Abstract
Given its population of CCR5-expressing, immunologically activated CD4+ T cells, the gastrointestinal (GI) mucosa is uniquely susceptible to human immunodeficiency virus (HIV)-1 infection. We undertook this study to assess whether a preferential depletion of mucosal CD4+ T cells would be observed in HIV-1–infected subjects during the primary infection period, to examine the anatomic subcompartment from which these cells are depleted, and to examine whether suppressive highly active antiretroviral therapy could result in complete immune reconstitution in the mucosal compartment. Our results demonstrate that a significant and preferential depletion of mucosal CD4+ T cells compared with peripheral blood CD4+ T cells is seen during primary HIV-1 infection. CD4+ T cell loss predominated in the effector subcompartment of the GI mucosa, in distinction to the inductive compartment, where HIV-1 RNA was present. Cross-sectional analysis of a cohort of primary HIV-1 infection subjects showed that although chronic suppression of HIV-1 permits near-complete immune recovery of the peripheral blood CD4+ T cell population, a significantly greater CD4+ T cell loss remains in the GI mucosa, despite up to 5 yr of fully suppressive therapy. Given the importance of the mucosal compartment in HIV-1 pathogenesis, further study to elucidate the significance of the changes observed here is critical.
Keywords: primary HIV-1, GALT, CD4+ T cells
Introduction
The gastrointestinal (GI) mucosa harbors the majority of the body's lymphocytes compared with the peripheral blood, which contains only 2–5% of all lymphocytes (1, 2). In addition to representing the largest lymphoid organ, certain characteristics of the mucosal compartment render it extremely permissive to HIV-1 infection and supportive of HIV-1 replication. Compared with circulating lymphocytes, a greater percentage of these mucosal CD4+ lymphocytes express the CCR5 chemokine coreceptor (3–8). The percentage of mucosal CD4+ cells that express the CXCR4 chemokine coreceptor is similar to that seen in the blood (8). Additionally, due to its proximity to the external environment and constant exposure to a myriad of food and microbial antigens, a predominant number of GI mucosal CD4+ T cells are activated and well differentiated with a memory phenotype (9, 10). Lastly, the GI mucosa is maintained in a state of physiological inflammation characterized by a high percentage of proinflammatory, HIV-1–stimulatory cytokines (11).
Increasing evidence suggests that virologic and immunologic events during acute and early HIV-1 infection have a crucial role in determining the rapidity of clinical progression in infected individuals (4, 12). Because the GI tract is an important route for HIV-1 entry and a potentially dominant site of HIV-1 replication, it is vital that early GI mucosal events be carefully examined to better understand the pathogenesis of acute and early HIV-1 infection. Owing to the difficulty in identifying patients during acute HIV-1 infection and the added complexity of obtaining GI biopsy samples in these patients, study of the mucosal events during acute/early HIV-1 infection has to date been lacking, and the majority of our current understanding is derived from the simian immunodeficiency (SIV)-macaque model. In the macaque model, acute SIV infection results in profound depletion of mucosal CD4+ T cells (2, 13–17) with relative preservation of CD4+ T cells in the peripheral blood and lymph nodes over the first 3 wk. Mucosal CD4+ T cell depletion is seen whether the inoculum was delivered parenterally (2, 3, 14) or mucosally (3, 15). A subset of CD4+ T cells that have a highly activated, memory phenotype are found to be the most significantly depleted (18). Because mucosal sites abound in CD4+ T cells with this phenotype, the majority of CD4+ T cell depletion in acute SIV infection is confined to the mucosal compartment. Although immunologic and virologic events during primary SIV infection have been well described, mucosal events have been described in only two primary HIV-1–infected human subjects. These patients, infected for 4 and 6 wk, respectively, showed depletion of mucosal CD4+ T cells (19).
We undertook this study to characterize changes in the GI tract in a cohort of patients with acute and early HIV-1 infection, and to understand the effects of treatment with highly active antiretroviral therapy (HAART).
Materials and Methods
Patients and Sample Acquisition.
Peripheral blood and rectosigmoid colonic mucosal tissue were collected from HIV-1–infected and –uninfected subjects. A total of 27 HIV-1–infected and 10 HIV-1–uninfected subjects were studied. Of the HIV-1 infected, 19 subjects were identified during primary HIV-1 infection. Patients were all viremic and staged as per the National Institutes of Health (NIH)-sponsored Acute and Early Disease Research Program (AIEDRP) as follows: enzyme-linked immunosorbent assay negative (stage Ia), Western blot indeterminate (stage Ib), or a detuned ELISA (20) nonreactive (stage II) or a documented negative within 6 mo of presentation (stage III). Eight HIV-1–infected subjects were studied during the chronic stage of HIV-1 infection. The primary infection cohort was subgrouped as follows (see Table I): group 1: HAART naive, newly diagnosed subjects (n = 11), as well as two subjects who were treated with HAART for 1 wk before biopsy; and group 2: HIV-1–infected subjects, identified during the primary infection stage and had received HAART for up to 5 yr (n = 8). Group 2 is comprised of subjects who had received HAART for 6 mo (n = 2), 1 yr (n = 2), 2 yr (n = 2), 3 yr (n = 1), and 5 yr (n = 1). There was no difference between groups 1 and 2 with respect to CD4+ T cell count on presentation (P = 0.5), log10 HIV-1 viral load on presentation (P = 0.7), or the estimated duration of infection on presentation (P = 0.1). The mean age for primary infection subjects was 35 yr. All subjects contracted HIV-1 sexually during same sex contact. Subjects were treated with a multidrug regimen consisting of combinations of nucleoside (nucleotide) reverse transcriptase inhibitors with either a ritonavir-boosted protease inhibitor and/or a nonnucleoside reverse transcriptase inhibitor.
Table I.
