Abstract
During aging, adaptive immunity is severely compromised, due in part to decreased production of B lymphocytes and loss of immunoglobulin (Ig) diversity. However, the molecular mechanisms that underlie age-associated diminished B cell production remain unclear. Using in vivo labeling, we find that this reduction in marrow pre–B cells reflects increased attrition during passage from the pro–B to pre–B cell pool. Analyses of reciprocal bone marrow chimeras reveal that the magnitude and production rates of pre–B cells are controlled primarily by microenvironmental factors, rather than intrinsic events. To understand changes in pro–B cells that could diminish production of pre–B cells, we evaluated rag2 expression and V(D)J recombinase activity in pro–B cells at the single cell level. The percentage of pro–B cells that express rag2 is reduced in aged mice and is correlated with both a loss of V(D)J recombinase activity in pro–B cells and reduced numbers of pre–B cells. Reciprocal bone marrow chimeras revealed that the aged microenvironment also determines rag2 expression and recombinase activity in pro–B cells. Together, these observations suggest that extrinsic factors in the bone marrow that decline with age are largely responsible for less efficient V(D)J recombination in pro–B cells and diminished progression to the pre–B cell stage.
Keywords: hematopoietic system, antibody formation, B lymphocytes, aging, gene rearrangement
Introduction
Advancing age is accompanied by compromised immune responses (1–3) and decreased vaccine efficacy (4, 5). These changes, in part, reflect age-associated decreases in B lymphocyte production and repertoire diversity (6–14), but the underlying molecular mechanisms remain unclear. RAG1 and RAG2 produce site-specific DNA double strand breaks that initiate V(D)J recombination (15, 16) as B cell progenitors transit differentiative stages associated with Ig gene rearrangement and B cell receptor assembly. Thus, age-associated reductions of RAG activity could contribute to diminution of pre–B cells in aged mice, and, indeed, rag1 and rag2 mRNA levels decline when assessed in total bone marrow preparations from aged mice (6, 9, 11). However, the age-associated reduction in pre–B cells alone might account for apparently reduced rag levels in total bone marrow, inasmuch as pre–B cells outnumber pro–B cells 3:1 in young mice, but are often equal to or less than the number of pro–B cells in aged mice (9, 17–19). It is similarly unclear whether the age-related reductions in marrow B lineage progenitors and rag expression levels reflect lineage-intrinsic versus microenvironment defects. For example, pro–B cells from aged mice yield diminished proliferative capacity in IL-7–supplemented cultures (17, 20, 21), yet stromal cell cultures established from aged mice support less in vitro proliferation of pro–B cells than cultures established from young mice (18, 20, 22).
To explore these questions, we have examined the magnitude and kinetics of each major B cell differentiative stage in aged versus young adults, coupled with single cell flow cytometric analyses of transgenic and knockin (KI) mice that afford direct assessments of RAG expression and activity (23–25). Our findings indicate that diminished pre–B cell numbers is a general feature of aging because it is observed in multiple stains and F1 combinations. Moreover, these changes are accompanied by reduction in RAG expression and activity within pro–B cells, whereas proliferative activity is unaffected, forging a link between reduced RAG activity and increased failure to transit the late pro–B cell stage. Finally, we show that these age-associated changes in population dynamics and RAG activity largely reflect microenvironmental changes because all of these properties are dictated by host age in reciprocal marrow chimeras.
Materials and Methods
Mice.
C57BL/6, CBA/J, FVBN/J, and B6.SJL-Ptprc aPep3b/BoyJ (CD45.1) were obtained from The Jackson Laboratory. Aged and young BALB/c, DBA/2, C57BL/6, (C57BL/6 × DBA/2)F1, and (C57BL/6 × Balb/c)F1 mice were obtained from the National Institute of Aging Repository. C57BL/6J (JAX) were also aged at the University of Massachusetts Medical School under specific pathogen-free conditions. NG transgenic mice (FVBN background) were obtained from M. Nussenzweig (The Rockefeller University, New York, NY; reference 23). NG mice were crossed to C57BL6/J mice for 6–9 generations and crossed to H2-SVEX mice and CD45.1 mice, or were crossed to CBA/J mice. Aged RAG2:GFP KI mice (129 × C57BL/6 background) were provided by F. Alt (Harvard Medical School, Boston, MA; reference 24). H2-SVEX mice (SB110 line; C57BL/6 background) were constructed by us (25). All animal husbandry and procedures were performed in accordance with the Animal Welfare Act.
Lymphocyte Suspensions.
Lymphocyte suspensions were prepared as described previously (26, 27). In brief, bone marrow cells were obtained from the two hind limbs of donor animals. Bone marrow cells were prepared by flushing the femurs and tibias with cold medium and filtered through nylon monofilament cloth to remove debris; erythrocytes were depleted with ammonium chloride-Tris for some analyses (see Fig. 1 and Table I).
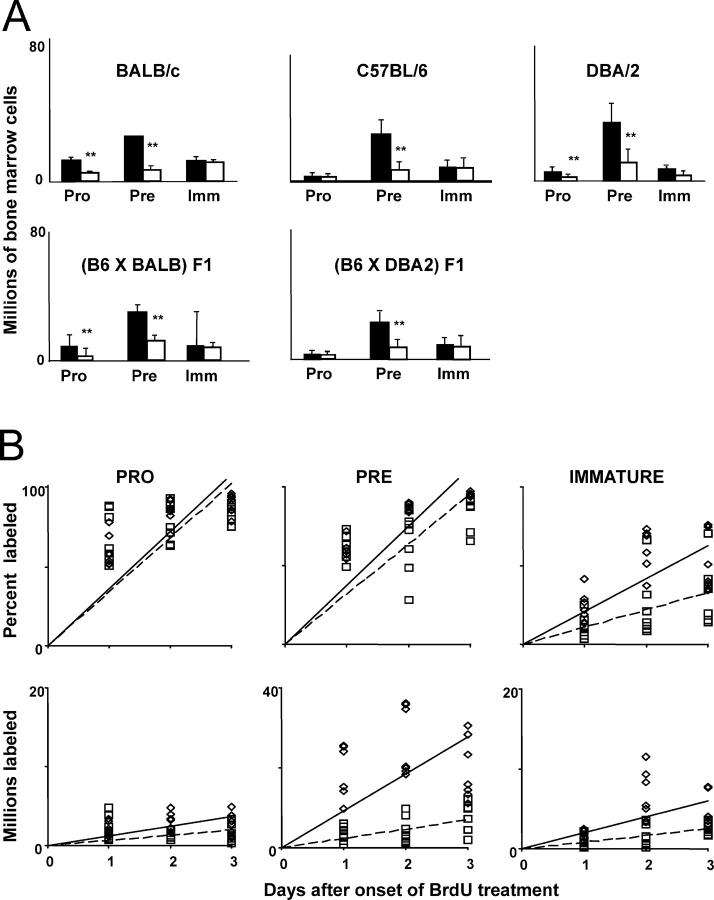
Figure 1.
Magnitude, renewal rates, and production rates of B lineage progenitor pools in aged and young adults. (A) Bone marrow was harvested from either young adult (black bars) or aged mice (white bars) of the strains and F1 combinations indicated and stained as described in Materials and Methods. The proportions of pro–B (IgM−B220+CD43+), pre–B (IgM−B220+CD43−), and immature B (IgM+B220LO) subsets were assessed using flow cytometry and multiplied by the marrow cell estimate of Osmond (reference 50) to obtain total numbers. Bars show mean ± SD of samples from 10 to 30 mice, depending on strain. **, statistical significance for young versus old (Student's t test; P < 0.05). (B) Young adult (diamonds) or aged (squares) C57BL/6 mice were injected with 0.6 mg BrdU at 12-h intervals. Bone marrow was harvested at various times after the onset of labeling and stained for surface phenotype and BrdU incorporation as described in Materials and Methods. The proportion of BrdU-labeled cells (top) was determined using flow cytometry, and the numbers of BrdU-labeled cells (bottom) were calculated by multiplying these proportions by the Osmond estimate of total marrow cells. Labeling among pro–B, pre–B, and immature B cell subsets is shown in the left, middle, and right plots, respectively. Each point represents an individual mouse. Solid and dashed lines (young and aged, respectively) are those determined by linear regression.
Table I.
