Abstract
The mechanisms underlying CD4+ T cell depletion in human immunodeficiency virus (HIV) infection are not well understood. Comparative studies of lymphoid tissues, where the vast majority of T cells reside, and peripheral blood can potentially illuminate the pathogenesis of HIV-associated disease. Here, we studied the effect of HIV infection on the activation and depletion of defined subsets of CD4+ and CD8+ T cells in the blood, gastrointestinal (GI) tract, and lymph node (LN). We also measured HIV-specific T cell frequencies in LNs and blood, and LN collagen deposition to define architectural changes associated with chronic inflammation. The major findings to emerge are the following: the GI tract has the most substantial CD4+ T cell depletion at all stages of HIV disease; this depletion occurs preferentially within CCR5+ CD4+ T cells; HIV-associated immune activation results in abnormal accumulation of effector-type T cells within LNs; HIV-specific T cells in LNs do not account for all effector T cells; and T cell activation in LNs is associated with abnormal collagen deposition. Taken together, these findings define the nature and extent of CD4+ T cell depletion in lymphoid tissue and point to mechanisms of profound depletion of specific T cell subsets related to elimination of CCR5+ CD4+ T cell targets and disruption of T cell homeostasis that accompanies chronic immune activation.
Keywords: HIV pathogenesis, CD4+ T cell depletion, lymph nodes, gastrointestinal tract, HIV-specific T cells
Introduction
The depletion of CD4+ T cells, the hallmark of HIV-1 infection, has largely been studied in the most accessible compartment, peripheral blood, to understand the mechanisms of this depletion, follow progression of infection, and determine the time to initiate antiretroviral therapy (1). However, for the reasons outlined below, we believe studies of lymphoid tissue are likely to prove more rewarding in an effort to better understand why CD4+ T cells are depleted, and thereby will enable a more rationally timed antiretroviral therapy. First, most CD4+ T cells reside within the gastrointestinal (GI) tract, LNs, and other lymphatic tissues rather than in peripheral blood (2). Second, there are large numbers of target cells in the GI tract that express CCR5, the HIV coreceptor for entry (3–7). Third, lymphoid tissue has been identified as a major site of HIV replication and a reservoir for HIV in vivo (8–15).
Indeed, both viral cytopathic effects and CTL killing of infected target cells could contribute to the depletion of CD4+ T cells from lymphoid tissue. In fact, substantial numbers of HIV or simian immunodeficiency (SIV)-specific CD8+ T cells reside within the GI tract of HIV/SIV-infected individuals (16–20) and there is one report suggesting that LNs contain a greater breadth of HIV-specific CD8+ T cells than peripheral blood (21). The relationship between infection of target cells and the cellular immune response is not clear, but it is known that host defenses do not prevent the nearly complete depletion of CD4+ T cells in the GI tract of SIV-infected macaques as early as 2 wk after infection (5, 6, 22, 23), or the loss of intestinal CD4+ T cells from the early to later stages of HIV-1 infection (24–27). Such lymphoid tissues are also likely to play an important role in the maintenance of CD4 T cell numbers throughout HIV infection. Indeed, the fibrosis and architectural disorganization documented in LN biopsies from HIV-infected individuals, which may reflect chronic inflammatory responses associated with viral replication, are likely to affect CD4+ T cell homeostasis. This is underscored by the highly significant correlation between collagen deposition, CD4+ T cell depletion (28), and CD4+ T cell repletion (unpublished data) on antiretroviral therapy.
Collectively, these studies suggest that a full understanding of the mechanisms relating to CD4+ T cell depletion and disease progression will likely require direct analysis of viral infection, the immune response, immune activation and pathology in lymphoid tissue compartments, and the relationship of the dynamics of infection in lymphoid tissue to peripheral blood. However, to date there have been no studies that directly compare T cell depletion, activation, or phenotypic composition in peripheral blood, LNs, and the GI tract from HIV-infected and -uninfected individuals. Here, we recruited 14 treatment-naive, HIV-infected individuals at different disease stages and 7 HIV-uninfected individuals and sampled inguinal LNs, ileal Peyer's patches and lamina propria, and venous blood. From each subject and within each compartment we examined (a) CD4+ T cell depletion; (b) relative levels of naive, effector–memory and central memory CD4+ and CD8+ T cells; (c) T cell activation based upon CCR5 and Ki67 expression; (d) the magnitude of HIV-specific CD4+ and CD8+ T cell responses; and (e) LN collagen composition. Taken together, our data reveal fundamental mechanisms underlying T cell depletion and disease progression in HIV-infected individuals.
Materials and Methods
Subjects.
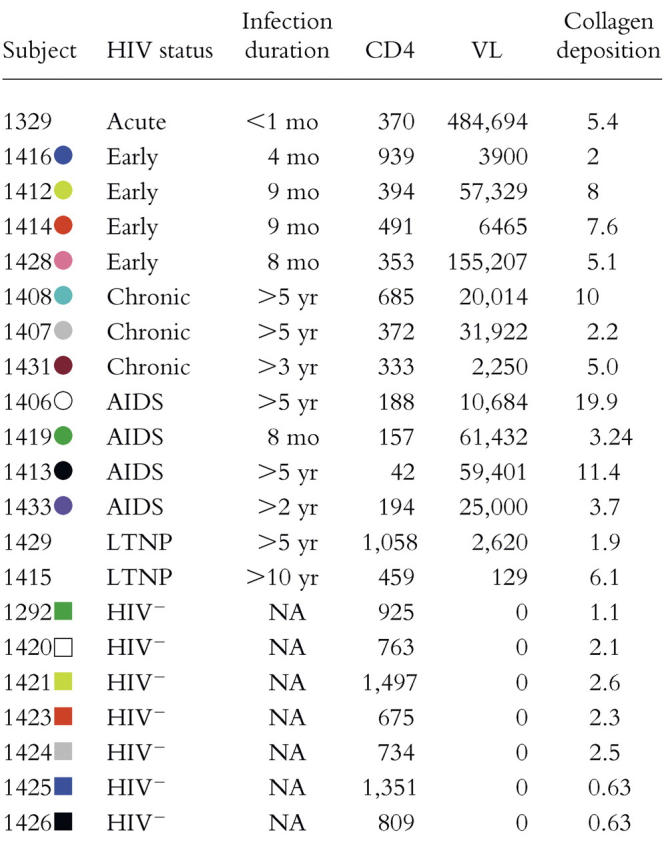
14 antiretroviral therapy–naive, HIV-infected and 7 HIV-uninfected, sexually active subjects that were high risk of becoming HIV+ were recruited for this study. Clinical details are shown in Table I. HIV+ individuals were classified as “early” based on HIV seropositivity for <1 yr with maintained CD4 counts of >300, “chronic” based on seropositivity for >1 yr with CD4 counts >200, and “AIDS” based on a CD4 count of <200 regardless of the duration of infection. HIV+ individuals were classified as long-term nonprogressors based on HIV infection of >5 yr without declines of blood CD4+ T cell counts and viral loads <5,000 copies/ml of plasma. Viral load was determined using either the Roche Amplicor Monitor assay or the Roche Ultradirect assay. The subjects all gave informed consent in compliance with the appropriate institutional review boards.
