenge, 106 peritoneal cells from killed mice were incubated with 2 × 106 PHA-activated human PBMCs in the presence of IL-2, and p24 was determined after 2 wk of coculture. Peritoneal cells were also analyzed by FACS® (EPICS Elite; Beckman Coulter) using FITC-labeled anti-CD45 and PE anti-CD4 antibody (BD Biosciences). Untreated or Lov-treated mice were reconstituted with CellTracker Green CMFDA (Molecular Probes)-stained PBMCs. At 3 and 7 d after reconstitution, peritoneal cells were obtained from two mice, pooled, and analyzed by FACS®.
Titration of Viral Production.
HEK 293T cells, cotransfected with pNL4-3.Luc.R.E. and cDNA encoding HIV-1ADA or VSV-G envelopes, were treated with Lov or Lov plus Mev. Viral stocks were harvested after 48 h and titrated by measuring luciferase activity after transduction of CD4-expressing HEK 293T cells. Values were normalized to luciferase activity from extracts of stock-producing cells.
LTR-driven Gene Expression.
Jurkat cells transfected with pLTR-luc (16), pcDNA-tat, and the promoterless renilla luciferase plasmid were treated at 4 h after transfection with inhibitors and metabolites at the indicated concentrations (see HIV-1 Infection). Relative luciferase units were calculated as the ratio between firefly and renilla activity after 48 h.
Cell Cholesterol Mass Determination.
Cholesterol content of untreated, Lov-, or Lov plus Mev–treated MT2-CCR5 cells was analyzed on a gas chromatograph (Chrompack; Hewlett-Packard) as described previously (17). The cholesteryl ester mass was calculated by subtracting free cholesterol from total cholesterol content.
Rho and Rac Activation Assay.
3 × 106 starved MT2-CCR5 cells treated with Lov or Lov plus GGPP were incubated with HIV-1 stocks. At the indicated times, cells were washed with ice-cold PBS and lysates were prepared using Rho or Rac activation assay kits (Upstate Biotechnology). GTP-bound Rho or Rac was precipitated with RBD or PBD agarose beads, respectively, and measured in pellets by Western blot with specific antibodies, using crude cell extracts for normalization. Densitometry was performed using NIH Image software.
gp120-induced Patching.
Unstimulated PBMCs plated into ICAM-2/Fc (R&D Systems)-coated chambers were incubated for 30 min at 12°C with recombinant gp120 (T cell line–adapted X4 virus, isolate IIIB; Intracel) in PBS/0.2% bovine serum albumin, followed by rabbit anti–gp120 and Cy2 anti–rabbit antibody (Jackson ImmunoResearch Laboratories). Cells were fixed with 3.7% paraformaldehyde/PBS on ice, and then incubated sequentially with biotinylated anti-CXCR4 (FAB172; R&D Systems) and streptavidin-Cy3. Cells were mounted in Vectashield medium (Vector Laboratories) and visualized by confocal microscopy (Leica).
Lov Effect on Ras Processing and Vesicle Fusion.
Lysates from MT2-CCR5 cells treated with Lov, Lov plus cholesterol, Lov plus FPP, GGTI, or FTI were resolved in 15% SDS-PAGE and blotted with an anti–pan-Ras antibody (Oncogene Research Products).
For vesicle fusion assays, HEK 293 cells were incubated for 9 min at 37°C with 1 mg/ml biotinylated peroxidase (horseradish peroxidase [HRP]) or 4 mg/ml avidin in Dulbecco's PBS with 1 mM CaCl2 and 1 mM MgCl2. After washing, cells were homogenized in 3 mM imidazole/HCl, pH 7.4, and 250 mM sucrose by several passages through a 23-gauge needle. Postnuclear supernatants containing avidin or biotin HRP-loaded endosomes were incubated for 1 h at 37°C in 10 mM Hepes-KOH, pH 7.0, 1.2 mM MgCl2, 50 mM KOAc, 0.8 mM DTT, biotin insulin (to quench free avidin), and an ATP-regenerating system. Control preincubations were performed in the absence of an ATP-regenerating system. Fusion was terminated by lysis with 0.25% Triton X-100, and HRP activity in the complex was detected in avidin immunoprecipitates.
