Abstract
Human immunodeficiency virus (HIV)-specific CD8+ T cells persist in high frequencies in HIV-infected patients despite impaired CD4+ T helper response to the virus, but, unlike other differentiated effector cytotoxic T lymphocytes, most continue to express the tumor necrosis factor receptor family member CD27. Because the ligand for CD27 (CD70) is also overexpressed in HIV-infected hosts, we examined the nature of expression and potential functional consequences of CD27 expression on HIV-specific CD8+ T cells. Analysis of CD27+ and CD27− T cells derived from the same HIV-specific clone revealed that retention of CD27 did not interfere with acquisition of effector functions, and that after T cell receptor stimulation, CD27+ cells that concurrently were triggered via CD27 exhibited more resistance to apoptosis, interleukin 2 production, and proliferation than CD27− T cells. After transfer back into an HIV-infected patient, autologous HIV-specific CD27− T cells rapidly disappeared, but CD27+ T cells derived from the same clone persisted at high frequency. Our findings suggest that the CD27–CD70 interaction in HIV infection may provide CD27+ CD8+ T cells with a survival advantage and compensate for limiting or absent CD4+ T help to maintain the CD8 response.
Keywords: adoptive immunotherapy, viruses, immunologic memory, TNFR, IL-2
Introduction
The induction and maintenance of a virus-specific cytotoxic CD8+ T cell response is sufficient for control of most chronic viral infections (1, 2), but is not adequate with HIV. Despite a numerically large HIV-specific CD8+ T cell response during much of the chronic phase of infection, viral replication persists (3–5) and ultimately infection progresses to AIDS (6–9). The failure of CD8+ cells to contain HIV reflects evasion by the virus, resulting in part from the high mutation rate and down-regulation of class I antigen expression in infected cells (10–12), as well as preferential depletion of the HIV-specific CD4+ T helper cell response, which is likely essential as demonstrated in other chronic viral infections to maintain an effective CD8+ response (13–16). Analysis of the responding CD8+ T cells (4, 17–19) has also suggested that abnormalities in function and differentiation of HIV-specific CD8+ T cells may contribute to inadequate control. HIV-specific CD8+ T cells isolated from peripheral blood of infected individuals do not directly ex vivo efficiently lyse target cells. The impaired cytolytic activity, which can be partially restored by exposure to exogenous IL-2 (17), is associated with decreased expression both of key signaling molecules such as CD3ζ and CD28, and of perforin (4, 18–20). The majority of HIV-specific effector cells also continue to express CD27, and it has been suggested that such incomplete differentiation of HIV-reactive CD8+ T cells may contribute to the observed functional impairment (4, 20–22).
A largely linear differentiation pathway of human CD8+ T cells from naive cells to memory and effector cells has been proposed, based in large part on expression of a panel of cell surface molecules (20, 23, 24). Naive CD8+ T cells are CD45RA+28+27+CCR7+ and respond to antigen stimulation by extensively proliferating, forming a population of “central” (central–memory T cells) or early memory cells that can efficiently localize in lymphoid nodes, express the CD45RO isoform, express low levels of granzyme but not perforin, and have high proliferative capacity but low cytolytic activity. Further stimulation of central–memory T cells leads to proliferation with both self-renewal and production of intermediate memory cells that have lost CCR7 and CD28 expression, and then to “effector” (effector–memory T cells) or late memory cells that lose CD27 expression, exhibit a lower proliferative capacity but high levels of granzyme and perforin, and are directly cytolytic. These CD45RO+28−27−CCR7− effector–memory T cells can be further driven by antigen stimulation to down-regulate CD45RO and reexpress CD45RA, becoming “terminally differentiated” effector cells (23, 25). Maturation of murine CD8+ T cells in response to model infections has revealed mostly similar phenotypic changes, with the precise effector functions of the originating central–memory T cells still controversial (26). This model has proven generally descriptive of the temporal evolution of human CD8+ responses, although some phenotypic exceptions have been identified (27). In HIV infection, however, the pattern appears disrupted. Despite persistent stimulation with virus, the majority of HIV-specific CD8+ T cells recoverable from peripheral blood have retained expression of CD27 and are CD45RO+28−27+ CCR7−, which has been interpreted as reflecting a failure to complete the differentiation process (20, 21). The basis for such a differentiation block, as well as the potential detrimental consequences of retained CD27 expression, still remain to be elucidated.
Although persistent CD27 expression may serve as a “marker” for impaired effector function of HIV-specific CTLs, recent analyses have provided evidence that the CD27–CD70 interaction provides a costimulatory signal to T cells. CD27 is a member of the TNFR family, and interaction with its transmembrane ligand CD70, which is expressed on activated T and B cells and potentially dendritic cells (28, 29), induces a costimulatory signal that activates NF-κB, promotes survival, enhances TCR-mediated proliferative signals, and increases effector function (30–32). CD70 is overexpressed in HIV-infected patients, and persistent signaling to CD27+ HIV-specific effector cells in infected hosts might theoretically lead either to improved T cell function (30–32) or to T cell dysfunction (32, 33), probably depending on the amount, duration, and timing of the CD70 signal provided. An alternative hypothesis for CD27 expression has been proposed in which differentiation of the responding CD8+ population may reflect unique properties of the particular viral infection (20). For example, in CMV infection the majority of virus-specific memory cells are CD27−, whereas in EBV the memory population contains CD27− cells but is enriched for CD27+ cells (20, 34). However, how different viruses could elicit or select distinct differentiation patterns and the phenotype of cells actually mediating the effector functions have not been resolved.
In this work, we have monitored CD27 expression after cloning and repetitive stimulation of initially CD27+ HIV-specific CD8+ T cells, and compared the CD27+ and CD27− subpopulations derived from the same parental clone. Ligation of surface CD27 by CD70 in association with a stimulatory TCR signal led to down-regulation of CD27, which was transient for most cells, but a substantial fraction of CD27+ cells permanently converted to CD27− cells with each successive stimulation. The cells retaining CD27 expression exhibited normal effector functions compared with the CD27− population. Moreover, the CD27+ cells produced IL-2 and exhibited improved survival and a greater proliferative response in vitro if a CD27 costimulatory signal was delivered with the TCR signal. After adoptive transfer of a mixed population into an HIV-infected individual, the CD27+ subset, but not the CD27− subset, derived from the same clone persisted in vivo. The results suggest that the predominance of CD27+ CD8+ HIV-reactive T cells in infected individuals may not reflect an abnormality in differentiation, but rather an appropriate compensatory event reflecting the survival and proliferative advantages of such cells after target recognition, particularly in a CD4-deficient environment, which likely serves to enhance and sustain the antiviral response.
Materials and Methods
Clinical Protocol and Patient Characteristics.
Patient number 15249 was a participant in Protocol 1313, which was approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, the Food and Drug Administration, and the Recombinant DNA Advisory Committee. Patient entry criteria included the following: HIV seropositive by Western blot, no prior history of opportunistic infections, CD4+ T cell count ≥200 cells/mm3 at the time T cell cultures were initiated, and maintenance of a stable regimen of antiretroviral therapy for at least 4 wk before study entry. Plasma HIV RNA was determined by quantitative PCR by the Roche Amplicor RNA PCR assay.
Isolation, Expansion, and Characterization of HIV-, EBV-, and CMV-specific CD8+ CTL Clones.