Table I.
Subject Cohort
Samples.
PBMCs were prepared from venous blood by density gradient centrifugation. Ileal Peyer's patches and lamina propria samples were acquired by endoscopy and biopsy of the terminal ileum. Patients received standard colonoscopy preparation and were sedated with versed and fentanyl. A colonoscope was passed through the large intestine and through the ileo–cecal valve. At least 8–10 biopsy samples were obtained with cold forceps using standard techniques. Four of the tissue samples were placed into formalin for histopathologic analysis and the remaining samples were placed into normal saline and kept on ice until digestion. Inguinal LNs were obtained as described previously (28). GI tract and LN samples were then dissected into 100-μL fragments and were digested in Iscove's media supplemented with 2 mg/ml Type II collagenase (Sigma-Aldrich) and 1 U/ml DNase I (Sigma-Aldrich) for 30 min at 37°C. After digestion, samples were passed through a 100-μm filter and were washed twice with RPMI media supplemented with 10% heat-inactivated fetal calf serum (R10; Sigma-Aldrich).
Flow Cytometric Analysis.
Six-parameter flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson). FITC, PE, peridin-chlorophyll protein (PerCP), and allophycocyanin (APC) were used as the fluorophores. At least 300,000 live lymphocytes were collected. The list-mode data files were analyzed using FlowJo (Tree Star Inc.).
HIV-specific T Cell Assay.
Stimulation was performed on fresh or frozen PBMCs as described elsewhere (29). Freshly isolated or freshly thawed PBMCs were resuspended at 106/ml in R10 supplemented with 1 μg/ml anti-CD28 and anti-CD49d antibodies. Peptides 15 amino acids in length, overlapping by 11 amino acids and encompassing HIV-1 gag, pol, and env (corresponding to the sequence of HXBc2), were used to stimulate HIV-specific T cells in the presence of 1 μg/ml brefeldin A (Sigma-Aldrich) for 5 h at 37°C. All cells were surface stained for phenotypic markers of interest and intracellularly stained for cytokines.
Monoclonal Antibodies, Tetrameric Complexes, and T Cell Phenotyping.
The following monoclonal antibodies were used for phenotypic and functional characterization of T cell subsets: anti-CD3 PerCP, anti-CD45RO FITC, anti-CD27 PE, anti-CD4 PerCP or PE, anti-CD8 APC or PerCP, anti-CCR5 PE, anti-Ki67 FITC, and anti–IFN-γ APC and anti–IL-2 APC (Becton Dickinson). As very few, if any, naive T cells express the activation markers Ki67 and CCR5, and as HIV-specific T cells are not detectable in the naive T cell pool, we report these data as percentages of memory T cells. We first determine the percentage of memory CD4+ and CD8+ T cells based upon characteristic expression patterns of CD45RO and CD27. We then separately determine the percentage of CD4+ and CD8+ T cells that express Ki67 or CCR5 or are HIV specific. The percentage of a defined population that are memory T cells is calculated by dividing the percentage of total T cells within that defined population by the fraction that are memory. The following tetrameric complexes were used to examine the frequency of HIV-specific CD8+ T cells: HLA-B57 KAFSPEVIPMF, HLA-A2 SLYNTVATL, HLA-B8 GGKKKYKL, and HLA-B8 FLKEKGGL.
Collagen Deposition.
Levels of collagen deposition within individual LN samples were determined as described previously (28). To identify collagen fibers, 5-μM sections were cut from the baseline tissue and stained with a trichrome stain using the Masson method. 18 images were obtained from the T cell zone of each tissue sample and imported into Adobe Photoshop 6.0. The color sampler tool was used to analyze representative shades and hues of the blue-stained collagen tissue. The area of the field containing collagen was selected and the background tissue was removed from the image. Collagen tissue was translated into a uniform color and the image was transferred into the Metamorph software (Universal Imaging) program to determine the percent area of tissue occupied by collagen as described previously (28).
Statistical Analysis.
Spearman's rank correlation, Wilcoxon matched pairs tests, and Mann-Whitney tests were performed using Prism 4.0 software.
Results
Comparative Analysis of CD4+ T Cell Percentages in the GI Tract, LNs, and Peripheral Blood of HIV+ and HIV− Individuals.
As it has been shown previously that SIV infection leads to preferential CD4+ T cell depletion in mucosal-associated lymphoid tissue compared with peripheral blood (6, 22, 23, 30), and early evidence suggests this holds for HIV infection (16, 24–26), we initially examined how HIV infection affects CD4+ T cell depletion in the GI tract, LNs, and peripheral blood by simple analysis of CD4+ T cell percentages in each tissue (Fig. 1). As expected, the frequencies of CD4+ T cells from HIV-infected individuals were significantly lower compared with HIV-uninfected individuals in each anatomical compartment (Fig. 1 A).
Figure 1.
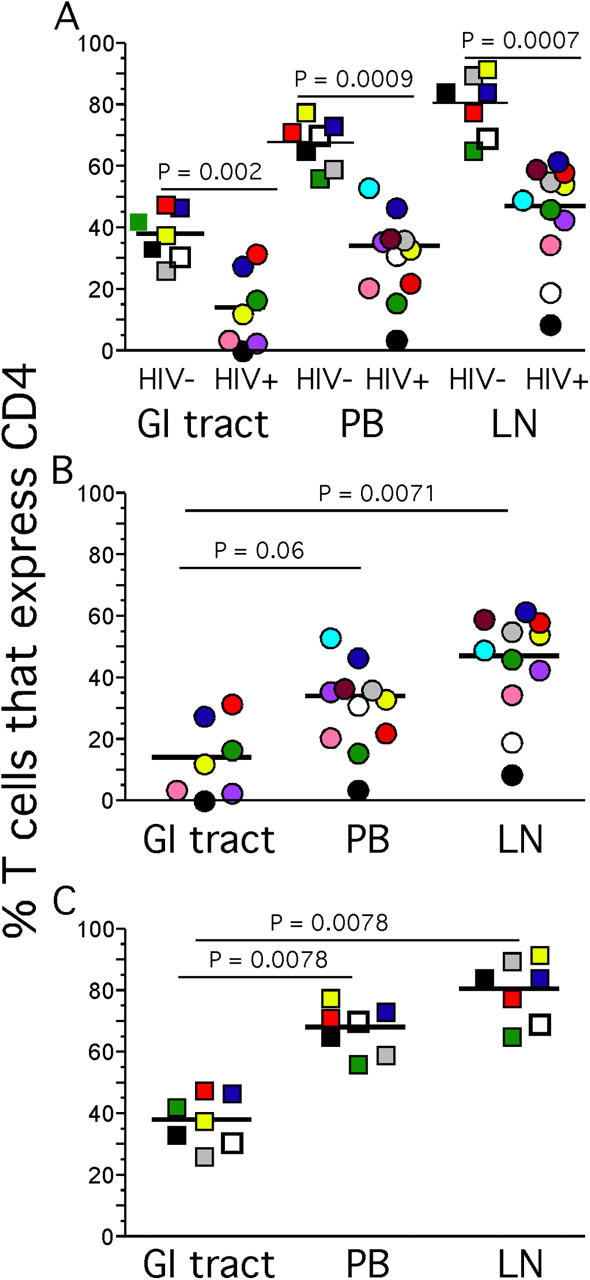
CD4+ T cell percentages in the GI tract, LNs, and peripheral blood. Lymphocytes from HIV+ (A and B) and HIV− (A and C) individuals obtained from the blood, LNs, and GI tract were stained with anti-CD3 and anti-CD4 antibodies. The percentage of T cells that express CD4 represents lymphocyte and CD3+ gated events that stain positively with anti-CD4. p-values represent the results of Mann-Whitney statistical significance calculations between sample cohorts (HIV+ compared with HIV−) or the results of Wilcoxon matched pairs for comparisons among compartments for individual cohorts.
