Abstract
Concomitant tumor immunity describes immune responses in a host with a progressive tumor that rejects the same tumor at a remote site. In this work, concomitant tumor immunity was investigated in mice bearing poorly immunogenic B16 melanoma. Progression of B16 tumors did not spontaneously elicit concomitant immunity. However, depletion of CD4+ T cells in tumor-bearing mice resulted in CD8+ T cell–mediated rejection of challenge tumors given on day 6. Concomitant immunity was also elicited by treatment with cyclophosphamide or DTA-1 monoclonal antibody against the glucocorticoid-induced tumor necrosis factor receptor. Immunity elicited by B16 melanoma cross-reacted with a distinct syngeneic melanoma, but not with nonmelanoma tumors. Furthermore, CD8+ T cells from mice with concomitant immunity specifically responded to major histocompatibility complex class I–restricted epitopes of two melanocyte differentiation antigens. RAG1 −/− mice adoptively transferred with CD8+ and CD4+ T cells lacking the CD4+CD25+ compartment mounted robust concomitant immunity, which was suppressed by readdition of CD4+CD25+ cells. Naturally occurring CD4+CD25+ T cells efficiently suppressed concomitant immunity mediated by previously activated CD8+ T cells, demonstrating that precursor regulatory T cells in naive hosts give rise to effective suppressors. These results show that regulatory T cells are the major regulators of concomitant tumor immunity against this weakly immunogenic tumor.
Keywords: melanocyte differentiation antigen, cancer immunity, immune suppression, GITR, cyclophosphamide
Introduction
Concomitant tumor immunity describes the immune response in a host bearing a progressive tumor that rejects an inoculum of the same tumor at a distant site. The phenomenon was first reported by Ehrlich (1) and Bashford et al. (2) in the early 1900's, although these early observations could be attributed to alloantigen recognition and transplantation immunity in outbred mice. Subsequently, the establishment of inbred mouse strains and syngeneic tumor transplants directly established the relevance of concomitant immunity to tumor immunity. More than 70 yr later, North et al. conducted a series of notable experiments demonstrating concomitant immunity in BALB/c mice bearing the chemically induced Meth A fibrosarcoma (3–7). Immunity, recognized as rejection of secondary tumors inoculated on days 6–9, was mediated by CD8+ (Ly-2+) T cells that were detected as the primary tumor grew. Subsequently, immunity was down-regulated by CD5+CD4+ (Ly-1+2−) suppressor cells that were induced as the primary tumor progressed (3, 8).
Although skepticism developed regarding the existence of suppressor cells, studies in recent years have confirmed a central role of suppressor cell populations in regulating immunity. Naturally occurring CD4+ suppressor T cells (renamed regulatory T cells) constitutively express the transcription factor FoxP3 (9, 10), CD25 (11), and glucocorticoid-induced TNF receptor (GITR; reference 12). They are selected in the thymus and comprise ∼5–10% of the peripheral CD4+ T cell repertoire in mice (11). Absence of CD4+CD25+ FoxP3+ T cells is associated with severe autoimmunity (9). It has been proposed that mechanisms underlying autoimmunity and tumor immunity are linked (13). In fact, carefully timed depletion of CD25+ T cells has been shown to enhance tumor immunity and autoimmunity in both immunized and naive mice (14, 15). In addition to naturally occurring CD4+CD25+ regulatory T cells, IL-10–induced Tr1-type cells (16) and NK T cells (17–19) have also been implicated in suppressor functions.
This is an appropriate time to readdress the role of suppressor T cells in the historical context of concomitant tumor immunity. Although strongly immunogenic, carcinogen-induced tumors elicit both concomitant immunity and immune suppression (3, 20), concomitant immunity has not been described for hosts bearing poorly immunogenic tumors of spontaneous origin. The goals of the present paper were twofold: to explore concomitant tumor immunity in the context of a poorly immunogenic tumor and to investigate a role for CD4+CD25+ regulatory T cells or other suppressor T cell populations in this model. For these experiments, we have used B16 melanoma, an extensively studied, poorly immunogenic tumor.
Materials and Methods
Mice and Tumors.
All mouse procedures were performed in accordance with institutional protocol guidelines at Memorial-Sloan Kettering Cancer Center (MSKCC) under an approved protocol. C57BL/6J, RAG1 −/− (B6.129S7-RAG11 tm1Mom/J), and IL-10 −/− (B6.129P2-Il10t m1Cgn) mice (males, 8–10 wk old) were obtained from The Jackson Laboratory. CD1 −/− mice (21) were donated by M. Exley and S. Balk (Harvard Medical School, Boston, MA) and bred at MSKCC. All tumors were of C57BL/6 origin. The B16F10 mouse melanoma cell line was originally obtained from I. Fidler (M.D. Anderson Cancer Center, Houston, TX) and passaged intradermally in mice four times to ensure reproducible and aggressive intradermal tumor growth (B16). Immunization with 5 × 107 irradiated B16 melanoma cells provides no tumor protection, and as few as 2 × 103 cells can give rise to tumors. B16-GMCSF was generated by I. Hara and A. Houghton at MSKCC by retroviral transduction of B16F10 with a retroviral vector encoding the gene for mouse GM-CSF, using procedures described previously (22). JBRH melanoma was provided by P. Livingston (MSKCC, New York, NY), and Lewis lung carcinoma was acquired from American Type Culture Collection. LiHa fibrosarcoma, a cutaneous tumor induced by dimethylbenzanthrene treatment of an INK4a −/− C57BL/6 mouse, was provided by T. Merghoub (MSKCC, New York, NY). Cells were cultured in RPMI 1640 medium containing 7.5% FBS. Growth medium for B16-GMCSF cells was supplemented with 1.0 mg/ml G418.
Cells were harvested after limited passage in vitro and were used only if viability was >96%. Tumors were generated by intradermal inoculation of 105 live cells (except for Lewis lung carcinoma, where 2 × 105 cells were used). For experiments requiring two tumors on the same mouse, primary tumors were given on the right flank and challenge tumors were given on left flank 6 d later, unless otherwise specified. Growth of secondary tumors was only monitored for those mice that supported growth of primary tumors. Tumor diameters were measured approximately every other day, and mice were killed when one of the tumors ulcerated, reached a maximum diameter of 1 cm, or when mice showed discomfort.
Monoclonal Antibody (mAb) and Drug Treatments.
All treatments were administered by intraperitoneal injection. Mice were depleted of CD4+ and CD8+ T cells by injection with 250 μg GK1.5 and 2.43 monoclonal antibodies, respectively (bioreactor supernatants). NK cells were depleted by injection with 500 μg PK136 mAb to NK1.1. Antibodies were injected on days 4, 10, and 17 after the primary tumor inoculation (unless otherwise specified), and flow cytometry was used to confirm >98% depletion of target cells for at least 7 d after injection. For in vivo GITR stimulation, 1 mg of affinity-purified DTA-1 mAb (DTA-1 hybridoma was a gift from S. Sakaguchi, Kyoto University, Kyoto, Japan) was injected at the specified time points. Cyclophosphamide (Sigma-Aldrich) in PBS was administered in a single dose of 150 mg/kg as described previously (23) at specified time points.
Peptides and ELISPOT Assay.
Peptides were synthesized by Genemed Synthesis, Inc. and used at >80% purity, as confirmed by HPLC. Peptides from dopachrome tautomerase/tyrosinase-related protein 2 (DCT): DCT181-188 (24) and DCT363-371 (unpublished data), are restricted by Kb. The gp100/pmel 17 peptide gp10025-33 (25) is restricted by Db. A nine–amino acid sequence from mouse prostate-specific membrane antigen was mutated at an anchor residue for optimized strong binding to Db and was used as an irrelevant peptide control.
