Abstract
The accumulation of immunoglobulin (Ig)A antibody-secreting cells (ASCs) in the lactating mammary gland leads to secretion of antibodies into milk and their passive transfer to the suckling newborn. This transfer of IgA from mother to infant provides transient immune protection against a variety of gastrointestinal pathogens. Here we show that the mucosal epithelial chemokine CCL28 is up-regulated in the mammary gland during lactation and that IgA ASCs from this tissue express CCR10 and migrate to CCL28. In vivo treatment with anti-CCL28 antibody blocks IgA ASC accumulation in the mammary gland, inhibiting IgA antibody secretion into milk and the subsequent appearance of antibody in the gastrointestinal tract of nursing neonates. We propose that CCL28 is a key regulator of IgA ASC accumulation in the mammary gland and thus controls the passive transfer of IgA antibodies from mother to infant.
Keywords: cell trafficking, common mucosal immune system, chemokine, passive immunity, milk
Introduction
The gut serves as a portal of entry for a myriad of pathogens. Several mechanisms of protection have evolved to protect the gut from microbes, including effector T cells and antibody-secreting cells (ASCs). IgA ASCs are of particular importance because IgA antibodies are secreted across the gut epithelium into the intestinal lumen where they can neutralize pathogens and toxins (1, 2). The gastrointestinal tract of the neonate is particularly vulnerable to infection because the newborn is immunologically naive for the first several days of life, until effector T cells and ASCs are generated and disseminated throughout the body.
The adaptive immune system of the mother can provide passive protection to the suckling newborn through antibodies ingested in the mother's milk (3, 4). During late pregnancy and lactation, maternal IgA ASCs primed in the gut and respiratory tract, home to the mammary gland (5–7), secreting antibody into the milk for passage to the gastrointestinal tract of the nursing neonate (8). Secretory IgA is resistant to gastrointestinal enzymes, allowing the passage of functional IgA through the infant's gastrointestinal tract (9). The transfer of maternal antibodies to the nursing neonate provides transient immune protection to pathogens previously encountered by the mother, and contributes to the dramatically reduced infant mortality levels in children who are breast fed compared with those who are formula fed in developing countries (10).
The participation of chemoattractants in the mammary gland IgA response is suggested by early studies reporting chemotactic activity for IgA ASCs in mouse colostrum (11). It is now clear that chemoattractant cytokines (chemokines) play a vital role in lymphocyte trafficking, and participate as key players in the multistep processes of lymphocyte recruitment from the blood into tissues (12). Recent studies have led to the hypothesis that the epithelial chemokines CCL25 and CCL28 mediate IgA ASC trafficking to gastrointestinal and respiratory mucosal sites (13–15). The role of these or other chemokines in IgA ASC migration to the mammary gland has not been examined. In regard to mammary gland homing, CCL28 is a particularly attractive candidate because most IgA ASCs in the body express the CCL28 receptor CCR10, migrate to CCL28 in vitro (13, 14), and CCL28 is found in milk (16).
In this report, we show that CCL28 is up-regulated in the mammary gland during lactation, and demonstrate that antibodies to CCL28 inhibit the accumulation of IgA-producing cells in the mammary gland, providing direct evidence that CCL28 can control local mucosal IgA ASC responses. Finally, we show that CCL28-mediated IgA ASC accumulation is required for efficient transfer of maternal IgA antibodies to the suckling neonate.
Materials and Methods
PCR.
Total RNA was collected from the mammary gland of BALB/c mice (the fourth abdominal mammary gland was used in all experiments) at various stages of pregnancy and lactation using the RNeasy kit (QIAGEN). All PCR reactions were performed using an RNA PCR core kit (Applied Biosystems) according to the manufacturer's recommendations. The following primers were used: CCL28: sense ATGCAGCAAGCAGGGCTCACACTC, antisense ACGAGAGGCTTCGTGCCTGTGTGT; GAPHD: sense CCATGGAGAAGGCTGGGG, antisense CAAAGTTGTCATGGATGACC; CCR10: sense CCCGAAAGCCTCACGCAGACTG, antisense GGAGCAGCCTCCGCAGGTCCCGGCGG; and CCR3: sense TCCACTGTACTCCCTGGTGT, antisense GACTGCAGGAAAACTCTCCA. PCR product was run on a 1.5% agarose gel and visualized with ethidium bromide staining.