HIV gag– and CMV-specific CD8+ CTL clones were isolated and characterized as described previously (35). PBMCs were stimulated twice with either autologous UV-inactivated vac/gag-infected adherent cells, CMV-infected fibroblasts, or dendritic cells pulsed with the HLA-A2–restricted EBV peptide (GLCTLVAML) from the BMFL1 protein, and responding cells were cloned by plating at 0.3 cells/well and stimulating with anti-CD3 and IL-2 in the presence of irradiated lymphoblastoid cell lines (LCLs) as accessory cells.
The antigen specificity and MHC class I restriction of the HIV gag–specific CTL clones was determined in a standard 5-h chromium release assay using as target cells autologous and partially class I MHC–matched allogeneic LCLs that were either mock infected, or infected with a vaccinia recombinant virus expressing either HIV gag or a control CMV protein. Samples were assayed at an effector–target ratio of 2.5:1. Epitope mapping was performed by pulsing autologous LCL target cells with overlapping 20- or 15-mer peptides, and then synthesizing smaller peptides to define the minimal epitope.
FACS Analysis of Antigen Expression, Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Dilution, and Apoptosis.
Anti–TCR-α/β-FITC, anti–CD45RA-PE, anti–CD45RO-PE, anti–CD38-PE, anti–CD69-PE, anti–Ki67-PE, anti–CCR7-PE, anti-CD62L, anti–Bcl-2–FITC, anti–CD95-PE, anti–CD28-APC, anti–annexin V–FITC, anti–perforin-PE, anti–granzyme A–PE, anti–CD27-PE, and anti–CD70-FITC antibodies were from BD Biosciences; anti–CD3ζ-FITC antibody was from Santa Cruz Biotechnology, Inc.; and anti–proliferating cell nuclear antigen antibody was from DakoCytomation. Permeabilization buffers were from BD Biosciences. Perforin staining was performed after methanol-fixation and triton permeabilization. Purified stimulatory anti-CD27 mAb and blocking anti-CD70 mAb were from Immunotech. Analyses of intracellular and surface staining were performed with a FACSCalibur (Becton Dickinson) and sorting was performed with a FACSVantage (Becton Dickinson).
T cells were labeled with CFSE (Sigma-Aldrich) in PBS at a concentration of 2 × 106 cells/ml for 10 min at 37°C, followed by blocking with 10% FCS. 30 ng/ml anti-CD3 and 10 mg/ml anti-CD27 mAb were adhered overnight to 96-well plates and CFSE-labeled CTL clones were added at 5 × 104 cells/well. Three wells with identical conditions were pooled on day 7 for FACS analysis. Similarly, 10 μg anti-CD3 mAb and 10 μg anti-CD27 were coated to 96-well tissue culture plates overnight. CD27+ and CD27− T cells were incubated in the coated plates for 16–18 h in the presence or absence of 2.5 μg/ml of soluble anti-FAS IgM (BD Biosciences).
Proliferation Assays.
96-well tissue culture plates were coated with the indicated amounts of anti-CD3 mAb (OKT3) and anti-CD27 antibody overnight, washed, and 5 × 104 cells were added to each well. For antigen-specific stimulation, autologous LCLs were inactivated with 50 ug/ml mitomycin C for 1 h at 37°C, washed three times, labeled with peptide for 3 h, and plated at 5 × 104 cells/well in 96-well plates. 2 × 105 responder cells were added in duplicate or triplicate wells and 5 μg/ml anti–IL-2 receptor β chain mAb (R&D Systems) was added to selected wells. Cultures were pulsed with 1 μCi [3H]TdR for the final 18 h of a 90-h assay.
Cytokine Production.
Cytokine production was assessed after stimulation as described above with either anti-CD3 mAb/anti-CD27 mAb stimulation or peptide stimulation. IL-2 production was determined after 36 h either by ELISA (R&D Systems) or by FACS using the CBA kit (BD Biosciences). Intracellular IFN-γ production was assessed after stimulation of T cells with autologous peptide-pulsed LCLs for 5 h in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich). 10 ng/ml PMA- and 1 μg/ml ionomycin-treated (both from Sigma-Aldrich) T cells were used as positive controls.
Telomere Length Analysis by Fluorescence In Situ Hybridization and Flow Cytometry.
The average length of telomere repeats in individual lymphocytes was measured by automated flow FISH as described previously (36). Cells were hybridized with or without 0.3 μg/ml of telomere-specific FITC-conjugated (C3TA2) PNA probe (provided by Applied Biosystems), washed, and counterstained with 0.01 μg/ml LDS 751 (Exciton Chemical Co. Inc.). To convert the specific fluorescence (fluorescence measured in cells hybridized with the FITC-labeled telomere PNA probe minus the autofluorescence of unstained cells) into kilobase telomere length, an internal standard (cow thymocytes) with a known telomere length was processed and analyzed simultaneously with each sample.
Quantification of T Cell Clone Frequency In Vivo.
T cell receptor variable gene usage was analyzed by multiplex PCR as described previously (37). Direct DNA sequencing was performed bidirectionally using either the TCRβc primer or the primer for the identified specific TCRVβ with Therma Sequenase (Amersham Biosciences). The resulting sequence was compared with known TCR sequences using BlastSearch to define the variable region.
A commercially available kit (TaqMan PCR core reagent kit; Applied Biosystems) was used for PCR mastermixes. The amplification mix and PCR conditions for a 50-μl reaction consisted of the following: 1X PCR buffer A; 0.5 U AmpErase (uracil glycosylase); 1.25 U AmpliTaq Gold polymerase; 3.5 mM MgCl2; 200 μM dATP, dGTP, dCTP, and dUTP; 41.5 μmol forward and reverse primers; and 10 μmol TaqMan probe (Synthegen), where FAM is 6-carboxy fluorescein (emission: 518 nm) and TAMRA is 6-carboxy tetramethyl rhodamine (emission: 582 nm). Reaction volumes were adjusted to 50 μl and transferred to a microtiter plate. The following primer and probes were used for patient number 15249: ATGTGAGCACCTTGGAGCTG, TTCAGTCCCCCCCAGACT, and 5′-FAM-CGCAAAGATAAAGGGCCGAGTCCC-TAMRA-3′. The concentration of the T cell clone in test samples was determined with β-globin used as an internal standard on an identical set of samples. The procedure used β-globin–specific primers (TGAAGGCTCATGGCAAGAAA and GCTCACTCAGTGTGGCAAAGG) and fluorogenic probe (5′-FAM-TCCAGGTGAGCCAGGCCATCACTA-TAMRA-3′). Target and internal control DNA were subjected to 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of amplification at 95°C for 20 s and 60°C for 1 min, using ABI PRISM 7700 sequence detector (PerkinElmer). The frequency of transferred T cells per total PBMC was calculated using the results obtained by the clonotypic PCR divided by the total number of PBMCs as assessed by the β-globin–specific PCR in each individual sample. The frequency per CD8+ T cells was calculated from the results of the white blood differential count and the analysis of T cell subsets. To assess surface expression of CD27 and annexin V of the transferred T cells, samples were stained with a fluorescent antibody (BD Biosciences) and sorted for positive and negative cells on a FACSVantage (Becton Dickinson). The quantity of transferred cells in positive and negative populations was assessed using the CDR3-specific quantitative PCR as described above.
Results
CD27 Expression on HIV-, CMV-, and EBV-specific T Cell Clones.