Production and Renewal Rates of Marrow B Cell Subsets in Bone Marrow Chimeras Determined from In Vivo BrdU Labeling a
| Untreated mice
|
Bone marrow chimeras
|
|||||
|---|---|---|---|---|---|---|
| Young | Aged | Young→Young | Young→Aged | Aged→Young | ||
| Pro | No. of cells (× 10−6) | 3.5 ± 1.3 | 2.1 ± 1.3b , c | 4.0 ± 1.2 | 2.3 ± 1.3d | 3.2 ± 1.0 |
| Renewal rate (% of pool/day)e | 35.8 | 34.1 | 31.9 | 33.0 | 38.5 | |
| Production rate (cells/day × 10−6)e | 1.2 | 0.7d | 1.2 | 0.8 | 1.4 | |
| Pre | No. of cells (× 10−6) | 28.0 ± 9.7 | 6.8 ± 4.3c | 33.3 ± 10.4 | 10.0 ± 5.9c | 30.4 ± 12.3 |
| Renewal rate (% of pool/day) | 37.3 | 32.0 | 38.0 | 31.7 | 35.8 | |
| Production rate (cells/day × 10−6) | 9.3 | 2.3c | 10.0 | 2.2c | 9.5 | |
| Immature | No. of cells (× 10−6) | 9.3 ± 3.2 | 7.8 ± 4.1 | 10.9 ± 2.4 | 4.7 ± 3.5 | 9.1 ± 4.0 |
| Renewal rate (% of pool/day) | 20 | 11d | 23.0 | 17.1 | 20.7 | |
| Production rate (cells/day × 10−6) | 2.0 | 0.8c | 1.5 | 0.7c | 1.7 | |
3 × 10−6 T- and B-depleted bone marrow cells from young or aged C57BL/6 mice were injected i.v. into young or aged (C57BL × DBA2)F1 mice. Adoptive hosts were allowed to reconstitute for >40 d. Chimeric bone marrow was harvested as described in Materials and Methods. The proportional representation of pro–, pre–, and immature B subsets was assessed by flow cytometry and multiplied by the marrow cell estimate of Osmond et al. (47) to obtain total numbers. Data for untreated mice are from young or aged C57BL6.
Means were compared (young vs. aged or reciprocal chimeras vs. control chimeras) using Student's t test.
P < 0.01.
P < 0.05.
BrdU labeling was performed in reciprocal young↔aged chimeric mice as described in Materials and Methods. The proportion of BrdU-labeled cells was determined by flow cytometry, and the numbers of BrdU-labeled cells calculated by multiplying these proportions estimates of total marrow cells. The regression coefficients of absolute and proportional labeling versus time provide estimates of production and renewal rates, respectively.
Flow Cytometric Analyses of Cell Surface Antigens and GFP.
For Fig. 1 and Table I, the following reagents were purchased from BD Biosciences: PE and FITC-labeled anti-CD24 (M1/69); PE-labeled anti-CD43 (S7); and allophycocyanin and PE-labeled anti-CD45R (B220; RA3-6B2). Biotin-labeled goat anti–mouse IgM, PE-labeled anti-IgD (SBA-1), and streptavidin-red 670 were purchased from Southern Biotechnology Associates, Inc. Cell surface staining and flow cytometry was done as described previously (26, 27).
For Figs. 2–5 and Tables II–IV, primary antibodies specific for the following were used: CD24 (30F1), Alexa594; CD24, cascade blue; CD43 (S7), PE; 493, biotin; CD45.1 (A20), cascade blue; AA4.1, Alexa594; AA4.1, biotin; B220 (RA3-6B2), allophycocyanin; B220, biotin; DX5, biotin; IgM (331), Alexa594; IgM, biotin; IgM, cascade blue; and Ly6C (AL21), biotin. Streptavidin-Cy5PE was used to reveal biotinylated reagents. Antibodies were purchased from BD Biosciences, eBioscience, or Caltag. CD24, CD45.1, AA4.1, 493, and IgM antibodies were purified and conjugated to cascade blue and Alexa594 (Molecular Probes, Inc.) or biotin in our laboratory using standard methods. Staining to reveal the AA4.1 epitope included both monoclonal antibodies AA4.1 and 493. Cell surface staining and evaluation of GFP and VEX was performed as described previously (25). Flow cytometry was performed on a three-laser (argon [488 nm], krypton [407 nm], and dye laser [tuned to 600 nm]), 10-parameter FACSVantage™ obtained from BD Biosciences Immunocytometry Systems. Post-hoc compensation and data analyses were performed using FlowJo software (Tree Star).
Figure 2.
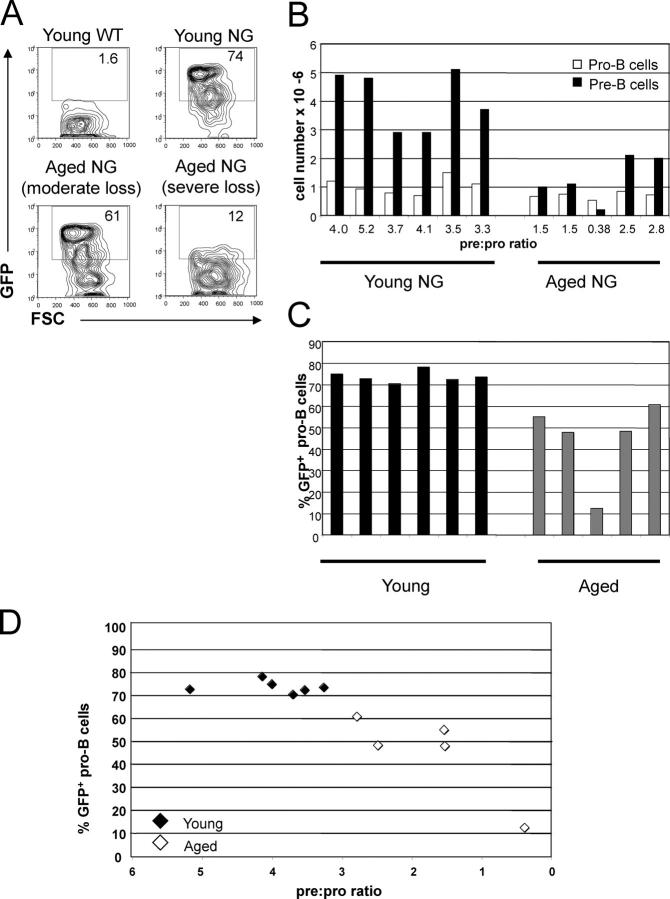
Reduced rag2 expression in pro–B cells is consistent with lower numbers of pre–B cells in aged mice. Bone marrow from young (2–3.5 mo) and aged (26–27 mo) NG mice and wild-type controls (both [FVBN × CBA]F1 background) was harvested and analyzed by flow cytometry as described in Materials and Methods. (A) Flow cytometric analysis of GFP expression (a reporter of rag2 expression in NG mice) and forward light scatter (FSC) within pro–B cells (B220LOCD43+AA4.1+) from young and old NG mice. The numbers within the gates depict the percent of pro–B cells that express GFP. Bone marrow pro–B cells from representative wild type, young NG, aged NG with a moderate loss of GFP expression, and an aged NG with a severe loss of GFP expression are displayed. (B) Numbers of pro–B (B220LOCD43+AA4.1+) and pre–B cells (B220LOCD43−AA4.1+CD242+) in bone marrow of young and aged NG mice. White bars represent pro–B cells, and black bars represent pre–B cells. The pre:pro ratio is calculated by dividing the number of pre–B cells by the number of pro–B cells. (C) Flow cytometric analysis of GFP in pro–B cells from young (black bars) and aged NG mice (gray bars). The percent of pro–B cells that are GFP+ are shown for the same mice depicted in B. (D) The percent of pro–B cells that are GFP+ and the pre:pro ratio are displayed for each NG mouse shown in B and C. Closed diamonds represent young mice, and open diamonds represent aged mice.
Figure 5.
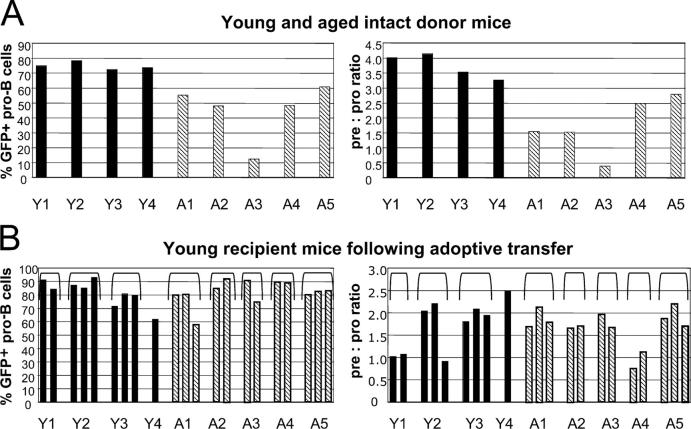
Pro–B cells derived from young and aged sources display similar rag2 expression and pre:pro ratios in young hosts. Bone marrow from young (3 mo) and aged (26–27 mo) NG mice were transferred into lethally irradiated young (2 mo at time of transfer) recipient mice. (A) GFP expression in pro–B cells (left) and pre:pro ratio (right) in young and aged donor mice at time of transfer. These mice are four of the six young and all of the aged mice shown in Fig. 1. Black bars represent young donor mice, and diagonally striped bars represent aged donor mice. To aid comparisons, each young donor mouse is given a designation, Y1-Y4, and each aged donor mouse was given A1-A5, depicted below each panel. (B) Analyses of bone marrow harvested from young recipient mice 5 wk after transfer of cells from either young or aged donors. Pro–B cells were defined as IgM−B220+CD43+AA4.1+ and pre–B cells were defined as IgM−B220+CD43−AA4.1+CD24+. The percentage of pro–B cells that are GFP+ and the pre:pro ratios are shown. The pre:pro ratio of two young unmanipulated NG mice used as controls in this experiment were 1.2 and 1.3. Each bar represents values obtained from one recipient mouse. The designations Y1-Y4 and A1-A5 reflect the source of the transferred bone marrow as shown in A, and brackets show the groups of recipients according to the donor designation.
