Abstract
T cell differentiation in the adult thymus depends on sequential interactions between lymphoid progenitors and stromal cells found in distinct regions of the cortex and medulla. Therefore, migration of T cell progenitors through distinct stromal environments seems to be a crucial process regulating differentiation and homeostasis inside the thymus.
Here we show that CCR7-deficient mice are distinguished by a disturbed thymic architecture, impaired T cell development, and decreased numbers of the thymocytes. Analysis of developing double negative (CD4−CD8−) pool of wild-type thymus reveals that CCR7 expression is restricted to a CD25intCD44+ subpopulation. Correspondingly, CCR7 deficiency results in an accumulation of this population in mutant thymus. Furthermore, immunohistology shows that in CCR7-deficient mice CD25+CD44+ cells accumulate at the cortico-medullary junction, suggesting that CCR7 signaling regulates the migration of early progenitors toward the outer thymic cortex, thereby continuing differentiation. Results obtained from mixed bone marrow chimeras support this view, since the development of CCR7-deficient thymocytes is also disturbed in a morphologically intact thymus. Thus, our findings establish an essential role for CCR7 in intrathymic migration and proper T cell development.
Keywords: chemokines, T cell development, cell migration, thymus, progenitor
Introduction
T cell development in adult mice initiates with the migration of lymphoid progenitors from the bone marrow into the thymus. Early intrathymic progenitor cells lack CD4 and CD8 expression and are referred to as double negative (DN) cells. DN progenitors enter the thymus at the cortico-medullary junction (CMJ) and subsequently migrate to the subcapsular zone (SCZ) (1–3). This migration is accompanied by a progressive differentiation of these progenitors indicating that differentiation-inducing signals locate to distinct cortical regions (4). Based on the expression of the surface molecules CD25 and CD44 on lineage-negative cells, four early differentiation stages (DN1-4) have been defined (5). Differentiation to the DN1 stage (CD25−CD44high) proceeds in proximity to the site of thymic entry (6), whereas the consecutive differentiation of stages DN2 (CD25+CD44high) and DN3 (CD25+CD44low) occur while cells migrate outwards of this region into the mid and outer cortex, respectively. DN3 cells accumulate in the SCZ where they differentiate to DN4 (CD25−CD44−), the pre–double positive (DP; CD4+CD8+) stage of development. Transition from the DN3 to the DN4 stage is accompanied by a reversion of the migration polarity, which finally guides the DP thymocytes across the cortex toward the medulla, although only positive selected cells will actually enter the medulla. Here functional maturation is completed. Finally, mature CD4+ or CD8+ single positive (SP) cells leave the thymus and enter the peripheral circulation (for review see reference 4).
Directional migration of developing cells through distinct thymic microenvironments is essential for proper T cell maturation, since this migration allows immature thymocytes to receive differentiation signals transmitted by different sets of epithelial cells that are located at distinct cortical regions. Interestingly, recent studies have shown that the generation and maintenance of intact cortical microenvironments also requires interaction of the stromal cells with developing thymocytes, establishing a feedback mechanism called “thymic cross talk” (for review see reference 7). Interaction of migrating precursors with stromal cells appears to be constitutive because the latter cells express counter-receptors for progenitor adhesion molecules and serve as a matrix for their migration (8). The molecular mechanisms that govern intrathymic migration are poorly understood. Although several studies suggested that chemokines and their receptors are involved in this process (for review see reference 9), the role of these molecules during T cell maturation in vivo has been only partially elucidated. A recent study has shown that signaling through CXCR4 is crucial for the entry of thymus-homing progenitors into the cortex, and consequently the development of cells lacking CXCR4 is arrested at the DN1 stage (10).
The present study demonstrates that signaling through CCR7 is essential for proper differentiation of DN thymocytes. We found that CCR7 is primarily expressed by a CD25intCD44high transitional DN cell population, herein named DN1-2 cells. Sustained development of DN cells is out of balance in CCR7-deficient mice, as part of the developing cells persists at the DN1-2 stage. Disturbed T cell development correlates with the accumulation of CD25+ CD44+ cells at the CMJ. Concomitantly, CCR7-deficient mice show altered thymic architecture and decreased absolute numbers of thymocytes. Our findings confirm the importance of coordinated intrathymic migration for the maintenance of thymic architecture and proper T cell development.
Materials and Methods
Mice.
The generation of CCR7−/− mice has been described elsewhere (11). CCR7-deficient mice were backcrossed for at least seven generations to the C57BL/6 genetic background. Some experiments were also performed on CCR7-deficient mice on the original 129SV × BALB/c background. plt/plt mice on a C57BL/6 genetic background were provided by Hideki Nakano (Toho University, Tokyo, Japan) (12). All animals were maintained under specific pathogen-free conditions and used at the age of 6 wk–8 mo as indicated in the figure legends. Wild-type C57BL/6 mice were obtained from Charles River.
Flow Cytometry.
Adult mice were killed by CO2 inhalation. Single cell suspensions of thymi were obtained by mincing the organs through a nylon mesh. Samples were washed with PBS supplemented with 3% FCS and counted using a Neubauer chamber. Cells were stained using the following antibodies: anti-CD3, anti-CD4, anti-CD8α, anti-CD25 (Caltag), anti-CD19 (Biosource), anti–TER-119, anti-CD11b, anti-CD11c, anti-CD44 and anti-Ly5G (BD Biosciences), anti-CD117 (Natutec), anti-Ly5.1, and anti-Ly5.2 (Cymbus Biotechnology). In some experiments, lineage-positive cells were stained with a cocktail of antibodies directed against CD3, CD4, CD8, CD19, CD11b, CD11c, Ly5G, and TER-119. To detect the expression of chemokine receptors, cells were stained with antibodies against CCR9 (13), CXCR4 (14) or with a CCL19–hIgG fusion protein followed by anti–human IgG antibody as described previously (15). Four-color flow cytometry was done with a FACS® Calibur (BD Biosciences).
Immunohistology.
After CO2 inhalation, the thymi were excised, rinsed in PBS, embedded in OCT compound, and frozen on dry ice as described (15). 6–10-μm-thin sections were prepared, air dried, and fixed for 15 min in ice-cold acetone. Sections were blocked with 10% mouse or rat serum and stained with antibodies against the indicated markers. In addition to antibodies used for flow cytometry, the following mAb and reagents were used: anti-CCL19, anti-CCL21, anti-CCL25, anti-CXCL12 (R&D Systems), anti–pan-cytokeratin (Sigma-Aldrich), and anti-MTS10 (BD Bioscience). Detection of anti–chemokine antibody binding was enhanced using the Tyramide Amplification system (NEN-DuPont). For systematic analysis of the cellular composition of thymi, composite images were automatically assembled using a motorized Axiovert 200M microscope (Carl Zeiss MicroImagaing, Inc.) and KS300 software (Carl Zeiss MicroImagaing, Inc.) as described (15).
Stable Bone Marrow Chimeras.
Bone marrow was prepared from the humerus and tibea of CCR7-deficient (C57BL/6), wild-type C57BL/6 or CD45.1 congenic mice (C57BL/6-Ly5.1; Charles River) followed by separation on Lympholyte® M (Cedarlane). Recipients for bone marrow transplantation were 6–8-wk-old sex-matched CD45.2 C57BL/6 CCR7-deficient or wild-type mice or CD45.1 congenic mice. Prior to transplantation, recipient mice were lethally irradiated (5.5 Gy) twice at an interval of 5 h. Approximately 1.5 × 107 bone marrow cells were transferred to irradiated recipients. 6–8 wk later, chimeric mice were killed, thymi and blood were harvested, and the degree of chimerism in the thymus was determined.
Results
Chemokine Receptor Expression on Developing Thymocytes.