Patient Characteristics
| Study | Stagea | CD4+ T cell count (cells/mm3) |
Log10 plasma HIV-1 (copies/ml) |
Estimated duration of infectionb
(days) |
Duration of HAARTprior to biopsy (days) |
||||
|---|---|---|---|---|---|---|---|---|---|
| At presentation | At biopsy | At presentation | At biopsy | At presentation | Prior to HAART initiation | ||||
| Group 1 | |||||||||
| 102 | Ia | 395 | 391 | 6.9 | 7.1 | 18 | NA | NA | |
| 100 | II | 610 | 595 | 5.8 | 5.5 | 45 | NA | NA | |
| 104 | II | 981 | 1,321 | 6.0 | 5.8 | 38 | NA | NA | |
| 107 | Ia | 456 | 426 | 6.1 | 5.6 | 24 | NA | NA | |
| 336 | II | 556 | 556 | 5.0 | 5.0 | 62 | NA | NA | |
| 109 | II | 340 | 340 | 6.3 | 6.3 | 19 | NA | NA | |
| 119 | Ia | 248 | 248 | 6.9 | 6.3 | 18 | NA | NA | |
| 122 | III | 285 | 400 | 5.1 | 4.9 | 72 | NA | NA | |
| 125 | II | 830 | 684 | 5.8 | 5.0 | 36 | NA | NA | |
| 127 | Ia | 390 | 350 | 4.8 | 4.9 | 23 | NA | NA | |
| 131 | Ia | 250 | 349 | 6.7 | 6.6 | 25 | NA | NA | |
| 105 | Ia | 374 | 462 | 7.1 | 4.7 | 27 | 7 | 7 | |
| 106 | Ia | 317 | 368 | 6.8 | 5.4 | 26 | 7 | 7 | |
| Group 2 | |||||||||
| 100-6m | II | 610 | 698 | 5.8 | <1.7 | 45 | 76 | 214 | |
| 336-6m | II | 556 | 996 | 4.9 | <1.7 | 48 | 90 | 229 | |
| 64 | Ia | 846 | 994 | 7.2 | <1.7 | 28 | 31 | 398 | |
| 78 | II | 175 | 460 | 7.4 | <1.7 | 26 | 28 | 392 | |
| 56 | II | 619 | 752 | 5.4 | <1.7 | 61 | 71 | 670 | |
| 132 | II | 366 | 657 | 6.1 | <1.7 | 19 | 26 | 763 | |
| 106 | III | 351 | 595 | 6.9 | <1.7 | 157 | 168 | 1,182 | |
| 923 | Ia | 698 | 939 | 5.1 | <1.7 | 201 | 204 | 1,932 | |
Subjects were staged as per the NIH-sponsored AIEDRP as follows: enzyme-linked immunosorbent assay negative (stage Ia), Western blot indeterminate (stage Ib), or a detuned ELISA nonreactive (stage II) or a documented negative within 6 mo of presentation (Stage III).
The approximate date of infection was calculated as follows: 2 wk before the onset of acute retroviral illness (97%); in cases where no acute retroviral illness symptoms were experienced, the midpoint between the last negative antibody result and first positive Western blot result was determined.
All eight subjects, five women and three men, identified during chronic HIV-1 infection were not receiving HAART at the time of biopsy. The mean age of this group was 38 yr. The risk factors for HIV acquisition in this group included heterosexual sex (n = 4), homosexual sex (n = 2), and intravenous drug abuse (n = 2). The mean CD4+ T cell count in this group was 216 cells/mm3, with a mean log10 plasma HIV-1 RNA level of 4.7 copies/ml. The 10 HIV-1–uninfected subjects were recruited from a population undergoing routine screening colonoscopy. This group was comprised of six men and four women. None of the HIV-1–infected or –uninfected subjects was found to have macroscopic evidence of GI mucosal disease, nor were any concomitant pathological processes found on histological examination. All enrolled subjects signed an informed consent form that was approved by the institutional review boards of The Rockefeller University, Bellevue Hospital Center, and Manhattan Veteran's Administration Center. All clinical investigation was conducted according to the principles expressed in the Helsinki Declaration.
Endoscopic biopsies were obtained from the colon from macroscopically normal mucosa at a standardized site in the rectosigmoid region 30–40 cm from the anal margin. This site was chosen for all sampling to avoid potential regional variation and the potential for confounding effects from infectious or traumatic proctitis that would be expected to occur distal to this location. Biopsies were taken using large-cup endoscopic biopsy forceps (outside diameter, 3.3 mm; Microvasive Radial Jaw; Boston Scientific) and (a) immediately placed in tissue culture medium (RPMI 1640; Mediatech Inc.), (b) placed into 2-ml prelabeled cryovials (Nalgene) and frozen in liquid nitrogen, or (c) placed in formalin to preserve tissue architecture. Formalin-fixed tissues were washed with PBS, transferred to 100% alcohol, and processed for immunohistochemistry and in situ hybridization. Phlebotomy was undertaken immediately before endoscopy.
Immediately after acquisition, mucosal mononuclear cells (MMCs) were enzymatically isolated from mucosal biopsies using a 30-min incubation in collagenase type II (Clostridiopeptidase A; Sigma-Aldrich) followed by mechanical separation through a blunt-end, 16-gauge needle. The digested cell suspension was strained through a 70-μm disposable plastic strainer. Immediately after isolation, cells were washed with PBS and resuspended in PBS containing antibodies for flow cytometry. PBMCs were prepared by centrifugation on a Ficoll-Hypaque density gradient (Mediatech). PBMCs were stained for flow cytometry immediately after isolation.
Flow Cytometry.
Cell surface expression of lymphocyte antigens was identified by monoclonal antibody staining of freshly isolated MMCs and PBMCs, followed by flow cytometry using a FACSCalibur (Becton Dickinson Immunocytometry Systems [BDIS]) with analysis using CELLQuest software (BDIS). Monoclonal antibodies used in this study included anti–human CD3 FITC (clone UCHT1; BDIS), anti–human CD3-PE (clone SK-7; BDIS), anti–human CD3–peridinin chlorophyll-a protein (clone SK-7; BDIS), anti–human CD4-allophycocyanin (clone RPA T4; BD Biosciences), anti–human CD8 PE (clone RPA T8; BD Biosciences), anti–human CXCR4-PE (clone 12G5; BD Biosciences), and anti–human CCR5-FITC (clone 2D7/CCR5; BD Biosciences). During flow cytometry, lymphocytes, initially identified by their forward and side scatter characteristics, were subject to phenotypic analysis. Dead cells were excluded from analysis using 7-aminoactinomycin D (Calbiochem). To determine the percentages of CD4+ and CD8+ cells in the T cell population, gated lymphocytes were initially examined for the expression of CD3. The CD3+ lymphocytes were then analyzed for expression of CD4 and CD8 receptors. To evaluate the expression of chemokine coreceptors, gated lymphocytes were initially examined for the expression of CD4 receptor. The CD4+ lymphocytes were further examined for the expression of chemokine coreceptors CCR5 and CXCR4.
Light Microscopy and Immunohistochemistry.
For light microscopic evaluation, tissues were fixed in 4% neutral-buffered formalin and embedded in paraffin. 5-μm thick sections were cut and stained with hematoxylin and eosin and Giemsa stains. Immunohistochemistry was also performed on paraffin-embedded sections after high temperature antigen retrieval as described previously (21). The following antibodies were used according to the manufacturer's instructions: NCL-CD4-IF6 (anti-CD4 antibody; Novocastra) and C8/144B (anti-CD8 antibody; Dakopatts). Binding of antibodies was visualized by the alkaline phosphatase/antialkaline phosphatase method using new fuchsin as a chromogen.
In Situ Hybridization.
The in situ hybridization was performed on paraffin sections as described previously (21). In brief, 5-μm sections were cut onto slides coated with 3-amino-propyl-trietho-silane. Dewaxed paraffin-embedded sections were either boiled in a domestic pressure cooker in citrate buffer (pH 6.0) for 5 min or treated with 0.01 mg/ml proteinase K for 8 min at room temperature and subjected to in situ hybridization to detect viral RNA. We used a 35S-labeled, single stranded antisense RNA probe of HIV-1 (Lofstrand Labs). The probe was composed of fragments of 1.4–2.7 kb, which collectively represent ∼90% of the HIV-1 genome (22). The specific activity of the probe was 2 × 106 dpm of probe/milliliter. The hybridization was performed overnight at 45°C in a moist chamber. The slides were washed, digested with RNase (Boehringer) at 37°C for 40 min, and washed again. The slides were then dipped into photo emulsion (NTB2; Eastman Kodak Co.), exposed for 7 d, developed, counterstained with hematoxylin, and mounted. As a positive control, paraffin-embedded sections from the spleen of an HIV-infected patient were used. As a negative control, sections were hybridized with a 35S-labeled sense probe.
