Abstract
Although it is widely supposed that chemokines play a role in the thymus, most existing evidence is circumstantial. In this issue, two groups provide direct evidence that the chemokine receptor CCR7 is required for normal thymocyte migration (Ueno, T., F. Saito, D. Gray, S. Kuse, K. Hieshima, H. Nakano, T. Kakiuchi, M. Lipp, R. Boyd, and Y. Takahama. 2004. J. Exp. Med. 200:493–505; Misslitz, A., O. Pabst, G. Hintzen, L. Ohl, E. Kremmer, H. T. Petrie, and R. Forster. 2004. J. Exp. Med. 200:481–491). The two papers focus on distinct and opposite migration events, an early outward migration and a later inward migration. Together these papers provide a fascinating picture of the complex role of CCR7 in orchestrating thymocyte migration.
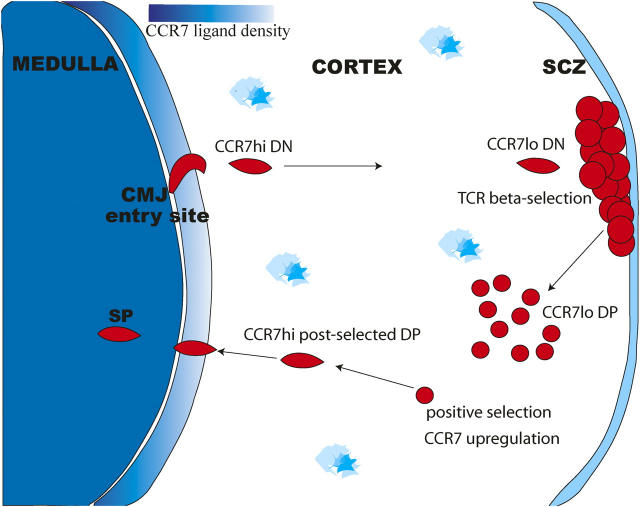
During T cell development, thymocytes undergo orchestrated migrations across the thymus covering thousands of microns before being released into the periphery as mature T cells. This movement of cells through the thymus is intimately tied to their developmental program resulting in a compartmentalized organ in which thymocytes representing particular developmental stages are found in distinct regions of the thymus (Fig. 1). Precursors to the T cell lineage enter the thymus at the cortico–medullary junction then move outward across the cortex to the subcapsulary zone (SCZ) as they undergo T lineage commitment and further differentiation (1). Upon reaching the SCZ, cells proceed through the TCRβ checkpoint followed by robust proliferative expansion (2, 3). At this time, polarity of migration is reversed and thymocytes move back into the cortex (4) where they undergo screening to ensure they express functional αβTCRs, a process known as positive selection (5). After positive selection, thymocytes then migrate from the cortex into the medulla where they complete their maturation before export to the periphery. Although the compartmentalization of the thymus is well appreciated, surprisingly little is known about how the orchestrated movement of thymocytes between compartments is achieved.
Figure 1.
Thymocyte migration and expression of CCR7 and its ligands in the adult thymus. A schematic diagram of the adult thymus, showing the major migratory paths taken by developing thymocytes and the proposed roles for CCR7 in these migrations. The density of CCR7 ligands, CCL19 and CCL21, is represented by blue shading, with highest levels in the medullary region, and additional sites of expression in the SCZ and scattered cells throughout the cortex (12, 24). Early DN thymocytes enter through venules located deep in the thymus at the cortical–medullary junction and migrate outward across the cortex toward the SCZ. CCR7 is highly expressed on a subset of these DN thymocytes (24). After TCRβ selection, thymocytes differentiate to DP stage and migrate into the cortex where they can undergo positive selection. Positive selection causes thymocytes to differentiate into either CD4 or CD8 SP thymocytes and migrate inward to the medulla. Positive selection induces up-regulation of CCR7 on a subset of DP thymocytes (23, 12, 25). Mature SP thymocytes also express high levels of CCR7.