Chemotaxis and Cell Staining.
Small intestine lamina propria lymphocytes and mammary gland lymphocytes were isolated by collagenase digestion of the tissue (after removal of Peyer's patches and lymph nodes, respectively) as described previously (14). All tissues were collected from lactating mice 9 d postpartum. Chemotaxis assays were performed and migrated lymphocytes were enumerated using a bead-counting method as described previously (14). IgA ASCs were identified and defined as described previously (14). CCL28–Ig binding was performed in the presence of 5 μg normal goat IgG using a mCCL28-hIgG chimera detected with PE-conjugated donkey anti–human IgG (Jackson ImmunoResearch Laboratories) as described previously (14). Negative controls were performed by inhibiting CCL28–Ig binding with 5 μg polyclonal goat anti-mCCL28 (R&D Systems). The following rat anti–mouse antibodies were used for staining: B220 (RA3-6B2), IgA (C10-3), and TCR-β (H57-597; all from BD Biosciences). Flow cytometry was performed on a FACSCalibur (BD Biosciences) using CELLQuest software.
In Vivo Anti-CCL28 Blockade.
Female BALB/c mice in their first pregnancy were used in all experiments. In antibody-blocking experiments 100 μg of monoclonal anti-CCL28 (R&D Systems) or IgG2b isotype control antibody was injected i.p. on days 1, 3, 5, and 7 postpartum. Mouse milk was collected on days 1 and 9. Anesthetized mice were injected i.p. with 2 U oxytocin (Sigma-Aldrich) and milk was collected using a suction powered milking apparatus, similar to that described previously (17). Milk was then centrifuged at 14,000 RPM for 5 min at room temperature, the fat was discarded, and the whey portion of the milk was stored at −20°C until use.
Immunohistology.
8-μm frozen sections were fixed in cold acetone for 10 min. After drying, slides were stained with FITC-labeled anti-IgA and PE-labeled anti–TCR-β. Staining was visualized using confocal microscopy. IgA staining lymphocytes were counted by photographing random mammary gland sections and visually analyzing photographs for the number of stained cells/field of view. Cell numbers were then scaled to reflect the number of cells/mm2 of mammary gland tissue and data were expressed as mean ± SEM. Multiple tissue sections from each of five mice were examined per treatment group.
ELISA.
ELISA plates (Nunc) were coated with 2 μg/ml of capture antibody diluted in PBS and coated overnight at 4°C. Milk samples were diluted in blocking buffer and incubated in ELISA plates for 2 h at room temperature. Alkaline phosphatase–conjugated secondary antibodies were used as detection reagents. Antibody concentrations were determined by constructing a standard curve of known values and calculating the microgram/milliliter of antibody in milk or the microgram/milligram of antibody in feces. Milk from five or more mice was used to determine antibody levels for each treatment group. 18 and 23 neonates were used to determine IgA levels in the feces of pups nursing on control- and anti-CCL28–treated mothers, respectively. Data are expressed as mean ± SEM.
Statistical Methods.
Student's t test was used to analyze the results, and P < 0.01 was considered significant.
Results and Discussion
CCL28 Is Up-regulated in the Mammary Gland during Lactation.
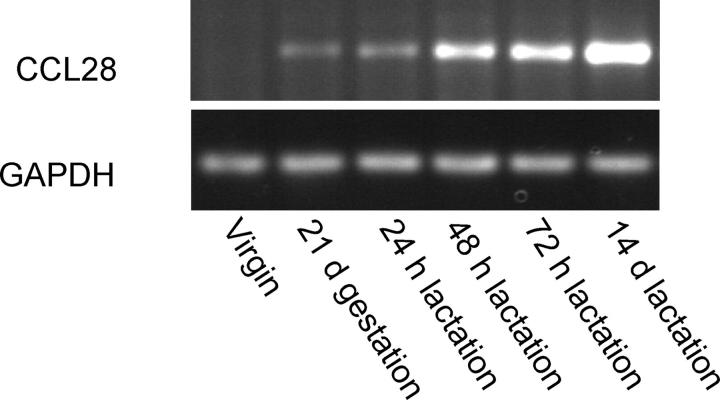
Few lymphocytes are present in the mammary glands of virgin mice and IgA ASCs are rare. IgA ASCs begin to appear late in pregnancy and increase dramatically in number soon after the start of lactation. By the third week of lactation, the number of IgA ASCs has increased by several hundredfold (6, 18). We determined if the level of CCL28 expression in the mammary gland correlates with the accumulation of IgA ASCs. In contrast to constitutive mucosal expression reported for salivary gland and colon (19), we found that CCL28 expression in the mammary gland is tightly regulated and intimately associated with the process of lactation. CCL28 message is not detected by semiquantitative RT-PCR in the mammary gland of virgin mice (Fig. 1). CCL28 message is slightly up-regulated during late pregnancy and early lactation, correlating with the beginning of IgA ASC accumulation. Approximately 48 h after the start of lactation, CCL28 expression rises dramatically and high levels of chemokine mRNA are maintained throughout lactation (Fig. 1). This remarkable up-regulation of CCL28 correlates well with the time course of IgA ASC appearance and accumulation.