Table II.
Statistical Analysis of Data Obtained from Young and Aged rag2 Reporter NG Transgenic Mice
| Significance of differences | Younga | Ageda | p-value |
|---|---|---|---|
| No. of pro–B cells (millions)b | 1.0 ± 0.29 | 0.70 ± 0.12 | 0.035 |
| No. of pre–B cells (millions)b | 4.1 ± 1.0 | 1.3 ± 0.79 | 0.001 |
| Pre:pro ratioc | 4.0 ± 0.67 | 1.7 ± 0.94 | 0.001 |
| % of pro–B cells that are CD24high | 61 ± 4.1 | 45 ± 10 | 0.005 |
| % of pro–B cells that are GFP+ | 74 ± 2.7 | 45 ± 19 | 0.026 |
| Correlations b/t characteristics | n | r | p-value |
| % of pro–B cells that are GFP+ and no. of pre–B cells | 11 | 0.80 | 0.003 |
| % of pro–B cells that are GFP+ and pre:pro ratio | 11 | 0.88 | <0.001 |
Bone marrow from young (2–3.5 mo) and aged (26–27 mo) NG mice, and wild-type controls (both [FVBN × CBA]F1 background) was harvested and analyzed by flow cytometry and statistical analyses as described in Materials and Methods. In the NG mice, GFP is a reporter of rag2 expression.
Means ± SD are presented.
Numbers of pro–B (B220LOCD43+AA4.1+) and pre–B cells (B220LOCD43−AA4.1+CD24++) in bone marrow of young and aged NG mice were determined by multiplying the frequency by the leg bone marrow cell number determined by counting to obtain total numbers.
The pre:pro ratio was calculated by dividing the number of pre–B cells by the number of pro–B cells.
n, no. of mice; r, correlation coefficient.
Continuous BrdU Labeling In Vivo and Analysis of BrdU-labeled Cells.
The rate of continuous in vivo BrdU labeling was assessed as described previously (26–28). Young and aged C57BL/6 mice were injected i.p. with 0.6 mg BrdU (Sigma-Aldrich) in 0.2 ml PBS at 12-h intervals for the duration of each experiment. The cells obtained from BrdU-treated mice were stained for three surface markers (PE, allophycocyanin, and biotin-conjugated Abs followed by streptavidin-Red670) as mentioned before and washed with cold PBS. FITC-labeled anti-BrdU (B44) was purchased from Becton Dickinson. The incorporated BrdU was analyzed according to previously published procedures (26, 29). Cytometric analysis was performed by gating on all nucleated cells as described previously (26). For each mouse, the percentage of BrdU-labeled cells in each subset was measured by flow cytometry and multiplied by the total cells in the subset to give the number of labeled cells. The mean ± SD values for these percentages and numbers were plotted as a function of time, and least squares regression analyses were performed to obtain the turnover and production rates, respectively.
Cell Cycle Analyses.
20,000–40,000 cells from each B-lineage subset were sorted directly into 70% ethanol using a Becton Dickinson FACStarPLUS. After an overnight incubation at 4°C, cells were pelleted and carefully resuspended in 300 μl staining solution containing 20 μg/ml propidium iodide and 100 U/ml RNase A in PBS with 1% glucose and incubated for 30 min at room temperature. Cells were analyzed on a Becton Dickinson FACScan™. Data were collected in doublet discrimination mode using CELLQuest™ software and analyzed with the cell cycle analysis program MacCycle.
Reciprocal Bone Marrow Chimeras.
Bone marrow chimeras (Fig. 1 and Table I) were constructed using methods described previously (26). In brief, 3 × 106 T- and B-depleted marrow cells from C57BL/6 mice were injected i.v. into adult (C57BL/6 × DBA2)F1 or (C57BL/6 × BALB/c)F1 mice that had received 900 rad of whole body irradiation 24 h previously. Chimeric mice were given drinking water supplemented with 0.05% neomycin sulfate and 100 U/ml polymyxin B sulfate (Sigma-Aldrich). In all recipients analyzed, >98% of all B220+ cells expressed H-2Kb, but <2% expressed H-2Kd, indicating complete chimerism. In addition, cell recoveries from the marrows and spleens did not differ from unirradiated controls, indicating reconstitution was largely complete and had achieved steady-state levels (unpublished data).
For additional transfers into both young and aged recipient mice, bone marrow was harvested and washed in HBSS. Young and aged recipient mice received lethal irradiation (850–1,000 rad) 20 h before adoptive transfer. Recipient mice received 3.5–6 × 106 cells via tail vein injection. Neomycin sulfate (N-6386; Sigma-Aldrich) and polymyxin B sulfate (P-4932; Sigma-Aldrich) were administered through drinking water starting 4 d before irradiation and ending 14 d after adoptive transfer. 5–6 wk after adoptive transfer, bone marrow was harvested from recipient mice for analysis. Gross inspection of young and aged mice was performed before and after euthanasia. Mice displaying signs of tumors or other abnormalities were eliminated from analysis.
Statistical Analyses (see Figs. 2–5 and Tables II–V).
Statistical analysis was performed using SPSS version 10.0 for Mac and Version 11.5 for PC. T tests were conducted using either the Welch-Aspin T test or the Student's t test based on the significance of Leven's test for equality of variances as follows: when significance was >0.1 the Welch-Aspin T test was used and when <0.1, a Student's t test was used. Correlations between variables were determined using Pearson correlations.
Results
Diminished Marrow Progenitor Compartments Are a Common Feature of Aging and Reflect Increased Pro–B Cell Attrition.
First, we analyzed the magnitude and kinetics of B lineage progenitor pools in several strains and F1 combinations. In accord with previous findings, the pre–B cell pool of aged individuals is markedly diminished in all strains examined (Fig. 1 A and Table I) . This approximately fourfold difference in pre–B cell numbers is sufficient to account for most of the age-associated reduction in newly formed B cell numbers. Surprisingly, the immature bone marrow B cell compartment shows less pronounced changes in magnitude, despite the striking reduction in their immediate precursor pool.
The kinetic changes underlying shifts in the size of B cell progenitor pools were determined by analyzing in vivo BrdU-labeling rates within the B cell developmental subsets of aged (18–24 mo old) versus young mice. Proportional and absolute labeling rates of C57BL/6 mice (Fig. 1 B and Table I) are representative of all strains and F1 combinations tested. Both the proportional and absolute labeling rates of pro–B cells are similar in young versus aged mice (Fig. 1 B). Both ages display a pro–B renewal rate of ∼35% per day, corresponding to a production rate of ∼106 cells/day (Table I). However, the number and production rate of pro–B cells is somewhat diminished in aged mice (Fig. 1 and Table I), in accord with previous papers (19, 30).
In contrast, labeling kinetics within the pre–B cell pools of aged versus young adult mice differ dramatically (Fig. 1 B, middle). Young adults generate 9–13 million pre–B cells daily, whereas aged individuals produce only 2–5 million pre–B cells per day (Table I). Nonetheless, the renewal rates of pre–B cells are very similar (32–42%/day), indicating that the residency time within the pool is unchanged in aged individuals (Table I). This fourfold reduction in the generation rate but unchanged renewal rate corresponds well with the fourfold diminution of the pre–B cell pool. Because immature marrow B cells are nondividing, labeling among these cells reflects transit from the pre–B cell pool. Surprisingly, the entry rate of immature marrow B cells in aged individuals is less severely affected than pre–B cell generation (Fig. 1 B and Table I). Thus, unlike young adults where ∼20–24% of the pre–B cells formed survive to reside within the immature pool, nearly 50% appear to do so in aged individuals. Furthermore, the renewal rate of immature marrow B cells is slower, indicating a lengthened residency in the immature compartment, and resulting in a compartment of similar magnitude despite the halved production rate.