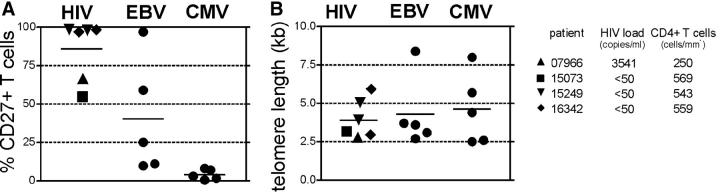
HIV-, CMV-, and EBV-specific CD8+ T cell clones were each independently generated from four different donors and one to two concurrently derived T cell clones from each donor were analyzed for CD27 surface expression. CD27 was detectable on all HIV- and most EBV-specific T cell clones, but not on any CMV-specific T cell clones (Fig. 1 A). All but one HIV-infected patient (no. 07966) had virus copy numbers below the detection limit. Surprisingly, CD27 expression on cells from several of the HIV- and EBV-specific T cell clonal populations was not uniform, with some cells CD27+ and others CD27−. Although the T cell clones were isolated after plating at 0.3 cells/well, this phenotypic heterogeneity raised the possibility that the cell populations might not be of clonal origin. Therefore, the TCR genes of the two heterogeneous HIV-specific T cell clones were sequenced and clone-specific primers were designed from within the hypervariable CDR3 region of the T cell receptor. Real-time PCR on DNA prepared from sorted CD27+ and CD27− subsets demonstrated usage in each instance of a single identical TCR, confirming that both subsets were derived from the same T cell (not depicted).
Figure 1.
CD27 surface expression and telomere length on HIV-, CMV-, and EBV-specific CD8 clones. All HIV-specific CD8 clones recognized the gag protein, with two restricted to the B57 allele, two to B8, and two to B13. Two independently generated clones were analyzed for patient numbers 15249 and 16342. Two CMV-specific T cell clones were A2 restricted and pp65 specific, one was specific for the immediate early protein with unknown restriction, and two were specific for unknown CMV proteins. All EBV-specific CD8 clones were A2-restricted and recognized the BMFL1 peptide. (A) Surface expression of CD8 clones was assessed by FACS, with the percentage of CD27+ cells of total T cells shown. (B) Telomere length of the same clones was analyzed by FACS. Virus copy number in plasma and CD4+ T cell count at the time point cells were obtained for cloning are indicated for HIV-infected patients.
The expression of CD27 on all HIV-, some EBV-, and no CMV-specific T cell clones is consistent with the reported in vivo phenotypes of polyclonal T cells specific for these viruses (20). Nevertheless, this result was surprising because all clones were derived by repetitive stimulation and extensive proliferation, which has been associated with telomere shortening and loss of CD27 expression (23). Therefore, we assessed if the populations with CD27+ cells had undergone fewer cell divisions before and during the cloning process than the CD27− populations by measuring with automated flow FISH telomere lengths in individual lymphocytes (36). The average telomere lengths of HIV-, EBV-, and CMV-specific T cell clones were comparably short (<5 kb), suggesting that independent of retention or loss of CD27 expression, these populations had all experienced a large number of cell divisions (Fig. 1 B). The T cells reactive with these viruses were likely all primed by professional APCs in vivo, but then experienced different in vivo events as well as disparate initial stimulation conditions in vitro, with CMV-specific T cells stimulated by infected fibroblasts in contrast to professional APCs for HIV and EBV, all of which may have influenced the resulting CD27 expression. However, all T cell clones were then expanded in the presence of LCLs, which express CD70, and all CD8+ cells transiently up-regulated CD70 in response to T cell receptor stimulation independent of CD27 expression (Fig. 2 A), suggesting that differences in the ability of CD27 to be ligated by CD70 during repetitive stimulations in vitro could not account for the observed distinct phenotypes of the HIV-, CMV-, and EBV-specific T cell clones.
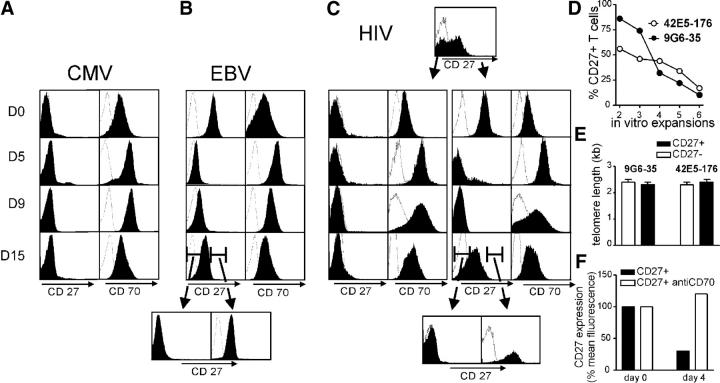
Figure 2.
CD27 expression after T cell activation. Virus-specific clones were expanded with anti-CD3 mAb in the presence of LCLs, irradiated feeder cells, and IL-2. At the time points indicated, (A) CD27− CMV-specific clone MT (and pp270, not depicted) and (B) CD27+ EBV-specific clone EG+4 (and RS+26, not depicted) were analyzed by FACS for CD27 and CD70 expression. (C) HIV gag–specific T cell clone 9G6-35 (and 42E5-176, not depicted), which initially contained both CD27+ and CD27− cells, were sorted and the fate of the CD27+ and CD27− T cells was separately analyzed. (B and C) At the end of a stimulation cycle of initially CD27+ EBV- and HIV-specific cells, the cells were sorted for CD27 expression and the CD27+ and CD27− cells were monitored through the next cycle. (D) The T cell clones 9G6-35 and 42E5-176 were repetitively expanded, and CD27 expression was assessed 14 d after anti-CD3 mAb stimulation. (E) Telomere lengths of CD27+ and CD27− cells at the end of the fifth in vitro expansion were compared. (F) CD27+ T cells from clone 42E5-176 were stimulated by plate-bound anti-CD3 mAb for 4 d in the presence or absence of 5 μg/ml anti-CD70 mAb. Surface expression of CD27 4 d after stimulation is shown.
The CD27+ and CD27− subpopulations from individual HIV-specific clones were isolated by FACS sorting after a stimulation cycle, and CD27 and CD70 expression by the subpopulations was then monitored after restimulation with anti-CD3 and low dose IL-2 (50 U/ml), and compared with the responses of CD27− CMV-specific and CD27+ EBV-specific clones. Both CD27+ and CD27− T cells transiently up-regulated CD70 expression, and the initially positive CD27 T cells lost surface CD27 expression by days 5–9 (Fig. 2, A–C). However, at the end of the 15-d stimulation cycle, CD70 expression was again low, the initially negative CD27 cells remained CD27−, and most of the initially positive CD27 cells reexpressed CD27. A small fraction of the initially positive CD27 cells failed to reexpress CD27, and restimulation and analysis of this CD27− population revealed that this loss was irreversible. Repetitive in vitro expansions of the HIV-specific T cell clones consisting of CD27+ and CD27− subsets led to progressive loss of CD27+ cells with each stimulation and accumulation of CD27− T cells (Fig. 2 D). The average lengths of telomere repeats in sorted CD27+ and CD27− subsets after completion of the fifth expansion cycle were both short (Fig. 2 E), again suggesting that both CD27+ and CD27− T cell subsets had extensively proliferated. The transient down-regulation of CD27 observed after stimulation of CD27+ T cells was dependent on ligation by CD70 because surface CD27 expression was stable on CD27+ cells after TCR stimulation in the presence of a blocking anti-CD70 antibody (Fig. 2 D).
The results demonstrate that effector–memory CD27+ CTLs gradually convert to a terminally CD27− phenotype with successive cell divisions, but that all CD27+ cells transiently lose CD27 surface expression after ligation of CD27. Thus, direct ex vivo analysis of CD27− cells can be potentially confounded by the presence of two very distinct populations: recently stimulated CD27+ CTLs and terminally differentiated CD27− CTLs.