Figure 1.
CCL28 expression in the mammary gland is up-regulated during lactation. RT-PCR was performed using primers specific for mouse CCL28 and GAPDH using mammary gland total RNA.
Mammary Gland IgA Cells Migrate to CCL28 and Express CCR10.
Next, we asked whether IgA ASCs from the lactating mammary gland can respond to CCL28 in in vitro chemotaxis assays (Fig. 2 A). Mammary gland IgA ASCs migrated approximately three times more efficiently to the CCR10 ligands CCL28 (mean migration: 36.2 ± 5.4% SEM) and CCL27 (not depicted), and less well to the small intestinal chemokine CCL25 (mean migration: 12.1 ± 3.2% SEM; P < 0.01), which has been implicated in the homing of CCR9-expressing IgA ASCs to the small intestine (Fig. 2 A; references 15, 20, and 21). In contrast, IgA ASCs isolated from the small intestines migrated well to both CCL28 and CCL25 (Fig. 2 A). A CCL28–Ig fusion protein bound specifically to the surface of most mammary gland IgA ASCs (Fig. 2 B), confirming expression of CCL28 receptor by the majority of IgA-expressing lymphocytes. The robust migration of mammary gland IgA ASCs to CCL28 but not CCL25 may indicate that mammary gland IgA ASCs comprise a population of lymphocytes derived primarily from antigen responses in sites such as the respiratory tract and large intestine. Small intestine–derived ASCs, which respond well to both chemokines, could represent a minor component of mammary ASCs. CCL28 has been shown to bind two receptors, CCR3 and CCR10 (19), but mammary gland IgA ASCs did not migrate to the to the CCR3 ligand eotaxin (not depicted). Moreover, IgA ASCs sorted from the mammary glands of mice 9 d postpartum showed strong expression of CCR10, but no expression of CCR3 by RT-PCR (Fig. 2 C). We conclude that mammary IgA ASCs, like IgA ASCs in the blood and other mucosal sites, express the CCL28 receptor CCR10 (22, 23).
Figure 2.
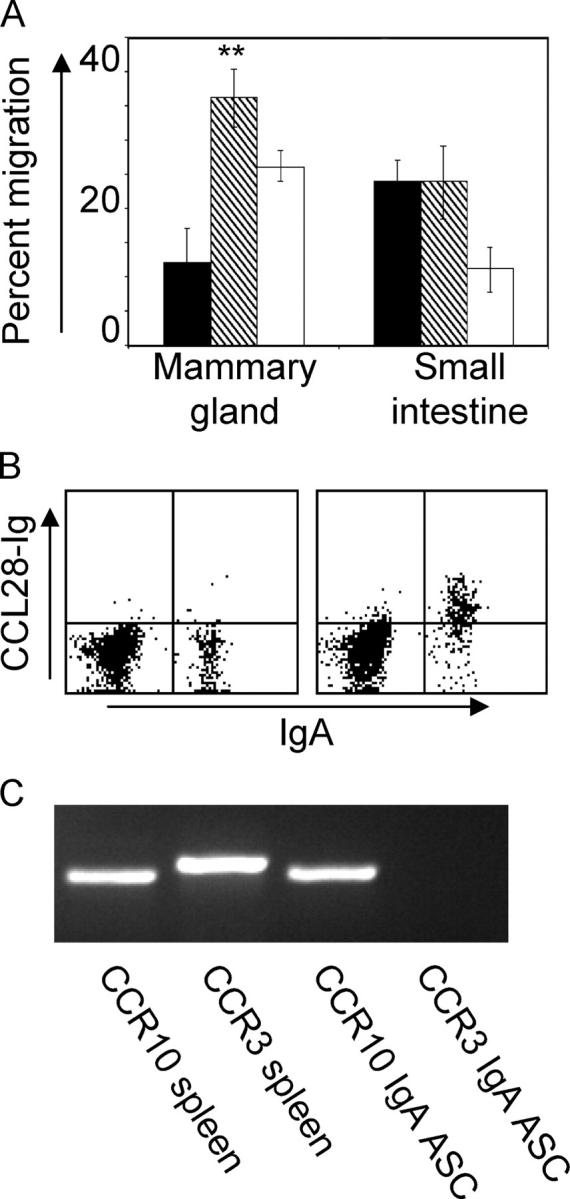