Fr. C′ pro–B cells and large pre–B cells undergo several rounds of division after successful heavy chain rearrangement and expression. Thus, the differences in pre–B cell production could either reflect failure to proliferate, or might instead indicate that fewer pro–B cells successfully pass the checkpoints imposed before this proliferative burst. Therefore, we performed cell cycle analysis in conjunction with staining combinations that delineate the marrow B cell fractions (31). We found that the proportion of cells in S and G2/M phases is similar for young and aged mice in each fraction of marrow development (unpublished data); statistical analyses indicate no significant differences between aged versus young marrow in the proportion of cycling cells within any fractions. Thus, because the C′ and D fractions show similar proportions of cells in S and G2/M phases, the proliferative burst associated with fraction C′ occurs in aged mice and, therefore, decreased proliferation does not underlie reduced pre–B cell production.
Host Age Dictates the Magnitude and Kinetics of the Pre–B Cell Pool in Reciprocal Bone Marrow Chimeras.
Both aged→young and young→aged bone marrow chimeras were generated using C57BL/6 donors and either (C57BL × BALB/c)F1 or (C57BL × DBA/2)F1 recipients. Donor marrow depleted of T cells and sIg+ cells was transferred to irradiated recipients. Young→young chimeras, as well as untreated F1 mice of appropriate age were analyzed to serve as controls. After ∼40 d of reconstitution, recipients were treated with BrdU and the magnitude, turnover, and renewal rates of each marrow B cell compartment were determined. The results are summarized in Table I.
When transferred to young recipients, both aged and young donor marrow produced newly formed B cell subpopulations of identical magnitude, turnover, and renewal rates (Table I). These results indicate that B lineage progenitors derived from aged marrow are fully capable of generation and survival rates associated with young marrow progenitors. In contrast, when transferred to aged recipients, young marrow yields a pre–B cell compartment different from that of young recipients, which mirrors the production and turnover rates observed in aged individuals (Table I). Together, these findings show that age-associated microenvironmental changes, rather than altered differentiative or survival capacity of B lineage progenitors themselves, underlie altered generation and attrition rates.
Age-associated Reduction in Pre–B Cells Is Correlated with a Reduction in the Percentage of Pro–B Cells That Express rag2.
We compared rag2 expression in the bone marrow of young (2–3.5 mo) and aged (26–27 mo) mice. Flow cytometric analysis of young and aged NG transgenic mice was used to determine the percentage of pro–B cells that express GFP as a reporter of rag2 expression (Fig. 2, A and C). The percentage of pro–B cells that are GFP+ is significantly lower in aged mice (Table II , mean: 45 vs. 74%). The mean fluorescent intensity of GFP in pro–B cells was similar between young mice and the four aged mice with moderate reduction in the percentage of GFP+ pro–B cells. This suggests that the levels of rag2 per cell are similar within these pro–B cells, even though fewer pro–B cells express rag2 in aged mice.
The loss of rag2 expression in pro–B cells was correlated with reduced numbers of pre–B cells; the percentage of pro–B cells that are GFP+ is correlated with both the number of pre–B cells and the pre:pro ratio (Fig. 2 D and Table II). This relationship is also evident in Fig. 2 A, as the aged mouse with the lowest percent of GFP+ pro–B cells is the same mouse that has the lowest number of pre–B cells and pre:pro ratio. The finding that the reduction in pre–B cell numbers in aged mice is correlated with a reduction in the percent of pro–B cells that express rag2 supports the hypothesis that reduced numbers of pre–B cells in aged mice are due to alterations that affect expression of rag2.
Reduced Numbers of Pre–B Cells in Aged Mice Are Correlated with Reduced RAG2 Protein Levels in Pro–B Cells.
Next, we analyzed B cell development in RAG2-GFP KI mice (24). These KI mice have two important advantages compared with NG mice: (a) the GFP reporter is located within the endogenous rag2 locus, and (b) GFP is expressed as a fusion protein with RAG2 and, thus, serves as a direct reporter of cellular RAG2 protein levels. The RAG2-GFP fusion protein maintains normal RAG enzymatic activity, and B cell development in these mice is not altered. GFP levels per cell and the percentage of pro–B cells that are GFP+ are lower in these mice compared with NG transgenic mice. This difference could be due to the single insertion of GFP in the KI compared with the multicopy NG transgene as well as differences in protein half-life for the RAG2-GFP fusion in the KI compared with GFP in the transgenic. We analyzed RAG2-GFP expression in pro–B cells harvested from young (4–7 mo) and aged (23–28 mo) RAG2-GFP KI mice. Results from flow cytometric analyses of pro–B cells and pre–B cells are shown in Fig. 3.
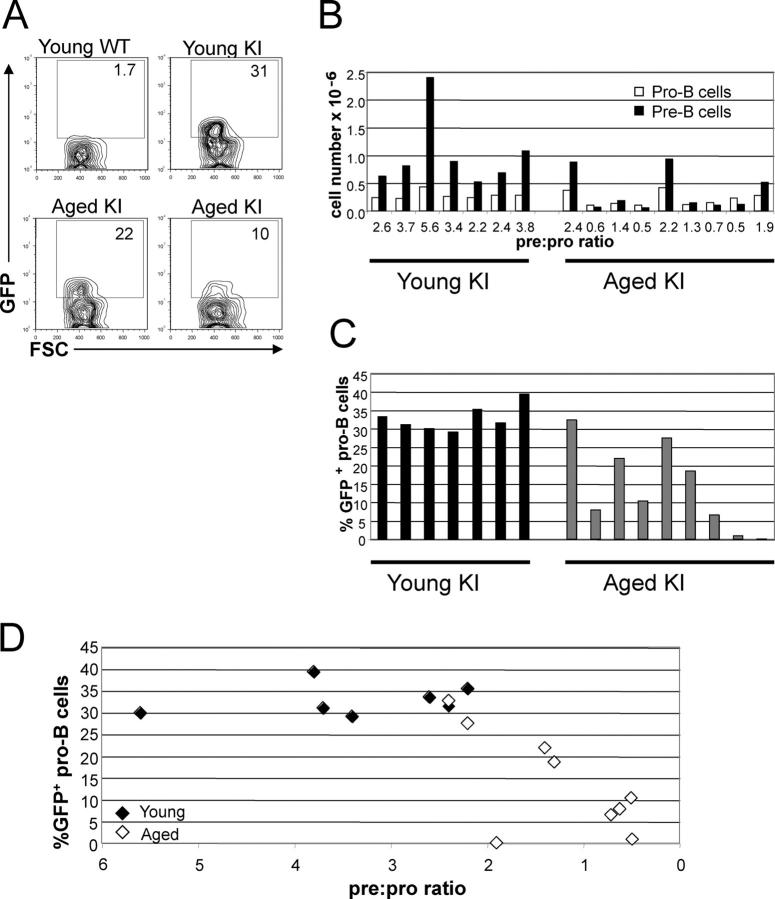
Figure 3.
Reduced RAG2 protein levels in pro–B cells are consistent with lower numbers of pre–B cells in aged RAG2-GFP KI mice. Bone marrow from young (4–7 mo) and aged (23–28 mo) RAG2-GFP KI mice was analyzed by flow cytometry. (A) Representative flow cytometric analysis of GFP in pro–B cells (Ly6C−DX5−IgM−B220+CD43+) of RAG2-GFP KI mice. Bone marrow from young wild type, young RAG2-GFP KI, aged RAG2-GFP KI with a moderate loss of GFP expression, and an aged RAG2-GFP KI with severe loss of GFP expression are displayed. The numbers within the gates depict the percentage of pro–B cells that express GFP. (B) Numbers of pro–B (Ly6C− DX5−IgM−B220+CD43+) and pre–B cells (Ly6C−DX5−IgM−B220+CD43−) in bone marrow of young and aged mice. White bars represent pro–B cells, and black bars represent pre–B cells. The pre:pro ratio was calculated by dividing the number of pre–B cells by the number of pro–B cells. (C) Analysis of GFP in pro–B cells of RAG2-GFP KI mice was conducted as in A. Black bars represent young mice, and gray bars represent aged mice. The percentage of pro–B cells that are GFP+ are displayed for the same mice shown in B. (D) The percentage of pro–B cells that are GFP+ and the ratio of pre–B to pro–B cells are displayed for each KI mouse shown in B and C. Closed diamonds represent young mice, and open diamonds represent aged mice.