Lov Treatment of HIV-1–infected Patients.
Six informed HIV-1–infected patients in A1 disease stage who did not receive HAART were treated with Lov (40 mg/d, oral) for 1 mo. Plasma HIV RNA copy number, circulating CD4+ T lymphocyte counts, and plasma cholesterol levels were measured before and immediately after treatment, as well as 3 mo after termination of Lov treatment, using standard clinical techniques.
Online Supplemental Material.
gp120-induced patching and the Lov effect on Ras processing and vesicle fusion are shown in Figs. S1 and S2, respectively. Figs. S1 and S2 are available at http://www.jem.org/cgi/content/full/jem.20040061/DC1.
Results and Discussion
Statins Inhibit HIV-1 Infection In Vitro and In Vivo.
It is suggested that statins have anti–HIV-1 effects (18). PHA-activated human PBMCs, pretreated for 48 h with 10 μM Lov, were exposed to X4 (NL4-3) or R5 (BaL) HIV-1 strains, and no cytotoxic effects were observed at this dosage (not depicted). Lov inhibited HIV-1 infection, as indicated by reduced p24 antigen production in X4- and R5-infected cultures (Fig. 1 A). This effect was reversed by coincubation of cells with l-Mev, the product of HMG-CoA reductase.
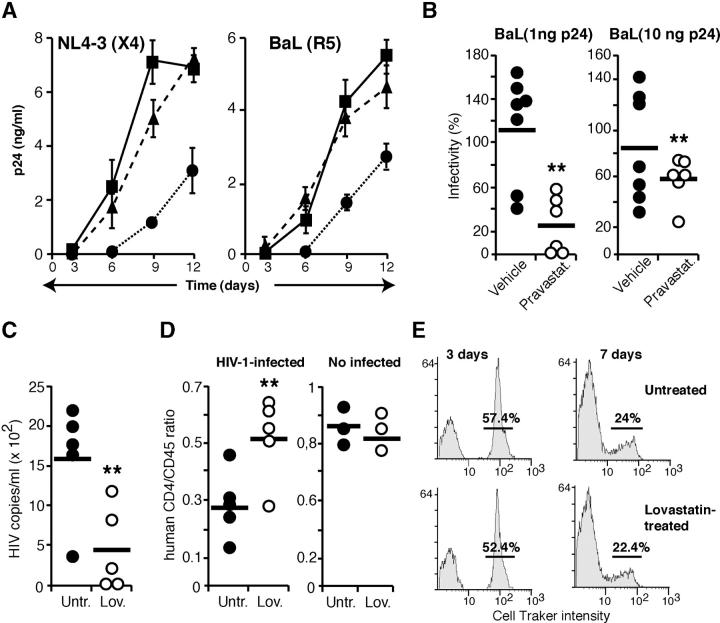
Figure 1.
Statins inhibit in vitro and in vivo HIV-1 infection of human PBMCs. (A) Infection of untreated (▪), Lov- (•), or Lov plus Mev–treated (▴) PHA-activated human PBMCs by X4 or R5 HIV-1 viral strains. Data are mean ± SD of triplicate points (n = 3). (B) PBMCs isolated from human volunteers, before and after vehicle or pravastatin treatment, were exposed to two doses of BaL HIV-1. Data are the ratio between post- and pretreatment p24 levels for PBMCs from each individual (**, P < 0.05). (C and D) Human PBMC-reconstituted SCID mice were Lov treated for 1 wk before HIV-1 infection. Viral load (C) and human CD4/CD45 ratio (D) was determined for each animal 1 wk after infection. One representative experiment out of two is shown (**, P < 0.05). The CD4+/CD45+ ratio was also determined in noninfected mice (D, right). (E) Lov-treated SCID mice were reconstituted with CellTracker-stained PBMCs and peritoneal cell labeling was examined at the indicated times. The numbers indicate the percentage of labeled cells.