We did not find that γδ T cells affected the frequencies of CD4+ or CD8+ αβ T cells in the GI tract. Indeed, their frequencies were always <10% of the total T cells, consistent with previous reports in humans (unpublished data; reference 31).
Although the lower percentages of CD4+ T cells in the GI tract of HIV-infected individuals (Fig. 1 B) are certainly consistent with preferential depletion in this compartment, preferential depletion cannot be established by simply comparing percentages of CD4+ T cells in each compartment because the GI tract of HIV-uninfected individuals also contained significantly lower percentages of CD4+ T cells compared with either peripheral blood or LNs (Fig. 1 C). Nonetheless, we were able to show that there is not only extensive depletion of CD4+ T cells, but also preferential depletion of particular CD4+ T cell subsets, including those specifically targeted by HIV in the GI tract, in the studies described next.
Endoscopic and Histological GI Tract Examination Suggests Profound CD4+ T Cell Depletion.
In these studies we sought additional measures of relative population sizes. In the GI tract we evaluated lymphoid tissue populations by endoscopy and immunohistology. Such analysis of subject 1421 (an HIV-uninfected individual) shows the typical gross anatomical appearance of terminal ileum with large lymphoid aggregates clearly apparent (Fig. 2 A). Similar lymphoid aggregates were observed in all seven healthy individuals. However, in subject 1329 (an acute HIV seroconverter) the terminal ileum is striking in its almost complete absence of discernible lymphoid tissue (Fig. 2 B). Indeed, the histological staining for CD4 confirmed the marked depletion of CD4+ T cells (Fig. 2 D; CD8+ T cells remained abundant; not depicted). Similar patterns were observed with the other subjects in our cohort; however, because CD4+ T cell populations are so spatially dispersed in the GI tract, we were unable to count sufficient numbers of cells in the small biopsies to accurately quantify the size of the total CD4+ T cell population. Nonetheless, these endoscopic and histologic analyses strongly suggested that CD4+ T cell depletion in the GI tract might be substantial even at very early stages of HIV infection.
Figure 2.
Endoscopic and histological analysis of the GI tract. Endoscopic photographs from subjects 1421 (A) and 1329 (B) were obtained by passing the colonoscope past the ileo–cecal junction into the terminal ileum. Immunohistological staining for CD4 was performed by sectioning biopsies from ileal lymphoid tissue, and then hematoxylin and eosin staining followed by staining for CD4 (C and D).
CCR5+ CD4+ T Cells Are Preferentially Depleted in the GI Tract.
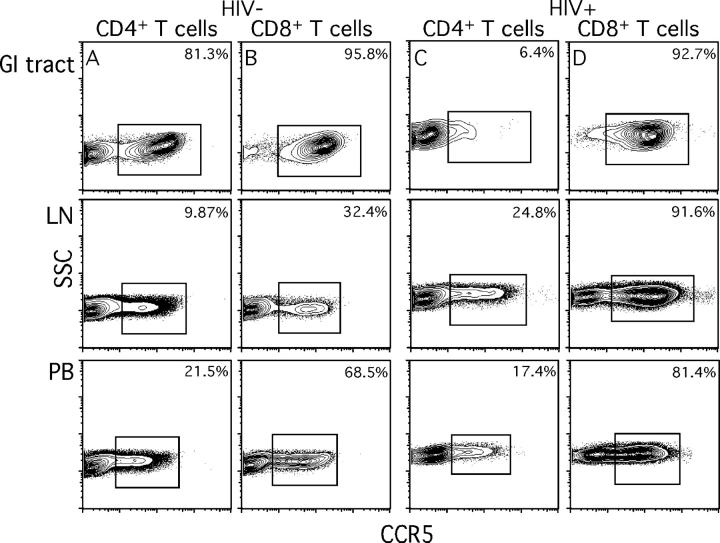
Although the endoscopic observations were striking, they could be considered anecdotal in isolation. Therefore, we sought to address CD4+ T cell depletion in each compartment more quantitatively, and investigate one mechanism for depletion related to target cell availability. We examined the frequency of CCR5+ CD4+ T cells and compared this with the frequency of CD8+ T cells (Fig. 3). In HIV-uninfected individuals, virtually all (>95%) CD8+ T cells in the GI tract expressed CCR5, whereas more than half of the GI tract CD4+ T cells expressed the HIV coreceptor (the raw data for one such individual are shown in Fig. 3, A and B), confirming previous reports (3, 4, 6). In contrast, only 5–10% of LNs and 10–30% of peripheral blood CD4+ and CD8+ T cells expressed CCR5, respectively (Fig. 3, A and B). In an HIV-infected, treatment-naive individual (1428; Fig. 3, C and D) and in four other HIV-infected individuals (1416, 1419, 1431, and 1431), we found a preferential and substantial depletion of GI tract CCR5+ CD4+ T cells, consistent with data found using SIV infection (6, 32). This depletion was specific to CD4+ T cells as all GI tract CD8+ T cells maintained CCR5 expression (Fig. 3 D). Importantly, the nearly complete depletion of CCR5+ CD4+ T cells was restricted to the GI tract. The percentage of CCR5+ CD4+ T cells in LNs and peripheral blood from HIV-infected individuals did not differ from those in HIV-uninfected individuals (Fig. 3). This was surprising, as immune activation, altered trafficking, and proliferation per se should result in accumulation of CCR5+ CD4+ and CD8+ T cells in peripheral blood (33), LNs, and GI tract. Although this was clearly the case for CD8+ T cells, CCR5+ CD4+ T cells do not appear to be elevated. Furthermore, as we next show and later discuss, Ki67+ CD4+ T cell frequency is not elevated in the GI tract. Taken together, the lack of expansion of activated CD4+ T cells and the preferential depletion of the CCR5+ CD4+ T cells in the GI tract could be the result of the different balance in lymphoid tissue compartments between target cell availability and death that reduce the numbers of these cells, and the recruitment and expansion of this subset.
Figure 3.