Quantitative Analysis of HIV RNA+ Cells, CD4+ Cells, and CD8+ Cells.
The autoradiographs were examined with a microscope equipped with epiluminescent illumination (Axiophot; Carl Zeiss MicroImaging, Inc.), a 3CD camera, and a PC-based image analysis system (KS 4000; Kontron). Cells were considered positive for viral gene expression if the grain count was more than six times the background. The positive cells were counted. The area occupied by lymphoid follicles was measured and the frequency of RNA-producing cells per mm2 of gut-associated lymphoid tissue (GALT) was calculated. To evaluate the number and distribution of T cell subsets, transmission light was used. Using a 40× objective, a standard area was set by the image analyzer. The number of positive cells within this unit area was determined by manual counting. For the lamina propria, a total of 10–15 consecutive nonoverlapping fields was analyzed for each staining. For the GALT, two to five representative areas were chosen. The individual values obtained with each T cell marker were then pooled and the mean numbers of positive cells per unit area of lamina propria or GALT were determined separately.
Statistical Methodology.
Values are expressed as mean ± standard deviation. Statistical comparisons were made between PBMCs and MMCs from individuals using a paired t test. Statistical comparisons were made between HIV-1–infected and control subjects using a two-sample, unequal variance t test. All reported p-values were two-sided at the 0.05 significance level using Windows software (SPSS 11.0; SPSS Inc.).
Results
Primary HIV-1 Infection Is Associated with a Preferential Depletion of mucosal CD4+ T Cells.
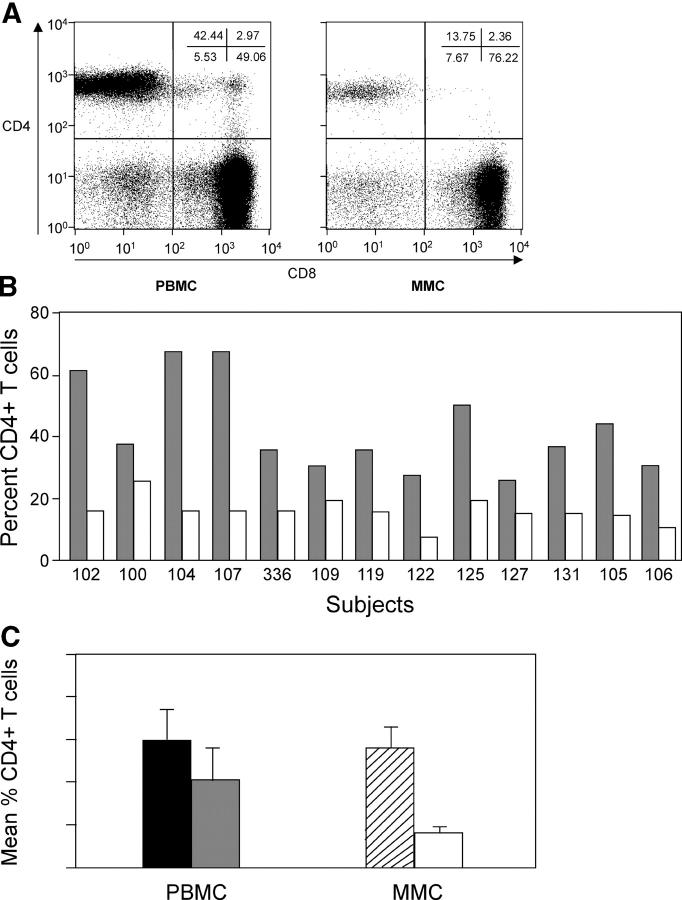
Isolated MMCs and PBMCs from all subjects were examined using flow cytometry to determine the percentage of CD4+ T cells (Fig. 1 A). The percentage of mucosal T cells expressing CD4 was significantly less than the percentage of CD4+ T cells in the peripheral blood in every patient in this group (Fig. 1 B). When cumulative data from group 1 was analyzed, the mean mucosal CD4+ T cell percentage was 15.7 ± 3.6, and was significantly less than the mean CD4+ T cell percentage in the peripheral blood (42.3 ± 14.7; P < 0.001). The mean CD4/CD8 ratio in the MMCs (0.2 ± 0.04) was also significantly lower than the mean CD4/CD8 ratio in the circulating PBMC compartment (0.9 ± 0.6; P = 0.002). In HIV-1–uninfected individuals, the percentage of CD4+ T cells in the mucosa (56.4 ± 8.8%) and blood (59.6 ± 14.3%) were not significantly different (P = 0.24), and the CD4/CD8 ratios in the mucosa and blood were 1.3 ± 0.5% and 1.7 ± 0.7%, respectively.
Figure 1.
CD4+ T cells are preferentially depleted in the GI tract in acute and early HIV-1 infection. PBMCs and MMCs from the rectosigmoid region from group 1, acute and early HIV-1 infection subjects (n = 13), and HIV-1–uninfected controls (n = 10) were analyzed by flow cytometry. CD3+ gated lymphocytes were analyzed for the expression of CD4 and CD8. (A) A representative flow plot from subject 105 is depicted. CD8+ T cells are shown on the x axis and CD4+ T cells are shown on the y axis. (B) Comparison of CD4+ T cells in the blood and GI tract of all 13 group 1 primary infection subjects. The percent of CD4+ T cells is shown in PBMCs (gray) and MMCs (white) per study subject. (C) Comparison of the mean percent of CD4+ T cells in the PBMCs of HIV-1–uninfected controls (black bar) and group1 subjects (gray bar), and MMCs of HIV-1–uninfected controls (hatched bar) and group 1 subjects (white bar).
Comparison of the percentages of CD4+ T cells in the mucosa of HIV-1–uninfected and newly diagnosed subjects with primary HIV-1 infection (group 1) revealed highly statistically significant differences with marked depletion in the HIV-1–infected group (P < 0.001; Fig. 1 C). As expected, depletion of peripheral blood CD4+ T cells was also observed when newly diagnosed, primary HIV-1–infected subjects were compared with HIV-1–uninfected subjects. However, the differences between the two groups were much less marked, though still significant (P = 0.01).
Primary HIV-1 Infection Is Associated with Preferential Depletion of CCR5-expressing Mucosal CD4+ T Cells.