Mammary gland IgA ASCs migrate to CCL28, bind CCL28–Ig chimera, and express CCR10. Lymphocytes were isolated from the mammary gland and small intestine of lactating mice. (A) Migration of mammary gland and small intestine IgA ASCs to CCL25 (black bars), CCL28 (hatched bars), and CXCL12 (white bars). **, differences were statistically significant (P < 0.01) between CCL28 and CCL25 migration. Data are expressed as mean ± SEM. (B) CCL28–Ig binding. Left, negative control; right, CCL28–Ig binding. (C) Total RNA was collected from sorted mammary gland IgA ASCs. RT-PCR analysis shows expression of the chemokine receptor CCR10 but not CCR3 on mammary gland IgA ASCs.
CCL28 Blockade Inhibits IgA ASC Accumulation to the Mammary Gland and IgA Accumulation in the Milk.
To determine whether CCL28 regulates IgA ASC accumulation in the mammary gland and secretory IgA levels in the milk, we treated mice with a function-blocking anti-CCL28 antibody and evaluated the number of IgA ASCs in the mammary gland as well as the level of IgA antibody in the milk on day 9 postpartum. Immunostaining of mammary tissue sections revealed an almost complete inhibition of IgA ASC accumulation in animals treated with anti-CCL28 (Fig. 3 A), with an average of 1.8 IgA ASCs/mm2 compared with 28.9 IgA ASCs/mm2 in isotype control mAb-treated mice (P < 0.001). Importantly, antibody blockade of CCL28 substantially inhibited IgA secretion into milk (Fig. 3 B). IgA antibody levels in milk collected 9 d postpartum from anti-CCL28–treated animals were ∼20 μg/ml, similar to IgA serum (not depicted) and milk levels on day 1 postpartum, at which time IgA ASCs in the mammary gland are infrequent and the majority of IgA antibody is derived from the blood (24). Conversely, IgA levels in the milk of control mice were >130 μg/ml (P < 0.001). Unlike IgA ASCs, most IgG and IgM secreting cells do not migrate to the chemokine CCL28 in vitro (14) and do not accumulate, in appreciable numbers, in the mammary gland (18). The small amount of IgG present in the milk is found at all stages of lactation and is likely serum derived (24, 25). Accordingly, levels of IgG1 and IgM in the milk were low in lactating mice and remained unaffected by anti-CCL28 treatment (Fig. 3 B).
Figure 3.