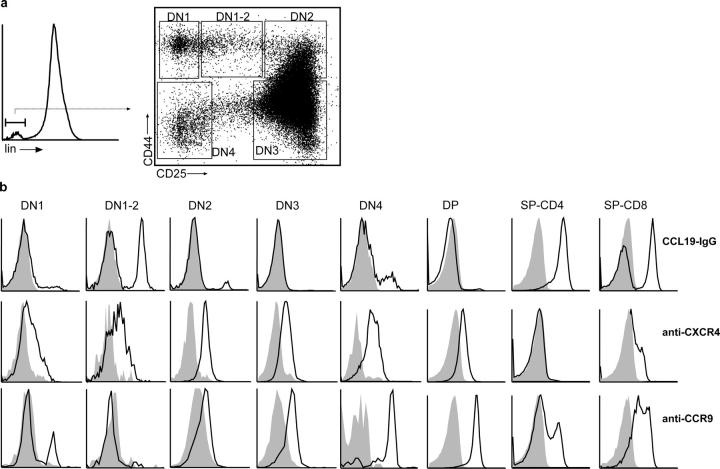
It has been suggested previously that chemokine receptors are differentially expressed during intrathymic T cell development (for review see reference 9). However, the majority of the data supporting this idea is based on either analysis of mRNA expression or on chemotactic properties of sorted thymocyte subpopulations in vitro. Therefore, we investigated the expression of CCR7 and CXCR4 and CCR9 on DN thymocytes of C57BL/6 mice by flow cytometry. For this purpose, expression of CD25 and CD44 in the lineage-negative cell population was determined to mark the DN1 (CD25−CD44high), DN2 (CD25+CD44high), DN3 (CD25+CD44low), and DN4 (CD25−CD44−) subpopulations (Fig. 1 a), and chemokine receptor expression was analyzed on these cells (Fig. 1 b). Consistent with previous reports (10, 16, 17), CXCR4 was expressed by all DN cell populations with elevated expression levels at DN2, DN3, and DN4 stages. In contrast, expression of CCR9 was first observed at the DN2 stage. Although expression of CXCR4 and CCR9 differed in the early developmental stages, their expression profiles resembled each other. Interestingly, expression of CCR7 on DN cells was unique since presence of this receptor was virtually restricted to a CD25intCD44high DN population that we refer to as DN1-2 stage (Fig. 1, a and b). At later stages of thymocyte differentiation, we found CXCR4 to be expressed on DP and few mature CD8+ SP cells, whereas CCR9 was present on all DP and CD8+ SP cells and on some CD4+ SP cells (Fig. 1 b) as described previously (14, 16, 18). In contrast, <1% of the DP cells did express CCR7. In addition, CCR7 was found on all CD4+ SP cells and a substantial proportion of the CD8+ SP cells. All CCR7-expressing SP cells were TCRαβ+ and CD24int or CD24− (not depicted), indicating that CCR7 is expressed on semimature and mature SP thymocytes. Therefore, it seems possible that CCR7 signaling plays a relevant role in the migration of maturing SP thymocytes through the medulla and, as suggested by others, for the egress of recently generated thymocytes (19).
Figure 1.
Differential expression of chemokine receptors during T cell development. (a) Thymocytes of adult C57BL/6 mice (6–8 wk old) were stained with a cocktail of biotinylated antibodies against the linage markers CD4, CD8, CD3, CD11c, CD11b, CD19, Ly5G, and Ter119 followed by Streptavidin-PerCP to allow gating of linage-negative cells (see histogram on the left) and with anti–CD44-FITC and anti–CD25-PE to identify DN populations as indicated. (b) Expression of CCR7 (CCL19-hIgG), CXCR4 (2B11), and CCR9 (7E7) on the DN populations shown in panel a, in DP and SP cells was revealed by goat anti–human-Cy5 (CCR7) or mouse anti–rat-Cy5 (CXCR4, CCR9). The CCL19–hIgG fusion protein did not bind to thymocytes of CCR7-deficient animals (not depicted). Shaded areas indicate binding of isotype controls.
Chemokine Expression in the Thymus.
It has been proposed that the chemokines CCL19 (ELC) and CCL21 (SLC), both ligands for the chemokine receptor CCR7, and CCL25 (TECK), a ligand for CCR9 and CXCL12 (SDF-1), which binds to CXCR4, are expressed in distinct microenvironments within the thymus, thus determining the chemotactic behavior of developing thymocytes (for review see reference 9). To confirm this hypothesis, we carefully examined the chemokine expression profile in the thymus of C57BL6 mice by three-color immunofluorescence microscopy.
This analysis revealed that although CCL19, CCL21, CCL25, and CXCL12 have distinct expression patterns, they are often expressed in overlapping regions of the thymus (Fig. 2). Expression of CCL19 and CCL21 was detected in the medulla, in the CMJ and adjacent areas, and in several cells scattering throughout the cortex. Interestingly, CCL21-positive cells in the cortex frequently did not stain for cytokeratins, and their morphology resembled DCs and macrophages (Fig. 2 a, arrows), whereas CCL19 expression was widely associated with blood vessels (Fig. 2 a, arrows). As expected, neither chemokine could be detected in thymi of the spontaneous mutant mouse strain, plt/plt (paucity of lymph node T cells), which lack expression of both CCL19 and the lymphoid tissue form of CCL21, CCL21-ser (20, 21) (Fig. 2 b). The expression of CCL25 was more ubiquitous, since medullary (MTS10+) and cortical (cytokeratin+) cells abundantly expressed this chemokine (Fig. 2 a and not depicted). In contrast to in situ hybridization data (10, 16), CXCL12 protein was predominantly detected in the medulla and CMJ but only in some cells of the cortex (Fig. 2 a). The discrepancy observed between immunofluorescence and in situ hybridization data could be due to the differential sensitivity of the applied methods and/or attributed to the detection of secreted chemokines bound to and enriched on nonproducing cells, a phenomenon reported for several chemokines including CCL25 and CCL19 (22, 23).
Figure 2.
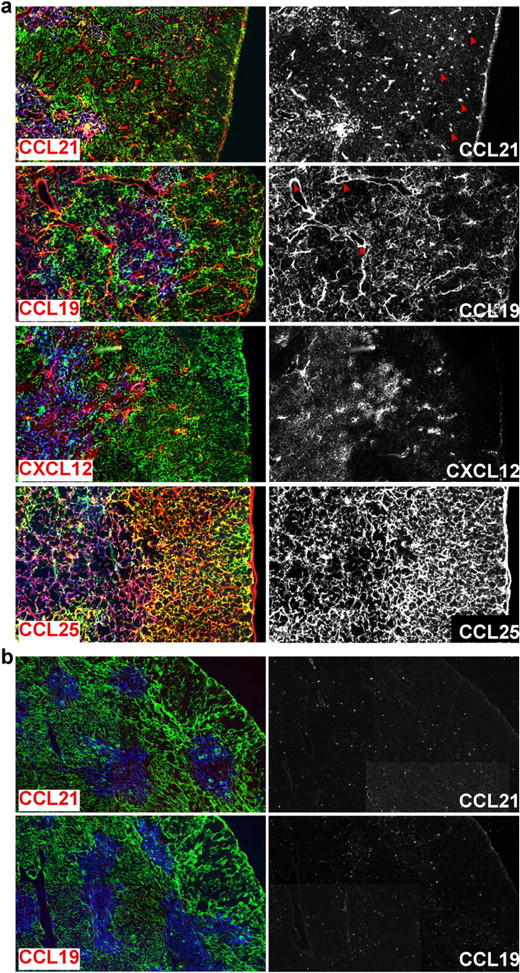
Localization of chemokine expression in the thymus of adult C57BL/6 mice. Cryosections from adult thymus of 6–8-wk-old C57BL/6 (a) or plt/plt (b) mice were stained with antibodies to cortical (pan-cytokeratin; clone C11; green) and medullary (MTS10; blue) thymic epithelial cells and to the chemokines CCL19, CCL21, CXCL12, and CCL25 as indicated (red, left). Chemokine staining was amplified using the Tyramide system. To facilitate visualization of chemokine expression, chemokine staining alone is also shown in the right panel. CCL19, CCL21, and CXCL12 expression was found in the medulla and on scattered cells in the cortex, whereas CCL25 was abundantly expressed in the whole thymus. plt/plt mice fail to express CCL19 and CCL21.