CCR5 expression by T cells in the GI tract, LNs, and peripheral blood. GI tract, LN, and peripheral blood lymphocytes from subject 1425 (A and B, HIV−) and 1428 (C and D, HIV+) were stained with anti-CD3, anti-CD4, anti-CD8, and anti-CCR5 antibodies. Plots represent lymphocyte, CD3+, and either CD4+ or CD8+ gated events. Values indicate the calculated percentage of memory CD4+ or CD8+ T cells that express CCR5 as described in Materials and Methods.
Different Activation Status of T Cells in the GI Tract, LNs, and Peripheral Blood.
It is well established that HIV-infected individuals contain higher percentages of activated T cells compared with uninfected individuals based on expression of a number of activation markers (34, 35), and that activated CD4+ T cells are the principal host cell in which virus replicates over much of the long course of infection (36). If HIV replicates in and causes the death mainly of activated CCR5+ CD4+ T cells, one might expect to see preferential depletion of such cells in the GI tract compared with other compartments based on their availability. We found that this was the case by comparing activation of T cell subsets in the peripheral blood, GI tract, and LNs by measuring expression of the nuclear antigen Ki67 (Fig. 4). In HIV-uninfected individuals, we found that the GI tract contained a significantly higher proportion of Ki67+ T cells compared with either LNs or peripheral blood (Fig. 4, A and B), in contrast to HIV-infected individuals where no one compartment contained a significantly higher proportion of either Ki67+ CD4+ or CD8+ T cells compared with any other compartment (Fig. 4, C and D). Consistent with immune activation in HIV infection, with the exception of the CD4+ T cells in the GI tract, both CD8+ and CD4+ T cells from HIV-infected individuals contained significantly higher proportions of Ki67+ T cells in every compartment examined. Ki67 expression by CD4+ T cells in GI tract of HIV-infected individuals was not increased compared with HIV-uninfected individuals (Fig. 4, E and F). This finding is consistent with the hypothesis that activated CCR5+ CD4+ T cells in the GI tract are continually depleted by ongoing infection by HIV. Furthermore, as we later discuss, the rapid depletion of the majority of GI tract CD4+ T cells that occurs during the acute phase of infection might be perpetuated in the chronic phase by a failure of immune reconstitution in this compartment.
Figure 4.
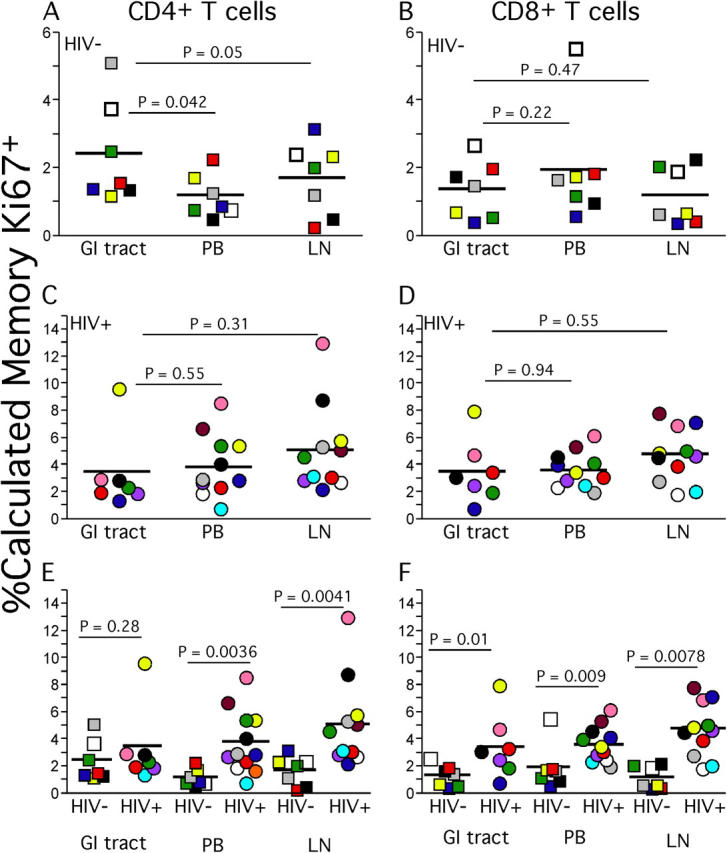
Ki67 expression by memory T cells in the GI tract, LNs, and peripheral blood. Lymphocytes from HIV− (A, B, E, and F) and HIV+ (C–F) individuals obtained from the peripheral blood, LNs, and GI tract were stained extracellularly with anti-CD3, anti-CD4 antibodies, and anti-CD8 antibodies followed by intracellular staining with anti-Ki67. The calculated percentage of memory T cells that express Ki67 represents lymphocyte gated, CD3+, and either CD4+ or CD8+ gated events that stain positively with anti–Ki67 and were then expressed as the percentage of memory CD4+ or CD8+ T cells as described in Materials and Methods.
Effector–Memory T Cells (TEM Cells) Accumulate Abnormally in HIV+ LNs.
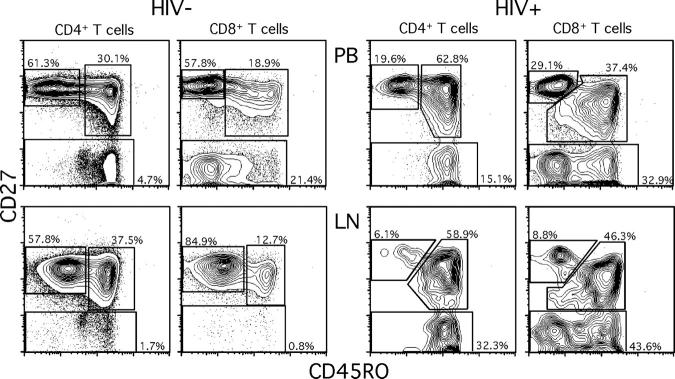
One hypothesis that readily accounts for the abnormally high levels of activated T cells we observed in LNs is that these cells reflect a general state of immune activation and recruitment of T cells to a site of ongoing viral replication. Because LNs are the site where virus is replicating, rather than peripheral tissues, we also hypothesized that effector-type T cells would be recruited to LNs, reversing the usual migration of activated T cells from LNs to peripheral tissues (37). We tested this hypothesis by examining expression patterns of CD27 and CD45RO that define at least three different T cell subsets (Fig. 5). Such distinct T cell subsets are not found in the GI tract samples, but T cells generally conform to a “memory” phenotype (unpublished data). The population that lacks CD27 expression represents a population of “effector” T cells (38) or TEM cells (37). We found a significantly higher proportion of CD27− CD4+ and CD27− CD8+ T cells from HIV-infected individuals compared with HIV-uninfected individuals in peripheral blood and LNs (Fig. 6, A–D).
Figure 5.
Naive, central memory, and TEM cell populations in LNs and peripheral blood. LN and peripheral blood lymphocytes from subject 1425 (HIV−) and 1413 (HIV+) were stained with anti-CD4, anti-CD8, anti-CD45RO, and anti-CD27 antibodies. Plots represent lymphocyte and either CD4+ or CD8+ gated events.