Multiscreen-IP plates (Millipore) were coated with 100 μl anti–mouse IFN-γ antibody (10 mg/ml; clone AN18; Mabtech) in PBS, incubated overnight at 4°C, washed with PBS to remove unbound antibody, and blocked with RPMI 1640 plus 7.5% FBS for 2 h at 37°C. CD8+ T cells were harvested from pooled spleen and inguinal lymph nodes of killed mice (10 mice/group), purified using anti-CD8 MACS magnetic beads (Miltenyi Biotec), and plated at a concentration of 2 × 105 cells/well. For antigen presentation, 2 × 104 irradiated B16 cells or EL-4 leukemia cells (American Type Culture Collection) that had been pulsed with 10 μg/ml peptide for 1 h were added to a final volume of 100 μl/well. After incubation for 20 h at 37°C, plates were extensively washed with PBS plus 0.05% Tween and incubated for 2 h at 37°C with 100 μl/well biotinylated antibody against mouse IFN-γ (2 mg/ml; clone R4-6A2; Mabtech). Spot development was performed as described previously (26). Spots were counted with an automated ELISPOT reader system with KS 4.3 software (Carl Zeiss MicroImaging, Inc.).
Concomitant Immunity in RAG1−/− Hosts, after Adoptive Transfer.
Naive T cell populations were freshly isolated from spleens of C57BL/6J untreated donor mice. All cells were purified using MACS magnetic beads, according to the manufacturer's instructions (Miltenyi Biotec). CD8+ and CD4+ populations were purified by positive selection, and CD4+CD25+ and CD4+CD25− populations were purified in two steps, by negative and positive selection (mouse regulatory T cell isolation kit; Miltenyi Biotec). Cell purity of >90% for all populations was confirmed by flow cytometry. Purified lymphocytes were transferred intravenously into five groups of 15 RAG1 −/− recipient mice/group. Recipients received either 6 × 106 CD8+ T cells alone, or 6 × 106 CD8+ T cells mixed with each of the following: 107 CD4+ T cells, 106 CD4+CD25+ T cells, or 9 × 106 CD4+CD25− T cells. 1 d after reconstitution, RAG1 −/− mice were challenged with 0.75 × 105 B16 cells in the right flank, followed 6 d later by an identical inoculum in the left flank.
Adoptive Transfer of Immunity and Suppression.
CD8+ T cell donor mice were inoculated with 105 live B16 cells in the right flank followed 6 d later by an identical inoculum in the left flank, and CD4 depletion on days 4 and 10. 12 d after primary tumor inoculation, CD8+ T cells were isolated from pooled spleen and tumor-draining lymph nodes. All cells were purified using MACS magnetic beads, as described before, and adoptive transfer was conducted with fresh unstimulated cells. CD8+ T cell purity was 93%, as determined by flow cytometry, and ∼107 CD8+ T cells were obtained from each immunized donor. For adoptive transfer of immunity, 107 CD8+ T cells were injected intravenously into RAG1 −/− recipients (10 mice/group). A separate group of mice received 107 CD8+ T cells taken from naive C57BL/6J mice, as a negative control.
Naive CD4+CD25+ T cells were isolated from spleens of untreated C57BL/6J mice, whereas tumor-bearing CD4+CD25+ and CD4+CD25− T cells were isolated from combined spleen and tumor-draining lymph nodes of mice bearing day-12 B16 tumors. Purity of CD4+CD25+ and CD4+CD25− T cells was 85 and 95%, respectively, as determined by flow cytometry. For adoptive transfer of suppression, groups of RAG1 −/− recipients (10 mice/group) were intravenously injected with 107 immune CD8+ T cells combined with each of the following putative suppressor populations: 8 × 105 naive CD4+CD25+ T cells, 8 × 105 CD4+CD25+ T cells from tumor-bearing mice, or 5 × 106 CD4+CD25− T cells from tumor-bearing mice. All mice were challenged with 105 B16 cells 1 d after adoptive transfer, and tumor growth was monitored for 60 d.
Statistical Calculations.
To determine significant differences between different groups of mice with respect to tumor-free survival, log rank analysis (comparisons pooled over strata) of Kaplan-Meier data was conducted using SPSS 10.0 software for Windows. Statistical differences between tumor sizes and groups of wells in ELISPOT assay were determined by two-tailed Student's t test.
Online Supplemental Material.
Fig. S1 shows growth of B16 primary and secondary tumors in CD1−/− and IL-10−/− mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20041130/DC1.
Results
Concomitant Immunity to Poorly Immunogenic B16 Can Be Elicited by Tumor GM-CSF Expression and/or CD4+ T Cell Depletion.
To determine if concomitant immunity is elicited by a poorly immunogenic tumor, B16 tumors were inoculated in the right flanks of C57BL/6 hosts, and 3, 6, 9, or 12 d later, an identical inoculum was given in the opposite flank. At all time points, mice with progressive primary tumors supported growth of challenge tumors at rates comparable to naive control mice. Out of a total of 156 mice bearing primary tumors, from six independent experiments, 148 supported normal growth of secondary tumors (Fig. 1 A and not depicted). A similar lack of immunity against a secondary tumor was observed when primary tumors were ligated or surgically excised, and when mice received primary and/or challenge inoculations in the footpad (unpublished data).
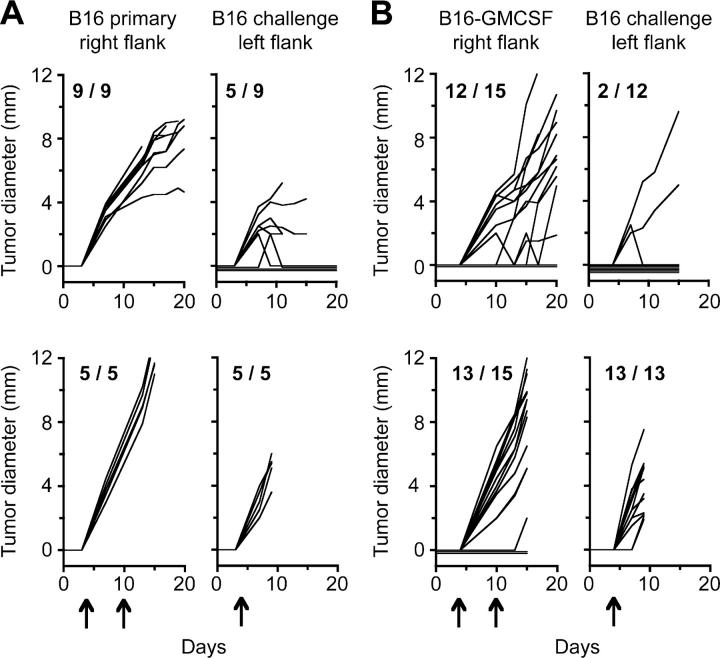
Figure 1.
Concomitant immunity to B16 melanoma can be induced by GM-CSF expression in tumor cells and/or by CD4+ T cell depletion. C57BL/6 mice (10–15/group) were inoculated with B16 cells (A and C) or B16-GMCSF cells (B and D) in the right flank. 6 d later, mice were challenged with B16 cells in the left flank, and growth of primary and challenge tumors was monitored. In each panel, the left graph represents growth of primary tumors, the middle graph depicts growth of challenge tumors, and the right graph shows growth of the challenge tumor inoculum given in naive mice. Mice were either left untreated (A and B) or treated (C and D) with GK1.5 CD4 depleting antibody on days 4, 10, and 17 relative to primary tumor inoculation (arrows). Significance for each group was determined by log rank analysis comparing growth of challenge tumors in tumor-bearing mice versus naive mice. (A) P = 0.145; (B) P = 0.014; (C) P = 0.001; (D) P = 0.0002. Results are shown for representative experiments.