Altered Thymic Architecture in CCR7-deficient Mice.
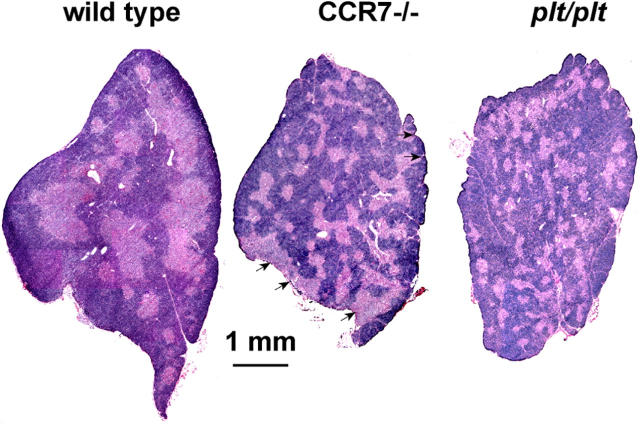
Since CCR7 expression was found at a very early developmental stage (DN1-2), we speculated that lack of CCR7 expression might interfere with the migration of early progenitors into the mid cortex leading to interruption or delay of the subsequent maturation process. Transition of DN1 to DN2 stage is known to represent an important checkpoint in the development of the cortical epithelium influencing thymic architecture (for review see reference 7). Therefore, it seemed possible that lack of CCR7 signaling might result in altered thymic morphology. To test this hypothesis, we performed a histological analysis of thymi from CCR7-deficient and plt/plt mice. Hematoxylin and eosin staining revealed strong morphological changes in the thymus of both CCR7-deficient and plt/plt mice compared with wild-type mice (Fig. 3). Although the cortex was still distinguishable from the medulla in both strains, the medullary areas in mutant thymi were smaller and more numerous than in wild-type mice. Furthermore, medullary areas were sometimes misplaced to the outer rim of the organ (Fig. 3, arrowheads). These morphological alterations were even more pronounced in thymi from aged mice (not depicted). These results indicate that CCR7 signaling is necessary for maintenance of proper thymic morphology.
Figure 3.
Altered thymus architecture in CCR7-deficient and plt/plt mice. Hematoxylin and eosin staining from representative sections of adult thymi of wild-type (left), CCR7-deficient (middle), and plt/plt (right) animals (6–8 wk old) are shown. Compared to wild-type animals, thymi of CCR7-deficient mice shown numerous small medullary areas distributed through the whole cortex. Note that medulla is even found in the subcapsular zone (arrows). The thymic morphology of plt/plt mice is very similar to that of CCR7-deficient animals.
Abnormal T Cell Development in CCR7-deficient Mice.
To investigate the functional consequences of lack of CCR7 signaling for T cell development, thymus abnormalities of CCR7-deficient mice were further characterized. The altered thymic architecture observed in these animals was accompanied by reduced thymic cellularity (Fig. 4). The absolute numbers of thymocytes in 6–8-wk-old CCR7-deficient mice are significantly lower than in age-matched control mice (1.1 ± 0.3 × 108 cells in mutants versus 2.1 ± 0.5 × 108 cells in wild type; mean ± SD, P < 0.01) (Fig. 4 a). This difference became even more pronounced when analyzing mice at the age of 6–8 mo (0.4 ± 0.2 × 108 cells in mutants versus 1.3 ± 0.3 × 108 cells in wild type; mean ± SD, P < 0.001). Despite of the reduced cellularity, T cell development in young CCR7−/− mice appears to proceed normally to some degree, since DP and mature SP cells were present at normal ratios in the mutant thymi (Fig. 4, b and c, and Fig. 5 d). In contrast, the majority of old animals analyzed showed a significant reduction of DP cells compared with age-matched wild-type controls (39% ± 29% in mutants versus 73.3% ± 3.5% in wild-type mice) (Fig. 4 b). Furthermore, in 30% of animals examined the reduction was strongly accentuated, resulting in an almost complete lack of DP cells (Fig. 4 b and Fig. 5, g and h). The proportion of mature SP thymocytes, however, increased slightly in old CCR7-deficient mice probably due to the reduced numbers of DP cells (Fig. 4 b and Fig. 5, g and h).
Figure 4.
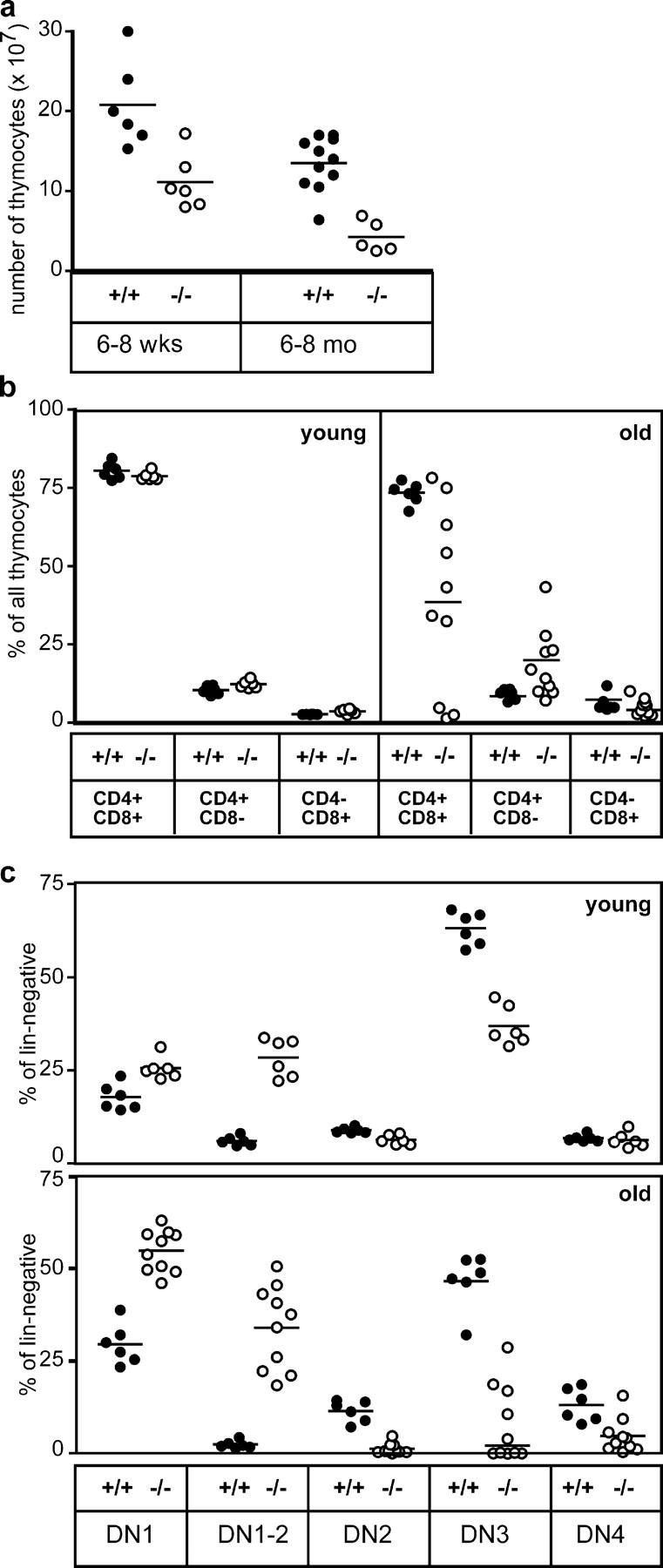
Reduced cellularity and impaired thymocyte development in CCR7-deficient mice. (a) Absolute numbers of thymocytes in young (6–8 wk old) and old (6–8 mo old) CCR7-deficient mice are reduced in comparison to age-matched controls. (b) Relative distributions of DP (CD4+CD8+) and SP (CD4+ and CD8+) cells in the thymus of young and old wild-type and mutant mice. Proportions of DP cells decreased with age in the majority of CCR7-deficient animals analyzed. (c) Proportions of cells belonging to distinct DN stages among the total DN population in the thymus of wild-type and mutant mice. Increased proportions of DN1 and DN1-2 cells and decreased proportions of DN3 cells were observed in young CCR7-deficient animals in comparison to age-matched controls. In older animals, the proportions of DN1 and DN1-2 cells increased further, whereas the proportions of DN2, DN3, and DN4 cells were markedly reduced in comparison to young animals. Note that ∼50% of the old animals analyzed have virtually no DN3 cells (c). Each dot relates to data obtained from a single animal; bars indicate means. Data for old CCR7-deficient mice shown in panels b and c were derived from each five animals of C57BL/6 and a mixed BALB/c × 129Sv/Ev genetic background. No differences were observed in the variation of the data obtained from mice of both genetic backgrounds (not depicted).