As with the NG mice, the percent of pro–B cells that express rag2 was significantly lower in aged mice as compared with young KI mice (mean: 14 vs. 33%; Fig. 3 C and Table III) . Both the NG and KI models demonstrate that rag2 expression in pro–B cells is lower in aged mice. Also, because the KI reporter is a RAG2-GFP fusion protein, the data suggest that protein levels of RAG2 are lower in the pro–B cells of aged mice. The number of pre–B cells was significantly lower in aged (mean: 0.33 × 106 cells) as compared with young KI mice (1.0 × 106 cells; Table III). In addition, the pre:pro ratio was lower in the aged compared with young mice (mean: 1.3 vs. 3.4; Table III). Similar to our data with the NG mice, these results suggest that the reduction in the numbers of pre–B cells in aged mice is not solely due to a reduction in the size of the population of pro–B cells, and may also reflect reduced development of pro–B cells and generation of pre–B cells. Furthermore, we observed that the percent of pro–B cells that express GFP was correlated with both the number of pre–B cells and the pre:pro ratio (Table III). Together, our analyses of both the NG transgenic and RAG2-GFP KI mice indicate that the reduction in numbers of pre–B cells is correlated with reduced rag2 expression in pro–B cells.
Table III.
Statistical Analysis of Data Obtained from Young and Aged RAG2-GFP KI Mice
| Significance of differences | Younga | Ageda | p-value |
|---|---|---|---|
| No. of pro–B cells (millions)b | 0.28 ± 0.07 | 0.21 ± 0.12 | NS |
| No. of pre–B cells (millions)b | 1.0 ± 0.64 | 0.33 ± 0.35 | 0.019 |
| Pre:pro ratioc | 3.4 ± 1.2 | 1.3 ± 0.75 | 0.001 |
| % of pro–B cells that are GFP+ | 33 ± 3.6 | 14 ± 12 | 0.001 |
| Correlations b/t characteristics | n | r | p-value |
| % of pro–B cells that are GFP+ and no. of pre–B cells | 16 | 0.575 | 0.020 |
| % of pro–B cells that are GFP+ and pre:pro ratio | 16 | 0.709 | 0.002 |
Bone marrow from young (4–7 mo) and aged (23–28 mo) RAG2-GFP KI mice was analyzed by flow cytometry and statistical analyses as described in Materials and Methods. In these mice, GFP is a reporter of RAG2 protein levels.
Means ± SD are presented.
Numbers of pro–B (Ly6C−DX5−IgM−B220+CD43+) and pre–B cells (Ly6C−DX5−IgM−B220+CD43−) in bone marrow of young and aged KI mice were determined by multiplying the frequency by the leg bone marrow cell number determined by counting to obtain total numbers.
The pre:pro ratio was calculated by dividing the number of pre–B cells by the number of pro–B cells.
n, no. of mice; r, correlation coefficient.
rag2 Expression, V(D)J Recombinase Activity, and the Pre:Pro Ratio Are Reduced in Aged Mice.
Next, we assessed whether reduced expression of rag2 in aged mice yields a corresponding decrease in recombinase activity using H2-SVEX transgenic mice (25). Cells that undergo V(D)J recombination of the transgenic recombination substrate express the GFP variant VEX (32, 33), are easily detected by flow cytometry, and are readily resolved from cells expressing conventional GFP (32, 33). In addition, the percent of VEX+ cells within a population reflects the level of rag expression (25, 34).
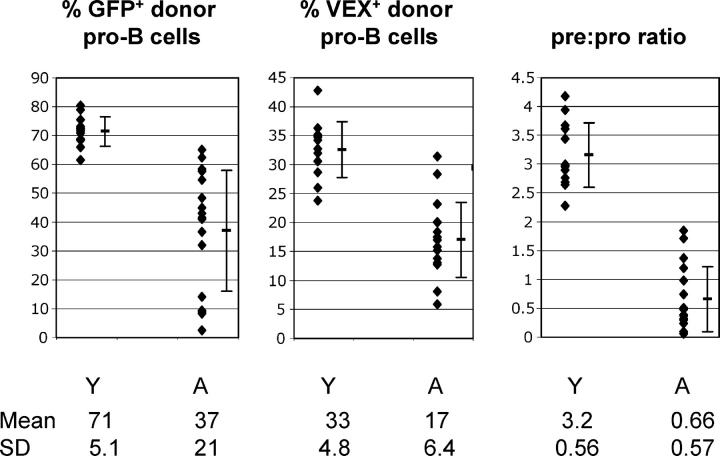
We crossed NG mice to H2-SVEX mice to generate double transgenic mice in which rag2 expression could be measured in conjunction with V(D)J recombinase activity at the single cell level. We used adoptive transfers to evaluate rag2 expression and recombinase activity in young versus aged recipient mice. This provided two advantages as follows: (a) we were able to isolate effects of the aged bone marrow microenvironment independent of cell-intrinsic defects, and (b) this was an expedient alternative to aging double transgenic mice. Whole bone marrow from young NG × H2-SVEX CD45.1 double transgenic mice was transferred into young (3 mo) and aged (28 mo) C57BL/6 CD45.2 irradiated recipient mice. 5–6 wk after transfer, we compared rag2 expression and recombinase activity in donor-derived pro–B cells and the pre:pro ratio of donor-derived cells. The percentage of donor-derived pro–B cells that express rag2 was significantly lower in aged recipients (mean: 37%) as compared with young recipients (mean: 71%; Fig. 4 and Table IV) . In addition, V(D)J recombinase activity, as indicated by the percent of pro–B cells that are VEX+, was also significantly lower in pro–B cells from aged recipients (mean, 17%) as compared with young recipients (mean, 33%; Fig. 4 and Table IV). This is the first analysis of recombinase activity in pro–B cells in aged mice and it suggests that the reduction in rag2 expression in pro–B cells of aged mice is sufficient to result in reduced recombinase activity.
Figure 4.
rag2 expression, V(D)J recombinase activity, and the pre:pro ratio are reduced in aged mice. CD45.1 NG × H2-SVEX double transgenic mice were generated (GFP serves as a reporter of rag2 expression and VEX serves as a reporter of V[D]J recombinase activity) and used as donors for adoptive transfer. Bone marrow was harvested from wild-type CD45.2 young (3–4 mo at time of harvest) and aged (23–29 mo at time of harvest) recipient mice 5–6 wk after adoptive transfer of bone marrow from NG × H2-SVEX CD45.1 mice. Pro–B (IgM−B220+CD43+) and pre–B cells (IgM−B220+CD43−) of donor origin were identified based on expression of CD45.1. The left panel displays the mean, standard deviation, and distribution of the percent of donor origin pro–B cells that are GFP+, whereas the middle panel depicts these values for VEX+ donor pro–B cells. The right panel displays the mean, standard deviation, and distribution of the pre:pro ratio. The pre:pro ratio was calculated by dividing the percent of bone marrow that was donor pre–B cells by the percent of bone marrow that was donor pro–B cells for each recipient mouse. Y, young mice; A, aged mice.
Table IV.
Statistical Analysis of Data Obtained from Adoptive Transfers of NG × H2-SVEX Transgenic Bone Marrow: Young→Aged and Young→Young
| Significance of differences | Young recipienta | Aged recipienta | p-value |
|---|---|---|---|
| % of donor pro–B cells that are GFP+ | 71 ± 5.1 | 37 ± 21 | <0.001 |
| % of donor pro–B cells that are VEX+ | 33 ± 4.8 | 17 ± 6.4 | <0.001 |
| Pre:pro ratiob | 3.2 ± 0.56 | 0.66 ± 0.56 | |
| Log of pre:pro ratioc | 1.1 ± 0.18 | −0.83 ± 1.0 | <0.001 |
| Correlations b/t characteristics, all mice (n = 30) |
log pre:pro ratio | % of pro–B cells that are GFP+ |
% of donor pro–B cells that are VEX+ |
| Log pre:pro ratio | r = 0.812, P < 0.001 | r = 0.860, P < 0.001 | |
| % of pro–B cells that are GFP+ | r = 0.812, P < 0.001 | r = 0.835, P < 0.001 | |
| % of pro–B cells that are VEX+ | r = 0.860, P < 0.001 | r = 0.835, P < 0.001 | |
| Correlations b/t characteristics, aged recipient mice (n = 17) |
log pre:pro ratio | % of pro–B cells that are GFP+ |
% of donor pro–B cells that are VEX+ |
| Log pre:pro ratio | r = 0.550, P = 0.022 | r = 0.670, P = 0.003 | |
| % of pro–B cells that are GFP+ | r = 0.550, P = 0.022 | r = 0.634, P = 0.006 | |
| % of pro–B cells that are VEX+ | r = 0.670, P = 0.003 | r = 0.634, P = 0.006 |
CD45.1 NG × H2-SVEX double transgenic mice (GFP serves as a reporter of rag2 expression, and VEX serves as a reporter of V(D)J recombinase activity) were used as donors for adoptive transfer. Bone marrow was harvested from wild-type young (3–4 mo at time of harvest) and aged (23–29 mo at time of harvest) CD45.2 recipient mice 5–6 wk after adoptive transfer of bone marrow from young (1–3 mo) NG × H2-SVEX CD45.1 mice. Pro–B (IgM−B220+CD43+) and pre–B cells (IgM−B220+CD43−) of donor origin were identified based on expression of CD45.1, using flow cytometry as described in Materials and Methods.