Because growth of B16 tumors did not induce concomitant immunity under any of these conditions, we used B16 cells stably transduced with GM-CSF (B16-GMCSF) as a potential positive control. Immunization of mice with irradiated B16 cells transduced with GM-CSF (including our B16-GMCSF cell line) has been shown to confer protection against B16, demonstrating that these cells are highly immunogenic compared with their wild-type counterparts (references 14, 27 and unpublished data). To determine if this strongly immunogenic tumor primes concomitant immunity to B16, 105 live B16-GMCSF cells were inoculated in mice, and wild-type B16 challenge tumors were given 6 d later on the opposite flank. As shown in Fig. 1 B, B16-GMCSF tumors protected ∼70% of mice from challenge tumors (in three independent experiments, a total of 30 out of 44 mice were protected), reinforcing the conventional belief that immunogenic tumors effectively prime concomitant immunity, and showing that a strongly immunogenic priming tumor can elicit immunity against a weakly immunogenic challenge tumor.
The studies by North et al. showed that growth of immunogenic Meth A fibrosarcoma induced potent concomitant immunity, followed by loss of immunity around day 12, due to appearance of CD4+ suppressor cells (3, 8). Because no concomitant immunity was observed at any time point in B16 growth, we hypothesized that a population of CD4+ suppressor cells might be functional at an earlier stage. To test this possibility, mice bearing primary tumors were depleted of CD4+ cells beginning 4 d after primary tumor inoculation and continuing throughout tumor growth. This approach could deplete putative suppressors, along with helper T cells and other CD4+ positive cells, while potentially preserving a window for early T cell help.
In mice bearing wild-type B16 primary tumors, CD4 depletion slightly reduced growth of primary tumors, but also induced significant concomitant immunity, protecting ∼70% of primary tumor-bearing mice from secondary tumors (Fig. 1 C); in a total of seven experiments, 59 out of 89 mice completely rejected secondary tumors, and 9 mice had secondary tumors that grew to a palpable size and regressed around day 12. CD4 depletion also augmented concomitant immunity induced by B16-GMCSF (in two experiments, 25 out of 30 mice were protected from secondary tumors), and retarded growth of primary B16-GMCSF tumors (Fig. 1 D). These data supported the existence of a CD4+ population, present after primary tumor inoculation, which suppresses concomitant immunity to B16.
CD8+ T Cells from Concomitantly Immune Mice Recognize Melanocyte Differentiation Antigens and Adoptively Transfer Tumor Immunity.
Because previous studies have shown that concomitant immunity is a CD8+ T cell–dependent phenomenon, we wanted to determine if this convention also held true for our models. Therefore, upon induction of concomitant immunity by tumor inoculation and CD4 depletion, we evaluated the effects of CD8 codepletion. Compared with immune controls, challenge tumors grew rapidly in CD8-depleted mice, at rates comparable to unimmunized mice, indicating that concomitant immunity is CD8 dependent (Fig. 2). This was true of mice bearing either B16 or B16-GMCSF primary tumors. Primary tumor growth rates also increased in CD8-depleted mice, demonstrating that effects on primary tumors are also CD8 dependent.
Figure 2.
Concomitant tumor immunity is dependent on CD8+ cells. C57BL/6 mice (5–15/group) were inoculated with B16 cells (A) or B16-GMCSF cells (B) in the right flank, followed 6 d later by B16 cells in the left flank. In addition, all mice received CD4-depleting antibody on days 4 and 10 to induce concomitant immunity. In each panel, the left graph depicts growth of primary tumors and the right graph depicts growth of challenge tumors in the same mice. The two bottom panels show tumor outgrowth in mice coinjected with CD8-depleting antibody (arrows). Significance for each group was determined by log rank analysis comparing growth of challenge tumors in CD8-depleted versus nondepleted mice. (A) P = 0.0892; (B) P = 0.0022.
At this point, having established concomitant immunity in the B16 and B16-GMCSF models, we conducted all subsequent experiments with wild-type B16 tumors. This model would allow the identification of relevant immune responses using unmanipulated tumor cells.
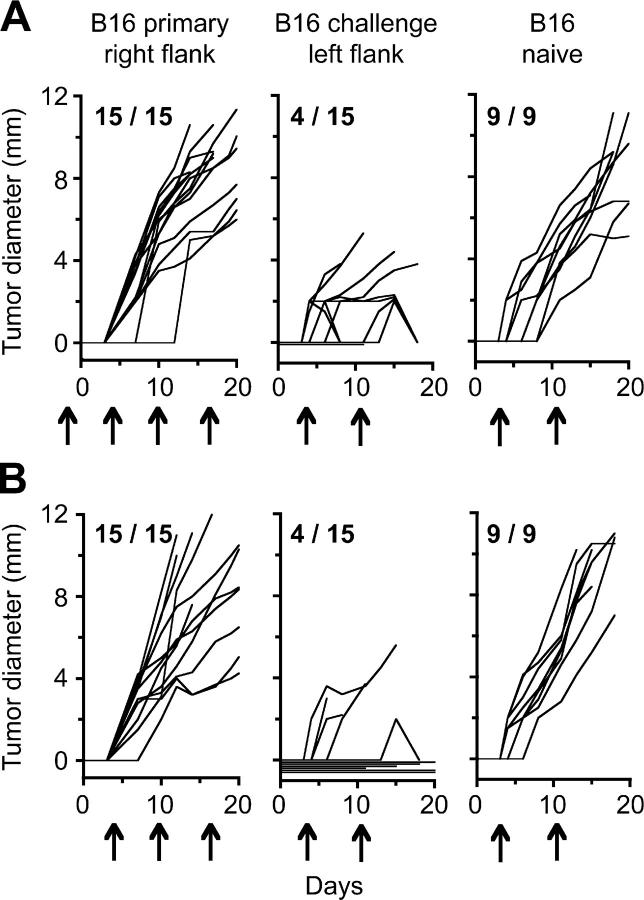
Our original schedule for CD4 depletion was based on the assumption that CD4 help might be required for early priming of concomitant immunity. To assess this possibility, the CD4-depleting antibody was also administered beginning 2 d before primary tumor inoculation, to ensure CD4+ T cell absence throughout tumor progression. Although rejection of secondary tumors by day 18 in these mice (Fig. 3 A) was similar to mice that did not receive the early CD4+ depletion (Fig. 3 B), a substantial proportion of challenge tumors (7 out of 15) in the early CD4-depleted mice grew to palpable size and later regressed. These results show that priming of concomitant immunity is still possible, but is less efficient in mice deficient in CD4 help at the time of primary tumor challenge.
Figure 3.
Priming of concomitant immunity is less effective in mice lacking CD4+ T cell help. Mice (15/group) were inoculated with B16 cells in the right flank, followed 6 d later by an identical inoculum in the left flank. Mice received intraperitoneal injections of CD4-depleting antibody on days −2, 4, 10, and 17 (A, arrows) or days 4, 10, and 17 (B). In each panel, the left graph represents growth of primary tumors, the middle graph represents growth of challenge tumors, and the right graph represents growth of the challenge tumor inoculum given in naive mice.