CD27+ and CD27− HIV gag–specific CD8+ T Cells Are Functionally Distinct.
The presence of CD27+ and CD27− cells derived from the same parental T cell clone after expansion permitted analysis of the function of CD27 on genetically identical HIV-specific T cells. Phenotypically, the two cell subsets appeared otherwise comparable, with no differences in levels of TCR chains or CD3ζ expression, and both populations were CD45RAlo, CD45ROhi, CD38hi, CD69hi, Ki67−, proliferating cell nuclear antigenlo, CCR7−, CD62L−, Bcl-2+, FAShi, and CD28− (not depicted). To facilitate functional analysis, the fine specificity of the clones was determined both by defining the restricting allele with partially matched targets, and by epitope mapping using overlapping gag peptides and autologous B cell LCLs as targets. Clone 9G6-35 from patient number 15249 was B57 restricted and recognized the peptide TSTLQEQIGW. Clone 42E5-176 from patient number 07966 was B13 restricted and recognized the peptide ERQANFLGKI.
The response of these HIV gag–specific CD8+ T cell clones was assessed after stimulation with autologous peptide-pulsed B-LCLs to mimic recognition of a target cell, and after stimulation with anti-CD3 and anti-CD27 to directly analyze the role of CD27 in the absence of other costimulatory signals. The proliferation index of CD27+ cells was twofold higher than CD27− cells after stimulation with peptide-pulsed autologous LCLs (Fig. 3 A). After stimulation with 30 ng of plate-bound anti-CD3, anti-CD27 had no effect on the proliferative response of CD27− cells, but, for CD27+ cells, proliferation was directly proportional to the level of CD27 ligation, with 10 μg anti-CD27 increasing [3H]thymidine incorporation up to 100-fold in CD27+ cells compared with CD27− cells (Fig. 3 B). Differences in [3H]thymidine incorporation between CD27+ and CD27− HIV-specific T cells were more pronounced after six cycles than three cycles of expansion (Fig. 3 C), suggesting that the CD27–CD70 interaction may become increasingly important for CD8+ responses as the CD8+ cells acquire a greater proliferative history.
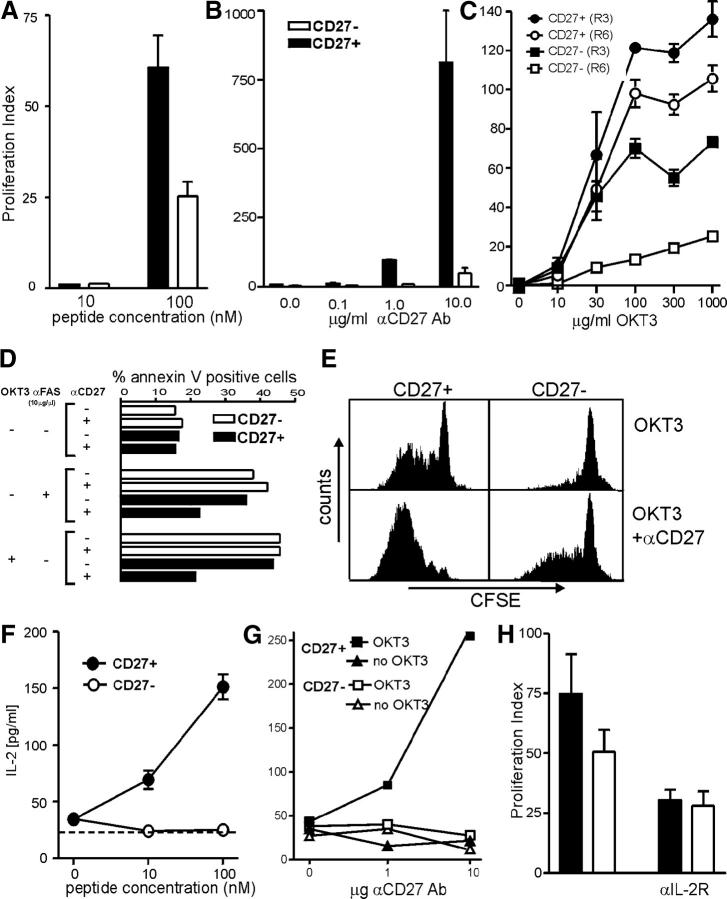
Figure 3.
Functional in vitro assays of CD27+ and CD27− CTLs. [3H]thymidine uptake of CD27+ and CD27− cells after sorting and in vitro expansion in response to (A) autologous LCLs pulsed with the noted concentrations of peptide, or to (B) titrated amounts of plate-bound anti-CD27 and 30 ng anti-CD3 mAb. (C) [3H]thymidine incorporation of CD27+ and CD27− T cells of the clone 9G6-35 after three (R3) or six (R6) in vitro expansions in response to titrated amounts of plate-coated anti-CD3 mAb (OKT3). Proliferation index was determined as [[3H]thymidine uptake of stimulated samples]/[[3H]thymidine incorporation of unstimulated samples]. The [3H]thymidine uptake in unstimulated CD27+ and CD27− subsets was comparable. (D) The percentage of annexin V+ cells is shown in CD27+ and CD27− T cell subsets after stimulation for 16–18 h with 10 μg/ml of plate-bound anti-CD3 mAb and/or 10 μg/ml of soluble anti-FAS mAb in the presence or absence of 10 μg/ml of coated anti-CD27mAb. (E) CFSE-labeled CD27+ and CD27− T cells of the clone number 9G6-35 were stimulated with 30 ng/ml of plate-bound anti-CD3 mAb (OKT3) in the presence or absence of 10 μg/ml of coated anti-CD27 mAb. CFSE expression is shown 7 d after stimulation. IL-2 production of CD27+ and CD27− cells was assessed after 36 h of stimulation with (F) peptide-pulsed autologous LCLs or (G) titrated amounts of plate-bound anti-CD27 and 30 ng anti-CD3 mAb. (H) [3H]thymidine incorporation of CD27+ and CD27− T cells of the clone 9G6-35 was measured after stimulation with 100 nM of autologous peptide-pulsed LCLs in the presence or absence of a blocking anti–IL-2Rβ mAb. The mean of triplicate samples is shown, with error bars indicating SEM. All in vitro stimulations presented in this figure were performed in the absence of IL-2 in the culture media.
Higher [3H]thymidine incorporation by a cell population may reflect increased cell division after CD27 signaling and/or enhanced cell survival. The susceptibility of CD27+ and CD27− T cells with the same TCR to activation-induced cell death and to FAS-induced apoptosis was examined at 15–18 d after the last stimulation by sorting CD27+ and CD27− subsets and incubating for 16 h in the presence of plate-bound anti-CD3 or soluble anti-FAS (Fig. 3 D). Concurrent signaling via CD27 reduced apoptosis induced by TCR triggering with anti-CD3 as well as apoptosis induced directly by anti-FAS. Proliferation of CD27+ and CD27− T cells in response to anti-CD3 was analyzed by examining dye dilution in cells labeled with CFSE. Ligation of CD27 with anti-CD27 increased CFSE dilution after TCR stimulation (Fig. 3 E). An increased number of CD27+ T cells divided compared with CD27− T cells, even in the absence of anti-CD27 antibody, potentially due to ligation of CD27 by the CD70 up-regulated on the surface of the T cells after activation (Fig. 2 A). CD27 ligation by coated antibody also increased, to a more limited extent, cell cycling of sorted CD27− T cells (Fig. 3 E), which likely reflects the presence of a small number of contaminating CD27+ cells and/or cells from the sorted CD27− that had only transiently down-regulated CD27 in the previous stimulation cycle.