Means ± SD are presented.
The pre:pro ratio was calculated by dividing the number of pre–B cells by the number of pro–B cells. Numbers of pro–B and pre–B cells in bone marrow were determined by multiplying the frequency by the leg bone marrow cell number determined by counting to obtain total numbers.
Log of the pre:pro–B ratio was used because its distribution was normal, whereas the ratio was not.
n, number of mice; r, correlation coefficient.
In addition, we analyzed the pre:pro ratio of CD45.1+ donor-derived cells as a measure of pre–B cell numbers in the young and aged recipients (Fig. 4). The donor pre:pro ratio was significantly lower in the aged (mean: 0.66) compared with the young recipient mice (mean: 3.2; Table IV). The age-associated reduction in rag2 expression, recombinase activity, and pre:pro ratio might be the result of independent age-associated defects, or these could be interrelated. To address this, we conducted statistical analyses to determine if these traits were correlated in the population of mice as a whole (n = 30), and if these correlations were also observed if the analysis was restricted to the aged recipient mice (n = 17). As reported in Table IV, all three parameters were correlated in both the entire population of mice and in the aged group.
The Bone Marrow Microenvironment Also Controls rag2 Expression and V(D)J Recombinase Activity in Pro–B Cells and the Pre:Pro Ratio.
We used adoptive transfers again to determine if these age-associated alterations are microenvironmental versus cell intrinsic. We observed that young donor-derived pro–B cells displayed lower rag2 expression and recombinase activity and there was a lower pre:pro ratio after bone marrow transfer into aged as compared with young recipient mice (Fig. 4). This indicates that age-associated alterations specific to the bone marrow microenvironment are sufficient to produce these defects in B cell development.
The presence of defects in B cell development due to changes in the bone marrow microenvironment does not preclude the existence of age-related, cell-intrinsic defects. To determine if cell-intrinsic defects affect rag2 expression in pro–B cells and the pre:pro ratio, we conducted adoptive transfer experiments in which bone marrow from aged and young donor mice was transferred into young recipient mice. The NG mice shown in Fig. 1 (four out of the six young and all of the aged) were used as sources of bone marrow for adoptive transfer into irradiated young wild-type recipient mice. Each recipient mouse received an adoptive transfer from either one young or one aged donor. Thus, progenitors from young and aged mice were evaluated after differentiation within the same microenvironment; the bone marrow of a young recipient.
Before adoptive transfer, all five of the aged donor mice displayed significantly lower pre:pro ratios (mean: 1.7) than those of the young donor mice (mean: 3.7; Fig. 5 A and Table V) . The percentage of pro–B cells that express rag2 was also significantly lower in the five aged donor mice (mean: 45%) than in the young donor mice (mean: 75%; Fig. 5 A and Table V). 5 wk after adoptive transfer, B cell development and rag2 expression in young recipient mice was assessed by flow cytometry. In this experiment, a marker of donor cells was not available. However, efficient engraftment must have occurred for the following reasons: (a) recipients survived lethal irradiation; (b) in comparable experiments, engraftment in young mice routinely resulted in >90% pro–B cells of donor origin; and (c) the expression of GFP in pro–B cells (which clearly identifies cells of NG transgenic donor origin) was high (60–90% of pro–B cells) in bone marrow harvested from recipient mice (Fig. 5 B). We found that rag2 expression in young recipient mice was not affected by the age of the bone marrow donors (Fig. 5 B and Table V). The mean percentage of GFP+ pro–B cells was 82% in mice that received bone marrow from young donors or from aged donors (Fig. 5 B and Table V). The degree of diminished rag2 expression in pro–B cells of particular aged donors was not reflected in recipient mice. Of the donors, mouse A3 displayed the lowest percentage of pro–B cells with rag2 expression (<15%; Fig. 5 A). In the two young mice that received bone marrow from A3, rag2 was expressed in >70% of pro–B cells and was comparable to that of other recipient young mice (Fig. 5 B). These observations indicate that the reduction in rag2 expression in pro–B cells of aged mice is not likely to be the result of defects that are intrinsic to the developing cells.
Table V.
Statistical Analysis of Data Obtained from Adoptive Transfer of NG Transgenic Bone Marrow: Aged→Young and Young →Young
| Significance of differences: donor micea | Young donorb | Aged donorb | p-value |
|---|---|---|---|
| Pre:pro ratio | 3.7 ± 0.41 | 1.7 ± 0.94 | 0.006 |
| % of pro–B cells that are GFP+ | 75 ± 2.5 | 45 ± 19 | 0.023 |
| Significance of differences: young recipient micec | |||
| Pre:pro ratio | 1.7 ± 0.58 | 1.7 ± 0.40 | NS |
| % of pro–B cells that are GFP+ | 82 ± 9.8 | 82 ± 9.3 | NS |
Bone marrow from young (3 mo) and aged (26–27 mo) NG mice were transferred into lethally irradiated young (2 mo at time of transfer) recipient mice.
GFP expression in pro–B cells and pre:pro ratio in young and aged donor mice at time of transfer. Pre–B cells (B220LOCD43−AA4.1+CD24++) and pro–B cells (B220LOCD43+AA4.1+) were evaluated by flow cytometry as described in Materials and Methods.
Mean ± SD are presented.
Analyses of bone marrow harvested from young recipient mice 5 wk after transfer of bone marrow cells from either young or aged donors. Pro–B cells were defined as IgM−B220+CD43+AA4.1+ and pre–B cells were defined as IgM−B220+CD43−AA4.1+CD24++. The pre:pro ratio of two young unmanipulated NG mice used as controls in this experiment were 1.2 and 1.3.
The pre:pro ratios were also compared in young reconstituted mice and were not significantly different due to the age of bone marrow donors (Table V). The mean pre:pro ratio was 1.7 in mice that received bone marrow from either aged or young donors (Fig. 5 B and Table V) In addition, the severity of the age-related defect in individual mice was not reflected after adoptive transfer into young hosts. Aged donor mice A1, A2, and A3 displayed the lowest pre:pro ratios (Fig. 5 A), yet the seven young recipients of bone marrow from these mice displayed pre:pro ratios that were as high as those observed in mice that received bone marrow from young donors (Fig. 5 B). This suggests that the age-related reduction in the pre:pro ratio is not the result of cell-intrinsic defects in hematopoietic precursors.
Discussion
These studies provide a direct assessment of turnover and production rates among B lineage progenitors in the marrow of aged mice. The results indicate that both the number and production rate of pre–B cells are reduced approximately fourfold compared with young adults. Because the proportion of cycling pre–B cells is similar in aged and young individuals, this difference reflects increased losses during passage from the pro–B to pre–B pool. Although pro–B cells from aged individuals clearly display impaired proliferative capacity in vitro (20), the similar proportions of cycling cells within fraction C′ suggest this impairment is unlikely the basis for reduced pre–B cell numbers in vivo. Furthermore, a shortened renewal pre–B cell rate was not observed (Table I), arguing against an increased attrition rate within the pre–B compartment. Thus, when considered together with the similar proportional labeling kinetics and reduced absolute production rates, our observations are most consistent with increased attrition as cells transit from the pro–B to pre–B compartment.
The transition into the pre–B compartment requires productive assembly of an IgH gene. Reduced expression of rag1 and rag2 could limit IgH assembly, although it was previously unclear whether the decline in rag1 and rag2 expression in total bone marrow reflects changes in gene expression or simply the loss of pre–B cells (6, 11, 13, 17, 18). Using a flow cytometric approach to evaluate rag2 gene expression at the single cell level, we found the frequency of pro–B cells that express rag2 is reduced, and that this is correlated with reduced numbers of pre–B cells, suggesting that the age-related reduction in pre–B cell numbers could be the direct result of diminished rag2 expression. We used H2-SVEX mice to measure V(D)J recombinase activity at the single cell level. Using NG × H2-SVEX double transgenic mice as donors, we demonstrate that recombinase activity is reduced in pro–B cells that develop in aged mice. This supports the hypothesis that reduced rag2 expression is of sufficient magnitude to reduce recombinase activity, potentially limiting heavy chain rearrangement, pre-BCR expression, and subsequent transit to the pre–B cell stage. Our findings of fewer pro–B cells and reduced rag2 expression and recombinase activity in pro–B cells from aged mice supports a model in which reduced recombination of IgH might compromise both the pro–B cell compartment and the pro–B to pre–B cell transition. Specific attrition of later-stage pro–B cells was first noted by Van der Put et al. (19) and pro–B cell reduction was recently demonstrated by Miller et al. (30). We also observed altered distribution of cells within the pro–B cell stage, as indicated by the reduced percentage of CD24high cells in aged mice (Table II), and decreases in the number of pro–B cells (Fig. 1 and Tables I and II). Failure to rearrange IgH is predicted to deplete CD24high pro–B cells and limit proliferation of pre–BCR+ Fr C′ cells.