Figure 5.
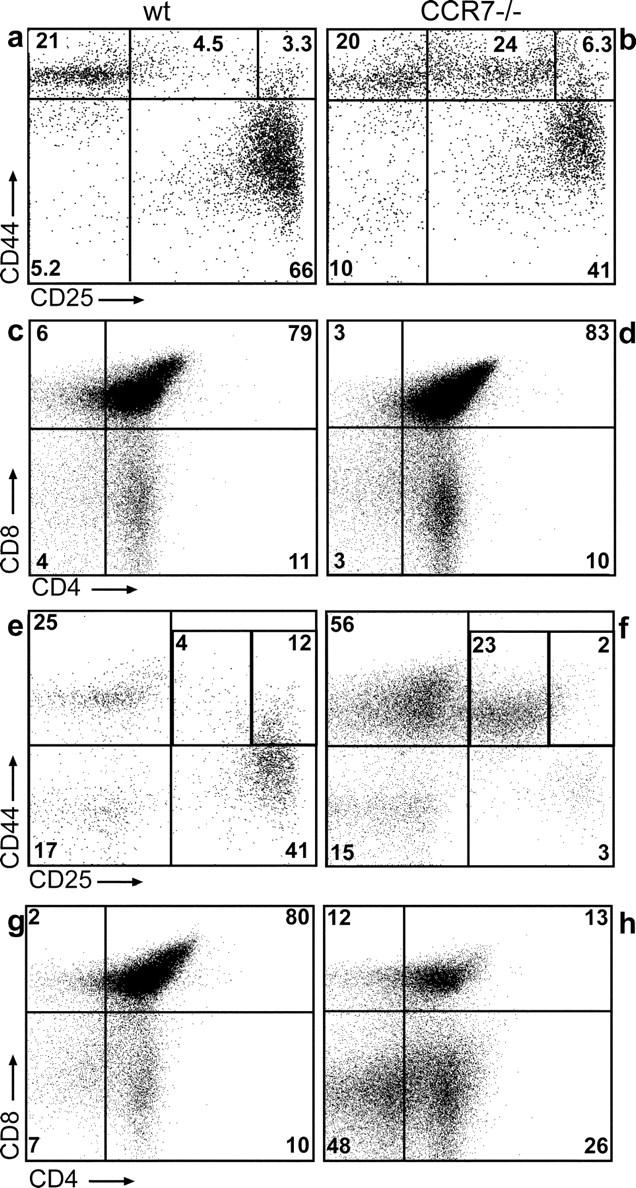
Accumulation of DN1-2 cells in CCR7-deficient animals. Thymocytes of young (6–8 wk; a–d) and old (6–8 mo; e–h) wild-type (a, c, e, and g) and mutant mice (b, d, f, and h) were stained as described for Fig. 1 a. Young mutants (b) have increased frequencies of DN1-2 cells compared with wild types (a), whereas no obvious difference regarding the distribution of SP and DP thymocytes could be observed (compare c and d) between both strains. Old mutants perform an aggravated phenotype (e and f) also showing decreased numbers of DP thymocytes (g and h). Numbers indicate the percentage of lin− cells within the corresponding gates for a, b, e, and f.
Altered thymic architecture and reduced cellularity in CCR7-deficient young mice correlated with an increased proportion of DN1-2 cells in these animals (28.4% ± 5.2% in CCR7−/− versus 6.0% ± 1.2% in wild-type mice) and decreased proportions of DN3 cells (36.9% ± 5.3 in mutants versus 63.2% ± 4.4% in controls; mean ± SD, n = 6), indicating that at least part of the developing cells arrested at the DN1-2 developmental stage or alternatively that transition from DN1 stage to DN2 is delayed (Fig. 4 c and Fig. 5, a and b). This phenotype aggravated with age leading to a strong accumulation of cells at the DN1 and DN1-2 stage, whereas the proportion of DN2, DN3, and DN4 cells was severely reduced in CCR7-deficient animals at the age of 6–8 mo compared with age-matched wild-type animals (Fig. 4 c and Fig. 5, e and f). The mean values observed for these cell populations were: DN1, 29.5 ± 5.5%; DN1-2, 2.4 ± 1.0%; DN2, 11.5 ± 2.9%; DN3, 46.6 ± 7.5% and DN4, 13.1 ± 4.5% in wild-type mice and DN1, 54.8 ± 5.7%; DN1-2, 34.0 ± 11.3%; DN2, 1.3 ± 1.4%; DN3, 7.9 ± 3.2% and DN4, 4.7 ± 4.6% in mutants (mean ± SD; wild type, n = 6; mutants, n = 10).
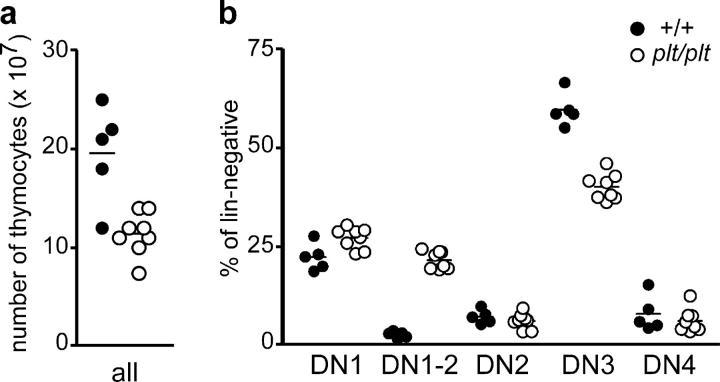
Similar to CCR7-deficient animals plt/plt mice have lower numbers of thymocytes than wild-type mice (1.1 ± 0.2 × 108 cells in plt/plt mice versus 2.0 ± 0.5 × 108 cells in controls) (Fig. 6 a). Furthermore, plt/plt mice also possess increased proportions of DN1-2 cells and decreased proportions of DN3 cells compared with wild-type animals: (DN1-2, 21.5 ± 2.3% versus 2.7 ± 0.8%; DN3, 40.1 ± 3.4% versus 59.6 ± 4.2%; mean ± SD; wild type, n = 5; plt/plt, n = 8) (Fig. 6 b). Together these results demonstrate that lack of CCR7 signaling is detrimental for proper differentiation of intrathymic progenitors. These data also suggest that lack of CCR7 can be partially compensated by yet unknown mechanisms, in particular in young animals, since CCR7-deficient and plt/plt mice are still capable of generating normal T cells in vivo although at significantly less numbers.