IL-2 is an important growth and survival factor, which is classically produced after ligation of the TCR and costimulatory molecule CD28. However, other costimulatory signals can promote IL-2 production, and we examined if the observed differences in proliferation of CD27+ and CD27− T cells might in part reflect IL-2 production (30, 31). HIV gag–specific CD8+ effector–memory T cells were stimulated by antigen (Fig. 3 F) or by titrating doses of anti-CD3 and anti-CD27 (Fig. 3 G). Only the CD27+ subset produced detectable amounts of IL-2, and IL-2 production increased with stronger CD27-mediated signaling. Proliferation of CD27+ cells, and to a lesser extent CD27− CD8+ T cells, was diminished by the addition of a blocking antibody to the anti–IL-2Rβ chain (Fig. 3 H). The observed incomplete blockade may reflect limitations of this antibody and/or contributions of IL-2–independent proliferative mechanisms. The limited reduction in proliferation of CD27− cells by antibody to IL-2Rβ despite failure to detect IL-2 in the culture supernatant may reflect consumption of low levels of IL-2 by proliferating cells or a nonspecific background effect of the antibody. Thus, although IL-2 production is not absolutely dependent on ligation of CD27, the CD27–CD70 interaction quantitatively increases IL-2 production.
Similar In Vitro Effector Functions of CD27+ and CD27− HIV gag–specific T Cells.
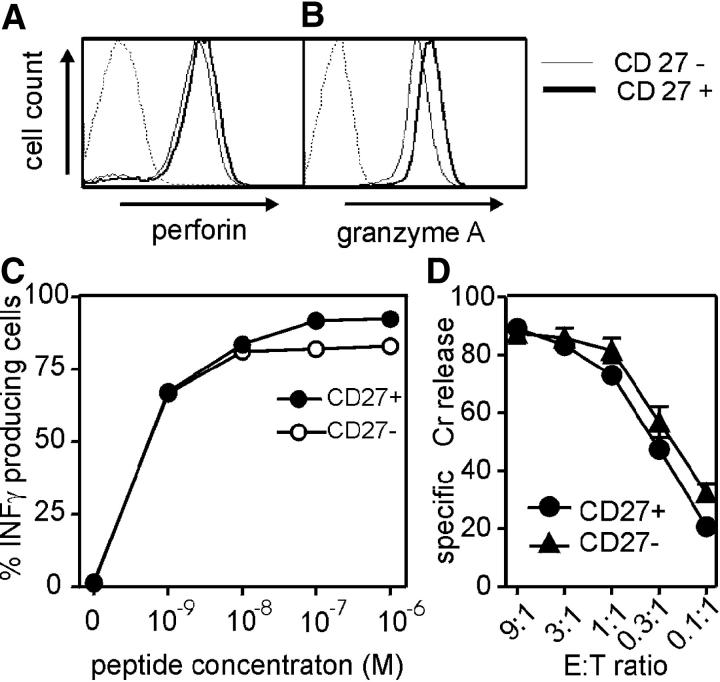
The persistence of CD27 expression on HIV-specific CD8+ T cells has been correlated with impaired effector function and deficient perforin expression (4, 19). However, intracellular staining and FACS analysis of CD27+ and CD27− subsets of the HIV gag–specific T cell clones 42E5-176 and 9G6-35 revealed comparable levels of perforin and granzyme A (Fig. 4, A and B). After stimulation with titrating amounts of peptide presented on autologous CD70+ LCLs, CD27+ and CD27− cells responded equivalently with IFN-γ production (Fig. 4 C) and lysed peptide-pulsed target cells with the same efficiency (Fig. 4 D). Although this seems to contrast with the reported differences in IFN-γ production and lytic activity of CD27+ and CD27− HIV-specific T cells analyzed directly ex vivo (17, 19, 21), in our experiments the cells were known to be stimulated and maintained identically, which cannot be controlled with cells of different phenotype obtained directly from an in vivo environment, particularly with variable viral burdens. Thus, retained CD27 expression is not intrinsically associated with impaired effector functions or failure to acquire such functions. In addition, as shown above, the direct ex vivo analysis of CD27− effector–memory T cells may include recently stimulated CD27+ (transiently CD27−) T cells that therefore express high perforin and IFN-γ levels with direct ex vivo cytolytic activity.
Figure 4.
Effector function of CD27+ and CD27− CTLs. Perforin (A) and granzyme A (B) expression of CD27+ and CD27− cells was examined after sorting and in vitro expansion. An isotype control mAb was included for comparison. (C) Intracellular IFN-γ staining of CD27+ and CD27− subsets from the clone 9G6-35 was evaluated after in vitro stimulation with LCLs pulsed with different peptide concentrations. (D) Standard 51Cr-release assay using CD27+ and CD27− effector cells of the clone 9G6-35 and 1 μM of peptide-pulsed autologous target cells at the effector–target ratios is indicated. One representative assay out of three is shown.
Long-Term Survival of Adoptively Transferred CD27+, But Not CD27−, HIV gag–specific CD8+ T Cells In Vivo.
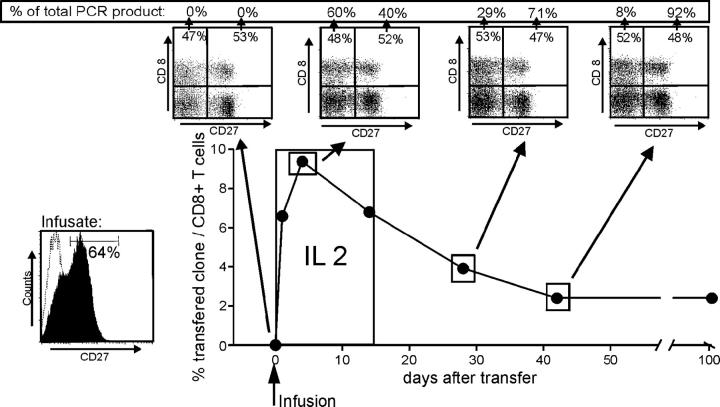
Our studies demonstrated that CD27+ HIV-specific T cells produce IL-2 after ligation of CD27, are more resistant to apoptosis, and proliferate better in response to antigen than CD27− cells with the same TCR derived from the same parental T cell. Analyzing the potential in vivo relevance of these observations in humans is difficult. However, we were able to take advantage of a patient previously entered on a protocol to evaluate augmentation of HIV immunity by adoptive transfer of autologous HIV-specific CD8+ clones expanded to large numbers in vitro, because this patient was treated with a single clone that in retrospect contained a mixture of 64% CD27+ and 36% CD27− cells (Fig. 5). Patient number 15249 was receiving anti-retroviral therapy at study entry with a plasma RNA level of <50 copies/ml, but HIV-RNA was regularly detectable in resting peripheral CD4+ T cells (not depicted). The patient received an infusion of 3.3 × 109 cells/m2 of the autologous in vitro–expanded HIV gag–specific T cell clone on day 0, followed by a low dose of IL-2 (2.5 × 105 U/m2) injected subcutaneously daily for 14 d to improve the engraftment of the adoptively transferred cells (38). As previously described, the clone administered, 9G6-35, contained both CD27+ and CD27− subsets that did not differ in respect to effector function or expression of activation or homing molecules, and had telomeres of equivalent length. The persistence of each subset was monitored after transfer by quantitative PCR of separated CD27+ and CD27− PBMCs for the clone-specific CDR3 region. Parental cells from which the clone was derived could not be detected by PCR in PBMCs before the cell infusion, but 1 h after the infusion the transferred cells represented 6% of all CD8+ T cells (Fig. 5). The transferred cells expanded to a maximum of 10% of all CD8+ T cells at 4 d after the infusion, declined in frequency over the next 40 d, and then stabilized at ∼2% of all CD8+ T cells. At each time point, PBMCs were sorted for CD8+ CD27+ and CD8+ CD27− T cells, and the number of infused T cells in each fraction was assessed by quantitative PCR. At the peak of the expansion of the transferred cells, which represented nearly 10% of total circulating CD8+ cells, the fraction of CD27− cells had increased (60%), which might reflect transient down-regulation of CD27 in responding CD27+ cells and/or preferential early expansion of CD27− cells in the presence of exogenous IL-2. After the peak, the percentage of CD27− cells continuously declined (29% at day 28 and 8% at day 42), and this predominantly CD27+ population remained engrafted long-term at 104 d. Although we cannot formally exclude that CD27− HIV-specific CTLs reexpress CD27 in vivo, such a change was never evidenced during in vitro analyses, and our results strongly suggest that infused CD27+ CTLs preferentially survived in this HIV-infected patient. Thus, HIV-specific CD8+ cells expressing CD27 appeared to have a survival advantage in vivo, and the transfer of CD27+ cells may permit establishment of a strong persistent response in HIV+ patients.