Figure 6.
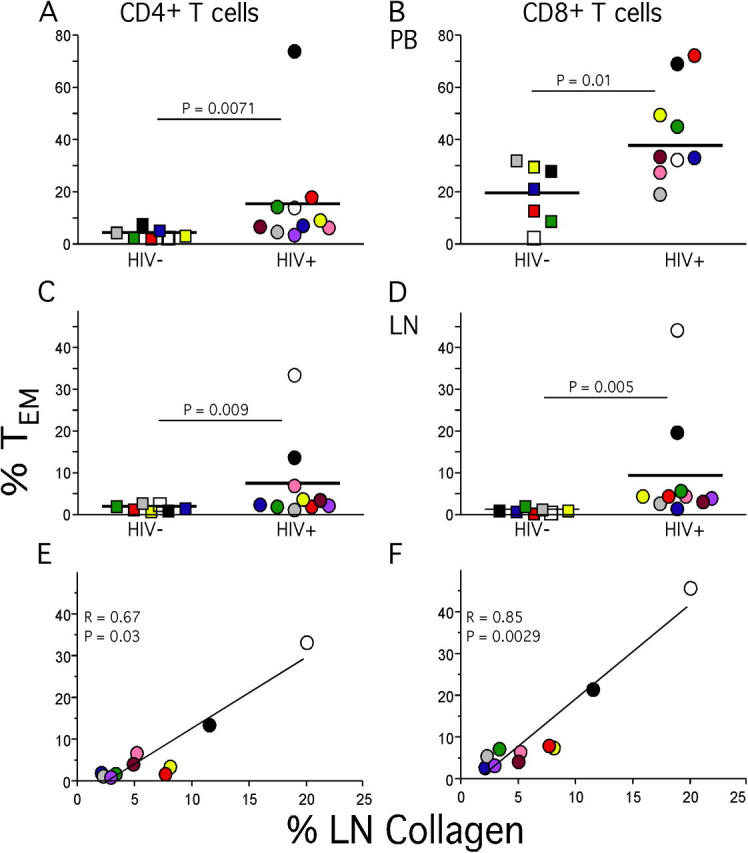
TEM cells and collagen deposition in LNs. Percentages of LN (A and B) and peripheral blood (C and D) CD4+ and CD8+ TEM cell populations were determined based upon expression patterns of CD27 and CD45RO. Collagen deposition levels were calculated as described in Materials and Methods and were then compared with percentage of CD4+ (E) or CD8+ T cells (F) of the TEM phenotype.
HIV-specific T Cells Do Not Account for all TEM Cells in LNs.
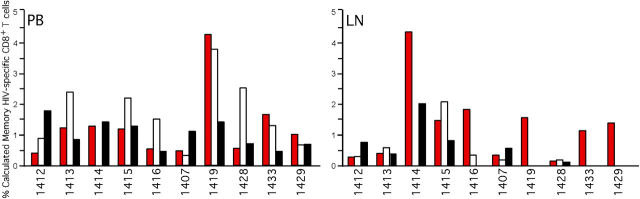
We then determined the contribution of HIV-specific T cells to the increase in the LN TEM cell population by intracellular cytokine staining after HIV peptide stimulation of LN and peripheral blood lymphocytes (Fig. 7). Consistent with previous reports, we found only a low frequency of HIV-specific CD4+ T cells in both compartments (29, 39, 40). However, we found detectable CD8+ T cells specific for multiple HIV antigens in LNs and peripheral blood (Fig. 7). In contrast to previous studies, we found that responses directed against particular HIV gene products in LNs were consistently found in peripheral blood; however, several individuals produced peripheral blood responses against specific HIV antigens that were clearly absent in LNs. These data suggest that the peripheral blood contains a greater breadth of HIV-specific CD8+ T cells compared with LNs. Importantly, the HIV-specific CD8+ T cell populations were not of sufficient magnitude to account for all CD8+ TEM cells in the LNs of HIV-infected individuals (Figs. 6 and 7). As our functional assay is likely to underestimate the frequency of HIV-specific CD8+ T cells (40), in two individuals we performed tetramer staining for defined immunodominant epitopes that allowed us to determine the frequency of such CD8+ T cells independent of function. These epitopes were selected because they are frequently immunodominant and encompass the majority of HIV-specific CD8+ T cells (40, 41). In subject 1429 (HLA-A2+, HLA-B57+) we examined the frequency of CD8+ T cells binding tetramers against the HLA-A2–restricted SLYTNVATL and the HLA-B57–restricted KAFSPEVIPMF epitopes. In this individual, a total of 0.84% of LN CD8+ T cells bound corresponding tetramers, whereas 3.6% of the CD8+ T cells in LNs were TEM. Similarly, in subject 1431 (HLA-A2+, HLA-B8+) a total of 2.1% of LN CD8+ T cells bound tetramers corresponding to HLA-A2–restricted SLYTNVATL and HLA-B8–restricted GGKKKYKL and FLKEKGGL epitopes, whereas 4.6% of CD8+ T cells in LNs were TEM. Therefore, the functional and physical analyses of LN HIV-specific CD8+ T cell frequency suggest that not all TEM cells are HIV specific.
Figure 7.
HIV-specific CD8+ T cells in LNs and peripheral blood. Lymphocytes from HIV+ individuals obtained from peripheral blood and LNs were stimulated with overlapping HIV peptides for gag, pol, and env, and costimulatory antibodies as described in Materials and Methods. Lymphocytes were then extracellularly stained with anti-CD3, anti-CD4, and anti-CD8 antibodies followed by intracellular staining with anti–IL-2 and anti–IFN-γ antibodies. The percentage of memory CD8+ T cells that are HIV specific was determined after lymphocyte, CD3+, and CD8+ gating followed by adjustment based upon the percentage of memory CD8+ T cells for each individual as described in Materials and Methods. Bars represent the frequency of memory CD8+ T cells that respond to pol, (red bars) gag (white bars), or env (black bars).
Immune Activation Correlates with Collagen Deposition in LNs.
The increased numbers of activated T cells and TEM cells in LNs of HIV-infected patients is consistent with the hypothesis that such increases reflect the in situ generation of these cells as well as their activation and recruitment to a site of active viral replication. We have also previously documented increased deposition of collagen in LNs, which we attributed to the inflammation associated with chronic immune activation. Because this would be a pathological process parallel to the increases in TEM cells, we looked for and found a significant positive correlation between LN collagen and percentage of TEM cells in LNs (Fig. 6, E and F). Consistent with the hypothesis of such parallel processes in LNs, these same correlations were not found with peripheral blood TEM cells (not depicted).
Discussion
Much that is understood regarding CD4+ T cell depletion, heightened T cell activation states, T cell dynamics, and HIV-specific T cells in HIV infection is derived from the analysis of peripheral blood lymphocytes. Although the importance of studies of how HIV infection affects lymphoid tissue is certainly appreciated (15, 42–44), there are few and only very recent studies of the GI tract (8, 20, 21, 24) due to the difficulty in obtaining tissue samples. Substantial ground has been gained through the SIV rhesus macaque model (5, 22, 23, 30). However, to date there have been no reports comparing CD4+ and CD8+ T cell populations in peripheral blood, GI tract, and LNs from HIV-infected and -uninfected individuals.