Figure 6.
Reduced cellularity and impaired thymocyte development in plt/plt mice. (a) Similar to CCR7-deficient animals, absolute numbers of thymocytes in young (6–8 wk old) plt/plt mice are significantly reduced in comparison to age-matched controls (P < 0.05). (b) Proportions of cells belonging to distinct DN stages among the total DN population in the thymus of young plt/plt mice. Increased proportions of DN1 (P < 0.05) and DN1-2 (P < 0.0001) cells and decreased proportions of DN3 cells (P < 0.0001) were observed in young plt/plt animals in comparison to age-matched controls.
Increased Proportions of DN1-2 Cells in CCR7-deficient Animals Correlate with Accumulation of CD25+CD44+ Cells at the CMJ.
Previous papers have shown that differentiation stages of DN cells occur at distinct regions of the thymus (for review see reference 4). Whereas cells at the DN1 stage are abundantly present in the proximity of the CMJ but rare in the SCZ, DN3 thymocytes are virtually absent from the CMJ but more frequently found in the outer third of the cortex accumulating at the SCZ (2, 3). To determine the microanatomic location of DN cells in the absence of CCL19/CCL21-mediated signals, thymus sections of CCR7-deficient and wild-type mice were stained with antibodies direct against CD25 and CD44. As expected, in wild-type mice CD25+CD44− (DN3) cells were abundantly found at the outer cortex and SCZ, whereas only few CD25+CD44+ (DN1-2 and DN2) cells were close to the CMJ (Fig. 7 a, arrows), reflecting regular development and distribution of DN cells. In contrast, in thymi of CCR7-deficient mice CD25+CD44− (DN3) cells were less abundant in the outer cortex and SCZ than in control mice. Importantly, a marked accumulation of CD25+CD44+ (DN1-2 and DN2) cells was observed at the CMJ (Fig. 7 b, arrows). A similar accumulation of CD25+CD44+ cells was also observed in thymus sections of plt/plt mice (not depicted). Together with the data obtained by flow cytometry these results suggest that the DN1-2 population get stuck at the CMJ due to their inability to migrate out of the peri-medullary cortex. Therefore, CCR7 signaling seems to be essential for efficient migration of DN1-2 progenitors into regions of the cortex where they continue to differentiate.
Figure 7.
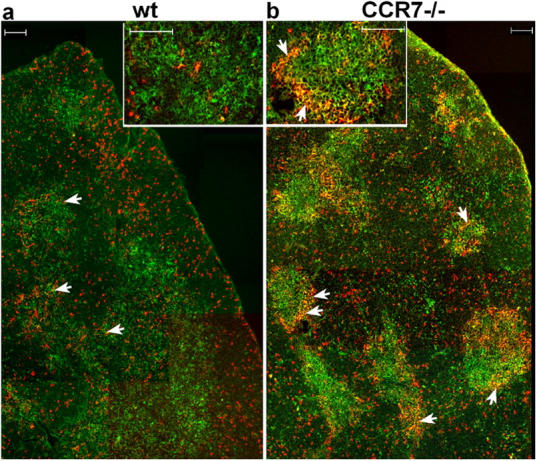
CD25+CD44+ cells accumulate at the CMJ of adult CCR7-deficient animals. Cryosections of thymus from 6–8-wk-old C57BL/6 wild-type and mutant mice were stained with antibodies to CD25 (red) and CD44 (green). DP cells accumulated at the CMJ from CCR7-deficient mice and appear in yellow (arrows). Inserts show higher magnification of CMJs. (Bars, 100 μm).
The Thymic Phenotype of CCR7-deficient Mice Can Be Induced in Bone Marrow Chimeras.
Data presented so far suggest that both normal thymus architecture and proper DN cell development depend on the expression of CCR7 on developing progenitors. To exclude the possibility that the phenotype observed in CCR7-deficient thymi is due to a lack of CCR7 expression on thymic stromal cells, we generated bone marrow chimeras. Chimeric animals were generated by transplanting bone marrow from CCR7-deficient mice into lethally irradiated CD45.1 congenic wild-type mice and bone marrow of CD45.1 wild-type mice into congenic CCR7-deficient or into congenic C57BL/6 wild-type recipients. 2 mo later, three to four thymi per group were analyzed by flow cytometry and immunohistology. In all cases, the degree of donor chimerism in the recipient thymi was >98% at the time of analysis (not depicted). Similar to CCR7-deficient mice, wild-type recipients reconstituted with CCR7−/− bone marrow showed reduced numbers of thymocytes compared with wild-type and CCR7−/− recipients reconstituted with wild-type bone marrow (0.3 ± 0.2 × 108 versus 1.1 ± 0.1 × 108 versus 1.0 ± 0.1 × 108, respectively; n = 3). In all recipients that received bone marrow of congenic wild-type donors, thymic DN cell development was normal and immunohistology was similar to that observed in wild-type mice (Fig. 8 a). In contrast, wild-type mice that received bone marrow of CCR7-deficient donors showed abnormal DN cell development and accumulation of CD25+CD44+ cells at the CMJ, similar to the phenotype observed in CCR7-deficient mice (Fig. 8 a compared with Fig. 7). Under these experimental conditions, CD4SP, CD8SP, and CD4CD8DP cells from CCR7-deficient donors showed no obvious alterations compared with wild-type donors (Fig. 8 a). The thymic morphology of wild-type recipient that received CCR7-deficient bone marrow showed considerable alterations since multiple “medullary islets” developed in the former cortical region of this organ. In addition, the thymic architecture of the mutant mice can be rescued by the transplantation of wild-type bone marrow (not depicted). These results demonstrate that the expression of CCR7 on hematopoietic progenitors but not on thymic stromal cells is relevant for maintenance of thymic morphology and normal DN cell development. Therefore, it seems likely that impaired DN cell development is caused by the inability of DN1-2 progenitors to migrate into the mid cortex. However, since wild-type mice that received bone marrow from CCR7-deficient animals showed altered thymic morphology, the data presented so far cannot exclude the possibility that lack of CCR7 results primarily in alterations of the thymic morphology, which then as a secondary effect impairs the development of DN cells. To address this possibility, we analyzed the differentiation of CCR7-deficient DN cells in a morphologically unaltered thymus. To obtain chimeric animals with a low degree of CCR7-deficient background, CD45.2 CCR7-deficient bone marrow was mixed with CD45.1 congenic wild-type bone marrow at a ratio from ∼1:10 to 1:40 and transplanted into lethally irradiated CD45.1 mice. 2 mo later, thymi of seven animals were analyzed by flow cytometry. At the time of analysis on average 2.5% (range, 1.4–4.6%) of the DN cells in the recipient thymi were of CD45.2-CCR7–deficient origin, resulting in unaltered thymic morphology (not depicted). Under these experimental conditions, increased proportions of DN1, DN1-2, and DN2 cells and decreased proportions of DN3 cells were observed for CCR7-deficient compared with wild-type cells (Fig. 8 b). These results demonstrate that expression of CCR7 on early intrathymic progenitors is needed for unimpaired T cell development supporting strongly the hypothesis that CCR7 is intrinsically involved in migration and positioning of progenitors within the thymus.