Chemokines are small (70–80 amino acids) diffusible polypeptides produced by a wide variety of cell types which signal through a subset of G protein–coupled receptors to stimulate cell motility and provide guidance cues to migrating cells. Chemokines are well known for their role in the emigration of leukocytes from the blood stream to sites of inflammation (6). Recent papers have shown that certain chemokines are constitutively expressed in lymphoid tissue in the absence of inflammation and help to orchestrate the cell migrations that underlie the normal organization of lymphoid tissues (7, 8). Within the thymus, various chemokines are expressed in distinct anatomical regions, and thymocytes can respond to chemotactic stimuli in vitro. In general, knockouts of thymic chemokine genes have not lead to major blocks in thymocyte development, although careful analysis has revealed some effects in the thymus (13–19).
Perhaps the strongest evidence for chemokine involvement in thymic development comes from studies of CCR7, a chemokine receptor well known for its role in peripheral lymphoid tissues (14, 20). CCR7 ligands are expressed in the thymus, with highest expression in the medulla and expression in the SCZ and from scattered cells throughout the cortex (9, 20) (Fig. 1). In addition, CCR7 itself is expressed on mature single-positive (CD4+CD8− or CD4−CD8+) (SP) thymocytes and is up-regulated on a small fraction of double-positive (DP) thymocytes expressing phenotypic markers indicative of TCR engagement (9). Overexpression of CCR7 on thymocytes led to a reduction in the numbers of DP (CD4+ CD8+) in the cortex with an accumulation of DP in the medulla (21). Collectively, these studies have led to the proposal that CCR7 signaling plays a role in the directed migration of post-selected DP from the cortex into the medulla.
A Paradoxical Role for CCR7 in Thymocyte Migration.
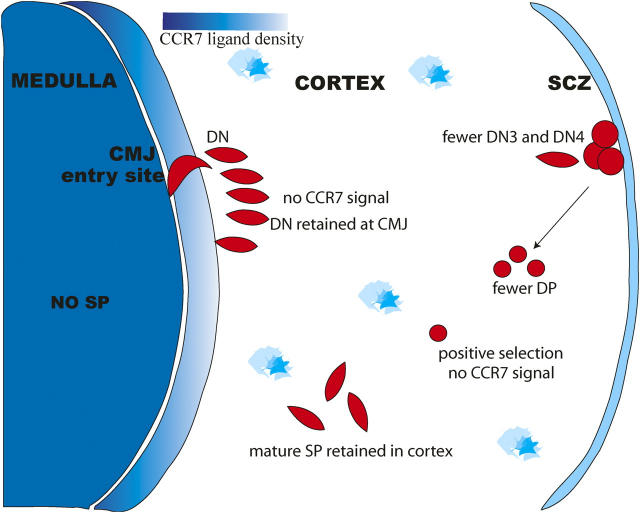
Initial reports of mice lacking CCR7 (CCR7−/−) or its ligands, CC19 and CCL21 (plt), focused on defects in peripheral immune cell trafficking and did not reveal any gross defects in thymic development (14, 18, 22). The papers in this issue (23, 24) reexamine the impact of these two mutations on the thymus by determining the location of different thymocytes populations within fixed thymic tissue sections. Although both groups report striking defects in the localization of thymocytes, the two papers focus on different stages of thymocyte development and provide very distinct perspectives on the function of CCR7 in the thymus (Fig. 2). The work of Ueno et al. (23) focuses on the inward migration of thymocytes after positive selection. Mutant mice lacking either CCR7 or its ligands show a marked reduction of SP thymocytes in the medulla and an accumulation of mature SP in the cortex. The authors also show up-regulation of CCR7 expression on DP thymocytes in response to TCR engagement, consistent with earlier studies (9, 25). Based on these results, Ueno et al. (23) conclude that CCR7 signaling is critical to the inward cortex–medulla migration of positively selected thymocytes. In contrast, the work of Misslitz et al. (24) focuses on the outward migration of early thymocytes. In situ studies of thymi from CCR7−/− or plt mice show an accumulation of DN2 (CD44+CD25+) thymocytes at or near the cortico–medullary junction. This migratory arrest is associated with an imbalance in the proportions of double-negative (CD4− CD8−) (DN) subsets, with an increase in what appeared to be DN1–DN2 transitional cells (referred to as DN1-2) and a decrease in DN3 and DN4 subsets. They also show that DN1-2 thymocytes expressed high levels of CCR7. Finally, they report a reduction in thymic cellularity, a defect that is exacerbated in older mutant mice. These results lead Misslitz et al. (24) to conclude that CCR7 signaling is essential for the outward migration and normal differentiation of DN cells.