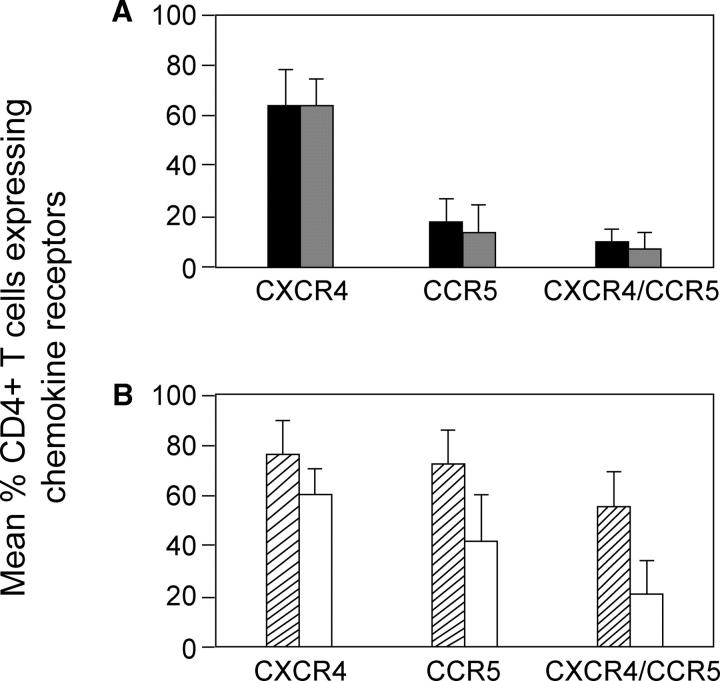
The majority of GI MMCs express CCR5, either alone or along with CXCR4 (4, 8). Given the high expression of CCR5 in the mucosa and the possibility that the mucosal environment plays an important role in the selection of the CCR5-tropic strains of HIV-1 that predominate in the blood during the primary infection period, we sought to determine the effect of primary HIV-1 infection on CCR5-expressing CD4+ MMCs. Using four-color flow cytometry, we quantified the percentage of CD3+/CD4+ MMCs and PBMCs that expressed CCR5, CXCR4, or coexpressed CCR5 and CXCR4. Comparison was made between HIV-1–uninfected subjects and the untreated primary HIV-1 infection subjects (group 1). In general there was no difference in levels of CD3+/CD4+ cells expressing CCR5, CXCR4, or both in the peripheral blood of uninfected controls when compared with subjects in group 1 (Fig. 2 A). Mean levels of CD4+ PBMC populations that expressed CXCR4 were 64.3 ± 15.2% in the HIV-1–uninfected subjects compared with 63.7 ± 11.8% in group 1 primary HIV-1 infection subjects (P = 0.6). Levels of CD3+/CD4+ PBMCs expressing CCR5 were 18.7 ± 9.0% in HIV-1–uninfected subjects compared with 14.8 ± 11.0% in group 1 (P = 0.4). Levels of CD3+/CD4+ PBMCs coexpressing CCR5 and CXCR4 were 9.6 ± 5.8% in HIV-1–uninfected subjects versus 6.9 ± 8.1% in group 1 primary HIV-1 infection subjects (P = 0.4). In comparison, analysis of CD4+ mucosal T cells revealed significant depletion in the HIV-1–infected subjects that was most prominent in the CD3+/CD4+ CCR5-expressing populations. Although there was only a minor degree of depletion of mucosal CD4+ T cells expressing CXCR4 (76.3 ± 14.4% in HIV-1–uninfected subjects vs. 61.7 ± 10.9 in group 1 subjects; P = 0.03), the percentage of cells expressing CCR5 alone was 73.5 ± 13.4% in HIV-1–uninfected subjects versus 42.5 ± 18.9% in group 1 (P = 0.001). The most significant depletion was noted in the population of CD4+ mucosal T cells that coexpressed CCR5 and CXCR4. In the uninfected individuals, 56.2 ± 13.9% of the CD4+ mucosal T cells coexpressed CCR5 and CXCR4 compared with just 21.1 ± 14.3% in the group 1 subjects (P < 0.001; Fig. 2 B).
Figure 2.
CD4+ T cells expressing CCR5 are preferentially depleted from the GI tract in acute and early HIV-1 infection. PBMCs (A) and MMCs (B) from the rectosigmoid region from individuals with acute and early HIV-1 infection (group 1, n = 13) and HIV-1–uninfected controls (n = 10) were analyzed by flow cytometry. CD3+/CD4+ gated lymphocytes were analyzed for the expression of CXCR4, CCR5, or CCR5/CXCR4. HIV-1–uninfected PBMCs (black bars), group 1 PBMCs (gray bars), HIV-1–uninfected MMCs (hatched bars), and group 1 MMCs (white bars) are shown. Mean percent of CD4+ T cells expressing chemokine receptors are depicted on the y axis and chemokine coreceptors CXCR4, CCR5, and CCR5/CXCR4 are grouped on the x axis.
CD4+ T Cells Are Preferentially Depleted from the Effector Sites in the GI Mucosa.
Having shown that the percentage of CD4+ T cells is decreased in acute and early HIV-1 infection, we went on to examine the absolute number of CD4+ T cells in the GI biopsies. Using immunohistochemistry, paraffin-embedded mucosal biopsies were compared between HIV-1–uninfected individuals and subjects with acute and early HIV-1 infection. To classify the anatomic subcompartment where CD4+ T cell depletion occurred, inductive and effector sites were examined separately.
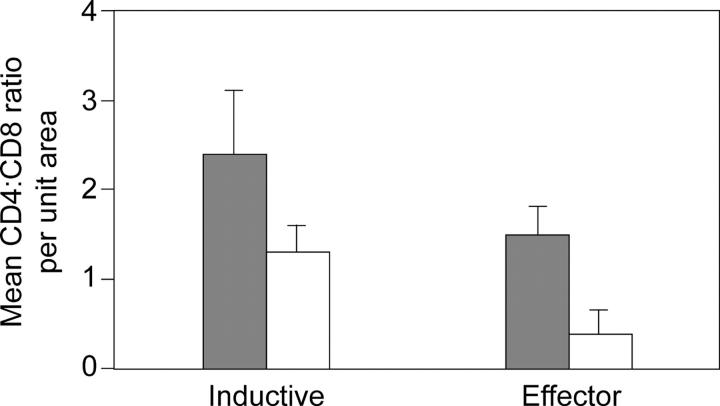
The inductive sites or lymphoid nodules consisted of a centrally located B cell–dependent area with or without a germinal center (Fig. 3 A). The follicle-associated epithelium and the dome area were noted in only a few of the sections examined. The B cell follicle was surrounded by the T-dependent zone, similar to that seen in the paracortex of a lymph node. In subjects with acute and early HIV-1 infection (Fig. 3 B) and in HIV-1–uninfected controls (Fig. 3 C), the T cell zone was densely populated with CD4+ T cells. In comparison, significantly more CD8+ T cells were noted in the subjects with acute and early HIV-1 infection (mean: 130 cells/unit area; range: 95–137 cells/unit area) compared with the HIV-1–uninfected controls (mean: 83 cells/unit area; range: 81–140 cells/unit area). The mean CD4/CD8 ratio in the inductive sites of HIV-1–uninfected controls was 2.4 (range 1.6–3.1). In comparison, the mean CD4/CD8 ratio in the inductive sites of subjects with acute and early HIV-1 infection was 1.3 (range 0.9–2.0). It is important to note that the lower CD4/CD8 ratio in HIV-1–infected subjects was due to an increase in the number of CD8+ T cells and not due to a depletion of the CD4+ T cells.