We investigated the specificity of concomitant immunity to B16. Mice bearing primary B16 tumors and depleted of CD4+ T cells were challenged with three different syngeneic tumor cell lines: Lewis lung carcinoma, LiHa fibrosarcoma, and JBRH melanoma. It has been shown previously that Lewis lung carcinoma is subject to immunological rejection after vaccination (28). CD4 depletion alone did not affect the growth of these tumors (Fig. 4). Similarly, CD4 depletion combined with B16 primary tumor growth did not detectably affect growth of Lewis lung carcinoma or LiHa fibrosarcoma (Fig. 4, A and B). However, growth of JBRH melanoma was halted for >2 wk, consistent with the notion that B16 tumors elicit immunity to shared melanoma antigens (Fig. 4 C).
Figure 4.
Concomitant immunity to B16 is shared with JBRH melanoma, but not with Lewis lung carcinoma or LiHa fibrosarcoma. Mice were inoculated (left flank) on day 0 with either Lewis lung carcinoma (A), LiHa fibrosarcoma (B), or JBRH melanoma (C). Mice were either untreated; depleted of CD4+ T cells on days −2, 4, and 11; or depleted of CD4+ T cells on days −2, 4, and 11 and inoculated with B16 tumors (right flank) on day −6 to induce concomitant immunity. Tumor diameter represents the mean of the greatest diameters (± SEM) for 15 mice/group. For JBRH tumors, the difference between mean sizes in naive versus B16 tumor-bearing mice was statistically significant (P < 0.05; by two-tailed Student's t test) at all time points between days 12 and 20.
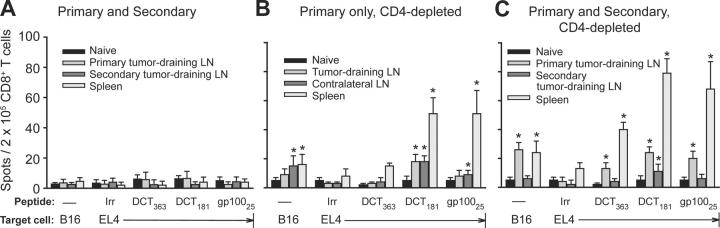
This observation led us to study epitope specificity of CD8+ T cells in mice with concomitant immunity. We currently know of 10 epitopes derived from melanocyte differentiation antigens that are presented by MHC class I molecules on B16 cells: 2 from DCT (reference 24 and unpublished data): 1 from gp100 (25), 3 from TYRP-1 (unpublished data), and 4 from tyrosinase (unpublished data). These epitopes served as a panel to survey CD8+ T cell specificity. For these experiments, CD8+ T cells were isolated from lymphoid organs of concomitantly immune mice and tested by IFN-γ ELISPOT analysis using either B16 cells or EL4 lymphoma cells (the latter pulsed with each of the 10 epitopes) as targets.
6 d after the primary tumor inoculation in CD4-depleted mice, no specific CD8+ T cells were detected. On day 12, the mice that did not receive CD4 depletion but bore both primary and secondary tumors still had no detectable response (Fig. 5 A). However, mice that received primary tumors and CD4 depletion had readily detectable CD8+ T cell responses against DCT181 and gp10025 peptides, as well as B16 cells (Fig. 5 B). Responding T cell numbers were similar for both epitopes and strongest in the spleen, although significant numbers of DCT181-specific T cells were also found in tumor-draining and contralateral lymph nodes. Inoculation of a secondary tumor on day 6 not only boosted day-12 T cell responses in spleen and tumor-draining lymph nodes, but also elicited recognition of a third epitope, DCT363 (Fig. 5 C). In no group were responses detected against any of the TYRP-1 or tyrosinase peptides.
Figure 5.
CD8+ T cells from concomitantly immune mice recognize epitopes from DCT and gp100 melanocyte differentiation antigens. Mice received (A) inoculation of B16 cells in the right flank followed 6 d later by an identical inoculum in the left flank, (B) inoculation of B16 cells in the right flank combined with CD4 depletion on days 4 and 10, or (C) inoculation of B16 cells in the right flank followed 6 d later by an identical inoculum in the left flank and CD4 depletion on days 4 and 10. 12 d after primary tumor inoculation, CD8+ T cells from spleen and inguinal lymph nodes were tested by IFN-γ ELISPOT analysis using as targets either B16 cells or peptide-pulsed EL4 lymphoma cells. Lymph node and spleen cells were pooled from groups of 5–10 mice. Values represent the mean number of spots (n = 3–4 replicates/point) ± SD. Asterisks depict statistically significant differences (P < 0.05; by two-tailed Student's t test) between T cell responses to target EL4 cells pulsed with relevant versus irrelevant (Irr) peptide; or, for target B16 cells, differences between responses from naive versus tumor-bearing mice.
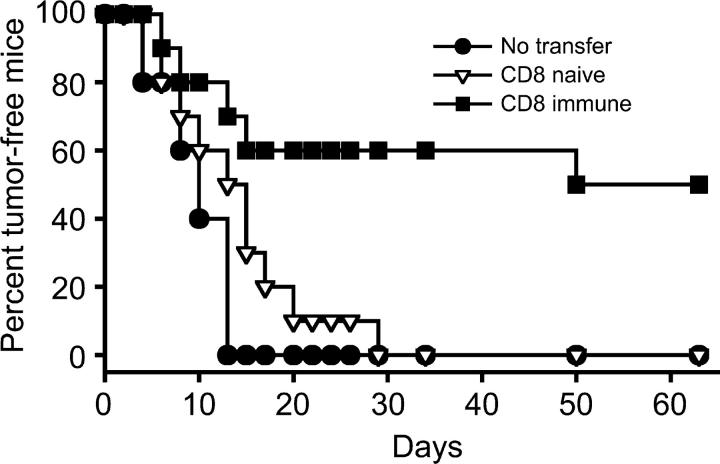
To determine if this day-12 CD8+ T cell population was sufficient for tumor protection, these immune cells were adoptively transferred into RAG1 −/− recipients, which were challenged with B16 on the following day. Although naive CD8+ T cells afforded no significant protection, immune CD8+ T cells clearly conferred immunity, protecting ∼50% of recipient mice from tumor growth (Fig. 6).
Figure 6.
CD8+ T cells from concomitantly immune donors adoptively transfer immunity to T cell–deficient hosts. Donor C57BL/6 mice were made concomitantly immune by inoculation of B16 cells in the right flank followed 6 d later by a secondary inoculation in the left flank and CD4 depletion on days 4 and 10. 12 d after the primary tumor inoculation, CD8+ T cells were harvested from spleens and tumor-draining lymph nodes of concomitantly immune mice (CD8 immune), or from spleens of naive C57BL/6 mice (CD8 naive). Cells were purified and adoptively transferred into RAG1 −/− recipients, which were challenged with an inoculum of B16 cells on the following day. Tumor growth was monitored in adoptively transferred recipients for 60 d. The difference between tumor incidence in mice receiving naive versus immune CD8+ T cells was statistically significant (P = 0.013), as determined by log rank analysis.
Naturally Occurring CD4+CD25+ Regulatory T Cells Are Required for Suppression of Concomitant Immunity in Mice Bearing Progressive B16 Tumors.
Our initial experiments with CD4 depletion indicated the presence of CD4+ suppressor T cells that block generation of CD8-dependent immunity. Nevertheless, the identity of these putative suppressors had not been established. CD4+ candidates included NK T cells, Tr1 regulatory cells, and CD4+CD25+ regulatory T cells. Significant concomitant immunity was not observed with B16 melanoma in CD1 −/− mice (12 out of 15 mice grew secondary tumors) or in mice depleted of NK cells (23 out of 37 mice grew secondary tumors), indicating that suppression did not require NK T cells (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20041130/DC1, and unpublished data). Concomitant immunity was also not observed in IL-10 −/− mice (12 out of 15 mice grew secondary tumors), showing that suppression was proceeding in the absence of IL-10 (Fig. S1). Therefore, we undertook a series of experiments to determine if the suppressors were CD4+CD25+ regulatory T cells.