Figure 2.
Thymocyte migration in the absence of CCR7 signaling. In the absence of CCR7 or its ligands, DN thymocytes are retained at the cortical–medullary junction, resulting in a modest block in the later stages of thymocyte development (24). In addition, post-selected DP thymocytes are not directed into the medulla but remain in the cortex where they develop into mature SP (23).
In both papers, effects of the mutations on the architecture of the thymus were noted. Although the medulla in wild-type mice forms a central organized structure, mice lacking CCR7 or its ligands had a reduced and fragmented medulla. Importantly, when CCR7 mutant thymocytes developed in thymus in which wild-type thymocytes were also present, normal thymic architecture was restored, but the migratory defects of the mutant thymocytes were still apparent. This indicated that impaired intrathymic migration was not due to abnormal thymic architecture but reflected an intrinsic defect in the mutant thymocytes. Whether the architectural abnormalities seen in CCR7−/− and plt mice were due to impaired outward migration of DN thymocytes, or the failure of SP thymocytes to accumulate in the medulla, or both is not yet clear. In any case, the interdependency between thymocyte and thymic epithelial development during ontogeny is well documented. In many studies, blocks were associated with profound defects in thymic epithelium, the severity of which depends on the timing of developmental arrest (26).
New Answers, More Questions.
Perhaps the most puzzling issue raised by these studies is how the same chemokines can be involved in simultaneously directing two thymocyte populations to migrate in opposite directions (Fig. 1). In particular, it seems paradoxical that CCR7 would be involved in directing early thymocytes to migrate away from the medulla, the site of the highest expression of CCR7 ligands. One possible explanation is that CCR7 ligands exert a repulsive effect on early thymocytes. Although most studies of chemokines in the immune system have focused on the attractive roles of chemokines, bifunctionality in chemotactic regulation is a common feature in the developing nervous system (27, 28), as well as myogenesis (29). Moreover, recent studies have demonstrated both attractive and repulsive effects of the chemokine CXCL12 (SDF-1) on T cells (30, 31). In these studies, high concentrations of CXCL12 mediated a strong repulsive effect in mature T cells, whereas lower concentrations elicited an attractive chemotactic response. In this regard, one could speculate that the high concentration of chemokines in the medulla is repulsive to early DN thymocytes, perhaps due to the combined effect of multiple chemokine receptors on these cells. In contrast, medullary chemokines may have an attractive effect on positively selected thymocytes due to the different combination of chemokine receptors expressed on positively selected thymocytes compared with DN thymocytes (9, 32). Alternatively, differences in the cellular machinery of DN thymocytes compared with positively selected thymocytes could also lead to distinct responses to the same chemokine gradient.
In the future, it will be important to determine the mechanism by which CCR7 exerts its effects of thymocyte trafficking. In addition to directing migration, chemokines can also stimulate the propulsion machinery of a cell in a random way (chemokinesis) without transducing any directional information (33). Thus, the migratory defects in mice deficient in CCR7 signaling could arise from a loss of directional guidance, or reduced motility, or both. It will also be important to determine whether the effects of CCR7 signaling on DN thymocytes are repulsive or attractive. Real-time analysis of the migratory behavior of CCR7−/− thymocytes in their native thymic environment may shed light on these questions. Recent advances in multiphoton imaging technology offers promise in addressing the migratory dynamics of cells in living tissues and will likely shed light on how lymphoid tissues conduct traffic (34, 35).
Another area for future investigation is the impact of anatomical location on TCR repertoire selection. Positive selection is believed to occur primarily in the cortex, whereas the medulla is an important site for negative selection (36, 37), and the cortex and the medulla provide specialized environments for these selection events to occur. Therefore, we might have expected the mislocalization of thymocytes to have some impact on thymocyte selection. However, Ueno et al. (23) found no evidence of alterations in positive or negative selection in CCR7 mutant mice. This is in line with an earlier study showing that mislocalization of DP thymocytes to the medulla by overexpressing CCR7 also does not lead to major effects on positive or negative selection (21). Of particular relevance is negative selection mediated by medullary epithelial cells to tissue-specific antigens driven by the transcription factor, AIRE (36, 38, 39). It seems likely that the failure of SP thymocytes to localize to the medulla in CCR7 mutant mice would impair AIRE-mediated negative selection. Although the deletion of thymocytes specific for AIRE-dependent antigens was not examined here, it is tempting to speculate that the slight but significant increase in the numbers of mature CD4 SP thymocytes reported by both groups could be due to the failure to delete cells that are reactive to peripheral self antigens driven by AIRE.