Figure 3.
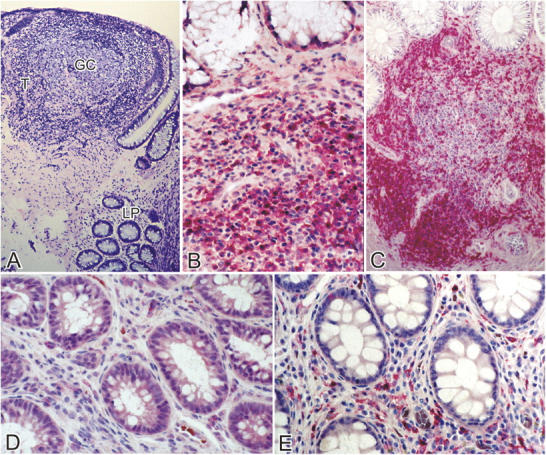
Effector sites (lamina propria) of the GI tract show the most pronounced CD4+ T cell depletion in acute and early HIV-1 infection. Immunohistochemical characterization of the effector and inductive sites in the rectal biopsies. Using a PC-based image analysis system (KS 4000; Kontron), a standard area was set by the image analyzer. For the lamina propria, a total of 10–15 consecutive nonoverlapping fields were analyzed for each staining. For the GALT, two to five representative areas were chosen. (A) At a magnification of 25, a Giemsa-stained section is shown depicting a lymphoid nodule (inductive site) where the germinal center (GC) and T cell zone (T) are indicated. The effector compartment represented by the lamina propria (LP) is seen in the lower part of the section. (B and C) The inductive sites are densely populated with CD4+ T cells (stained red) in both group 1 (B) and HIV-1–uninfected (C) subjects. A magnification of 100. (D and E) Effector sites in acute and early HIV-1 infection (D) show significant depletion of CD4+ T cells (stained red) compared with HIV-1–uninfected controls (E). A magnification of 100. (F) In the inductive and effector compartments, the mean of CD4+ and CD8+ T cells per unit area was determined and the CD4/CD8 ratio was calculated. A comparison of the mean CD4/CD8 ratio (represented on the y axis) between HIV-1–uninfected (gray bars) and group 1 subjects (white bars) is shown.
Significant CD4+ T cell depletion was noted in the effector sites in subjects with acute and early HIV-1 infection (Fig. 3 D) compared with HIV-1–uninfected controls (Fig. 3 E). In this site, the mean CD4/CD8 ratio was 0.4 (range 0.1–0.7) in the HIV-1–infected subjects and 1.5 (1.1–2) in the HIV-1–uninfected controls (Fig. 3 F).
HIV-1 RNA Is Located within the Inductive Sites of the GI Mucosa.
Having shown that depletion of mucosal CD4+ T cells was primarily noted in the effector compartment, we sought to determine whether this anatomic subcompartment was also the principal site of viral replication. In situ hybridization was performed to localize HIV-1 RNA within the effector and inductive subcompartments of the GI mucosa. Cells expressing HIV-1 RNA were predominantly localized to the organized lymphoid follicles or the inductive sites of the GI mucosa (Fig. 4, A and B), present both in the T cell zone and the germinal centers. Even though CD4+ T cells were depleted in the effector sites, of the CD4+ T cells that remained, productively infected cells were either absent or very rare. Interestingly, no viral trapping by follicular dendritic cells was noted in the biopsies of the group 1 primary infection subjects.
Figure 4.
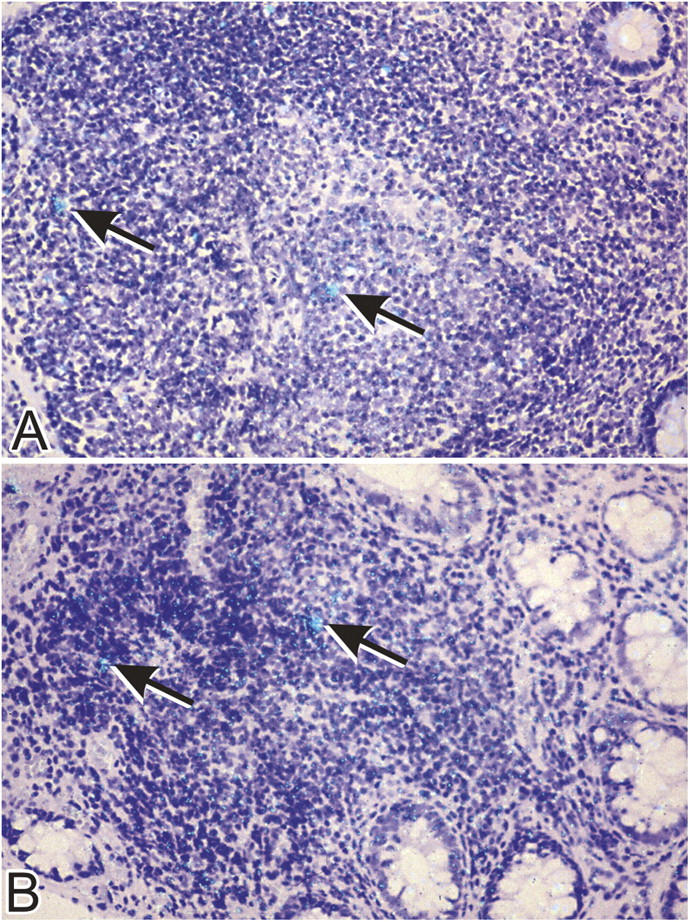
HIV-1 RNA is localized in the organized lymphoid follicles of the GI tract in subjects with acute and early HIV-1 infection. In situ hybridization was performed using a 35S-labeled, single stranded antisense RNA probe as described in Materials and Methods. Cells expressing HIV-1 RNA (arrows) are present in the germinal center and T cell zone. A magnification of 40.
Reconstitution of CD4+ MMCs Is Incomplete Despite Suppressive Antiretroviral Therapy.