To further assess the statin-induced anti–HIV-1 effect, we compared susceptibility to R5 virus infection of PBMCs from vehicle- or pravastatin-treated human volunteers isolated before and after the treatment. PBMCs isolated before and after vehicle treatment were equally infected by R5 viruses (Fig. 1 B; P = 0.812 for 1 ng of p24/106 cells and P = 0.218 for 10 ng of p24/106 cells, two-tailed Wilcoxon Signed-Rank test). In contrast, PBMCs isolated from pravastatin-treated volunteers were more resistant to HIV infection than PBMCs from the same individuals before treatment (Fig. 1 B; P = 0.032 for both virus doses, two-tailed Wilcoxon Signed-Rank test).
We tested whether statins blocked HIV-1 replication in vivo in the SCID-hu-PBMC model (19) by injecting Lov before HIV-1 NL4-3 challenge. Mean viral load was significantly reduced in Lov-treated mice (P = 0.028, two-tailed Mann-Whitney test) compared with vehicle-treated animals (Fig. 1 C). Viral RNA was undetectable in plasma of 4 out of 10 Lov-treated mice, and coculture of peritoneal cells from 2 of these mice with PHA-activated human PBMCs did not rescue virus. At 1 wk after infection, Lov-treated SCID-hu-PBMC mice showed higher CD4+ T cell counts than controls, and the average CD4+/CD45+ ratio was 51% in Lov- and 28% in vehicle-treated mice (Fig. 1 D), indicating specific CD4+ cell loss in controls (P = 0.048, two-tailed Mann-Whitney test). In non-HIV–challenged controls, the CD4+/CD45+ ratio was similar between untreated and Lov-treated mice. Lov affected neither CCR5 (69.7% untreated, 76.1% Lov treated) or CXCR4 levels (56.5% untreated, 64.3% Lov treated). To determine whether statins affected viability or proliferation of grafted human cells, SCID mice were reconstituted with fluorescent-labeled, PHA-activated human PBMCs and were Lov treated as described above. As the label is distributed in each cell division, a deleterious Lov effect on grafted PBMCs would produce a difference in label intensity or in the number of labeled cells compared with untreated animals. We found no difference in the number of labeled cells or in labeling intensity (Fig. 1 E).
Statins Affect the HIV-1 Replicative Cycle by Reducing Geranylgeranylation.
We analyzed infection of untreated or Lov-treated cells using the replication-defective HIV-1 NL4-3.Luc variant pseudotyped with HIV or VSV envelopes. As the virus cannot replicate in the cell, luciferase activity correlates with the ability of the virus to enter target cells. Single-round infection experiments showed that Lov inhibited entry of HIV-1 NL4-3.Luc pseudotyped with HIV-R5 (Fig. 2 A) or X4 (not depicted) envelopes, but not that of viruses pseudotyped with the VSV-G envelope. The inhibitory effect of statin on HIV-1 entry was dose dependent (Fig. 2 B). Lov treatment also reduced HIV-1-X4–pseudotyped viral production, but not that of VSV-G–pseudotyped viruses, by HEK 293T cells transfected with replication-defective NL4-3.Luc DNA (Fig. 2 C). It is unlikely that the specific Lov-induced reduction in HIV-1–pseudotyped viral production is due to differential Gag synthesis and processing because HIV-1 and VSV pseudotypes share the same viral genome. Nonetheless, Lov increased HIV-1 LTR-driven promoter activity (Fig. 2 D), as monitored in cells transfected with a luciferase-encoding reporter under the control of HIV-LTR (pLTR-luc), suggesting that the drug regulates the activity of nuclear factors involved in HIV transcription. These results indicate that Lov has both pro-HIV (increasing viral genome transcription) and anti-HIV effects (inhibiting virus entry into and virus production by the target cell). Both pro– and anti–HIV-1 Lov-induced effects were mediated through the Mev pathway, as they were reversed by coincubation of cells with l-Mev (Fig. 2, A, C, and D).