References
- 1.Lind, E.F., S.E. Prockop, H.E. Porritt, and H.T. Petrie. 2001. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J. Exp. Med. 194:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley, E.C., H.T. Petrie, L.M. Shah, M.J. Owen, and A.C. Hayday. 1994. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1:83–93. [DOI] [PubMed] [Google Scholar]
- 3.Penit, C., B. Lucas, and F. Vasseur. 1995. Cell expansion and growth arrest phases during the transition from precursor (CD4-8-) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J. Immunol. 154:5103–5113. [PubMed] [Google Scholar]
- 4.Penit, C. 1988. Localization and phenotype of cycling and post-cycling murine thymocytes studied by simultaneous detection of bromodeoxyuridine and surface antigens. J. Histochem. Cytochem. 36:473–478. [DOI] [PubMed] [Google Scholar]
- 5.Starr, T.K., S.C. Jameson, and K.A. Hogquist. 2003. Positive and negative selection of T cells. Annu. Rev. Immunol. 21:139–176. [DOI] [PubMed] [Google Scholar]
- 6.Johnston, B., and E.C. Butcher. 2002. Chemokines in rapid leukocyte adhesion triggering and migration. Semin. Immunol. 14:83–92. [DOI] [PubMed] [Google Scholar]
- 7.Ansel, K.M., and J.G. Cyster. 2001. Chemokines in lymphopoiesis and lymphoid organ development. Curr. Opin. Immunol. 13:172–179. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, D.J., C.H. Kim, and E.C. Butcher. 2003. Chemokines in the systemic organization of immunity. Immunol. Rev. 195:58–71. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, J.J., J. Pan, and E.C. Butcher. 1999. Cutting edge: developmental switches in chemokine responses during T cell maturation. J. Immunol. 163:2353–2357. [PubMed] [Google Scholar]
- 10.Uehara, S., K. Song, J.M. Farber, and P.E. Love. 2002. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3(high)CD69+ thymocytes and gammadeltaTCR+ thymocytes preferentially respond to CCL25. J. Immunol. 168:134–142. [DOI] [PubMed] [Google Scholar]
- 11.Kim, C.H., L.M. Pelus, J.R. White, and H.E. Broxmeyer. 1998. Differential chemotactic behavior of developing T cells in response to thymic chemokines. Blood. 91:4434–4443. [PubMed] [Google Scholar]
- 12.Bleul, C.C., and T. Boehm. 2000. Chemokines define distinct microenvironments in the developing thymus. Eur. J. Immunol. 30:3371–3379. [DOI] [PubMed] [Google Scholar]
- 13.Plotkin, J., S.E. Prockop, A. Lepique, and H.T. Petrie. 2003. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J. Immunol. 171:4521–4527. [DOI] [PubMed] [Google Scholar]
- 14.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 99:23–33. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Lopez, C., A. Varas, R. Sacedon, E. Jimenez, J.J. Munoz, A.G. Zapata, and A. Vicente. 2002. Stromal cell-derived factor 1/CXCR4 signaling is critical for early human T-cell development. Blood. 99:546–554. [DOI] [PubMed] [Google Scholar]
- 16.Mori, S., H. Nakano, K. Aritomi, C.R. Wang, M.D. Gunn, and T. Kakiuchi. 2001. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 193:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uehara, S., A. Grinberg, J.M. Farber, and P.E. Love. 2002. A role for CCR9 in T lymphocyte development and migration. J. Immunol. 168:2811–2819. [DOI] [PubMed] [Google Scholar]
- 18.Ueno, T., K. Hara, M.S. Willis, M.A. Malin, U.E. Hopken, D.H. Gray, K. Matsushima, M. Lipp, T.A. Springer, R.L. Boyd, et al. 2002. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 16:205–218. [DOI] [PubMed] [Google Scholar]
- 19.Wurbel, M.A., M. Malissen, D. Guy-Grand, E. Meffre, M.C. Nussenzweig, M. Richelme, A. Carrier, and B. Malissen. 2001. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 98:2626–2632. [DOI] [PubMed] [Google Scholar]