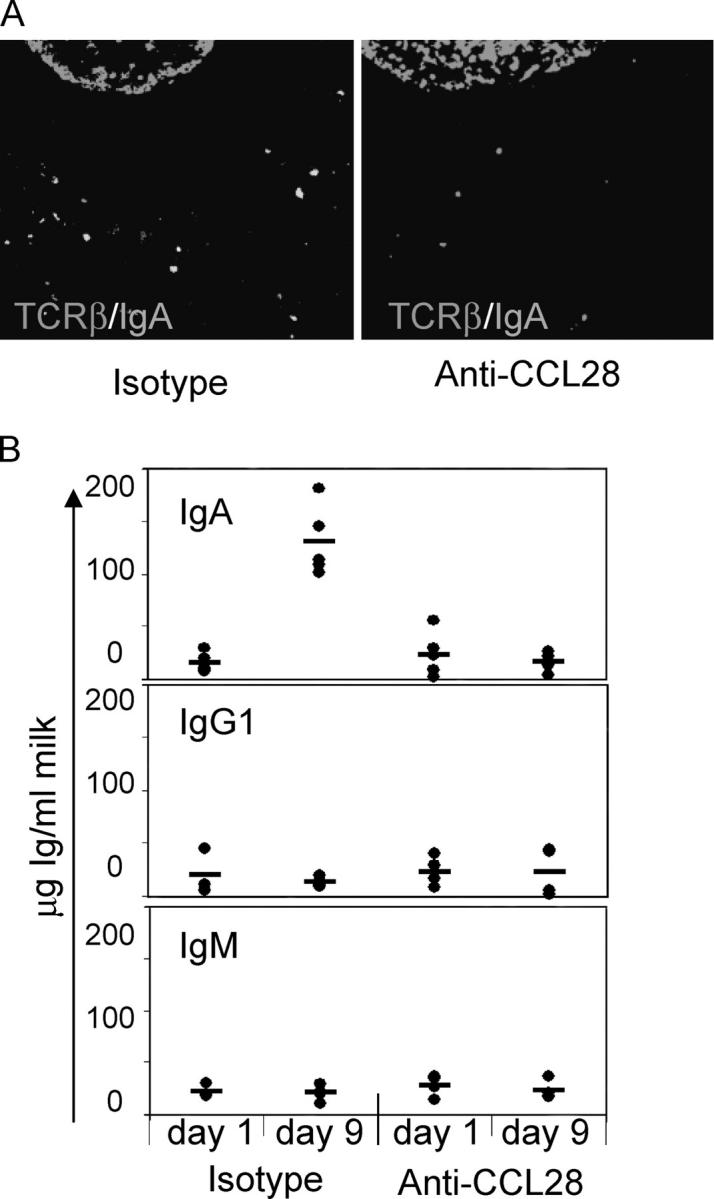
Anti-CCL28 inhibits IgA ASC homing to the mammary gland and IgA antibody accumulation in the milk. (A) Tissue sections from the mammary gland of 9-d postpartum mice treated with anti-CCL28 function-blocking antibody or isotype control antibody. Tissue sections were stained with anti-IgA (green) and anti–TCR-β (red) antibodies. The subiliac lymph node is included in the top region of each photograph as a reference point. A magnification of 200. (B) Milk was collected on days 1 and 9 postpartum from mice treated with anti-CCL28 or isotype control antibody. IgA, IgG1, and IgM levels in the milk were determined. Horizontal bars represent the average of each group. Differences between IgA ASCs and IgA antibody accumulation between control and anti-CCL28 treatment groups were statistically significant (P < 0.001).
CCL28 Blockade in the Mother Inhibits Passive Immune Transfer to the Neonate.
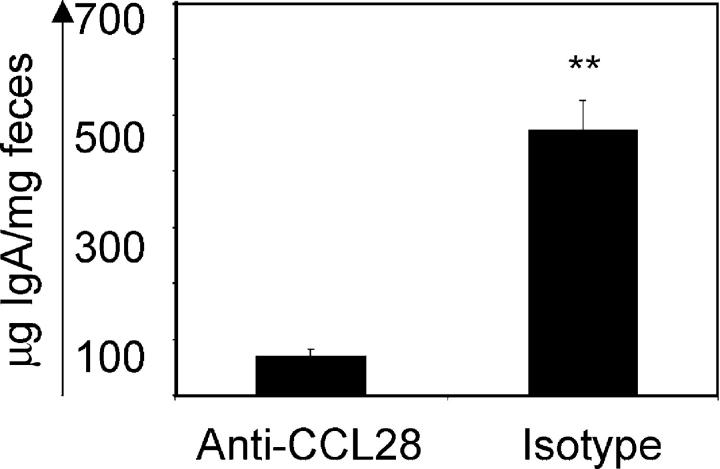
Next, we asked whether CCL28 blockade in the mother significantly reduced maternal IgA levels in the gastrointestinal tract of the neonate. Mothers were treated with control antibody or anti-CCL28, and the amount of IgA in the feces of nursing pups was determined (Fig. 4). Newborn mice nursing on mothers treated with isotype control antibodies had high levels of IgA antibody in their stool (mean: 472.7 ± 53.7 μg antibody/mg feces). Conversely, newborn mice nursing on mothers treated with anti-CCL28 antibody had approximately sevenfold lower levels of IgA in the stool (mean: 70.3 ± 14.1 μg antibody/mg feces; P < 0.001).