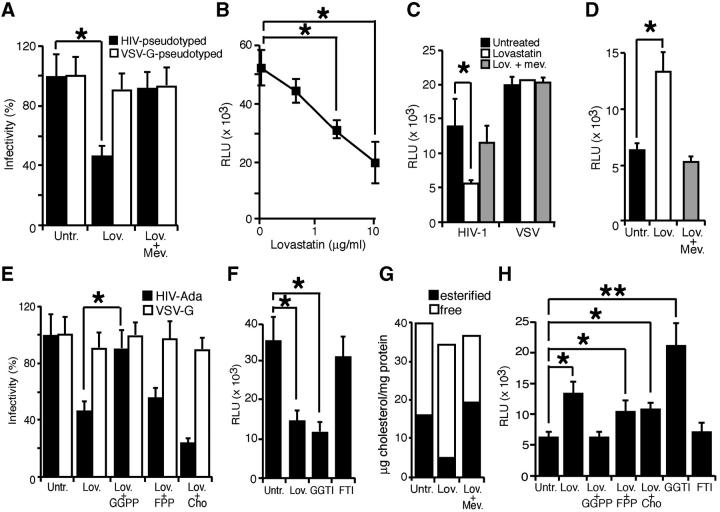
Figure 2.
Statins inhibit HIV-1 entry and exit. (A) Single-round infections were performed in untreated, Lov-, and Lov plus Mev–treated MT2-CCR5 cells using a replication-defective NL4-3 virus bearing the luciferase reporter pseudotyped with HIV-1Ada or VSV-G envelopes. Cell infection was normalized using untreated cells as 100%. (B) MT2-CCR5 cells were Lov treated (0.4, 2, or 10 μM), and then exposed to NL4-3 virus pseudotyped with HIV-1Ada envelope. The x axis is in log scale. (C) Virus production was measured by titration of viral stocks produced in untreated, Lov-, and Lov plus Mev–treated HEK-293T cells transfected with replication-defective NL4-3 virus. Relative luciferase units were calculated after normalization with luciferase activity from extracts of stock-producing cells. (D) LTR-driven gene expression was analyzed in untreated, Lov-, or Lov plus Mev–treated Jurkat cells transfected with pLTR-Luc, pcDNA-tat, and promoterless renilla for normalization. (E) Single-round infection experiments were performed using replication-defective NL4-3 virus in MT2-CCR5 cells treated with Lov or Lov plus the indicated compounds. Cell infection was normalized considering untreated cells as 100%. (F) Single-round infections performed with the HIV-1Ada–pseudotyped virus in MT2-CCR5 cells treated with Lov, GGTI, or FTI. (G) Free or esterified cholesterol levels in untreated, Lov-, or Lov plus Mev–treated MT2-CCR5 cells. One representative experiment out of two is shown. (H) LTR-driven gene expression in MT2-CCR5 cells treated with Lov, Lov plus the indicated compounds, or with GGTI-286 or FTI-277. (A–E, G, and H) Data are mean ± SD of duplicates (n = 3). Significant differences are indicated: *, P < 0.05; **, P < 0.01. Kruskal-Wallis test.