Figure 8.
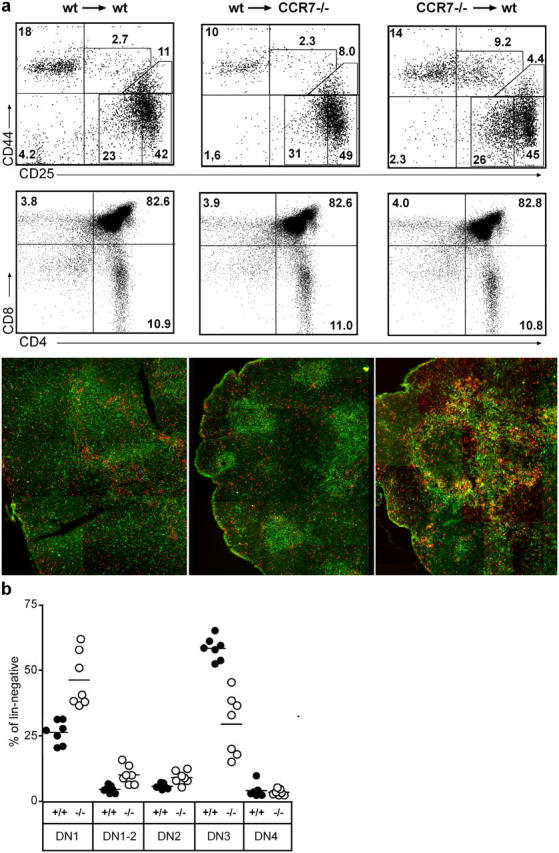
Requirement for CCR7 for proper T cell differentiation. Stable bone marrow chimeras were prepared, as described in Materials and Methods, resulting in donor chimerism >98% (a) or <5% (b). The phenotype of chimeric thymi was analyzed 2 mo later by flow cytometry and immunohistology (red, CD25; green, CD44) using mAbs as indicated. (a) Transfer of wild-type bone marrow in irradiated congenic wild-type recipients resulted in a phenotype similar to that observed in wild types (left). CCR7 mutant mice that received bone marrow of congenic wild-type mice also developed a thymus phenotype similar to that of wild-type mice (middle), whereas the thymic phenotype of CCR7-deficient was induced in wild-type recipients after transfer of congenic mutant bone marrow (right). Data shown are representative for three to four mice of each group. The numbers shown in the top panels indicate the percentage of lin− cells within the corresponding gates, whereas the numbers in the middle panels refer to the percentage of all thymocytes. (b) Bone marrow of wild-type (CD45.1) and CCR7-deficient (CD45.2) donors was mixed and transferred to irradiated wild-type recipients. Low levels of mutant donor chimerism in the thymus of wild-type recipients confirms impaired development of CCR7-deficient DN cells compared with that of wild-type DN cells isolated from the same organs.
Discussion
T cell development in the postnatal thymus depends on the interaction of developing thymocytes with epithelial and stromal cells. Migration of progenitor cells through distinct zones of the thymus enables these interactions (for review see reference 4). It has been shown previously that the movement of progenitors within the thymus is a coordinated event and that the localization of developing thymocytes in distinct regions of the thymus correlates with their stage of development (2, 3).
A role for chemokines and chemokine receptors in coordinating the migration process has been repeatedly suggested, but so far there is a considerable lack of experimental evidence to support this idea. Several studies have demonstrated that CCR9 and its ligand CCL25 play only minor roles in postnatal T cell development (24, 25). The participation of CXCR4 and CXCL12 was discussed more controversially because CXCR4- and CXCL12-deficient mice die before or right after birth, thus hampering studies addressing the role of CXCR4/CXCL12 signaling directly in conventionally gene-targeted mice. Although no significant defects in T cell development have been reported in embryos of either strain, adult irradiated wild-type recipients reconstituted with fetal liver or bone marrow cells from CXCR4-deficient mice showed reduced numbers of mature donor-derived thymocytes, indicating a possible involvement of CXCR4 in T cell development (17, 26–30). More recently, this supposition was confirmed by using chimeric mice reconstituted with adult bone marrow cells of lck[Cre]/CXCR4loxP/loxP mice, which become deficient for CXCR4 after entry in the thymus. This study showed that CXCR4 signaling is required to enable the migration of DN1 progenitors outwards of the CMJ and that any cells lacking CXCR4 are unable to differentiate past the DN1 stage (10).
In the present study, we demonstrate expression of the chemokine receptors CCR9, CXCR4, and CCR7 at distinct stages of thymocyte differentiation. Additionally, immunohistological analyses revealed that the ligands for these receptors are expressed in distinct but frequently overlapping regions of the thymus. CCL25 is abundantly expressed in the cortex and medulla, whereas CXCL12 is found in the medulla including the CMJ and few cortical cells. Of interest, CCL19 and CCL21 are mostly expressed in the medulla but also in cortical areas adjacent to the CMJ and by several cells scattered throughout the cortex. The latter cells might be the source for those chemokines, which direct the migration of developing cells into the mid cortex. Of interest, all chemokines analyzed here were found in both medulla and cortex, indicating that the time-regulated expression of chemokine receptors on developing cells is the key regulator of intrathymic migration. It seems also reasonable that early DN progenitors express more than one chemokine receptor and that the sum of received signals might help to determine the migration direction. A similar well-balanced response of B cells to the chemokines CXCL13 (expressed in B cell follicles) versus CCL19/CCL21 (expressed in T cell area) could be attributed to differential expression of CCR7 (31). Together these results indicate that not only the differential expression of chemokines in distinct thymic microenvironments, but in particular, the temporary and stage-specific, regulated expression of chemokine receptors on thymocytes themselves might coordinate their directional migration within the thymus.
Early studies have shown that CCR7 is involved in the migration of hematopoietic precursors in the parathyroid and thymic anlagen of mouse embryos and in the emigration of newly generated T cells from newborn thymus (19, 32). However, the role of CCR7 in intrathymic migration and T cell development has not been addressed in these studies. More recently, a role for CCR7 in the migration of DP cells into the medulla has been implicated by using transgenic mice overexpressing CCR7 at the DP stage (33). However, since only a minute proportion (<1%) of DP cells express CCR7 in wild-type mice the physiological role of CCR7 in this process remains obscure. Since CCR7 is also expressed on a considerable proportion of SP thymocytes, it seems possible that CCR7 signaling plays a relevant role in guiding maturing SP cells through the medulla.
Data presented here demonstrate that CCR7 plays an important role in intrathymic differentiation and maintenance of thymic homeostasis, since CCR7 deficiency results in decreased thymic cellularity and altered thymic architecture. It is of note that the former observation disagrees with the data of Ueno et al. (19), which reported increased numbers of thymocytes in adult CCR7-deficient animals. The reasons for this discrepancy are not known, but it is unlikely due to differences in the genetic background of the mice analyzed in our work (C57BL/6) and that of Ueno et al. (19) (mixed 129S6/SvEv and BALB/c), since we obtained similar results from mice of the mixed 129S6/SvEv × BALB/c background (not depicted) as we have shown here for C57BL/6 mice.
Interestingly, analysis of DN populations revealed that CCR7 protein expression was virtually restricted to a CD25intCD44+ post-DN1 population. Since these cells most probably represent a transitional stage of differentiation between the DN1 and DN2, we termed this stage DN1-2. Lack of CCR7 partially blocks or delays the transition from DN1 to DN2, thus inducing an accumulation of DN1-2 cells in the thymus of CCR7-deficient mice.