Figure 4.
Anti-CCL28 blockade inhibits passive transfer of maternal IgA to the neonate. Feces were collected from 9-d-old neonates nursing on mothers treated with anti-CCL28 or isotype control antibody, and IgA levels were determined. **, statistically significant difference (P < 0.01). Data are expressed as mean ± SEM.
In summary, we show that the mucosal epithelial chemokine CCL28 regulates the accumulation of IgA ASCs in the lactating mammary gland, and is required for transfer of maternal IgA antibodies to the suckling infant. In contrast to its constitutive expression by intestinal, respiratory, and salivary gland epithelium (19), CCL28 is not highly expressed in the resting mammary gland, but instead is dramatically up-regulated postpartum in association with the onset of lactation. The up-regulation of CCL28 correlates with an influx of large numbers of IgA-secreting cells. Mammary gland ASCs, like IgA ASCs in other mucosal sites and in blood, migrate efficiently to CCL28 in vitro and express CCR10. In vivo, anti-CCL28 antibodies block the postpartum accumulation of IgA-secreting cells in the mammary gland, supporting the hypothesized role of CCL28 in the tissue recruitment of IgA plasma cells (14, 22, 26). This blockade results in dramatically reduced levels of IgA antibody in milk and in the gastrointestinal tract of the nursing infant.
It has been proposed that IgA ASCs homing to diverse mucosal surfaces creates a common mucosal immune system (27, 28). Our data indicates that the chemokine CCL28 can play an integral role in such a common mucosal immune system by linking homing mechanisms between the gut, respiratory tract, and the mammary gland. The “redirection” of IgA cells to the mammary gland is controlled through the regulated expression of CCL28 during lactation, a process that enables the passive transfer of maternal IgA antibody from the mother to the gut of the immunologically naive newborn.
Acknowledgments
The authors thank B. Johnston, D.J. Campbell, and E. O'Hara for advice and technical assistance.
E. Wilson is supported by a National Research Service Award (5F32HD042356). This work was also supported by National Institutes of Health (grants AI47822 and GM37734), by the FACS Core facility of the Stanford Digestive Disease Center (under DK56339), and a Merit Award from the Veterans Administration to E.C. Butcher.
The authors have no conflicting financial interests.
References
- 1.Williams, R.C., and R.J. Gibbons. 1972. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 177:697–699. [DOI] [PubMed] [Google Scholar]
- 2.Russell, M.W., M. Kilian, and M.E. Lamm. 1999. Biological activities of IgA. Mucosal Immunology. P.L. Ogra, J. Mestecky, M.E. Lamm, W. Strober, J. Bienenstock, and J.R. McGhee, editors. Academic Press, San Diego. 225–240.
- 3.Holmgren, J., L.A. Hanson, B. Carlson, B.S. Lindblad, and J. Rahimtoola. 1976. Neutralizing antibodies against Escherichia coli and Vibrio cholerae enterotoxins in human milk from a developing country. Scand. J. Immunol. 5:867–871. [DOI] [PubMed] [Google Scholar]
- 4.Stoliar, O.A., R.P. Pelley, E. Kaniecki-Green, M.H. Kkaus, and C.C. Carpenter. 1976. Secretory IgA against enterotoxins in breast-milk. Lancet. 1:1258–1261. [DOI] [PubMed] [Google Scholar]
- 5.Roux, M.E., M. McWilliams, J.M. Phillips-Quagliata, P. Weisz-Carrington, and M.E. Lamm. 1977. Origin of IgA-secreting plasma cells in the mammary gland. J. Exp. Med. 146:1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanneau, G.M., L. Hibrand-Saint Oyant, C.C. Chevaleyre, and H.P. Salmon. 1999. Differential recruitment of T- and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. J. Histochem. Cytochem. 47:1581–1592. [DOI] [PubMed] [Google Scholar]
- 7.Hanson, L.A., and E. Telemo. 1999. Immunobiology and epidemiology of breastfeeding in relation to prevention of infections from a global perspective. Mucosal Immunology. P.L. Ogra, J. Mestecky, M.E. Lamm, W. Strober, J. Bienenstock, and J.R. McGhee, editors. Academic Press, San Diego. 1501–1510.