Inhibition of the Mev pathway diminishes cholesterol biosynthesis, but also reduces cell pools of GGPP and FPP, both of which are involved in posttranslational protein modification. We found that Lov-induced inhibition of HIV-1 entry into permissive cells was reversed by the coaddition of GGPP, but not of FPP or cholesterol (Fig. 2 E). This suggests that Lov inhibits HIV-1 infection by blocking protein geranylgeranylation rather than by preventing farnesylation or reducing cholesterol biosynthesis. R5-pseudotyped virus entry was inhibited by cell treatment with a geranylgeranyl transferase-I inhibitor (GGTI-286), but not a farnesyl transferase inhibitor (FTI-277; Fig. 2 F). These drugs did not affect VSV-G–pseudotyped virus entry (not depicted). Measurement of cell cholesterol content indicated comparable free cholesterol levels in Lov-treated and untreated cells. Nonetheless, the drug drastically reduced the cholesteryl ester mass (Fig. 2 G), probably due to concomitant inhibition of acyl-CoA:cholesterol acyltransferase (20). Although we cannot exclude a role for esterified cholesterol in HIV-1 infection, the finding that GGPP reverses Lov-induced inhibition of virus entry suggests that Lov effects are mediated mainly by impairment of protein geranylgeranylation. Supplementation with GGPP, but not FPP or free cholesterol, also reversed the Lov-induced increase in LTR-driven transcription (Fig. 2 H). Cell treatment with a geranylgeranyl transferase inhibitor also increased HIV transcription (Fig. 2 H), suggesting a general molecular mechanism for Lov mediation of pro– and anti–HIV-1 effects.
Statins Inhibit HIV-1 Infection by Down-regulating Rho Activation.
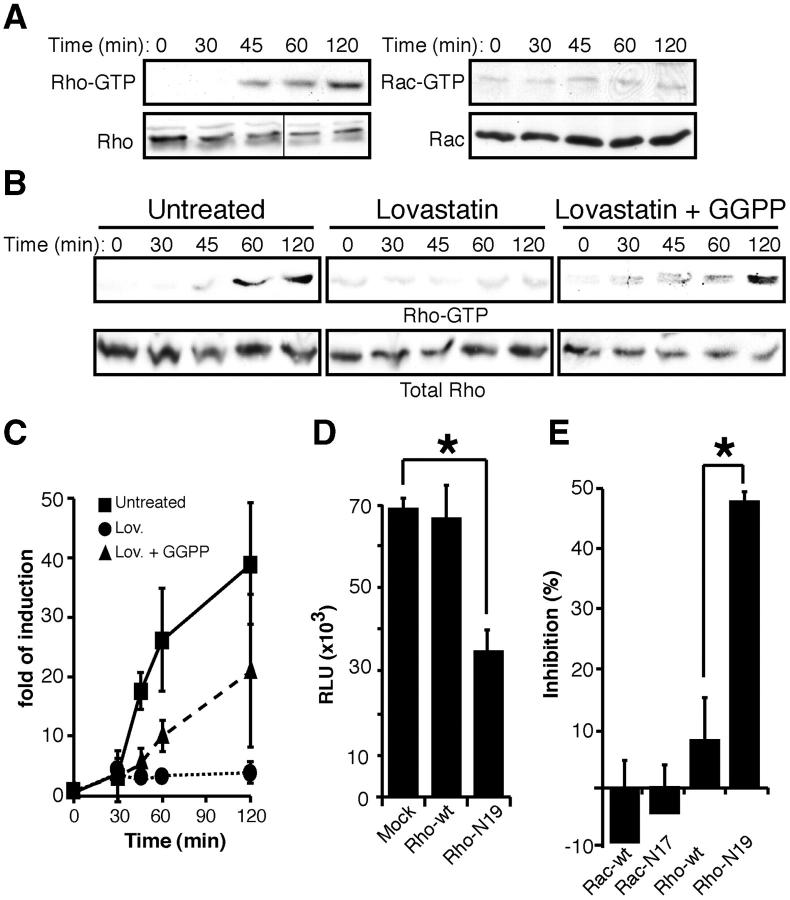
Geranylgeranylation is needed for posttranslational lipid modification of several proteins anchored to the inner membrane leaflet, including the Rho GTPases (15). Moreover, gp120 binding to target cells modifies Rho molecular mass and increases Cdc42 expression (21). Target cell incubation with HIV-1 resulted in activation of Rho, but not Rac (Fig. 3 A) or Cdc42 (not depicted). Cell incubation with Lov before virus exposure inhibited HIV-1–induced Rho activation, which was reversed when cells were coincubated with GGPP (Fig. 3, B and C), indicating that Lov prevented HIV-1–induced Rho activation by a geranylgeranylation-dependent mechanism. Virus-induced Rho activation is required for virus entry because infection by R5-pseudotyped HIV-1 was reduced in dominant negative RhoN19 mutant-expressing cells (Fig. 3 D). RhoN19 expression also specifically prevented HIV-1 envelope fusion with target cell membrane in a cell–cell fusion assay (Fig. 3 E). The results suggest that Lov inhibits HIV-1 entry into target cells, at least in part, by preventing Rho activation. Rho inhibition has been associated with an increase in HIV-1 transcription (22), suggesting that Lov-induced pro– and anti–HIV-1 effects might be Rho mediated.