It should be noted that it is currently unclear to what extent DN1-2 cells contribute to the pool of developing T cells. Although the lineage potential of various DN progenitor populations remains a unsolved issue (34), it is largely accepted that T cell progenitors are c-kit+. Since more than two third of the CCR7+ DN1-2 population express c-kit (not depicted), it is likely that at least part of these population give rise to T cells. Furthermore, lack of CCR7 results in reduction of DN3 cells, which are currently considered to be committed to the T cell lineage, further implicating a direct contribution for CCR7+ progenitors in T cell development.
Lack of CXCR4 is apparently more deleterious for T cell development than lack of CCR7, since CXCR4-deficient progenitors in chimeric animals completely arrest at the DN1 stage (10), whereas T cell development was only partially impaired in CCR7-deficient and chimeric animals, which received bone marrow of CCR7−/− mice. Because the DN1-2 population described here also expresses low levels of CXCR4, it is tempting to speculate that signaling through CXCR4 partially compensates for the absence of CCR7 signaling, rescuing part of the DN1-2 population from CCR7-deficient mice. This mechanism could explain why a part of the CCR7-deficient cells develops normally.
Immunohistological analysis of thymi of CCR7-deficient and chimeric mice indicates that accumulation of DN1-2 cells correlates with the inability of these cells to leave the CMJ. Although it is likely that the altered thymic morphology of these animals prevents per se the migration of DN1-2 progenitors into the cortex, the analysis of mixed chimeric animals demonstrates clearly that CCR7-deficient progenitors are unable to differentiate normally, even in a morphologically unaltered thymus, suggesting that CCR7 participates intrinsically in the migration and positioning of progenitors in the thymic cortex. Together the data provided by Plotkin et al. (10) and the observations of this work indicate that the successful transition and the concomitant migration of early progenitors require signaling through both CXCR4 and CCR7.
Alterations of thymic architecture associated with thymocyte developmental arrest have been observed earlier, including hCD3ɛ26 transgenic and Rag2-deficient mice. Transgenic hCD3ɛ26 mice show a profound developmental arrest at the DN1 stage that correlates with severe abnormalities in cortex organization and a poor demarcation of cortical and medullary areas. In contrast, Rag2-deficient mice with a later arrest at the DN3 stage have a well-developed cortex but possess a disorganized medulla, indicating that cortex organization requires interactions of epithelial cells with thymocytes that have gone past the DN1 stage yet not passed the DN3 stage (35, 36). Although CCR7-deficient mice show alterations at an early developmental stage, their morphologic phenotype is less severe than that observed in hCD3ɛ26 mice, most likely due to the fact that part of the CCR7-deficient progenitors are still able to settle the cortex, allowing to some degree interactions between thymocytes and epithelial cells. These interactions are, however, insufficient to assure normal thymus morphology.
A further intriguing finding of our work is the observation that the phenotype of CCR7-deficient mice aggravates with age. It has been shown recently that recruitment of T cell progenitors from the blood into the postnatal thymus is a gated event occurring with a periodicity of ∼4 wk (37). Based on this observation, it can be expected that thymocytes developing during the first 4 wk of life are derived from fetal progenitors. However, fetal progenitors might have other migratory requirements than postnatal progenitors, since fetal progenitors enter the thymus at a time point where the organ is neither structured nor vascularized extensively (38). After this refractory period of ∼4 wk, new progenitors would be imported into a now structured organ, where CCR7 expression becomes more crucial for enabling migration of DN1-2 progenitors outwards of the peri-medullary region and therefore for proper differentiation of DN1 to DN2 cells. Additionally, a recent kinetic study has shown that DN cells spend a period of 9–10 d at the DN1 stage in a very restricted region near the sites of thymic entry (3). Therefore, functional consequences of lack of CCR7 expression on maturing thymocytes would become more evident in mice older than 5–6 wk.
It has been shown previously that cross talk between developing thymocytes and epithelial cells is not essential for the initial patterning of the fetal thymic rudiment (which also supports T cell commitment), but is indispensable to maintain organization of the adult thymus (36, 39–41). Therefore, lack of CCR7 expression on adult DN1-2 thymocytes might eventually result in progressive lost of thymic organization, which could explain the aggravated phenotype of older mice. Alternatively, it has been suggested that CCR9 expression is reduced in thymocytes of adult compared with newborn mice and that the response of thymocytes to CCL25 decreases with age (18). Therefore, it seems also possible that decreasing compensatory responses to chemokines such as CCL25 in aged mice could contribute indirectly to the more severe phenotype observed in older CCR7-deficient mice.
Although the inefficiency of CCR7-deficient cells to migrate into the cortex might cause the phenotype observed in mutant mice, we cannot exclude the involvement of other functions of CCR7 signaling than migration contributing to the phenotype described here. Interestingly, mice deficient for Gfi1, a transcriptional repressor expressed in T cell precursors, show a phenotype similar to that of CCR7-deficient mice, since lack of Gfi1 expression also results in accumulation of C25intCD44+ cells and decreased thymic cellularity. The phenotype of Gfi1-deficient mice was associated with increased apoptosis of DN1 and DN2 cells, and consequently Gfi1 was thought to be involved in survival and proliferation of early T cell progenitors (42). Since a role for G protein–coupled receptor signaling, including chemokine receptors, in inhibition of apoptosis and induction of cell survival and proliferation has been demonstrated (for review see reference 43), it would be possible that CCR7 is directly involved in the transmission of survival signals to developing thymocytes. In summary, our findings identify a new function of CCR7 in regulating the migration of thymic progenitors assuring proper differentiation of thymocytes in adult mice.
Acknowledgments
We would like to thank Eva Stüwe for excellent technical assistance, Günter Bernhardt for valuable suggestions on the article, Martin Lipp (Max-Delbrück-Center for Molecular Medicine, Berlin, Germany) for providing CCR7-deficient animals on a mixed 129Sv × BALB/c background, and Hideki Nakano (Toho University, Tokyo, Japan) for providing plt/plt mice.
This work was supported by Deutsche Forschungsgemeinschaft grants Fo334/1-1 and SFB621/A1 to R. Förster.
The authors have no conflicting financial interests.
A. Misslitz and O. Pabst contributed equally to this work.
Abbreviations used in this paper: CMJ, cortico-medullary junction; DN, double negative; DP, double positive; SCZ, subcapsular zone; SP, single positive.