From our current study, the following five major points emerged: (a) the GI tract has the most substantial CD4+ T cell depletion at all stages of HIV disease; (b) this depletion occurs preferentially within the CCR5+ CD4+ T cell subset, which accounts for the majority of GI tract CD4+ T cells; (c) HIV-associated immune activation results in an accumulation of effector/TEM cells within LNs; (d) HIV-specific T cells residing in LNs do not, alone, account for the inflammatory T cell response within HIV-infected LNs; and (e) T cell activation in LNs is associated with collagen deposition.
Quantitative analysis of CD4+ T cell depletion in LNs and the GI tract is substantially more challenging than in peripheral blood. LNs and the GI tract are not homogeneous organs and histological sections sampled for analysis may not be representative; therefore, other parameters must be examined. Perhaps the most obvious variables to quantify are either CD4+/CD8+ T cell ratios or CD4+ T cell percentages by flow cytometry. Indeed, such analysis suggests that the GI tract is substantially more depleted of CD4+ T cells than either peripheral blood (24) or LNs. However, examination of the same anatomical compartments from healthy individuals demonstrates that even under normal circumstances, the GI tract contains a lower percentage of CD4+ T cells compared with either peripheral blood or LNs. Thus CD4+ T cell percentages cannot be used to estimate CD4+ T cell depletion in tissue. Therefore, we examined three other, independent parameters to demonstrate that the GI tract was preferentially depleted of CD4+ T cells. Measurement of CCR5+ CD4+ and CD8+ T cells, endoscopic and histological examination of the GI tract, and measurement of activated T cells in each compartment suggested that the GI tract was significantly depleted of CD4+ T cells compared with either peripheral blood or LNs and that this occurred even at very early time points after infection.
Interpretation of the changes in T cell subsets that differ between lymphoid tissue compartments is complicated by the nature of the analysis—“snap-shots” of tissues—and by the potential mechanisms to explain these changes that include preferential infection and killing of T cell subsets, and the redistribution, proliferation, activation-induced T cell death, and alterations in the lymphoid tissue milieu that accompany immune activation. We believe that compartment-specific variations in these processes account for the differences we observed, particularly in the CD4+ T cell population in the GI tract.
The majority of GI tract CD4+ T cells expresses CCR5 and comprises a higher frequency of activated T cells than peripheral blood or LNs in HIV-uninfected individuals. Thus, they represent ideal targets for HIV replication. It is likely that direct infection of this population results in its profound depletion during the acute phase of HIV disease. Ongoing direct infection and sustained death might explain the continued depletion of GI tract CCR5+ CD4+ T cells that is only partially offset by proliferation of CD4+ T cells in the GI tract, and resulting in apparently normal levels of CD4+ T cell activation. On the other hand, in LNs, there is a smaller proportion of activated CCR5+ CD4+ T cell targets and thus proliferation and recruitment of CD4+ T cells might sufficiently exceed direct killing for the heightened state of immune activation to be evident in this compartment. In addition, it is possible that during the chronic phase of the disease, the disruption of the homeostatic processes that maintain total body T cell numbers (44) would in itself hinder CD4+ T cell reconstitution in lymphoid tissue. The consequence of this would be particularly damaging in the GI tract as, in contrast to LNs, there is only a negligible resident naive CD4+ T cell pool available to become activated, expand, and supply the already profoundly depleted memory CD4+ T cell pool.
Alternatively, the decrease in the frequency of CCR5+ CD4+ T cells in the GI tract in HIV infection might arise from altered migration of activated CCR5+ CD4+ T cells into the GI tract, or from recruitment of CCR5− CD4+ and CCR5+ CD8+ T cells to the GI tract. However, the latter explanation requires specific infiltration of two unrelated T cell subsets, CCR5+ CD8+ T cells and CCR5− CD4+ T cells, even though total GI tract lymphoid tissue appears to be dramatically decreased overall. Therefore, we believe the most likely explanation is that direct infection and killing, either by HIV or by HIV-specific T cells, of GI tract CCR5+ CD4+ T cells leads to their profound depletion in acute infection, and that this depletion is maintained during the chronic phase of the disease. In addition, it is possible that the few CCR5+ CD4+ T cells in GI tract that persist represent resting CCR5+ CD4+ T cells that have never seen antigen and might be resistant to HIV-mediated lysis (45, 46). Importantly, we chose to biopsy terminal ileum as this site has the greatest concentration of GI tract T cells. Although our samples contained a combination of lamina propria and Peyer's patch lymphoid tissue, the majority of our biopsies contained a low frequency of naive T cells, suggesting that they consisted predominantly, but not exclusively, of lamina propria. Because we cannot distinguish between these lymphoid compartments, it is possible that CD4+ T cell depletion is more substantial in lamina propria compared with Peyer's patches, and we may have observed more dramatic CD4+ T cell depletion if we had also biopsied jejunum or colon, which lack Peyer's patches (45–47). Hence, we may have even underestimated the extent of CD4+ T cell depletion from the entire GI tract.
It has been suggested that immune activation associated with HIV infection would lead to increased percentages of CCR5+ CD4+ T cells in peripheral blood, as seen in acute EBV infection (33). However, although HIV-infected individuals contain increased frequencies of peripheral blood CCR5+ CD8+ T cells, a corresponding increase in peripheral blood CCR5+ CD4+ T cells is not observed. One explanation for this observation is that there might be redistribution of CCR5+ CD4+ T cells from peripheral blood to LNs or GI tract in HIV infection (48–50). However, upon examination of CD4+ T cells in the LNs and GI tract, we found no increases in CCR5+ CD4+ T cell percentages. Therefore, we believe our data suggest that accumulation of CCR5+ CD4+ T cells resulting from immune activation associated with HIV infection is offset by their preferential death rather than redistribution, whereas activated CD8+ T cells accrue. Importantly, as the percentage of infected peripheral blood CD4+ T cells is usually <1% in chronic infection (39, 51), it seems unlikely that direct infection of activated CD4+ T cells is solely responsible for the death of CCR5+ CD4+ T cells, and other factors likely contribute. Taken together, the findings of substantial CD4+ T cell depletion in the GI tract and lack of evidence for redistribution to LNs, suggest that peripheral blood CD4+ T cell counts may actually underestimate the degree of overall T cell depletion in acute and chronic HIV infection.