Figure 3.
Statins inhibit HIV-1 infection by down-regulating Rho activation. (A) Serum-starved MT2-CCR5 cells were incubated with HIV-1 and cell lysates were assayed for active Rho or Rac. Total Rho or Rac was analyzed in parallel in crude cell extracts as a protein loading control. One experiment out of three is shown. Black line indicates that different sections of the same gel were juxtaposed. (B) Active Rho was determined in untreated, Lov-, or Lov plus GGPP–treated cells, as described above. Total Rho in crude cell extracts is shown as a loading control. One representative experiment out of three is shown. (C) Western blots from three independent experiments as in B were quantified by densitometry and values were normalized using Rho in crude cell extracts as a loading control. Data points are plotted relative to mean values of cells not exposed to virus (time 0) for each condition. (D) Single-round infections of MT2-CCR5 cells transfected with mock, wild-type Rho, or mutant Rho-N19 using an HIV-1Ada–pseudotyped, replication-defective virus. *, P < 0.05. Kruskal-Wallis test. (E) HeLa-CD4 cells transfected with wild-type Rac, wild-type Rho, Rac-N17, or Rho-N19 were mixed with HIV gp160–expressing BSC40 cells. Cell fusion events were measured and normalized relative to mock transfected cells. *, P < 0.05. Kruskal-Wallis test. (D and E) Data are mean ± SD of duplicate points (n = 3).
Statins Reduce Plasma HIV RNA Copy Number in Chronically Infected Individuals.
Statins are used for treatment of HAART-associated lipodystrophy. Based on our in vitro results, we tested the potential use of statins for in vivo treatment of HIV patients. In a preliminary study for proof of concept, six A1 stage HIV-1–infected, non-HAART–treated patients with stable viral load for at least 6 mo were Lov treated for 1 mo as sole therapy. Short-term statin treatment induced a clear reduction in serum viral RNA loads in all patients (Table I). Discontinuation of statin treatment caused a rebound in viral load (Table I). The data suggest that statins can inhibit HIV-1 replication in chronically infected individuals, and support future clinical studies of statins as possible antiretroviral agents.
Table I.
Clinical Parameters of HIV-1–infected, Statin-treated Patients
| Patient ID | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Female | Male | Male | Male | |
| Age | 53 | 23 | 33 | 24 | 42 | 39 | |
| Virus transmission | Sexual | Sexual | IVDUa | Sexual | IVDU | IVDU | |
| Diagnosis date | 1997 | 2000 | 1996 | ND | 1998 | 1996 | |
| HAART | No | No | No | No | No | No | |
| HCVb coinfection | No | No | Yes | No | Yes | Yes | |
| Other | Ethylism, pancreatitis |
Asthma | Methadone treatment |
No | Methadone treatment |
Methadone treatment |
|
| Viral loadc | Before | 16,800 | 19,500 | 50,100 | 84,000 | 37,300 | 46,400 |
| After | 2,330 | 9,940 | 12,138 | 3,590 | 21,600 | 26,300 | |
| Reboundd | 16,100 | 56,100 | 64,000 | 26,400 | 26,400 | 32,600 | |
| CD4+ (count/ml) | Before | 798 | 520 | 513 | 760 | 465 | 538 |
| After | 940 | 560 | 540 | 1,010 | 487 | 552 | |
| Rebound | 690 | 550 | 501 | 501 | 478 | 560 |
Intravenous drug use.