Before our investigation, it was unclear if altered B cell development in aged mice was due to changes in the bone marrow hematopoietic microenvironment or to intrinsic changes in developing precursor cells. The reciprocal bone marrow chimera experiments herein show that the aged marrow microenvironment is sufficient to produce reduced rag2 activity in pro–B cells, reduced pre:pro ratios, and reduced pre–B cell generation rates, whereas these parameters were similar when developing in young hosts regardless of donor age. These experiments indicate that the age-associated defects in rag2 expression, recombinase activity in pro–B cells, and the generation of pre–B cells are the result of alterations in the bone marrow microenvironment and are unlikely to result from cell-intrinsic alterations. In these and additional studies (unpublished data), cells from old mice transferred to young hosts express normal levels of rag2 and generate normal numbers of pre–B cells and mature peripheral B cell subsets. It will be of interest to determine whether this restoration also recapitulates complete B cell function and Ig repertoires. The bone marrow microenvironment includes factors derived from bone marrow stromal cells that are essential to the development of B cell precursors. Specific extrinsic factors that induce rag expression have yet to be identified; the age-related decrease in rag2 expression in pro–B cells might reflect attenuation of yet unknown inductive signals. Reduced numbers of either stromal cells or specific cellular niches for B cell development (35) could also contribute to reduced pre–B cell generation rates. In aged mice, the percentage of pro–B cells that express rag2 is reduced. However, in the pro–B cells that do express rag2, the level of expression per individual cell is similar to that of young mice. This could indicate that fewer pro–B cells receive signals and factors from the stromal microenvironment, but those that do receive the required factors develop normally. We cannot exclude the possibility that additional host-derived factors contribute to the marrow environment. However, in contrast with a published paper (11), we find B cell development and recombinase activity are normal in completely T cell–deficient mice (TCRβ−/−δ−/−H2-SVEX mice; unpublished data). Therefore, it seems unlikely that the decline in peripheral T cells with age limits bone marrow environmental support of B cell development.
Reduced numbers of pre–B cells in aged mice may also stem from a reduced expression of essential transcription factors during the pro–B cell stage. At this stage, pax5, E2A, and EBF govern commitment and development by controlling expression of B cell–specific genes that are important for the pro–B to pre–B transition. In particular, the E2A gene product E47 is essential for survival and proliferation of B lineage cells. E47 binds to the Erag enhancer required for expression of rag1 and rag2 in pro–B cells (36). E47 also regulates expression of λ5 (37). Protein levels of E47 are reduced in pro–B cells of aged mice, and this reduction is correlated with a reduction in λ5 expression (21, 38). It is possible that reduced E2A expression could attenuate B cell development due to reduced expression of both rag and λ5. (39)
Our results indicate that fewer cells in the pro–B cell stage of development have an active V(D)J recombinase in aged mice. Consistent with this, Szabo et al. noted fewer cleaved DFL16.1 signal ends in pro–B cells from aged mice (40). Thus, reduced recombinase activity could contribute to reduced diversity of IgH chains and, thus, compromise the Ig repertoire in aged mice. Previous work has shown that receptor diversity is reduced in aged mice (3, 9, 14, 41–46). Interestingly, lower enzymatic activity of RAG in rag “core-only” KI mice resulted in reduced VH to DJH joining and an altered repertoire (47–49). Alternatively, the reduced rag2 expression and V(D)J recombinase activity could support formation of a normal repertoire, but with decreased efficiency in the generation of pre–B cells. The immature B cell pool in aged mice is somewhat reduced, but not to the extent that might be predicted based on the diminution in the pre–B cell pool. This appears to reflect lengthened average residency time within this pool because the immature B cell renewal rate is twofold slower (Fig. 1 and Table I). Together, our data best support a model in which reduced V(D)J recombinase activity in the pro–B cell stage alters the formation and possibly the diversity of the IgH repertoire.
Our results demonstrate that the previously documented reduction in numbers of pre–B cells is linked to reduced generation of pre–B cells that occur concurrent with changes in pro–B cells that include reduced rag2 expression and V(D)J recombinase activity. Alterations in the aged bone marrow microenvironment are sufficient to produce these defects independent of cell-intrinsic alterations, although the nature of these changes remains to be determined.
Acknowledgments
We are grateful to M. Nussenzweig for providing NG transgenic mice and F. Alt for supplying RAG2:GFP knockin mice. We thank B. Blomberg, R. Woodland, L. Schmidt, L. Borghesi, and J. Stavnezer for helpful discussions and critical reading of the paper, and the University of Massachusetts Medical School (UMMS) Flow Cytometry Core for their expert assistance.
This work was supported in part by a grant from the Lucille P. Markey Charitable trust (to M.P. Cancro); and U.S. Health Public Service grants AG15623 (to M.P. Cancro), AI043534 and AG19042 (to R.M. Gerstein), and National Institute of Diabetes and Digestive and Kidney Diseases 5 P30 DK32520 to the UMMS Diabetes and Endocrinology Center.
The authors have no conflicting financial interests.
Abbreviation used in this paper: KI, knockin.
References
- 1.Sanchez, M., K. Lindroth, E. Sverremark, A. Gonzalez Fernandez, and C. Fernandez. 2001. The response in old mice: positive and negative immune memory after priming in early age. Int. Immunol. 13:1213–1221. [DOI] [PubMed] [Google Scholar]
- 2.Lu, Y.F., and J. Cerny. 2002. Repertoire of antibody response in bone marrow and the memory response are differentially affected in aging mice. J. Immunol. 169:4920–4927. [DOI] [PubMed] [Google Scholar]
- 3.Miller, R.A. 1996. The aging immune system: primer and prospectus. Science. 273:70–74. [DOI] [PubMed] [Google Scholar]
- 4.Looney, R.J., M.S. Hasan, D. Coffin, D. Campbell, A.R. Falsey, J. Kolassa, J.M. Agosti, G.N. Abraham, and T.G. Evans. 2001. Hepatitis B immunization of healthy elderly adults: relationship between naive CD4+ T cells and primary immune response and evaluation of GM-CSF as an adjuvant. J. Clin. Immunol. 21:30–36. [DOI] [PubMed] [Google Scholar]
- 5.Lucas, A.H., and D.C. Reason. 1998. Aging and the immune response to the Haemophilus influenzae type b capsular polysaccharide: retention of the dominant idiotype and antibody function in the elderly. Infect. Immun. 66:1752–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Yehuda, A., P. Szabo, R. Dyall, and M.E. Weksler. 1994. Bone marrow declines as a site of B-cell precursor differentiation with age: relationship to thymus involution. Proc. Natl. Acad. Sci. USA. 91:11988–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Yehuda, A., P. Szabo, and M.E. Weksler. 1994. Age-associated changes in the B-cell repertoire: effect of age on RAG-1 gene expression in murine bone marrow. Immunol. Lett. 40:287–289. [DOI] [PubMed] [Google Scholar]
- 8.Hu, A., D. Ehleiter, A. Ben-Yehuda, R. Schwab, C. Russo, P. Szabo, and M.E. Weksler. 1993. Effect of age on the expressed B cell repertoire: role of B cell subsets. Int. Immunol. 5:1035–1039. [DOI] [PubMed] [Google Scholar]
- 9.LeMaoult, J., P. Szabo, and M.E. Weksler. 1997. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol. Rev. 160:115–126. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk-Hard, I., I. Soderstrom, S. Feld, D. Holmberg, and I. Lundkvist. 1997. Age-related impaired affinity maturation and differential D-JH gene usage in human VH6-expressing B lymphocytes from healthy individuals. Eur. J. Immunol. 27:1381–1386. [DOI] [PubMed] [Google Scholar]