References
- 1.Ceredig, R., and M. Schreyer. 1984. Immunohistochemical localization of host and donor-derived cells in the regenerating thymus of radiation bone marrow chimeras. Thymus. 6:15–26. [PubMed] [Google Scholar]
- 2.Lind, E.F., S.E. Prockop, H.E. Porritt, and H.T. Petrie. 2001. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J. Exp. Med. 194:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porritt, H.E., K. Gordon, and H.T. Petrie. 2003. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J. Exp. Med. 198:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrie, H.T. 2003. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat. Rev. Immunol. 3:859–866. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey, D.I., J. Kennedy, T. Suda, and A. Zlotnik. 1993. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 150:4244–4252. [PubMed] [Google Scholar]
- 6.Dunon, D., D. Courtois, O. Vainio, A. Six, C.H. Chen, M.D. Cooper, J.P. Dangy, and B.A. Imhof. 1997. Ontogeny of the immune system: gamma/delta and alpha/beta T cells migrate from thymus to the periphery in alternating waves. J. Exp. Med. 186:977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson, G., and E.J. Jenkinson. 2001. Lymphostromal interactions in thymic development and function. Nat. Rev. Immunol. 1:31–40. [DOI] [PubMed] [Google Scholar]
- 8.Prockop, S.E., S. Palencia, C.M. Ryan, K. Gordon, D. Gray, and H.T. Petrie. 2002. Stromal cells provide the matrix for migration of early lymphoid progenitors through the thymic cortex. J. Immunol. 169:4354–4361. [DOI] [PubMed] [Google Scholar]
- 9.Savino, W., D.A. Mendes-da-Cruz, J.S. Silva, M. Dardenne, and V. Cotta-de-Almeida. 2002. Intrathymic T-cell migration: a combinatorial interplay of extracellular matrix and chemokines? Trends Immunol. 23:305–313. [DOI] [PubMed] [Google Scholar]
- 10.Plotkin, J., S.E. Prockop, A. Lepique, and H.T. Petrie. 2003. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J. Immunol. 171:4521–4527. [DOI] [PubMed] [Google Scholar]
- 11.Förster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 12.Nakano, H., S. Mori, H. Yonekawa, H. Nariuchi, A. Matsuzawa, and T. Kakiuchi. 1998. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 91:2886–2895. [PubMed] [Google Scholar]
- 13.Pabst, O., L. Ohl, M. Wendland, M.A. Wurbel, E. Kremmer, B. Malissen, and R. Förster. 2004. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J. Exp. Med. 199:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schabath, R., G. Muller, A. Schubel, E. Kremmer, M. Lipp, and R. Förster. 1999. The murine chemokine receptor CXCR4 is tightly regulated during T cell development and activation. J. Leukoc. Biol. 66:996–1004. [DOI] [PubMed] [Google Scholar]
- 15.Ohl, L., G. Henning, S. Krautwald, M. Lipp, S. Hardtke, G. Bernhardt, O. Pabst, and R. Förster. 2003. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 197:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki, G., H. Sawa, Y. Kobayashi, Y. Nakata, K. Nakagawa, A. Uzawa, H. Sakiyama, S. Kakinuma, K. Iwabuchi, and K. Nagashima. 1999. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J. Immunol. 162:5981–5985. [PubMed] [Google Scholar]
- 17.Ara, T., M. Itoi, K. Kawabata, T. Egawa, K. Tokoyoda, T. Sugiyama, N. Fujii, T. Amagai, and T. Nagasawa. 2003. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J. Immunol. 170:4649–4655. [DOI] [PubMed] [Google Scholar]
- 18.Carramolino, L., A. Zaballos, L. Kremer, R. Villares, P. Martin, C. Ardavin, A.C. Martinez, and G. Marquez. 2001. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood. 97:850–857. [DOI] [PubMed] [Google Scholar]
- 19.Ueno, T., K. Hara, M.S. Willis, M.A. Malin, U.E. Hopken, D.H. Gray, K. Matsushima, M. Lipp, T.A. Springer, R.L. Boyd, et al. 2002. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 16:205–218. [DOI] [PubMed] [Google Scholar]
- 20.Vassileva, G., H. Soto, A. Zlotnik, H. Nakano, T. Kakiuchi, J.A. Hedrick, and S.A. Lira. 1999. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 190:1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luther, S.A., H.L. Tang, P.L. Hyman, A.G. Farr, and J.G. Cyster. 2000. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA. 97:12694–12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurbel, M.A., J.M. Philippe, C. Nguyen, G. Victorero, T. Freeman, P. Wooding, A. Miazek, M.G. Mattei, M. Malissen, B.R. Jordan, et al. 2000. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30:262–271. [DOI] [PubMed] [Google Scholar]
- 23.Baekkevold, E.S., T. Yamanaka, R.T. Palframan, H.S. Carlsen, F.P. Reinholt, U.H. von Andrian, P. Brandtzaeg, and G. Haraldsen. 2001. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uehara, S., A. Grinberg, J.M. Farber, and P.E. Love. 2002. A role for CCR9 in T lymphocyte development and migration. J. Immunol. 168:2811–2819. [DOI] [PubMed] [Google Scholar]
- 25.Wurbel, M.A., M. Malissen, D. Guy-Grand, E. Meffre, M.C. Nussenzweig, M. Richelme, A. Carrier, and B. Malissen. 2001. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 98:2626–2632. [DOI] [PubMed] [Google Scholar]
- 26.Nagasawa, T., S. Hirota, K. Tachibana, N. Takakura, S. Nishikawa, Y. Kitamura, N. Yoshida, H. Kikutani, and T. Kishimoto. 1996. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 382:635–638. [DOI] [PubMed] [Google Scholar]
- 27.Ma, Q., D. Jones, and T.A. Springer. 1999. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 10:463–471. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana, K., S. Hirota, H. Iizasa, H. Yoshida, K. Kawabata, Y. Kataoka, Y. Kitamura, K. Matsushima, N. Yoshida, S. Nishikawa, et al. 1998. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 393:591–594. [DOI] [PubMed] [Google Scholar]
- 29.Zou, Y.R., A.H. Kottmann, M. Kuroda, I. Taniuchi, and D.R. Littman. 1998. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 393:595–599. [DOI] [PubMed] [Google Scholar]
- 30.Kawabata, K., M. Ujikawa, T. Egawa, H. Kawamoto, K. Tachibana, H. Iizasa, Y. Katsura, T. Kishimoto, and T. Nagasawa. 1999. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc. Natl. Acad. Sci. USA. 96:5663–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reif, K., E.H. Ekland, L. Ohl, H. Nakano, M. Lipp, R. Förster, and J.G. Cyster. 2002. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 416:94–99. [DOI] [PubMed] [Google Scholar]
- 32.Bleul, C.C., and T. Boehm. 2000. Chemokines define distinct microenvironments in the developing thymus. Eur. J. Immunol. 30:3371–3379. [DOI] [PubMed] [Google Scholar]
- 33.Kwan, J., and N. Killeen. 2004. CCR7 directs the migration of thymocytes into the thymic medulla. J. Immunol. 172:3999–4007. [DOI] [PubMed] [Google Scholar]
- 34.Porritt, H.E., L.L. Rumfelt, S. Tabrizifard, T.M. Schmitt, J.C. Zuniga-Pflucker, and H.T. Petrie. 2004. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 20:735–745. [DOI] [PubMed] [Google Scholar]
- 35.Hollander, G.A., B. Wang, A. Nichogiannopoulou, P.P. Platenburg, W. van Ewijk, S.J. Burakoff, J.C. Gutierrez-Ramos, and C. Terhorst. 1995. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 373:350–353. [DOI] [PubMed] [Google Scholar]
- 36.van Ewijk, W., G. Hollander, C. Terhorst, and B. Wang. 2000. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 127:1583–1591. [DOI] [PubMed] [Google Scholar]
- 37.Foss, D.L., E. Donskoy, and I. Goldschneider. 2001. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J. Exp. Med. 193:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suniara, R.K., E.J. Jenkinson, and J.J. Owen. 1999. Studies on the phenotype of migrant thymic stem cells. Eur. J. Immunol. 29:75–80. [DOI] [PubMed] [Google Scholar]
- 39.Klug, D.B., C. Carter, E. Crouch, D. Roop, C.J. Conti, and E.R. Richie. 1998. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc. Natl. Acad. Sci. USA. 95:11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klug, D.B., C. Carter, I.B. Gimenez-Conti, and E.R. Richie. 2002. Cutting edge: thymocyte-independent and thymocyte-dependent phases of epithelial patterning in the fetal thymus. J. Immunol. 169:2842–2845. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson, W.E., E.J. Jenkinson, and G. Anderson. 2003. Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J. Exp. Med. 198:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yucel, R., H. Karsunky, L. Klein-Hitpass, and T. Moroy. 2003. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J. Exp. Med. 197:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youn, B.S., K.Y. Yu, J. Oh, J. Lee, T.H. Lee, and H.E. Broxmeyer. 2002. Role of the CC chemokine receptor 9/TECK interaction in apoptosis. Apoptosis. 7:271–276. [DOI] [PubMed] [Google Scholar]