Hepatitis C virus.
Viral load is expressed as HIV-1 RNA copies/ml.
Measurements after 3 mo without treatment.
Statins may have several immune cell targets (23, 24). We show that statin-induced inhibition of HIV-1 entry and virion production, as well as the increase in viral transcription, is mediated via Mev pathway inhibition. HIV-1 entry and budding are cooperative processes that require protein coaggregation at the host cell surface: CD4 and the chemokine coreceptors for entry, and Gag and gp160 for budding (1). It is suggested that these processes are mediated by protein association with lipid rafts (2–5, 25–27) and driven by the actin cytoskeleton (11–13). Raft clustering entails actin cytoskeleton reorganization, for which Rho might be a key effector (28). Statins can inhibit HIV-1 infection in part by reducing Rho prenylation, essential for Rho localization and function, including the cytoskeletal reorganization required for virus entry and exit. Indeed, although Lov did not affect gp120 binding to CD4, colocalization between gp120 and CXCR4 was drastically reduced in Lov-treated cultures. Mev addition restored gp120-CXCR4 colocalization in Lov-treated cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20040061/DC1). Nonetheless, statins are general inhibitors of protein prenylation. We found that Lov treatment inhibited Ras farnesylation and ∼25% vesicle fusion, a process mediated by prenylated Rab GTPases (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20040061/DC1). Although our results establish a role for Rho in HIV-1 entry, we cannot exclude the involvement of other prenylated proteins.
In summary, we provide evidence that statins prevent HIV-1 infection in cultured primary cells, in animal models, and in chronically infected individuals. We show that at the cellular level, statins inhibit viral entry and budding by preventing Rho geranylgeranylation. Based on the ability of statins to lower viral load in HIV-1–infected individuals, we suggest that these compounds have direct antiretroviral effects and might be appropriate drugs for more accessible treatment of the AIDS pandemic.
Acknowledgments
We would like to thank J. Stein for the critical reading of the manuscript, F. Ortego for help with statistics, L. Gómez for animal handling, and C. Mark for editorial assistance.
S.J. Baranda is the recipient of a pre-doctoral fellowship from the Spanish Programa de Formación de Personal Universitario. This work was supported by grants from the Spanish MEyC, the CSIC, and the Spanish MSyC.
The authors have no conflicting financial interests.
G. del Real and S. Jiménez-Baranda contributed equally to this work.
G. del Real's present address is Centro de Investigación en Sanidad Animal (CISA-INIA), Valdeolmos, E-28130 Madrid, Spain.
References
- 1.Mañes, S., G. del Real, and C. Martínez-A. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557–568. [DOI] [PubMed] [Google Scholar]
- 2.Mañes, S., G. del Real, R. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and C. Martinez-A. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen, D., and J. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono, A., and E. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA. 98:13925–13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Real, G., S. Jiménez-Baranda, R. Lacalle, E. Mira, P. Lucas, C. Gómez-Moutón, A. Carrera, C. Martínez-A., and S. Mañes. 2002. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J. Exp. Med. 196:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popik, W., T. Alce, and W. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 76:4709–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen, D., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121–4126. [DOI] [PubMed] [Google Scholar]
- 8.Guyader, M., E. Kiyokawa, L. Abrami, P. Turelli, and D. Trono. 2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol. 76:10356–10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, S., S. Crowe, and J. Mak. 2002. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS. 16:2253–2261. [DOI] [PubMed] [Google Scholar]