- 11.Szabo, P., K. Zhao, I. Kirman, J. LeMaoult, R. Dyall, W. Cruikshank, and M.E. Weksler. 1998. Maturation of B cell precursors is impaired in thymic-deprived nude and old mice. J. Immunol. 161:2248–2253. [PubMed] [Google Scholar]
- 12.Szabo, P., S. Shen, and M.E. Weksler. 1999. Age-associated defects in B lymphocyte development. Exp. Gerontol. 34:431–434. [DOI] [PubMed] [Google Scholar]
- 13.Klinman, N.R., and G.H. Kline. 1997. The B-cell biology of aging. Immunol. Rev. 160:103–114. [DOI] [PubMed] [Google Scholar]
- 14.Kline, G.H., T.A. Hayden, and N.R. Klinman. 1999. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J. Immunol. 162:3342–3349. [PubMed] [Google Scholar]
- 15.Oettinger, M.A., D.G. Schatz, C. Gorka, and D. Baltimore. 1990. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 248:1517–1523. [DOI] [PubMed] [Google Scholar]
- 16.Schatz, D.G., M.A. Oettinger, and D. Baltimore. 1989. The V(D)J recombination activating gene, RAG-1. Cell. 59:1035–1048. [DOI] [PubMed] [Google Scholar]
- 17.Riley, R.L., M.G. Kruger, and J. Elia. 1991. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin. Immunol. Immunopathol. 59:301–313. [DOI] [PubMed] [Google Scholar]
- 18.Stephan, R.P., V.M. Sanders, and P.L. Witte. 1996. Stage-specific alterations in murine B lymphopoiesis with age. Int. Immunol. 8:509–518. [DOI] [PubMed] [Google Scholar]
- 19.Van der Put, E., E.M. Sherwood, B.B. Blomberg, and R.L. Riley. 2003. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation and apoptosis. Exp. Gerontol. 38:1137–1147. [DOI] [PubMed] [Google Scholar]
- 20.Stephan, R.P., D.A. Lill-Elghanian, and P.L. Witte. 1997. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J. Immunol. 158:1598–1609. [PubMed] [Google Scholar]
- 21.Sherwood, E.M., W. Xu, A.M. King, B.B. Blomberg, and R.L. Riley. 2000. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech. Ageing Dev. 118:45–59. [DOI] [PubMed] [Google Scholar]
- 22.Stephan, R.P., C.R. Reilly, and P.L. Witte. 1998. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 91:75–88. [PubMed] [Google Scholar]
- 23.Yu, W., Z. Misulovin, H. Suh, R.R. Hardy, M. Jankovic, N. Yannoutsos, and M.C. Nussenzweig. 1999. Coordinate regulation of RAG1 and RAG2 by cell type-specific DNA elements 5′ of RAG2. Science. 285:1080–1084. [DOI] [PubMed] [Google Scholar]
- 24.Monroe, R.J., K.J. Seidl, F. Gaertner, S. Han, F. Chen, J. Sekiguchi, J. Wang, R. Ferrini, L. Davidson, G. Kelsoe, and F.W. Alt. 1999. RAG2:GFP knockin mice reveal novel aspects of RAG2 expression in primary and peripheral lymphoid tissues. Immunity. 11:201–212. [DOI] [PubMed] [Google Scholar]
- 25.Borghesi, L., L.Y. Hsu, J.P. Miller, M. Anderson, L. Herzenberg, M.S. Schlissel, D. Allman, and R.M. Gerstein. 2004. B lineage–specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J. Exp. Med. 199:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allman, D.M., S.E. Ferguson, V.M. Lentz, and M.P. Cancro. 1993. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J. Immunol. 151:4431–4444. [PubMed] [Google Scholar]
- 27.Lentz, V.M., M.P. Cancro, F.E. Nashold, and C.E. Hayes. 1996. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J. Immunol. 157:598–606. [PubMed] [Google Scholar]
- 28.Allman, D.M., S.E. Ferguson, and M.P. Cancro. 1992. Peripheral B cell maturation. I. Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J. Immunol. 149:2533–2540. [PubMed] [Google Scholar]
- 29.Sprent, J., and D.F. Tough. 1994. Lymphocyte life-span and memory. Science. 265:1395–1400. [DOI] [PubMed] [Google Scholar]
- 30.Hardy, R.R., C.E. Carmack, S.A. Shinton, J.D. Kemp, and K. Hayakawa. 1991. Resolution and characterization of pro–B and pre-pro–B cell stages in normal mouse bone marrow. J. Exp. Med. 173:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson, M.T., N. Baumgarth, R.P. Haugland, R.M. Gerstein, T. Tjioe, and L.A. Herzenberg. 1998. Pairs of violet-light-excited fluorochromes for flow cytometric analysis. Cytometry. 33:435–444. [DOI] [PubMed] [Google Scholar]
- 32.Anderson, M.T., I.M. Tjioe, M.C. Lorincz, D.R. Parks, L.A. Herzenberg, and G.P. Nolan. 1996. Simultaneous fluorescence-activated cell sorter analysis of two distinct transcriptional elements within a single cell using engineered green fluorescent proteins. Proc. Natl. Acad. Sci. USA. 93:8508–8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borghesi, L., and R.M. Gerstein. 2004. Developmental separation of V(D)J recombinase expression and initiation of IgH recombination in B lineage progenitors in vivo. J. Exp. Med. 199:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J.P., and D. Allman. 2003. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J. Immunol. 171:2326–2330. [DOI] [PubMed] [Google Scholar]
- 35.Tokoyoda, K., T. Egawa, T. Sugiyama, B.I. Choi, and T. Nagasawa. 2004. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 20:707–718. [DOI] [PubMed] [Google Scholar]
- 36.Hsu, L.Y., J. Lauring, H.E. Liang, S. Greenbaum, D. Cado, Y. Zhuang, and M.S. Schlissel. 2003. A conserved transcriptional enhancer regulates RAG gene expression in developing B cells. Immunity. 19:105–117. [DOI] [PubMed] [Google Scholar]
- 37.Sigvardsson, M., M. O'Riordan, and R. Grosschedl. 1997. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 7:25–36. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood, E.M., B.B. Blomberg, W. Xu, C.A. Warner, and R.L. Riley. 1998. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. J. Immunol. 161:4472–4475. [PubMed] [Google Scholar]
- 39.Frasca, D., E. Van Der Put, R.L. Riley, and B.B. Blomberg. 2004. Age-related differences in the E2A-encoded transcription factor E47 in bone marrow-derived B cell precursors and in splenic B cells. Exp. Gerontol. 39:481–489. [DOI] [PubMed] [Google Scholar]
- 40.Szabo, P., S. Shen, W. Telford, and M.E. Weksler. 2003. Impaired rearrangement of IgH V to DJ segments in bone marrow pro-B cells from old mice. Cell. Immunol. 222:78–87. [DOI] [PubMed] [Google Scholar]
- 41.Ghia, P., E. ten Boekel, A.G. Rolink, and F. Melchers. 1998. B-cell development: a comparison between mouse and man. Immunol. Today. 19:480–485. [DOI] [PubMed] [Google Scholar]
- 42.Ghia, P., F. Melchers, and A.G. Rolink. 2000. Age-dependent changes in B lymphocyte development in man and mouse. Exp. Gerontol. 35:159–165. [DOI] [PubMed] [Google Scholar]
- 43.Sambhara, S., A. Kurichh, R. Miranda, O. James, B. Underdown, M. Klein, J. Tartaglia, and D. Burt. 2001. Severe impairment of primary but not memory responses to influenza viral antigens in aged mice: costimulation in vivo partially reverses impaired primary immune responses. Cell. Immunol. 210:1–4. [DOI] [PubMed] [Google Scholar]
- 44.Callahan, J.E., J.W. Kappler, and P. Marrack. 1993. Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 151:6657–6669. [PubMed] [Google Scholar]
- 45.Zheng, B., S. Han, Y. Takahashi, and G. Kelsoe. 1997. Immunosenescence and germinal center reaction. Immunol. Rev. 160:63–77. [DOI] [PubMed] [Google Scholar]
- 46.Riley, S.C., B.G. Froscher, P.J. Linton, D. Zharhary, K. Marcu, and N.R. Klinman. 1989. Altered VH gene segment utilization in the response to phosphorylcholine by aged mice. J. Immunol. 143:3798–3805. [PubMed] [Google Scholar]
- 47.Dudley, D.D., J. Sekiguchi, C. Zhu, M.J. Sadofsky, S. Whitlow, J. DeVido, R.J. Monroe, C.H. Bassing, and F.W. Alt. 2003. Impaired V(D)J recombination and lymphocyte development in core RAG1-expressing mice. J. Exp. Med. 198:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akamatsu, Y., R. Monroe, D.D. Dudley, S.K. Elkin, F. Gartner, S.R. Talukder, Y. Takahama, F.W. Alt, C.H. Bassing, and M.A. Oettinger. 2003. Deletion of the RAG2 C terminus leads to impaired lymphoid development in mice. Proc. Natl. Acad. Sci. USA. 100:1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang, H.E., L.Y. Hsu, D. Cado, L.G. Cowell, G. Kelsoe, and M.S. Schlissel. 2002. The “dispensable” portion of RAG2 is necessary for efficient V-to-DJ rearrangement during B and T cell development. Immunity. 17:639–651. [DOI] [PubMed] [Google Scholar]
- 50.Opstelten, D., and D.G. Osmond. 1983. Pre-B cells in mouse bone marrow: immunofluorescence stathmokinetic studies of the proliferation of cytoplasmic mu-chain-bearing cells in normal mice. J. Immunol. 131:2635–2640. [PubMed] [Google Scholar]