GITR is constitutively expressed on CD4+CD25+ suppressor T cells and its ligation by the stimulatory antibody DTA-1 down-regulates suppressor function in vivo (12). Consequently, enhancement of concomitant immunity by DTA-1 would be consistent with a suppressive role for CD4+CD25+ T cells. Mice treated with DTA-1 on days 1 and 7 after primary tumor inoculation demonstrated progressive growth of primary tumors, while mounting concomitant immunity (∼85% protection) against challenge tumors (Fig. 7 A). Similar results were observed when DTA-1 was administered on days 3 and 9 (unpublished data). Interestingly, DTA-1 administration on days 0 and 7 (Fig. 7 B) was especially potent, protecting 50% of mice from primary tumors and 100% of mice from challenge tumors. Although these results suggested that DTA-1 was blocking the function of suppressor T cells in our model, alternate mechanisms involving binding to recently activated CD8+ or CD4+CD25− T cells, which also express GITR, could not be ruled out.
Figure 7.

GITR stimulation induces potent concomitant immunity. Mice (10–15/group) were inoculated with B16 cells in the right flank followed 6 d later with an identical inoculum in the left flank. In each panel, the left graph represents growth of primary tumors, the middle graph depicts growth of challenge tumors, and the right graph shows growth of the challenge tumor inoculum given to naive mice. 1 mg anti-GITR antibody (clone DTA-1) was administered intraperitoneally on days 1 and 7 (A) or days 0 and 7 (B) relative to primary tumor inoculation. (C) Mice received injections of 1 mg rat IgG isotype control antibody. Arrows indicate time of antibody administration. The difference between challenge tumor incidence in mice receiving rat control IgG versus DTA-1 was statistically significant for both treatment schedules as determined by log rank analysis. (A) P = 0.0002; (B) P = 0.0050.
Cyclophosphamide has been implicated in selective toxicity to suppressor T cells induced during tumor growth (23, 29, 30) and, more recently, to CD4+CD25+ T cells (31). Therefore, cyclophosphamide-induced concomitant immunity would also support involvement of CD4+ CD25+ suppressor T cells. The drug was tested at various early time points, according to the doses used by North et al. (23), to decrease collateral toxicity to CD8+ effectors. Administration of cyclophosphamide 4 d before inoculation of B16 primary tumors induced concomitant immunity (90% protection) and had no effect on growth of primary tumors or control tumors in naive mice (Fig. 8 A). Effects on concomitant immunity were schedule dependent, as treatment on day 2 only induced 40% protection and treatment on day 0 induced no protection (Fig. 8 B).
Figure 8.
Single-dose cyclophosphamide induces concomitant immunity when administered 4 d before primary tumor inoculation. (A) Mice (10/group) were treated with cyclophosphamide on day −4, inoculated with B16 cells in the right flank on day 0, and reinoculated with B16 in the left flank on day 6. The left graph represents growth of primary tumors, the middle graph depicts growth of challenge tumors, and the right graph shows growth of the challenge tumor inoculum in naive mice that were treated with cyclophosphamide according to the same schedule. (B) Incidence of B16 secondary tumors (left flank) was measured in mice bearing day-6 primary tumors and receiving the following: no treatment or cyclophosphamide treatment on days −4, −2, or 0 relative to primary tumor inoculation. The difference between challenge tumor incidence in untreated mice and those that received cyclophosphamide on day −4 was statistically significant (P = 0.002) as determined by log rank analysis.
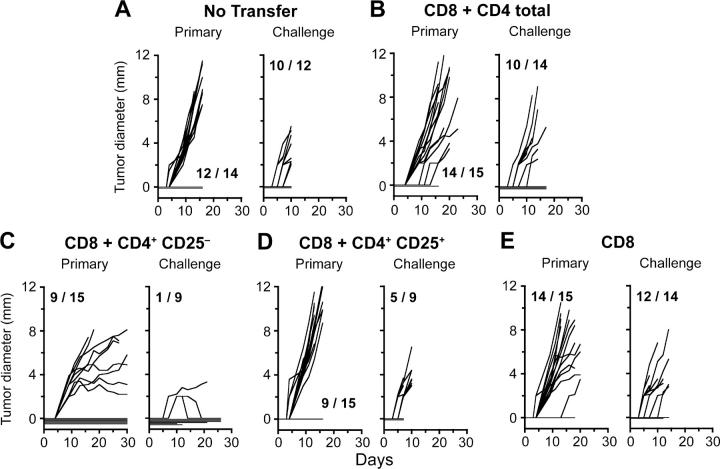
To confirm that CD4+CD25+ T cells are the suppressors of concomitant immunity, RAG1 −/− hosts were partially reconstituted with naive CD8+ T cells mixed with various purified CD4+ populations from naive C57BL/6 mice. Concomitant immunity was assessed by inoculation of B16 primary and challenge tumors. As predicted, similar to mice that had received no cell transfer (Fig. 9 A), mice reconstituted with CD8+ cells combined with the entire CD4+ compartment did not mount immunity (Fig. 9 B), consistent with the presence of active suppressors. In contrast, mice that had received a CD4+ population depleted of CD25+ cells primed strong immunity (89% protection) against secondary tumors (Fig. 9 C). Immunity in this group was potent enough that it also affected growth of the primary tumors: 6/15 primary tumors were rejected and 7/15 demonstrated reduced growth after day 12. Conversely, mice reconstituted with CD8+ T cells and the CD4+CD25+ T cell population had very rapid growth of primary tumors and required early sacrifice (Fig. 9 D). These results confirm the suppressive role of CD4+CD25+ T cells in priming of concomitant immunity. Interestingly, mice transferred with naive CD8+ T cells alone were not able to mount concomitant immunity, indicating a requirement for CD4+ T cell help in RAG1 −/− hosts, in contrast with wild-type C57BL/6 hosts (see CD8+ T Cells from Concomitantly Immune Mice Recognize Melanocyte Differentiation Antigens and Adoptively Transfer Tumor Immunity).
Figure 9.
CD4+CD25+ T cells suppress priming of concomitant immunity. RAG1 −/− mice were adoptively transferred with various populations of naive T cells from C57BL/6J donor mice as follows: (A) no cells, (B) CD8+ and CD4+ T cells, (C) CD8+ and CD4+CD25− T cells, (D) CD8+ and CD4+CD25+ T cells, or (E) CD8+ T cells alone. Adoptively transferred mice were inoculated with primary B16 tumors on day 1, followed by challenge tumors on day 7. In each panel, the left graph depicts growth of primary tumors and the right graph depicts growth of challenge tumors in the same mice. In D, the experiment was terminated 8 d after tumor challenge because growth of primary tumors was too rapid. Five out of nine tumors had already appeared by day 9.