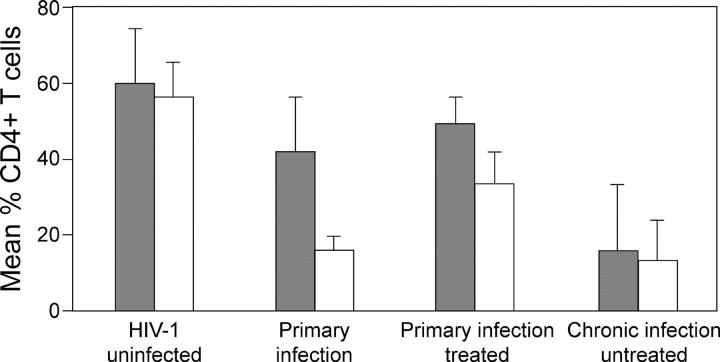
To examine whether apparently suppressive HAART would result in reconstitution of CD4+ MMCs, as has been described among CD4+ PBMCs, subjects in whom HAART was initiated during acute and early HIV-1 infection were examined. We performed a cross-sectional analysis of primary HIV-1–infected subjects who had been treated with regimens containing three or four drug combinations for varying durations (n = 8), and eight untreated HIV-1–infected subjects biopsied during chronic HIV-1 infection. The primary HIV-1 infection cohort included two subjects from group 1 who had been treated with suppressive HAART for 6 mo, two subjects who had been treated with suppressive HAART for 1 yr, two subjects who had been treated with suppressive HAART for 2 yr, and two subjects each treated for 3 and 5 yr, respectively. The mean percentage of CD4+ T cells in the peripheral blood in this group was 51.3 ± 11.3%, whereas in the GI mucosa, it was 35.4 ± 7.8% (P = 0.008; Fig. 5). When the percentage of CD4+ T cells of these subjects was compared with that from HIV-1–uninfected subjects, there was a significant difference in the mucosal compartment (P < 0.001), even though the difference in the blood was no longer statistically significant (P = 0.06). The mean CD4/CD8 ratio in the mucosal compartment (0.6 ± 0.2) continued to be significantly lower than the CD4/CD8 ratio in the blood compartment (1.3 ± 0.7) of HAART-treated primary infection subjects (P = 0.03).
Figure 5.
Prolonged HAART results in only partial reconstitution of the GI tract CD4+ T cells. PBMCs and MMCs from the rectosigmoid region from HIV-1–uninfected individuals (n = 10), subjects with acute and early HIV-1 infection (n = 13), subjects with HIV-1 infection treated during the primary infection stage (n = 8), and chronically infected HIV-1–untreated subjects (n = 8) were analyzed by flow cytometry. The mean percent of CD4+ T cells is shown in PBMCs (gray bars) and MMCs (white bars) per study group.
To better understand the effect of HAART in this diverse group, we analyzed each patient individually (Table II). In the two subjects (100-6m and 336-6m) followed longitudinally for 6 mo, the CD4/CD8 ratio in the blood showed significant improvement over baseline with treatment (0.6 in the untreated stage to 1.1 after 6 mo of HAART in both subjects). However, the CD4/CD8 ratio in the GI tract continued to be low (0.4 and 0.5, respectively). In subjects 78 and 132, the CD4/CD8 ratio in the blood and GI tract equalized but continued to be lower than the mean CD4/CD8 ratio in the blood (1.7 ± 0.6) and mucosa (1.3 ± 0.4) of HIV-1–uninfected controls. It is of interest that these two subjects had initiated HAART after 28 and 26 d, respectively, of their estimated dates of infection (Table I). In subjects 106 and 923, the CD4/CD8 ratio in the GI tract continued to be significantly lower compared with the blood, despite treatment with HAART for 3 and 5 yr, respectively. These two subjects had initiated HAART after 168 and 204 d, respectively, of their estimated date of infection (Table I). Notably for subject 56, the CD4/CD8 ratio in the blood normalized to 2.9 with fully suppressive HAART for 2 yr. In comparison, the CD4/CD8 ratio in the GI tract remained considerably lower at 0.8.
Table II.
Comparison of Blood and GI Tract Reconstitution with HAART
| Subject | CD4/CD8 ratioin the blood | CD4/CD8 ratioin the GI tract | Duration of HAARTat the time of biopsy |
|---|---|---|---|
| 100-6m | 1.1 | 0.4 | 6 mo |
| 336-6m | 1.1 | 0.5 | 6 mo |
| 64 | 1.6 | 0.6 | 1 yr |
| 78 | 0.7 | 0.7 | 1 yr |
| 56 | 2.9 | 0.8 | 2 yr |
| 132 | 0.7 | 0.8 | 2 yr |
| 106 | 0.9 | 0.5 | 3 yr |
| 923 | 1 | 0.5 | 5 yr |
As expected, in chronic, untreated HIV-1–infected subjects, the mean CD4+ T cell percentage continued to decline in both the blood (15.3 ± 17%) and mucosal (12.5 ± 11%) compartments (Fig. 5).
Discussion
Being that the GI tract is the largest lymphoid organ in the body, it would follow that the potential for deleterious effects of HIV-1 infection would be great at this site. Although the effect of primary HIV-1 infection on lymphocyte populations in the peripheral blood has been well characterized, the events associated with primary HIV-1 infection in GI mucosal lymphocyte populations in man has to date remained largely unknown. Given the strong selection pressure for CCR5 coreceptor using viruses during transmission of HIV-1 (23), it would be likely that the sizable population of CCR5-expressing, immunologically activated CD4+ T cells in the GI mucosa would be more supportive of viral replication and susceptible to its deleterious effects as compared with the peripheral blood where the T cells are predominantly CCR5− and naive (8). We undertook this study to assess whether a preferential depletion of mucosal CD4+ T cells would be observed in primary HIV-1–infected subjects as has been observed in the SIV-macaque model. We went on to examine the anatomic subcompartment from which these cells are depleted and examine whether suppressive HAART therapy could result in complete immune reconstitution in the mucosal compartment.
Previous studies of the effect of HIV-1 on the GI mucosal CD4+ T cells during the primary infection period have been limited to a single report, with two subjects studied during early HIV-1 infection. In both of these individuals, identified after 4 and 6 wk of the estimated date of infection, mucosal CD4+ T cell depletion was noted (19). The remaining data suggesting the GI tract as the preferred site for virus replication and CD4+ T cell depletion are derived from studies examining primary infection of macaques with SIV (2, 3, 13, 15, 17, 18, 24, 25).
Here we have described 13 subjects who are identified and studied extremely early in the course of HIV-1 infection. We have confirmed that indeed, significant mucosal CD4+ T cell depletion occurs in subjects during primary HIV-1 infection, before changes seen in the peripheral blood. However, human mucosal CD4+ T cell depletion appears to be less marked than that described in primary SIV infection, where the percentage of CD4+ T cells in the macaque small bowel was reduced to <10% by 3 wk after infection (13). This is likely explained by a difference in the site sampled. All the biopsies in our studies were obtained from the rectal mucosa, which has both effector and inductive lymphoid tissue. In the macaque models described, the biopsies are usually obtained from the proximal jejunum, which is devoid of immune inductive sites (26). As a result, samples obtained from the jejunum in macaque models may demonstrate a much higher degree of CD4+ T cell depletion compared with what is noted in this study. There may also be other factors responsible. SIV is introduced in large inocula to attempt to guarantee infection. Simian AIDS is characterized by an accelerated natural history of the disease (27, 28) and is accompanied by higher peak plasma viral levels (29, 30) when compared with HIV-1 infection in man. Also, SIV is known to use alternative and additional coreceptors when compared with HIV-1 (31, 32), which may account for apparent differences in replication dynamics. Yet unexplained differences may also exist in host susceptibility to the two infections.