- 10.Zheng, Y., A. Plemenitas, C. Fielding, and B. Peterlin. 2003. Nef increases the synthesis of and transports cholesterol to lipid rafts and HIV-1 progeny virions. Proc. Natl. Acad. Sci. USA. 100:8460–8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyengar, S., J. Hildreth, and D. Schwartz. 1998. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J. Virol. 72:5251–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viard, M., I. Parolini, M. Sargiacomo, K. Fecchi, C. Ramoni, S. Ablan, F. Ruscetti, J. Wang, and R. Blumenthal. 2002. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76:11584–11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steffens, C., and T. Hope. 2003. Localization of CD4 and CCR5 in living cells. J. Virol. 77:4985–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature. 420:629–635. [DOI] [PubMed] [Google Scholar]
- 15.Koch, G., C. Benz, G. Schmidt, C. Olenik, and K. Aktories. 1997. Role of Rho protein in lovastatin-induced breakdown of actin cytoskeleton. J. Pharmacol. Exp. Ther. 283:901–909. [PubMed] [Google Scholar]
- 16.Schwartz, O., J. Virelezier, L. Montagnier, and U. Hazan. 1990. A microtransfection method using the luciferase-encoding reporter gene for the assay of human immunodeficiency virus LTR activity. Gene. 88:197–205. [DOI] [PubMed] [Google Scholar]
- 17.Llaverias, G., M. Jove, M. Vázquez-Carrera, R. Sánchez, C. Díaz, G. Hernández, J. Laguna, and M. Alegret. 2002. Avasimibe and atorvastatin synergistically reduce cholesteryl ester content in THP-1 macrophages. Eur. J. Pharmacol. 451:11–17. [DOI] [PubMed] [Google Scholar]
- 18.Maziere, J., J. Landureau, P. Giral, M. Auclair, L. Fall, A. Lachgar, A. Achour, and D. Zagury. 1994. Lovastatin inhibits HIV-1 expression in H9 human T lymphocytes cultured in cholesterol-poor medium. Biomed. Pharmacother. 48:63–67. [DOI] [PubMed] [Google Scholar]
- 19.del Real, G., M. Llorente, L. Bosca, S. Hortelano, A. Serrano, P. Lucas, L. Gomez, J. Toran, C. Redondo, and C. Martínez-A. 1998. Suppression of HIV-1 infection in linomide-treated SCID-hu-PBL mice. AIDS. 12:865–872. [DOI] [PubMed] [Google Scholar]
- 20.Kam, N., E. Albright, S. Mathur, and F. Field. 1990. Effect of lovastatin on acyl-CoA: cholesterol O-acyltransferase (ACAT) activity and the basolateral-membrane secretion of newly synthesized lipids by CaCo-2 cells. Biochem. J. 272:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicala, C., J. Arthos, S. Selig, G. Dennis, D. Hosack, D. Van Ryk, M. Spangler, T. Steenbeke, P. Khazanie, N. Gupta, et al. 2002. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc. Natl. Acad. Sci. USA. 99:9380–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, L., H. Zhang, P. Solski, M. Hart, C. Der, and L. Su. 2000. Modulation of HIV-1 replication by a novel RhoA effector activity. J. Immunol. 164:5369–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano, M., L. Diomede, M. Sironi, L. Massimiliano, M. Sottocorno, N. Polentarutti, A. Guglielmotti, D. Albani, A. Bruno, P. Fruscella, et al. 2000. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Invest. 80:1095–1100. [DOI] [PubMed] [Google Scholar]
- 24.Kwak, B., F. Mulhaupt, S. Myit, and F. Mach. 2000. Statins as a newly recognized type of immunomodulator. Nat. Med. 6:1399–1402. [DOI] [PubMed] [Google Scholar]
- 25.Wang, J.-K., E. Kiyokawa, E. Verdi, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA. 97:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindwasser, O., and M. Resh. 2001. Multimerization of human immunodeficiency virus tipe 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mañes, S., R. Lacalle, C. Gomez-Mouton, G. del Real, E. Mira, and C. Martinez-A. 2001. Membrane raft microdomains in chemokine receptor function. Semin. Immunol. 13:147–157. [DOI] [PubMed] [Google Scholar]
- 28.Mañes, S., R. Lacalle, C. Gómez-Moutón, and C. Martínez-A. 2003. From rafts to crafts: membrane asymmetry in moving cells. Trends Immunol. 24:320–326. [DOI] [PubMed] [Google Scholar]