Figure 5.
Frequency and phenotype of HIV gag–specific CD8+ T cell clones after adoptive transfer. Autologous 3.3 × 109/m2 clonal CD8+ T cells were infused on day 0 (clone 9G6-35 from patient no. 15249) followed by a low dose of IL-2 (2.5 × 105 U/m2) injected subcutaneously daily for 14 d. The frequency of the transferred T cells was followed by quantitative PCR specific for the CDR3 region as described in Materials and Methods. At the time points indicated, CD8+ PBMCs were sorted for surface expression of CD27 followed by quantitative clonotypic PCR of the sorted samples. The numbers in the FACS dot plots indicate the percentage of CD8+ T cells, and the numbers given as percent PCR product indicate the percentage of the transferred T cell clone in the CD27+ or CD27− subpopulation.
Discussion
The TNFR family member CD27 is expressed by naive human CD8+ T cells and has been reported to be lost during clonal expansion and normal differentiation to an effector cell. Thus, the CD27− CD8+ population appears to evolve from continued stimulation of CD27+ precursors and presumably represents a more differentiated antigen-experienced cell with a more extensive proliferative history and more complete effector functions than CD27+ CD8+ cells (24). However, CD27 expression by virus-specific CD8+ T cells that persist after infection with different viruses has appeared more varied than predicted by such an ordered linear differentiation schema, despite no clear biological basis for such differences (4, 20, 34, 39). In this work, we have examined the nature of the relationship between CD27 expression and CD8+ T cell differentiation and function by directly comparing in vitro and in vivo CD27+ and CD27− cells derived from the same parental cell, and the results suggest that the current model requires modification.
Panels of clones were independently derived for this analysis from 12 individuals infected with HIV, CMV, and/or EBV. Despite the extensive in vitro proliferation required for cloning, the phenotypes of the clones were typical of freshly isolated CD8+ T cells reactive with these viruses. In particular, most HIV-specific clones were CD27+, a fraction of EBV-specific clones were CD27+, and all CMV-specific clones were CD27−. Analysis of the HIV- and EBV-specific CD8+ clones derived from single progenitor cells surprisingly also revealed heterogeneity in several “clones” with regard to CD27 expression, potentially mimicking the progression expected of an in vivo response. Sorting of the CD27− population followed by restimulation revealed that most of these cells had irreversibly lost CD27 expression. By contrast, dynamic changes were evident with the sorted CD27+ population, with all the cells down-regulating expression of CD27 shortly after stimulation and the majority reexpressing CD27 at the end of the proliferative cycle (transiently CD27− CTLs), but a small fraction converting to stably CD27−. With repeated stimulations of CD27+ cells, the fraction of stably CD27− cells progressively increased. The telomere lengths we measured in the CD27+ and CD27− progeny of a proliferating HIV-specific CD27+ parental clone suggested both populations had experienced extensive proliferation. These results confirm and extend earlier studies demonstrating that polyclonal PBMCs irreversibly lose CD27 expression after multiple in vitro stimulations and that the telomere lengths of RA+ CD27− effector cells and RA− CD27+ memory cells measured directly ex vivo are comparable, but 1–3 kb shorter than those of naive RA+ CD27+ cells (23). Taken together, this suggests that CD27+ and CD27− antigen-experienced CD8+ T cells have a comparable proliferative history. Analysis of telomere length in HIV-specific CD8+ T cells obtained directly from PBMCs has also demonstrated that expression of CD27 does not necessarily imply limited prior proliferation (40–42).
With the propensity of CD27+ cells to yield with continued proliferation to CD27− cells, the presence of predominantly CD27+ cells in vivo in the response to some chronic viral infections suggests that such cells may have a selective advantage in those particular settings. Signals delivered through CD27 could potentially provide a selective advantage by enhancing proliferation and survival (30, 31, 43). Our finding that both CD27+ and CD27− CD8+ T cells were present within the same HIV-specific “clonal” populations made it possible to directly assess the functional consequences of retention or loss of CD27 expression. CD27+ cells proliferated better in response to antigen or direct TCR triggering, with the increased proliferation dependent on ligation of CD27. The increased proliferative response reflected both a decrease in apoptosis, likely reflecting prosurvival signals delivered via NF-κB activation by CD27 (44, 45), and the production of IL-2 as a result of a costimulatory signal from CD27. The presence of such signals emanating from CD27 might be particularly helpful for promoting the survival and persistence of HIV-specific CD8+ T cells for several reasons. First, HIV-specific effector–memory cells isolated from PBMCs have generally lost expression of CD28, which might otherwise provide an alternative and more potent costimulatory signal for IL-2 production (46). Indeed, we have recently shown that enforced reexpression of CD28 in HIV-specific CD28− CD8+ effector T cells by genetic modification restores this costimulatory signal and IL-2 production (47), but such reexpression does not naturally occur in CD28− CD8+ T cells. Second, the CD4+ response to HIV is generally lost early in infection, eliminating a usual source of IL-2 for supporting CD8+ responses to viruses (13, 48). Third, the proapoptotic environment observed for CD8+ cells in HIV-infected individuals likely increases the importance of delivery of prosurvival signals to T cells (49). Finally, HIV-specific CD8+ T cells exist in a milieu rich in cells expressing CD70, including activated T, B, and dendritic cells (50). Thus, what emerges in HIV infection is a setting in which the subset of HIV-specific CD8+ T cells that retain CD27 expression might be afforded a strong in vivo selective advantage, and may help explain how a robust CD8+ response can persist in infected individuals despite the absence of adequate CD4 help.