In addition to confirming the numerical decrease in mucosal CD4+ T cell percentage, we have gone on to characterize the relationship between CD4+ T cell depletion and HIV-1 RNA localization within specific anatomic subcompartments of the GI mucosa. Like the small intestine, the immune compartment in the rectal mucosa is also divided into inductive and effector arms (24, 33). The organized lymphoid tissue with a high content of CD68+ MHC class II+ macrophages and cells expressing costimulatory molecules CD86 and CD40 serves as an antigen-presenting site (24). The majority of lymphocytes present in the inductive compartment are antigen naive. However, once activated and primed by antigen-presenting cells such as dendritic cells, GI mucosal lymphocytes can home back to the mucosa to perform effector functions (34). The lamina propria lymphocytes, which are comprised of a high percentage of differentiated effector cells (9, 10), serve as the effector arm of the mucosal immune system (33). A limited number of studies have described the effects of SIV infection (24–26) and chronic and advanced HIV-1 infection (35) on these distinct, anatomic subcompartments. Our results suggest that as early as the primary infection stage, there is a depletion of CD4+ T cells from the effector compartment of the GI mucosa. Given the activated and differentiated phenotype of these cells, direct cytopathic effects of the HIV-1 virus could be one mechanism explaining this lesion. To assess this, we performed in situ hybridization for HIV-1 RNA to localize the sites of HIV-1 replication in the GI mucosa. We observed that HIV-1 RNA was localized in the inductive compartment of the mucosa. These findings are consistent with the SIV-macaque model where during early infection, most of the SIV-infected cells are located in the inductive sites. In a recent study, Veazey et al. (26) noted that very early in the course of infection, i.e., from 7 to 14 d after infection, SIV-infected cells could be localized in the effector compartment as well. However, once effector CD4+ T cells were depleted, SIV RNA was predominantly seen in the inductive compartment. Our patients, although studied extremely early in the course of HIV-1 infection, had already developed profound CD4+ T cell depletion in the effector sites. Thus, one possible explanation for the localization of HIV-1 RNA only in the inductive compartment is the loss of target cells from the effector sites by the time the biopsies were performed.
Further studies are underway to better characterize the dynamics of CD4+ T cell loss noted in the effector compartment. Some of the possibilities include decreased local proliferation, increased cell death in the effector compartment due to apoptosis or activation-induced cell death (36), reduced homing of cells to the effector site from the periphery after antigen recognition due to either direct cytopathic effects of HIV-1 or immune activation-induced cell death, or perhaps combinations of the above (37).
By studying subjects in whom treatment was initiated during acute and early HIV-1 infection, we sought to understand whether HAART would result in mucosal CD4+ T cell reconstitution. Guadalupe et al. (19) showed that in one subject, mucosal CD4+ T cell reconstitution was attained when HAART was initiated within 6 wk of the estimated date of infection. In comparison, we did not see immune reconstitution in the GI mucosa with HAART in our study population. It is to be noted that all eight patients studied initiated therapy in the primary infection stage, remained compliant with treatment, and continued to have undetectable plasma HIV-1 RNA levels for the entire duration of the study period. The cohort remains small and we plan to study more subjects longitudinally to better assess the degree of immune reconstitution and the factors that may predispose to a greater or lesser response.
The consequence of early depletion and incomplete reconstitution of mucosal CD4+ T cells is unclear. Although opportunistic infections remain rare until the peripheral CD4+ T cell count falls during the chronic infection period, the long-term outcome of treated HIV-1 infection remains unknown. Furthermore, the nature of the CD4+ T cell loss requires additional study. Theoretically, the loss of specific clones of CD4+ T cells in the mucosa may predispose to accelerated immune senescence, more rapid than that seen as a consequence of aging, which in turn may result in the increased incidence of malignancies, be they lymphomas or solid tumors such as adenocarcinomas.
Given the importance of the mucosal compartment in HIV-1 pathogenesis, our findings may have important implications with regard to treatment. Perhaps efforts to specifically inhibit T cell activation in the GI tract as well as drugs to more specifically interfere with HIV-1 entry (38, 39) and replication in the GI mucosa during the primary infection period may result in lowering of the viral set point and in conjunction with HAART, result in improvement in clinical outcome. Such investigations are clearly needed as the goal of optimized treatment of HIV-1 infection is yet to be attained.
Acknowledgments
We would like to thank the patients for their participation. We acknowledge the nursing staff at The Rockefeller University and Bellevue Hospital for their clinical assistance. We would like to thank Wendy Chen for help with the graphs and figures; Petra Meyer, Birgit Raschdorff, and Gudrun Großschupff for their technical assistance with immunohistochemistry and in situ hybridization; and Dr. Gerard Eberl for helpful discussion.
This work was supported in part by the following grants: The AIEDRP (AI-41534) and the Virology Core of the Columbia-Rockefeller Center for AIDS Research (P30 AI42848-05), AI-01668 and AI-46964 from the National Institute of Allergy and Infectious Diseases, the General Clinical Research Center grant from the National Center for Research Resources at the NIH (M01-RR00102), and the German Ministry of Education and Research (BMBF) Contract KompNet 01KI0211 (to P. Racz and K. Tenner-Racz).
The authors have no conflicting financial interests.
S. Mehandru and M.A. Poles contributed equally to this work.
A portion of this data was presented in abstract forms at Digestive Disease Week, May 2004 (New Orleans, LA) and the second international workshop for acute and early HIV-1 infection, May 2004 (Bethesda, MD). No similar paper has been or will be submitted elsewhere.
Abbreviations used in this paper: GALT, gut-associated lymphoid tissue; GI, gastrointestinal; HAART, highly active antiretroviral therapy; MMC, mucosal mononuclear cell; SIV, simian immunodeficiency.