Having demonstrated that CD4+CD25+ T cells could suppress priming of concomitant immunity, we wished to determine if this suppressor population required preexposure to tumor for function. For this experiment, preactivated CD8+ effectors were isolated from concomitantly immune donors and combined with each of three putative CD4+ T cell suppressor populations: CD4+CD25+ cells from naive C57BL/6 donors, CD4+CD25+ cells from day-12 tumor-bearing donors, or CD4+CD25− cells from day-12 tumor-bearing donors. These individual populations were cotransferred into RAG1 −/− recipients, which were challenged with B16 tumor cells 1 d later. Although we observed moderate tumor protection (33%) in mice receiving immune CD8+ T cells alone, immunity was completely abolished in mice cotransferred with naive CD4+ CD25+ T cells, at a suppressor/effector ratio of 1:12, demonstrating that regulatory T cell precursors are naturally occurring in naive C57BL/6 host (Fig. 10). Interestingly, CD4+CD25+ T cells from tumor-bearing mice did not suppress with the same efficiency as naive CD4+CD25+ T cells, although this difference did not reach statistical significance (Fig. 10 A). In addition, CD4+CD25− T cells taken from tumor-bearing mice did not function as suppressors, but rather slightly enhanced tumor rejection (Fig. 10, A and B).
Figure 10.

CD4+CD25+ T cells from naive mice suppress preactivated effectors of concomitant immunity. CD8 immune T cells were generated as described in Fig. 6. (A) Incidence of B16 tumors in RAG1 −/− mice that had been previously (day −1) reconstituted with CD8 immune T cells or CD8 immune T cells mixed with various potential suppressor populations as follows: naive CD4+CD25+ T cells, CD4+CD25+ T cells from day-12 tumor-bearing mice, or CD4+CD25− T cells from day-12 tumor-bearing mice. As determined by log rank analysis, the difference between tumor incidence in mice receiving immune CD8 cells alone and those receiving immune CD8 cells combined with naive CD4+CD25+ T cells was statistically significant (P = 0.0026). However, there was no statistical difference for mice cotransferred with tumor-bearing CD4+ CD25− T cells (P = 0.992) or tumor-bearing CD4+CD25+ T cells (P = 0.677). Naive versus tumor-bearing CD4+CD25+ T cells did not reach statistical significance (P = 0.0729). (B) Tumor growth curves depicting growth rates of individual tumors in each group of adoptively transferred mice.
Discussion
Historically, concomitant tumor immunity has been described for hosts bearing immunogenic tumors, including tumors from outbred animals (1, 2, 32), methylcholanthrene-induced tumors (4, 33–35), and virally transformed models (36, 37). This response was shown to be complex, with immunity decaying when primary tumors reached large sizes (3, 33, 34, 36). The work of North et al. ∼20 yr ago carefully characterized the immune response to Meth A fibrosarcoma as an equilibrium between tumor-induced CD8+ effector and CD4+CD5+ suppressor T cells (3, 8). Nevertheless, concomitant immunity has not been characterized for poorly immunogenic tumors of spontaneous origin. Resistance to a second inoculum of Madison lung carcinoma and Lewis lung carcinoma has been examined (20, 38), but immunological mechanisms were not directly implicated, and it remained unclear if growth of such tumors could prime host immunity.
In addition to extending this phenomenon to a poorly immunogenic tumor, the present description of concomitant immunity to B16 melanoma demonstrates possible fundamental differences between strongly and poorly immunogenic tumors. Concomitant immunity to B16 does not occur as a result of progressive tumor growth alone, but rather only as a result of progressive tumor growth in the absence of CD4+ suppressor T cells. These suppressors come from naturally occurring CD4+CD25+ regulatory T cells present de novo in the naive host and are capable of blocking concomitant immunity at both priming and effector phases. GM-CSF production by otherwise poorly immunogenic tumor cells can induce resistance to a second tumor inoculum, although this phenomenon is likely to occur by mechanisms other than simply overcoming suppression, such as enhancing APC recruitment and/or functionality. This supposition is supported by the observation that depletion of CD4+ suppressors further augments the immune response in mice bearing B16-GMCSF primary tumors. Growth of primary B16-GMCSF tumors is strongly dependent on the presence of CD4+ T cells, with CD4 depletion resulting in CD8-dependent rejection of even primary tumors. Therefore, B16-GMCSF may require a basal level of suppressor T cell function to evade immune recognition.
Before this paper, concomitant tumor immunity models had not been characterized with regard to antigen specificity. In the B16 model, we demonstrate that progressive tumor growth elicits immunity to melanocyte differentiation autoantigens. The number of individual CD8+ T cells recognizing any single epitope was modest compared with what might be achieved through active vaccination, and certainly much less than observed after immunity to viruses or bacteria. However, the gp10025 and DCT181 epitopes have been described previously as B16 tumor rejection antigens (24, 25, 39, 40), and it is most likely that the entire population of CD8+ T cells recognizing multiple shared tumor antigens mediates the potent concomitant immunity that we observe. Furthermore, the presence of cross-reactive immunity to a different melanoma, but not to nonmelanomas, supports the argument that rejection antigens include shared melanocyte differentiation antigens. The fact that JBRH melanoma is not completely rejected suggests that immunity is also directed against antigens specific to B16 and that B16-specific CD8+ T cells are required for complete tumor rejection.
Because these experiments were terminated within ∼3 wk due to primary tumor size, we were not able to further characterize autoimmunity (manifested as hypopigmentation of coat) or immunological memory. Memory T cell responses against melanoma differentiation autoantigens have been characteristically difficult to obtain through vaccination (unpublished data). Surgical resection of primary tumors may facilitate future studies of autoimmunity and memory by prolonging the lifespan of tumor-bearing hosts. Postsurgical tumor immunity has been described for highly immunogenic tumors, in many cases with potency exceeding concomitant immunity (20). This comparison remains to be defined for a poorly immunogenic tumor.
Unexpectedly, priming of concomitant immunity in immune competent mice was only partially reduced in the absence of CD4+ T cell help. However, this result may reflect the regeneration of small numbers of CD4+ T cells between antibody depletions because RAG1 −/− mice lacking CD4+ T cells were not able to mount concomitant immunity. Alternatively, local help may be provided by inflammation at the tumor site, and the lack of immunity in RAG1 −/− hosts may reflect a difference in antigen-presenting capabilities between these two strains.
Depletion of CD25+ cells by treatment with PC61 mAb has been shown previously to break immunological unresponsiveness to highly immunogenic and, to a lesser extent, poorly immunogenic tumors (41, 42). PC61 pretreatment of naive mice has been reported to significantly reduce growth of B16 tumors by mechanisms that appeared to involve combinations of NK cells and CD8+ T cells (15) or CD4+ and CD8+ T cells (41); however, it remains unclear if relevant CD8+ populations were nonspecifically activated. Our model of concomitant immunity illustrates that, in addition to CD4+CD25+ T cell elimination, progressive tumor growth is required for generation of tumor-specific CD8+ T cells and tumor immunity.
In our hands, pretreatment of mice with PC61 mAb was associated with variable growth of primary tumors and CD4+ T cell–mediated rejection of ∼20–40% of control tumors, which made it difficult to assess the role of primary tumor growth in rejection of secondary tumors (unpublished data). In addition, treatment with PC61 mAb at later time points was ineffective presumably because of the depletion of activated CD8+ effectors (14). Rather than PC61, we used GK1.5 mAb, DTA-1 mAb, and cyclophosphamide as tools to block or deplete suppressor T cells. We were interested in DTA-1 mAb because it has been shown previously to block CD4+CD25+ suppressor T cell function in vivo (12). However, more recently, in an allogeneic bone marrow transplant setting, DTA-1 mAb has been shown to act directly on both effector CD4+ and CD8+ T cell populations (43). Therefore, although our data are collectively consistent with the notion that DTA-1 acts to inhibit CD4+CD25+ suppressor T cells, potential activation of effector cells is possible, and further experiments are needed to determine which cell populations are responding to DTA-1 in the B16 concomitant immunity model.