One potential caveat with this model as beneficial for the anti-HIV response is the proposed relationship between loss of CD27 expression and acquisition of effector function by CD8+ cells (4, 20, 21), which would render the retention of CD27+ cells of limited value to the host. However, our in vitro analysis of the function of CD27+ and CD27− cells derived from the same clonal population suggests that acquisition of effector function is not intimately linked with loss of CD27 expression. CD27+ cells expressed similar levels of perforin and granzyme as CD27− cells, produced equivalent levels of IFN-γ, and demonstrated identical cytolytic activity. How may these differences to the reported impaired effector function of CD27+ CTLs be explained? First, direct ex vivo analysis of CD27− cells from infected individuals will include recently stimulated CD27+ CTLs that are only transiently CD27−. Due to the recent TCR stimulation, such cells might be expected to exhibit full effector functions. Second, the functional abnormalities observed in freshly isolated HIV-specific CD27+ CD8+ may reflect consequences of the in vivo environment of an HIV-infected host, such as limiting T help in the context of a high antigen burden rather than aberrant differentiation, as supported by the observed acquisition of IFN-γ production and cytolytic activity after overnight culture in IL-2 (17). Thus, the in vitro results may reflect the effector potential of CD27+ cells if properly stimulated.
A model in which persistent CD27 expression in HIV-specific CD8+ T cells reflects selection due to a survival advantage rather than representing a differentiation defect is also consistent with the phenotypes observed in other infections. The dominant virus-specific memory CD8+ population is CD27− in chronic CMV infection, a setting in which a strong CD4+ response that can provide IL-2 is present and in which the targets such as infected fibroblasts are CD70− and would not provide CD27+ cells with a selective advantage. An intermediate phenotype with both CD27− and CD27+ cells present is observed in EBV infection, a setting in which the CD4+ response can support the CD27− population that evolves with CD8 proliferation, but the presence of CD70 on some infected targets such as B cells can impart an advantage to CD27+ cells. Moreover, in HIV-infected individuals who develop EBV-related lymphoma, a loss of EBV-specific CD27− CD8+ cells and an accumulation of CD27+ CD8+ cells has been observed (21). Although these changes have been interpreted, because these lymphomas are not well-controlled, as providing further evidence that abnormal differentiation of the CD8+ response is permissive for EBV disease, the lymphomas typically occur late in the course of HIV infection. An alternative explanation suggested by our data is that the malignant expansion of CD70+ B cells and global loss of CD4+ T helper responses with progressive HIV infection result, respectively, in preferential selection of the CD27+ population and loss of the helper-dependent CD27− population, even though this may still eventuate in a quantitatively inadequate response.
Ultimately, in vivo studies are necessary to resolve these issues, but such studies are difficult to perform in humans. Recent data from influenza infection of CD27-deficient mice revealed that the CD27–CD70 interaction mediates a prosurvival signal essential for maintaining the CD8 response at the site of infection, with the contribution of CD27 signals in proliferation unclear because the mice had an intact CD4 response (45). Our analysis of CD27 expression on transferred T cells from a patient in a clinical trial that we had performed examining adoptive transfer of autologous, in vitro–expanded HIV-specific CD8+ T cells as a means to augment virus-specific immunity in infected individuals (35) has provided evidence supporting a positive role for CD27 signals in maintaining the human CD8 response. After the patient received a clonal HIV-specific CD8+ population consisting of both CD27+ and CD27− subclones, cells of both phenotypes could be detected shortly after transfer, but the fraction of CD27− cells in the persisting transferred cells began declining shortly thereafter, becoming largely undetectable by day 42 despite the persistence of large numbers of CD27+ cells. At the time of infusion after in vitro expansion, both CD27+ and CD27− cell subsets were CCR7− and CD62L−, suggesting that the detection of predominantly CD27+ cells over time in PBMCs did not reflect differential homing of CD27− cells to secondary lymphoid organs. The observed survival advantage of the transferred HIV-specific CD27+ subpopulation of CD8+ cells after infusion into an HIV-infected host mimics what we would predict for an endogenous response. Although future studies might be able to indirectly address this issue in humans in vivo by monitoring the phenotypic and functional evolution of virus-specific CD8+ responses during the course of viral infections, the unique opportunity to directly compare HIV-specific CD27+ and CD27− progeny of the same cell both in vitro and in vivo in this work strongly supports the hypothesis that the accumulation of CD27+ effector–memory cells in certain chronic viral infections reflects a functional adaptive response by the host rather than a reflection of aberrant differentiation.
Acknowledgments
This work was supported by National Institutes of Health grants AI43650, AI054334, AI27757, AI29524, CA33084, and CA18029. A.F. Ochsenbein was supported by the Swiss National Science Foundation grants SSMBS-1078 and 632-066020, and G.M. Baerlocher was supported by a fellowship from the Swiss National Science Foundation.
The authors have no conflicting financial interests.
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate succinimidyl ester; LCL, lymphoblastoid cell line.
References
- 1.Walter, E.A., P.D. Greenberg, M.J. Gilbert, R.J. Finch, K.S. Watanabe, E.D. Thomas, and S.R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038–1044. [DOI] [PubMed] [Google Scholar]
- 2.Wodarz, D., J.P. Christensen, and A.R. Thomsen. 2002. The importance of lytic and nonlytic immune responses in viral infections. Trends Immunol. 23:194–200. [DOI] [PubMed] [Google Scholar]
- 3.Altman, J.D., P.A. Moss, P.J. Goulder, D.H. Barouch, M.G. McHeyzer-Williams, J.I. Bell, A.J. McMichael, and M.M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274:94–96. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood. 98:1667–1677. [DOI] [PubMed] [Google Scholar]
- 5.Gea-Banacloche, J.C., S.A. Migueles, L. Martino, W.L. Shupert, A.C. McNeil, M.S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, et al. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082–1092. [DOI] [PubMed] [Google Scholar]
- 6.Miedema, F., and M.R. Klein. 1996. AIDS pathogenesis: a finite immune response to blame? Science. 272:505–506. [DOI] [PubMed] [Google Scholar]
- 7.Safrit, J.T., and R.A. Koup. 1995. The immunology of primary HIV infection: which immune responses control HIV replication? Curr. Opin. Immunol. 7:456–461. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael, A., X. Jin, P. Sissons, and L. Borysiewicz. 1993. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)–specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 177:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein, M.R., B.C. van, A.M. Holwerda, G.S. Kerkhof, R.J. Bende, I.P. Keet, J.K. Eeftinck-Schattenkerk, A.D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips, R.E., S. Rowland-Jones, D.F. Nixon, F.M. Gotch, J.P. Edwards, A.O. Ogunlesi, J.G. Elvin, J.A. Rothbard, C.R. Bangham, and C.R. Rizza. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 354:453–459. [DOI] [PubMed] [Google Scholar]