Another mechanism contributing to the loss of CD4+ T cells in HIV infection is likely to involve disturbance of normal lymphoid tissue homeostatic processes. Peripheral LNs are structurally organized to promote interaction between antigens, chemokines, growth factors, and lymphocytes to generate an immunologic response and maintain populations of CD4+ and CD8+ T cells (52). It is likely that the inflammation and tissue remodeling that accompany local innate and adaptive immune responses to HIV replication lead to destruction of LN architecture observed in HIV disease (28), which, because of the particular dependency of CD4+ T cells on the LN milieu (53), contributes to decreased survival and depletion of the CD4+ T cell subset. We interpret our data on the sequestration or retention of TEM cells that are not normally found in LNs as evidence of the chronic proinflammatory responses generated as a result of ongoing viral replication in the LN. Furthermore, B cell activation, characterized by germinal center hyperplasia, might add to LN architectural damage. Importantly, trafficking of T cells to the follicular dendritic cell network is likely to depend on an intact LN architecture, and such trafficking might be consequently impaired in a fibrosed LN. Hence, what was an organ of antigen presentation and homeostasis in health becomes an organ of inflammation and fibrosis in HIV infection. Furthermore, our data continue to suggest that in individuals with significant LN collagen deposition, there might be therapeutic benefit from antiinflammatory and/or antifibrotic agents, singly or in combination with antiretroviral therapy, to prevent further or reduce LN damage.
Finally, our data also demonstrate that the majority of TEM cells sequestered to or retained in LNs are not HIV specific. One possibility is that they are elicited by subclinical opportunistic infections secondary to the immunosuppressive effects of HIV infection (46). In fact, the LNs actually had a smaller breadth of HIV-specific T cells compared with peripheral blood, suggesting that HIV-specific T cells are not preferentially recruited to LNs. Indeed, although we have not determined the cytotoxic capacity of HIV-specific T cells in LNs against the resident viral quasispecies, the low frequency of T cells responding at a major site of virus replication may point to one more example of the failure of immune defenses in fully controlling HIV replication.
The results also imply that LNs are not a continuous source of peripheral blood HIV-specific T cells. Moreover, the breadth of HIV-specific T cells was not the only variable we measured that differed between anatomical compartments. There was no clear correlation between the GI tract, LNs, and peripheral blood for any of the parameters we measured. Hence, data obtained from peripheral blood cannot be used to predict or model immunological processes or HIV pathogenesis in the GI tract or LNs, and such compartments should be directly sampled and studied.
The substantial depletion of GI tract CCR5+ CD4+ T cells implies that total body CD4+ T cell numbers are severely reduced, even in very early infection. This, by itself, would impose a considerable homeostatic strain on the maintenance of the memory CD4+ T cell pool. Immune activation that defines the chronic phase of the infection results in destruction of lymphoid tissue architecture that, in turn, impacts upon the ability of lymphoid tissue to support normal lymphocyte homeostasis and antigen presentation. Taken together, our data suggest that HIV infection directly and indirectly causes damage to many immune compartments and a combination of such deleterious effects ultimately leads to immune failure and AIDS. Understanding the relative contribution of each of the factors that we have described can provide a rational framework upon which future therapeutic interventions can be based.
Acknowledgments
We would like to thank Drs. Frank Rhame, Alan Lifson, Susan Kline, Margarat Simpson, and Leslie Baker for patient referral.
T.W. Schacker is supported by NIH grants RO1 AI54232, K24 AIO56986, and RO1 DE-12934. A.T. Haase is supported by NIH grant R437 AI028246.
The authors have no conflicting financial interests.
Abbreviations used in this paper: GI, gastrointestinal; SIV, simian immunodeficiency; TEM cell, effector–memory T cell.
References
- 1.Dybul, M., A.S. Fauci, J.G. Bartlett, J.E. Kaplan, and A.K. Pau. 2002. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. MMWR Recomm. Rep. 51:1–55. [PubMed] [Google Scholar]
- 2.Mowat, A., and J. Viney. 1997. The anatomical basis of intestinal immunity. Immunol. Rev. 156:145–166. [DOI] [PubMed] [Google Scholar]
- 3.Anton, P.A., J. Elliott, M.A. Poles, I.M. McGowan, J. Matud, L.E. Hultin, K. Grovit-Ferbas, C.R. Mackay, I.S.Y. Chen, and J.V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 14:1761–1765. [DOI] [PubMed] [Google Scholar]
- 4.Agace, W.W., A.I. Roberts, L. Wu, C. Greineder, E.C. Ebert, and C.M. Parker. 2000. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur. J. Immunol. 30:819–826. [DOI] [PubMed] [Google Scholar]
- 5.Veazey, R.S., M. DeMaria, L.V. Chalifoux, D.E. Shvetz, D.R. Pauley, H.L. Knight, M. Rosenzweig, R.P. Johnson, R.C. Desrosiers, and A.A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 280:427–431. [DOI] [PubMed] [Google Scholar]
- 6.Veazey, R.S., P.A. Marx, and A.A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187:769–776. [DOI] [PubMed] [Google Scholar]
- 7.Moore, J.P., S.G. Kitchen, P. Zhong, and J.A. Zack. 2004. The CCR5 and CXCR4 coreceptors-central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retroviruses. 20:111–126. [DOI] [PubMed] [Google Scholar]
- 8.Hufert, F.T., J. van Lunzen, G. Janossy, S. Bertram, J. Schmitz, O. Haller, P. Racz, and D. von Laer. 1997. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 11:849–857. [DOI] [PubMed] [Google Scholar]
- 9.Heath, S.L., J.G. Tew, J.G. Tew, A.K. Szakal, and G.F. Burton. 1995. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 377:740–744. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel, H., H. Herbst, G. Niedobitek, H.D. Foss, and H. Stein. 1992. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am. J. Pathol. 140:15–22. [PMC free article] [PubMed] [Google Scholar]
- 11.Tenner-Racz, K., H.J. Stellbrink, J. van Lunzen, C. Schneider, J.P. Jacobs, B. Raschdorff, G. Grosschupff, R.M. Steinman, and P. Racz. 1998. The unenlarged lymph nodes of HIV-1–infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J. Exp. Med. 187:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Embretson, J., M. Zupancic, J.L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A.T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 362:359–362. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo, G., C. Graziosi, J.F. Demarest, L. Butini, M. Montroni, C.H. Fox, J.M. Orenstein, D.P. Kotler, and A.S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 362:355–358. [DOI] [PubMed] [Google Scholar]
- 14.Haase, A.T., K. Henry, M. Zupancic, G. Sedgewick, R.A. Faust, H. Melroe, W. Cavert, K. Gebhard, K. Staskus, Z.Q. Zhang, et al. 1996. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 274:985–989. [DOI] [PubMed] [Google Scholar]