References
- 1.Cerf-Bensussan, N., and D. Guy-Grand. 1991. Intestinal intraepithelial lymphocytes. Gastroenterol. Clin. North Am. 20:549–576. [PubMed] [Google Scholar]
- 2.Smit-McBride, Z., J.J. Mattapallil, M. McChesney, D. Ferrick, and S. Dandekar. 1998. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4(+) T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J. Virol. 72:6646–6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey, R.S., K.G. Mansfield, I.C. Tham, A.C. Carville, D.E. Shvetz, A.E. Forand, and A.A. Lackner. 2000. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellors, J.W., C.R. Rinaldo Jr., P. Gupta, R.M. White, J.A. Todd, and L.A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 272:1167–1170. [DOI] [PubMed] [Google Scholar]
- 5.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 326:711–713. [DOI] [PubMed] [Google Scholar]
- 6.Orenstein, J.M., C. Fox, and S.M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science. 276:1857–1861. [DOI] [PubMed] [Google Scholar]
- 7.Poles, M.A., J. Elliott, P. Taing, P.A. Anton, and I.S. Chen. 2001. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75:8390–8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anton, P.A., J. Elliott, M.A. Poles, I.M. McGowan, J. Matud, L.E. Hultin, K. Grovit-Ferbas, C.R. Mackay, I.S.Y. Chen, and J.V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 14:1761–1765. [DOI] [PubMed] [Google Scholar]
- 9.Kim, S.K., D.S. Reed, W.R. Heath, F. Carbone, and L. Lefrancois. 1997. Activation and migration of CD8 T cells in the intestinal mucosa. J. Immunol. 159:4295–4306. [PubMed] [Google Scholar]
- 10.Schieferdecker, H.L., R. Ullrich, H. Hirseland, and M. Zeitz. 1992. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J. Immunol. 149:2816–2822. [PubMed] [Google Scholar]
- 11.McGowan, I.M., J. Elliott, M. Fuerst, P. Taing, J. Boscardin, M.A. Poles, and P.A. Anton. 2004. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. In press. [DOI] [PubMed] [Google Scholar]
- 12.Lefrere, J.J., F. Roudot-Thoraval, M. Mariotti, M. Thauvin, J. Lerable, J. Salpetrier, and L. Morand-Joubert. 1998. The risk of disease progression is determined during the first year of human immunodeficiency virus type 1 infection. J. Infect. Dis. 177:1541–1548. [DOI] [PubMed] [Google Scholar]
- 13.Veazey, R.S., M. DeMaria, L.V. Chalifoux, D.E. Shvetz, D.R. Pauley, H.L. Knight, M. Rosenzweig, R.P. Johnson, R.C. Desrosiers, and A.A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 280:427–431. [DOI] [PubMed] [Google Scholar]
- 14.Harouse, J.M., A. Gettie, R.C. Tan, J. Blanchard, and C. Cheng-Mayer. 1999. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 284:816–819. [DOI] [PubMed] [Google Scholar]
- 15.Kewenig, S., T. Schneider, K. Hohloch, K. Lampe-Dreyer, R. Ullrich, N. Stolte, C. Stahl-Hennig, F.J. Kaup, A. Stallmach, and M. Zeitz. 1999. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 116:1115–1123. [DOI] [PubMed] [Google Scholar]
- 16.Mattapallil, J.J., Z. Smit-McBride, M. McChesney, and S. Dandekar. 1998. Intestinal intraepithelial lymphocytes are primed for gamma interferon and MIP-1beta expression and display antiviral cytotoxic activity despite severe CD4(+) T-cell depletion in primary simian immunodeficiency virus infection. J. Virol. 72:6421–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise, C., C.J. Miller, A. Lackner, and S. Dandekar. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169:1116–1120. [DOI] [PubMed] [Google Scholar]
- 18.Veazey, R.S., I.C. Tham, K.G. Mansfield, M. DeMaria, A.E. Forand, D.E. Shvetz, L.V. Chalifoux, P.K. Sehgal, and A.A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen, R.S., G.A. Satten, S.L. Stramer, B.D. Rawal, T.R. O'Brien, B.J. Weiblen, F.M. Hecht, N. Jack, F.R. Cleghorn, J.O. Kahn, et al. 1998. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 280:42–48. [DOI] [PubMed] [Google Scholar]
- 21.Tenner-Racz, K., H.J. Stellbrink, J. van Lunzen, C. Schneider, J.P. Jacobs, B. Raschdorff, G. Grosschupff, R.M. Steinman, and P. Racz. 1998. The unenlarged lymph nodes of HIV-1–infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J. Exp. Med. 187:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox, C.H., K. Tenner-Racz, P. Racz, A. Firpo, P.A. Pizzo, and A.S. Fauci. 1991. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J. Infect. Dis. 164:1051–1057. [DOI] [PubMed] [Google Scholar]
- 23.Paxton, W.A., R. Liu, S. Kang, L. Wu, T.R. Gingeras, N.R. Landau, C.R. Mackay, and R.A. Koup. 1998. Reduced HIV-1 infectability of CD4+ lymphocytes from exposed-uninfected individuals: association with low expression of CCR5 and high production of beta-chemokines. Virology. 244:66–73. [DOI] [PubMed] [Google Scholar]
- 24.Vajdy, M., R.S. Veazey, H.K. Knight, A.A. Lackner, and M.R. Neutra. 2000. Differential effects of simian immunodeficiency virus infection on immune inductive and effector sites in the rectal mucosa of rhesus macaques. Am. J. Pathol. 157:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vajdy, M., R. Veazey, I. Tham, C. deBakker, S. Westmoreland, M. Neutra, and A. Lackner. 2001. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J. Infect. Dis. 184:1007–1014. [DOI] [PubMed] [Google Scholar]
- 26.Veazey, R.S., J.D. Lifson, I. Pandrea, J. Purcell, M. Piatak Jr., and A.A. Lackner. 2003. Simian immunodeficiency virus infection in neonatal macaques. J. Virol. 77:8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy, J.A. 1996. The value of primate models for studying human immunodeficiency virus pathogenesis. J. Med. Primatol. 25:163–174. [DOI] [PubMed] [Google Scholar]
- 28.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, et al. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 248:1109–1112. [DOI] [PubMed] [Google Scholar]
- 29.Staprans, S.I., P.J. Dailey, A. Rosenthal, C. Horton, R.M. Grant, N. Lerche, and M.B. Feinberg. 1999. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J. Virol. 73:4829–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith, S.M., B. Holland, C. Russo, P.J. Dailey, P.A. Marx, and R.I. Connor. 1999. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res. Hum. Retroviruses. 15:1691–1701. [DOI] [PubMed] [Google Scholar]
- 31.Deng, H.K., D. Unutmaz, V.N. KewalRamani, and D.R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 388:296–300. [DOI] [PubMed] [Google Scholar]
- 32.Alkhatib, G., F. Liao, E.A. Berger, J.M. Farber, and K.W. Peden. 1997. A new SIV co-receptor, STRL33. Nature. 388:238. [DOI] [PubMed] [Google Scholar]
- 33.Cheroutre, H., and L. Madakamutil. 2004. Acquired and natural memory T cells join forces at the mucosal front line. Nat. Rev. Immunol. 4:290–300. [DOI] [PubMed] [Google Scholar]
- 34.Mora, J.R., M.R. Bono, N. Manjunath, W. Weninger, L.L. Cavanagh, M. Rosemblatt, and U.H. Von Andrian. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 424:88–93. [DOI] [PubMed] [Google Scholar]
- 35.Clayton, F., G. Snow, S. Reka, and D.P. Kotler. 1997. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin. Exp. Immunol. 107:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krammer, P.H. 2000. CD95's deadly mission in the immune system. Nature. 407:789–795. [DOI] [PubMed] [Google Scholar]
- 37.McCune, J.M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 410:974–979. [DOI] [PubMed] [Google Scholar]
- 38.Veazey, R.S., P.J. Klasse, T.J. Ketas, J.D. Reeves, M. Piatak Jr., K. Kunstman, S.E. Kuhmann, P.A. Marx, J.D. Lifson, J. Dufour, et al. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian–human immunodeficiency virus infection. J. Exp. Med. 198:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veazey, R.S., R.J. Shattock, M. Pope, J.C. Kirijan, J. Jones, Q. Hu, T. Ketas, P.A. Marx, P.J. Klasse, D.R. Burton, et al. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346. [DOI] [PubMed] [Google Scholar]