- 11.Klenerman, P., S. Rowland-Jones, S. McAdam, J. Edwards, S. Daenke, D. Lalloo, B. Koppe, W. Rosenberg, D. Boyd, A. Edwards, et al. 1994. Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature. 369:403–407. [DOI] [PubMed] [Google Scholar]
- 12.Collins, K.L., B.K. Chen, S.A. Kalams, B.D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 391:397–401. [DOI] [PubMed] [Google Scholar]
- 13.Pitcher, C.J., C. Quittner, D.M. Peterson, M. Connors, R.A. Koup, V.C. Maino, and L.J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518–525. [DOI] [PubMed] [Google Scholar]
- 14.Matloubian, M., R.J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun, J.C., and M.J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 300:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shedlock, D.J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 300:337–339. [DOI] [PubMed] [Google Scholar]
- 17.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 96:3094–3101. [PubMed] [Google Scholar]
- 18.Trimble, L.A., and J. Lieberman. 1998. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 91:585–594. [PubMed] [Google Scholar]
- 19.Appay, V., D.F. Nixon, S.M. Donahoe, G.M. Gillespie, T. Dong, A. King, G.S. Ogg, H.M. Spiegel, C. Conlon, C.A. Spina, et al. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appay, V., P.R. Dunbar, M. Callan, P. Klenerman, G.M. Gillespie, L. Papagno, G.S. Ogg, A. King, F. Lechner, C.A. Spina, et al. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379–385. [DOI] [PubMed] [Google Scholar]
- 21.van Baarle, D., S. Kostense, E. Hovenkamp, G. Ogg, N. Nanlohy, M.F. Callan, N.H. Dukers, A.J. McMichael, M.H. van Oers, and F. Miedema. 2002. Lack of Epstein-Barr virus- and HIV-specific CD27− CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS. 16:2001–2011. [DOI] [PubMed] [Google Scholar]
- 22.Kern, F., E. Khatamzas, I. Surel, C. Frommel, P. Reinke, S.L. Waldrop, L.J. Picker, and H.D. Volk. 1999. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur. J. Immunol. 29:2908–2915. [DOI] [PubMed] [Google Scholar]
- 23.Hamann, D., S. Kostense, K.C. Wolthers, S.A. Otto, P.A. Baars, F. Miedema, and R.A. van Lier. 1999. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int. Immunol. 11:1027–1033. [DOI] [PubMed] [Google Scholar]
- 24.Hamann, D., M.T. Roos, and R.A. van Lier. 1999. Faces and phases of human CD8 T-cell development. Immunol. Today. 20:177–180. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 26.Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. [DOI] [PubMed] [Google Scholar]
- 27.Hislop, A.D., N.H. Gudgeon, M.F. Callan, C. Fazou, H. Hasegawa, M. Salmon, and A.B. Rickinson. 2001. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 167:2019–2029. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin, R.G., M.R. Alderson, C.A. Smith, R.J. Armitage, T. VandenBos, R. Jerzy, T.W. Tough, M.A. Schoenborn, T. Davis-Smith, K. Hennen, et al. 1993. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 73:447–456. [DOI] [PubMed] [Google Scholar]
- 29.Tesselaar, K., Y. Xiao, R. Arens, G.M. van Schijndel, D.H. Schuurhuis, R.E. Mebius, J. Borst, and R.A. van Lier. 2003. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J. Immunol. 170:33–40. [DOI] [PubMed] [Google Scholar]
- 30.Kobata, T., K. Agematsu, J. Kameoka, S.F. Schlossman, and C. Morimoto. 1994. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J. Immunol. 153:5422–5432. [PubMed] [Google Scholar]
- 31.Hintzen, R.Q., S.M. Lens, K. Lammers, H. Kuiper, M.P. Beckmann, and R.A. van Lier. 1995. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J. Immunol. 154:2612–2623. [PubMed] [Google Scholar]
- 32.Arens, R., K. Tesselaar, P.A. Baars, G.M. van Schijndel, J. Hendriks, S.T. Pals, P. Krimpenfort, J. Borst, M.H. van Oers, and R.A. van Lier. 2001. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 15:801–812. [DOI] [PubMed] [Google Scholar]
- 33.Tesselaar, K., R. Arens, G.M. van Schijndel, P.A. Baars, M.A. van der Valk, J. Borst, M.H. van Oers, and R.A. van Lier. 2003. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 4:49–54. [DOI] [PubMed] [Google Scholar]
- 34.Wills, M.R., G. Okecha, M.P. Weekes, M.K. Gandhi, P.J. Sissons, and A.J. Carmichael. 2002. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J. Immunol. 168:5455–5464. [DOI] [PubMed] [Google Scholar]
- 35.Brodie, S.J., D.A. Lewinsohn, B.K. Patterson, D. Jiyamapa, J. Krieger, L. Corey, P.D. Greenberg, and S.R. Riddell. 1999. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat. Med. 5:34–41. [DOI] [PubMed] [Google Scholar]
- 36.Baerlocher, G.M., and P.M. Lansdorp. 2003. Telomere length measurements in leukocyte subsets by automated multicolor flow-FISH. Cytometry. 55A:1–6. [DOI] [PubMed] [Google Scholar]
- 37.Akatsuka, Y., E.G. Martin, A. Madonik, A.A. Barsoukov, and J.A. Hansen. 1999. Rapid screening of T-cell receptor (TCR) variable gene usage by multiplex PCR: application for assessment of clonal composition. Tissue Antigens. 53:122–134. [DOI] [PubMed] [Google Scholar]
- 38.Yee, C., J.A. Thompson, D. Byrd, S.R. Riddell, P. Roche, E. Celis, and P.D. Greenberg. 2002. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA. 99:16168–16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, D., P. Shankar, Z. Xu, B. Harnisch, G. Chen, C. Lange, S.J. Lee, H. Valdez, M.M. Lederman, and J. Lieberman. 2003. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 101:226–235. [DOI] [PubMed] [Google Scholar]
- 40.Wolthers, K.C., S.A. Otto, G.B. Wisman, S. Fleury, P. Reiss, R.W. ten Kate, A.G. van der Zee, and F. Miedema. 1999. Normal T-cell telomerase activity and upregulation in human immunodeficiency virus-1 infection. Blood. 93:1011–1019. [PubMed] [Google Scholar]
- 41.Effros, R.B., R. Allsopp, C.P. Chiu, M.A. Hausner, K. Hirji, L. Wang, C.B. Harley, B. Villeponteau, M.D. West, and J.V. Giorgi. 1996. Shortened telomeres in the expanded CD28− CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 10:F17–F22. [DOI] [PubMed] [Google Scholar]
- 42.Palmer, L.D., N. Weng, B.L. Levine, C.H. June, H.C. Lane, and R.J. Hodes. 1997. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J. Exp. Med. 185:1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendriks, J., L.A. Gravestein, K. Tesselaar, R.A. van Lier, T.N. Schumacher, and J. Borst. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 1:433–440. [DOI] [PubMed] [Google Scholar]
- 44.Akiba, H., H. Nakano, S. Nishinaka, M. Shindo, T. Kobata, M. Atsuta, C. Morimoto, C.F. Ware, N.L. Malinin, D. Wallach, et al. 1998. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J. Biol. Chem. 273:13353–13358. [DOI] [PubMed] [Google Scholar]
- 45.Hendriks, J., Y. Xiao, and J. Borst. 2003. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 198:1369–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenschow, D.J., T.L. Walunas, and J.A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233–258. [DOI] [PubMed] [Google Scholar]
- 47.Topp, M.S., S.R. Riddell, Y. Akatsuka, M.C. Jensen, J.N. Blattman, and P.D. Greenberg. 2003. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J. Exp. Med. 198:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho, D.D., A.U. Neumann, A.S. Perelson, W. Chen, J.M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 373:123–126. [DOI] [PubMed] [Google Scholar]
- 49.Mueller, Y.M., S.C. De Rosa, J.A. Hutton, J. Witek, M. Roederer, J.D. Altman, and P.D. Katsikis. 2001. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity. 15:871–882. [DOI] [PubMed] [Google Scholar]
- 50.Wolthers, K.C., S.A. Otto, S.M. Lens, D.N. Kolbach, R.A. van Lier, F. Miedema, and L. Meyaard. 1996. Increased expression of CD80, CD86 and CD70 on T cells from HIV-infected individuals upon activation in vitro: regulation by CD4+ T cells. Eur. J. Immunol. 26:1700–1706. [DOI] [PubMed] [Google Scholar]