- 15.Haase, A.T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625–656. [DOI] [PubMed] [Google Scholar]
- 16.Shacklett, B.L., C.A. Cox, J.K. Sandberg, N.H. Stollman, M.A. Jacobson, and D.F. Nixon. 2003. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattapallil, J.J., Z. Smit-McBride, M. McChesney, and S. Dandekar. 1998. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1beta expression and display antiviral cytotoxic activity despite severe CD4+ T-cell depletion in primary simian immunodeficiency virus infection. J. Virol. 72:6421–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vingert, B.C., R. Le Grand, and A. Venet. 2003. Heterogeneity of the simian immunodeficiency virus (SIV) specific CD8+ T-cell response in mucosal tissues during SIV primary infection. Microbes Infect. 5:757–767. [DOI] [PubMed] [Google Scholar]
- 19.Evans, D.T., L.M. Chen, J. Gillis, K.C. Lin, B. Harty, G.P. Mazzara, R.O. Donis, K.G. Mansfield, J.D. Lifson, R.C. Desrosiers, et al. 2003. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 77:2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shacklett, B.L., O. Yang, M.A. Hausner, J. Elliott, L. Hultin, C. Price, M. Fuerst, J. Matud, P. Hultin, C. Cox, et al. 2003. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J. Immunol. Methods. 279:17–31. [DOI] [PubMed] [Google Scholar]
- 21.Altfeld, M., J. van Lunzen, N. Frahm, X.G. Yu, C. Schneider, R.L. Eldridge, M.E. Feeney, D. Meyer-Olson, H.J. Stellbrink, and B.D. Walker. 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Invest. 109:837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kewenig, S., T. Schneider, K. Hohloch, K. Lampe-Dreyer, R. Ullrich, N. Stolte, C. Stahl-Hennig, F.J. Kaup, A. Stallmach, and M. Zeitz. 1999. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 116:1115–1123. [DOI] [PubMed] [Google Scholar]
- 23.Vajdy, M., R. Veazey, I. Tham, C. deBakker, S. Westmoreland, M. Neutra, and A. Lackner. 2001. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J. Infect. Dis. 184:1007–1014. [DOI] [PubMed] [Google Scholar]
- 24.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton, F., G. Snow, S. Reka, and D.P. Kotler. 1997. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim, S.G., A. Condez, C.A. Lee, M.A. Johnson, C. Elia, and L.W. Poulter. 1993. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullrich, R., W. Schmidt, T. Zippel, T. Schneider, M. Zeitz, and E.O. Riecken. 1998. Mucosal HIV infection. Pathobiology. 66:145–150. [DOI] [PubMed] [Google Scholar]
- 28.Schacker, T.W., P.L. Nguyen, G.J. Beilman, S. Wolinsky, M. Larson, C. Reilly, and A.T. Haase. 2002. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J. Clin. Invest. 110:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitcher, C.J., C. Quittner, D.M. Peterson, M. Connors, R.A. Koup, V.C. Maino, and L.J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518–525. [DOI] [PubMed] [Google Scholar]
- 30.Smit-McBride, Z., J.J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayday, A.C. 2000. γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18:975–1026. [DOI] [PubMed] [Google Scholar]
- 32.Veazey, R.S., K.G. Mansfield, I.C. Tham, A.C. Carville, D.E. Shvetz, A.E. Forand, and A.A. Lackner. 2000. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaunders, J.J., G.R. Kaufmann, P.H. Cunningham, D. Smith, P. Grey, K. Suzuki, A. Carr, L.E. Goh, and D.A. Cooper. 2001. Increased turnover of CCR5+ and redistribution of CCR5− CD4 T lymphocytes during primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 183:736–743. [DOI] [PubMed] [Google Scholar]
- 34.Bentwich, Z., A. Kalinkovich, Z. Weisman, and Z. Grossman. 1998. Immune activation in the context of HIV infection. Clin. Exp. Immunol. 111:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baier-Bitterlich, G., D. Fuchs, and H. Wachter. 1997. Chronic immune stimulation, oxidative stress, and apoptosis in HIV infection. Biochem. Pharmacol. 53:755–763. [DOI] [PubMed] [Google Scholar]
- 36.Stebbing, J., B. Gazzard, and D.C. Douek. 2004. Where does HIV live? N. Engl. J. Med. 350:1872–1880. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 38.Hamann, D., P.A. Baars, M.H. Rep, B. Hooibrink, S.R. Kerkhof-Garde, M.R. Klein, and R.A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douek, D.C., J.M. Brenchley, M.R. Betts, D.R. Ambrozak, B.J. Hill, Y. Okamoto, J.P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, et al. 2002. HIV preferentially infects HIV-specific CD4+ T-cells. Nature. 417:95–98. [DOI] [PubMed] [Google Scholar]
- 40.Betts, M., D. Ambrozak, D. Douek, S. Bonhoeffer, J. Brenchley, J. Casazza, R. Koup, and L. Picker. 2001. Analysis of total HIV-specific CD4+ and CD8+ T cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Addo, M.M., X.G. Yu, A. Rathod, D. Cohen, R.L. Eldridge, D. Strick, M.N. Johnston, C. Corcoran, A.G. Wurcel, C.A. Fitzpatrick, et al. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, Z.-Q., D. Notermans, G. Sedgewick, W. Cavert, S. Sietgrefe, M. Zupancic, S. Gebhard, K. Henry, L. Boies, Z. Chen, et al. 1998. Kinetics of CD4+ T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc. Natl. Acad. Sci. USA. 95:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pantaleo, G., C. Graziosi, and A.S. Fauci. 1993. New concepts in the immunopathogenesis of human imunodeficiency virus infection. N. Engl. J. Med. 328:327–335. [DOI] [PubMed] [Google Scholar]
- 44.Douek, D.C., L.J. Picker, and R.A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265–304. [DOI] [PubMed] [Google Scholar]
- 45.Veazey, R.S., J.D. Lifson, I. Pandrea, J. Purcell, M. Piatak Jr., and A.A. Lackner. 2003. Simian immunodeficiency virus infection in neonatal macaques. J. Virol. 77:8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veazey, R.S., P.A. Marx, and A.A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626–633. [DOI] [PubMed] [Google Scholar]
- 47.Veazey, R.S., P.J. Klasse, T.J. Ketas, J.D. Reeves, M. Piatak Jr., K. Kunstman, S.E. Kuhmann, P.A. Marx, J.D. Lifson, J. Dufour, et al. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian–human immunodeficiency virus infection. J. Exp. Med. 198:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pakker, N., D. Notermans, R. De Boer, M. Roos, F. De Wolf, A. Hill, J. Leonard, S. Danner, F. Miedema, and P. Schellekens. 1998. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat. Med. 4:208–214. [DOI] [PubMed] [Google Scholar]
- 49.Autran, B., G. Carcelain, T. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 277:112–116. [DOI] [PubMed] [Google Scholar]
- 50.Bucy, R.P., R.D. Hockett, C.A. Derdeyn, M.S. Saag, K. Squires, M. Sillers, R.T. Mitsuyasu, and J.M. Kilby. 1999. Initial increase in blood CD4+ lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J. Clin. Invest. 103:1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley, J.M., B.J. Hill, D.R. Ambrozak, D.A. Price, F.J. Guenaga, J.P. Casazza, J. Kuruppu, J. Yazdani, S.A. Migueles, M. Connors, et al. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Andrian, U.H., and T.R. Mempel. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878. [DOI] [PubMed] [Google Scholar]
- 53.Dai, Z., and F.G. Lakkis. 2001. Cutting edge: secondary lymphoid organs are essential for maintaining the CD4, but not CD8, naive T cell pool. J. Immunol. 167:6711–6715. [DOI] [PubMed] [Google Scholar]