- 20.Ngo, V.N., H.L. Tang, and J.G. Cyster. 1998. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J. Exp. Med. 188:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan, J., and N. Killeen. 2004. CCR7 directs the migration of thymocytes into the thymic medulla. J. Immunol. 172:3999–4007. [DOI] [PubMed] [Google Scholar]
- 22.Nakano, H., S. Mori, H. Yonekawa, H. Nariuchi, A. Matsuzawa, and T. Kakiuchi. 1998. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 91:2886–2895. [PubMed] [Google Scholar]
- 23.Ueno, T., F. Saito, D.H.D. Gray, S. Kuse, K. Hieshima, H. Nakano, T. Kakiuchi, M. Lipp, R.L. Boyd, and Y. Takahama. 2004. CCR7 signals are essential for cortex–medulla migration of developing thymocytes. J. Exp. Med. 200:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misslitz, A., O. Pabst, G. Hintzen, L. Ohl, E. Kremmer, H.T. Petrie, and R. Forster. 2004. Thymic T cell development and progenitor localization depend on CCR7. J. Exp. Med. 200:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, Y.H., D. Li, A. Winoto, and E.A. Robey. 2004. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc. Natl. Acad. Sci. USA. 101:4936–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill, J., M. Malin, J. Sutherland, D. Gray, G. Hollander, and R. Boyd. 2003. Thymic generation and regeneration. Immunol. Rev. 195:28–50. [DOI] [PubMed] [Google Scholar]
- 27.Hopker, V.H., D. Shewan, M. Tessier-Lavigne, M. Poo, and C. Holt. 1999. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 401:69–73. [DOI] [PubMed] [Google Scholar]
- 28.Pasquale, E. 2000. Neurobiology. Turning attraction into repulsion. Science. 289:1308–1310. [DOI] [PubMed] [Google Scholar]
- 29.Kramer, S.G., T. Kidd, J.H. Simpson, and C.S. Goodman. 2001. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science. 292:737–740. [DOI] [PubMed] [Google Scholar]
- 30.Poznansky, M.C., I.T. Olszak, R.H. Evans, Z. Wang, R.B. Foxall, D.P. Olson, K. Weibrecht, A.D. Luster, and D.T. Scadden. 2002. Thymocyte emigration is mediated by active movement away from stroma-derived factors. J. Clin. Invest. 109:1101–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poznansky, M.C., I.T. Olszak, R. Foxall, R.H. Evans, A.D. Luster, and D.T. Scadden. 2000. Active movement of T cells away from a chemokine. Nat. Med. 6:543–548. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, G., Y. Nakata, Y. Dan, A. Uzawa, K. Nakagawa, T. Saito, K. Mita, and T. Shirasawa. 1998. Loss of SDF-1 receptor expression during positive selection in the thymus. Int. Immunol. 10:1049–1056. [DOI] [PubMed] [Google Scholar]
- 33.Bignold, L.P. 1988. Measurement of chemotaxis of polymorphonuclear leukocytes in vitro. The problems of the control of gradients of chemotactic factors, of the control of the cells and of the separation of chemotaxis from chemokinesis. J. Immunol. Methods. 108:1–18. [DOI] [PubMed] [Google Scholar]
- 34.Bousso, P., and E. Robey. 2004. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by 2-photon microscopy. Immunity. In press. [DOI] [PubMed] [Google Scholar]
- 35.Cahalan, M.D., I. Parker, S.H. Wei, and M.J. Miller. 2002. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat. Rev. Immunol. 2:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 37.Sprent, J., and H. Kishimoto. 2002. The thymus and negative selection. Immunol. Rev. 185:126–135. [DOI] [PubMed] [Google Scholar]
- 38.Liston, A., S. Lesage, J. Wilson, L. Peltonen, and C.C. Goodnow. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4:350–354. [DOI] [PubMed] [Google Scholar]
- 39.Klein, L., and B. Kyewski. 2000. “Promiscuous” expression of tissue antigens in the thymus: a key to T-cell tolerance and autoimmunity? J. Mol. Med. 78:483–494. [DOI] [PubMed] [Google Scholar]