Cyclophosphamide has been described previously to deplete suppressor T cells induced by progressive Meth A tumors and other immunogenic tumors (23, 29, 30). More recently, a selective reduction in the proportion of CD4+ CD25+ T cells has been reported in spleens of cyclophosphamide-treated rats (31). We have observed a similar decline in this population in mice on days 4–10 after cyclophosphamide treatment, although other T cell populations are also affected (unpublished data). Because cyclophosphamide had profound effects on concomitant immunity when administered 4 d before the primary tumor, this evidence supports the role for cyclophosphamide through a mechanism of selective toxicity against CD4+CD25+ T cells. However, effects on T cell homeostasis are likely, and further studies are required to examine the exact mechanism of cyclophosphamide function.
The Meth A sarcoma has been shown previously to induce a suppressor population beginning after day 9 of tumor growth (3), and it has been shown recently that human melanoma can be recognized by a line of CD4+ CD25+ regulatory T cells derived from the tumor (44). In addition to suppressor T cell populations, myeloid cells and soluble molecules (e.g., IL-10 and TGF-β) have been shown to contribute to suppression of tumor immunity. Although our studies do not rule out the possibility that B16 growth also generates a CD4+CD25+ regulatory T cell population, suppressive CD4+CD25+ or CD4+CD25− T cells could not be transferred from mice bearing day-12 tumors. In fact, CD4+CD25+ T cells taken from these mice appeared to be inefficient suppressors compared with their naive counterparts. This suggests that tumor growth primes a population of activated CD4+ T cells that participates in tumor immunity, implying that CD4+CD25+ T cells isolated from tumor-bearing mice contain a mixed population with different functions. The fact that CD4+CD25− T cells from tumor-bearing mice did not significantly enhance tumor rejection also supports the conclusion that T helper cells were activated and, therefore, were present in the CD4+CD25+ population. In any case, CD4+CD25+ T cells from naive mice were potent suppressors in vivo after adoptive transfer, without requiring preexposure to tumor. The antigen specificity of the relevant CD4+CD25+ T cells within this population remains to be determined, but the finding that regulatory T cell expansion is driven by presentation of self peptides (45) presents the intriguing possibility that these cells may recognize melanocyte differentiation antigens. In this case, precursor regulatory T cells might not require a priori activation by tumor because these antigens are already expressed by normal cutaneous melanocytes in hair follicles.
One fundamental characteristic of concomitant immunity to tumors and pathogens is the observation that progression of primary tumors or lesions is unaffected by the developing host immune response. For instance, in the case of concomitant immunity to Leishmania major, CD4+CD25+ T cells resident in the primary lesion prevent local immune reactions, while maintaining antigen persistence for priming of systemic immunity (46). In contrast, concomitant immunity to B16 only proceeds in the absence of preexisting host suppressor cells and, therefore, the presence of a local suppressive environment involving CD4+CD25+ T cells only within primary but not challenge tumors is unlikely. Furthermore, significant numbers of tumor-reactive, specific CD8+ T cells are present in lymph nodes draining primary tumors, suggesting that primary tumors can be vulnerable to host immunity under the right circumstances. Indeed, we have observed decreased growth of primary tumors in mice with certain types of concomitant immunity, particularly in those treated with DTA-1 mAb and in T cell–reconstituted RAG1 −/− mice lacking only the CD4+CD25+ population. DTA-1 mAb might activate effector cells in addition to inhibiting suppressors, whereas in the case of RAG1 −/− recipients, emerging T cell homeostasis could skew the response strongly to tumor immunity. Although participation of local CD4− suppressors (such as tumor-associated macrophages) cannot be ruled out, our observation that most primary tumors continue to grow in the face of rejection of challenge tumors likely reflects the fact that these tumors are established with stroma and vasculature before CD8 immunity is primed and, therefore, are more formidable targets for host immunity compared with tumor cells given on day 6. In summary, these studies illustrate the requirements for generating concomitant immunity to a progressive, poorly immunogenic tumor. Our finding that B16 tumors rapidly immunize hosts that lack the CD4+CD25+ regulatory T cell population demonstrates the central role played by these cells in the blockade of tumor immunity in this melanoma model. The fact that this concomitant immune response recognizes melanocyte differentiation antigens is consistent with an important role for shared, unaltered self antigens in rejection of poorly immunogenic, spontaneous tumors.
Acknowledgments
The authors thank T. Merghoub, H. Uchi, and M.L. Palomba for creation of the LiHa tumor cell line; T. Merghoub and M.Y. Tan for breeding of CD1 −/− mice; and R. Stan for creating this paper.
Support for this work was provided by the National Institutes of Health (NIH) grants R01 CA56821, P01 CA33049, CA59350, and CA47179 (A.N. Houghton). M.J. Turk was supported by NIH training grant T32 CA09149. M.E. Engelhorn was the recipient of a fellowship award from the Cancer Research Institute. J.A. Guevara-Patino received support from Mr. W.H. Goodwin, Mrs. A. Goodwin, the Commonwealth Cancer Foundation for Research, and the Experimental Therapeutics Center of MSKCC, and A.N. Houghton has Damon Runyon/Eli Lilly mentorship support. We are grateful to Swim Across America, the Louis & Anne Abrons Foundation, and the Mr. and Mrs. Quentin J. Kennedy Fund.
The authors have no conflicting financial interests.
Abbreviations used in this paper: GITR, glucocorticoid-induced TNF receptor; mAb, monoclonal antibody.
References
- 1.Ehrlich, P. 1906. Collected Studies on Immunity. J. Wiley & Sons, London, England.
- 2.Bashford, E., J. Murray, and M. Haaland. 1908. Resistance and susceptibility to inoculated cancer. Third Scientific Report on the Investigations of the Imperial Cancer Research Fund. E. Bashford, editor. Taylor & Francis, London, England. 359–397.
- 3.North, R.J., and I. Bursuker. 1984. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J. Exp. Med. 159:1295–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berendt, M.J., R.J. North, and D.P. Kirstein. 1978. The immunological basis of endotoxin-induced tumor regression. Requirement for a pre-existing state of concomitant antitumor immunity. J. Exp. Med. 148:1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berendt, M.J., and R.J. North. 1980. T-cell–mediated suppression of antitumor immunity. An explanation for progressive growth of an immunogenic tumor. J. Exp. Med. 151:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bursuker, I., and R.J. North. 1985. Suppression of generation of concomitant antitumor immunity by passively transferred suppressor T cells from tumor-bearing donors. Cancer Immunol. Immunother. 19:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bursuker, I., and R.J. North. 1986. Immunological consequences of tumor excision: from active immunity to immunological memory. Int. J. Cancer. 37:275–281. [DOI] [PubMed] [Google Scholar]
- 8.DiGiacomo, A., and R.J. North. 1986. T cell suppressors of antitumor immunity. The production of Ly-1-,2+ suppressors of delayed sensitivity precedes the production of suppressors of protective immunity. J. Exp. Med. 164:1179–1192. (published erratum appears in J. Exp. Med. 1986. 164:2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 10.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 12.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142. [DOI] [PubMed] [Google Scholar]
- 13.Turk, M.J., J.D. Wolchok, J.A. Guevara-Patino, S.M. Goldberg, and A.N. Houghton. 2002. Multiple pathways to tumor immunity and concomitant autoimmunity. Immunol. Rev. 188:122–135. [DOI] [PubMed] [Google Scholar]
- 14.Sutmuller, R.P., L.M. van Duivenvoorde, A. van Elsas, T.N. Schumacher, M.E. Wildenberg, J.P. Allison, R.E. Toes, R. Offringa, and C.J. Melief. 2001. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 194:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu, J., S. Yamazaki, and S. Sakaguchi. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218. [PubMed] [Google Scholar]
- 16.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 17.Terabe, M., S. Matsui, N. Noben-Trauth, H. Chen, C. Watson, D.D. Donaldson, D.P. Carbone, W.E. Paul, and J.A. Berzofsky. 2000. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1:515–520. [DOI] [PubMed] [Google Scholar]
- 18.Moodycliffe, A.M., D. Nghiem, G. Clydesdale, and S.E. Ullrich. 2000. Immune suppression and skin cancer development: regulation by NKT cells. Nat. Immunol. 1:521–525. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda, K.H., and J. Stein-Streilein. 2002. Ocular immune privilege and CD1d-reactive natural killer T cells. Cornea. 21:S33–S38. [DOI] [PubMed] [Google Scholar]
- 20.Gorelik, E. 1983. Concomitant tumor immunity and the resistance to a second tumor challenge. Adv. Cancer Res. 39:71–120. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda, K.H., M. Exley, S. Snapper, S.P. Balk, and J. Stein-Streilein. 1999. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 190:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hara, I., H. Nguyen, Y. Takechi, B. Gansbacher, P.B. Chapman, and A.N. Houghton. 1995. Rejection of mouse melanoma elicited by local secretion of interleukin-2: implicating macrophages without T cells or natural killer cells in tumor rejection. Int. J. Cancer. 61:253–260. [DOI] [PubMed] [Google Scholar]
- 23.Awwad, M., and R.J. North. 1988. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 24.Bloom, M.B., D. Perry-Lalley, P.F. Robbins, Y. Li, M. el-Gamil, S.A. Rosenberg, and J.C. Yang. 1997. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J. Exp. Med. 185:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overwijk, W.W., A. Tsung, K.R. Irvine, M.R. Parkhurst, T.J. Goletz, K. Tsung, M.W. Carroll, C. Liu, B. Moss, S.A. Rosenberg, and N.P. Restifo. 1998. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 188:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheibenbogen, C., K.H. Lee, S. Stevanovic, M. Witzens, M. Willhauck, V. Waldmann, H. Naeher, H.G. Rammensee, and U. Keilholz. 1997. Analysis of the T cell response to tumor and viral peptide antigens by an IFNgamma-ELISPOT assay. Int. J. Cancer. 71:932–936. [DOI] [PubMed] [Google Scholar]
- 27.Dranoff, G., E. Jaffee, A. Lazenby, P. Golumbek, H. Levitsky, K. Brose, V. Jackson, H. Hamada, D. Pardoll, and R.C. Mulligan. 1993. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 90:3539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima, T., K. Yamazaki, Y. Tamura, S. Ogura, K. Tani, J. Konishi, N. Shinagawa, I. Kinoshita, N. Hizawa, E. Yamaguchi, H. Dosaka-Akita, and M. Nishimura. 2003. Granulocyte-macrophage colony-stimulating factor gene-transduced tumor cells combined with tumor-derived gp96 inhibit tumor growth in mice. Hum. Gene Ther. 14:715–728. [DOI] [PubMed] [Google Scholar]
- 29.Awwad, M., and R.J. North. 1989. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 49:1649–1654. [PubMed] [Google Scholar]
- 30.North, R.J. 1982. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 155:1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghiringhelli, F., N. Larmonier, E. Schmitt, A. Parcellier, D. Cathelin, C. Garrido, B. Chauffert, E. Solary, B. Bonnotte, and F. Martin. 2004. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 34:336–344. [DOI] [PubMed] [Google Scholar]
- 32.Gershon, R.K., R.L. Carter, and K. Kondo. 1967. On concomitant immunity in tumour-bearing hamsters. Nature. 213:674–676. [DOI] [PubMed] [Google Scholar]
- 33.Belehradek, J., Jr., G. Barski, and M. Thonier. 1972. Evolution of cell-mediated antitumor immunity in mice bearing a syngeneic chemically induced tumor. Influence of tumor growth, surgical removal and treatment with irradiated tumor cells. Int. J. Cancer. 9:461–469. [DOI] [PubMed] [Google Scholar]
- 34.Chandradasa, K.D. 1973. The development and specific suppression of concomitant immunity in two syngeneic tumour-host systems. Int. J. Cancer. 11:648–662. [DOI] [PubMed] [Google Scholar]
- 35.Deckers, P.J., B.W. Edgerton, B.S. Thomas, and Y.H. Pilch. 1971. The adoptive transfer of concomitant immunity to murine tumor isografts with spleen cells from tumor-bearing animals. Cancer Res. 31:734–742. [PubMed] [Google Scholar]
- 36.Howell, S.B., J.H. Dean, and L.W. Law. 1975. Defects in cell-mediated immunity during growth of a syngeneic simian virus-induced tumor. Int. J. Cancer. 15:152–169. [DOI] [PubMed] [Google Scholar]
- 37.Zarling, J.M., and S.S. Tevethia. 1973. Transplantation immunity to simian virus 40-transformed cells in tumor-bearing mice. II. Evidence for macrophage participation at the effector level of tumor cell rejection. J. Natl. Cancer Inst. 50:149–157. [DOI] [PubMed] [Google Scholar]
- 38.Gorelik, E., S. Segal, and M. Feldman. 1981. On the mechanism of tumor “concomitant immunity”. Int. J. Cancer. 27:847–856. [DOI] [PubMed] [Google Scholar]
- 39.Bowne, W.B., R. Srinivasan, J.D. Wolchok, W.G. Hawkins, N.E. Blachere, R. Dyall, J.J. Lewis, and A.N. Houghton. 1999. Coupling and uncoupling of tumor immunity and autoimmunity. J. Exp. Med. 190:1717–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold, J.S., C.R. Ferrone, J.A. Guevara-Patino, W.G. Hawkins, R. Dyall, M.E. Engelhorn, J.D. Wolchok, J.J. Lewis, and A.N. Houghton. 2003. A single heteroclitic epitope determines cancer immunity after xenogeneic DNA immunization against a tumor differentiation antigen. J. Immunol. 170:5188–5194. [DOI] [PubMed] [Google Scholar]
- 41.Gallimore, A., and S. Sakaguchi. 2002. Regulation of tumour immunity by CD25+ T cells. Immunology. 107:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onizuka, S., I. Tawara, J. Shimizu, S. Sakaguchi, T. Fujita, and E. Nakayama. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128–3133. [PubMed] [Google Scholar]
- 43.Muriglan, S.J., T. Ramirez-Montagut, O. Alpdogan, T.W. van Huystee, J.M. Eng, V.M. Hubbard, A.A. Kochman, K.H. Tjoe, C. Riccardi, P.P. Pandolfi, et al. 2004. GITR activation induces an opposite effect on alloreactive CD4+ and CD8+ T cells in graft-versus-host disease. J. Exp. Med. 200:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, H.I., D.A. Lee, G. Peng, Z. Guo, Y. Li, Y. Kiniwa, E.M. Shevach, and R.F. Wang. 2004. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 20:107–118. [DOI] [PubMed] [Google Scholar]
- 45.Cozzo, C., J. Larkin III, and A.J. Caton. 2003. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J. Immunol. 171:5678–5682. [DOI] [PubMed] [Google Scholar]
- 46.Belkaid, Y., C.A. Piccirillo, S. Mendez, E.M. Shevach, and D.L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507. [DOI] [PubMed] [Google Scholar]