- 8.Goldblum, R.M., S. Ahlstedt, B. Carlsson, L.A. Hanson, U. Jodal, G. Lidin-Janson, and A. Sohl-Akerlund. 1975. Antibody-forming cells in human colostrum after oral immunisation. Nature. 257:797–798. [DOI] [PubMed] [Google Scholar]
- 9.Lindh, E. 1975. Increased risistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J. Immunol. 114:284–286. [PubMed] [Google Scholar]
- 10.Feachem, R.G., and M.A. Koblinski. 1984. Interventions for the control of diarrhoeal diseases among young children: promotion of breastfeeding. Bull. World Health Organ. 62:271–291. [PMC free article] [PubMed] [Google Scholar]
- 11.Czinn, S.J., and M.E. Lamm. 1986. Selective chemotaxis of subsets of B lymphocytes from gut-associated lymphoid tissue and its implications for the recruitment of mucosal plasma cells. J. Immunol. 136:3607–3611. [PubMed] [Google Scholar]
- 12.Butcher, E.C., and L.J. Picker. 1996. Lymphocyte homing and homeostasis. Science. 272:60–66. [DOI] [PubMed] [Google Scholar]
- 13.Kunkel, E.J., C.H. Kim, N.H. Lazarus, M.A. Vierra, D. Soler, E.P. Bowman, and E.C. Butcher. 2003. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Invest. 111:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarus, N.H., E.J. Kunkel, B. Johnston, E. Wilson, K.R. Youngman, and E.C. Butcher. 2003. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 170:3799–3805. [DOI] [PubMed] [Google Scholar]
- 15.Pabst, O., L. Ohl, M. Wendland, M.A. Wurbel, E. Kremmer, B. Malissen, and R. Forster. 2004. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J. Exp. Med. 199:411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hieshima, K., H. Ohtani, M. Shibano, D. Izawa, T. Nakayama, Y. Kawasaki, F. Shiba, M. Shiota, F. Katou, T. Saito, and O. Yoshie. 2003. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 170:1452–1461. [DOI] [PubMed] [Google Scholar]
- 17.Nagasawa, H. 1979. A device for milk collection from mice. Lab. Anim. Sci. 29:633–635. [PubMed] [Google Scholar]
- 18.Weisz-Carrington, P., M.E. Roux, and M.E. Lamm. 1977. Plasma cells and epithelial immunoglobulins in the mouse mammary gland during pregnancy and lactation. J. Immunol. 119:1306–1307. [PubMed] [Google Scholar]
- 19.Pan, J., E.J. Kunkel, U. Gosslar, N. Lazarus, P. Langdon, K. Broadwell, M.A. Vierra, M.C. Genovese, E.C. Butcher, and D. Soler. 2000. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J. Immunol. 165:2943–2949. [DOI] [PubMed] [Google Scholar]
- 20.Zabel, B.A., W.W. Agace, J.J. Campbell, H.M. Heath, D. Parent, A.I. Roberts, E.C. Ebert, N. Kassam, S. Qin, M. Zovko, et al. 1999. Human G protein–coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowman, E.P., N.A. Kuklin, K.R. Youngman, N.H. Lazarus, E.J. Kunkel, J. Pan, H.B. Greenberg, and E.C. Butcher. 2002. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J. Exp. Med. 195:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunkel, E.J., and E.C. Butcher. 2003. Plasma-cell homing. Nat. Rev. Immunol. 3:822–829. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama, T., K. Hieshima, D. Izawa, Y. Tatsumi, A. Kanamaru, and O. Yoshie. 2003. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J. Immunol. 170:1136–1140. [DOI] [PubMed] [Google Scholar]
- 24.Halsey, J.F., C.S. Mitchell, and S.J. McKenzie. 1983. The origins of secretory IgA in milk: a shift during lactation from a serum origin to local synthesis in the mammary gland. Ann. N. Y. Acad. Sci. 409:452–460. [DOI] [PubMed] [Google Scholar]
- 25.Halsey, J.F., C. Mitchell, R. Meyer, and J.J. Cebra. 1982. Metabolism of immunoglobulin A in lactating mice: origins of immunoglobulin A in milk. Eur. J. Immunol. 12:107–112. [DOI] [PubMed] [Google Scholar]
- 26.Kunkel, E.J., and E.C. Butcher. 2002. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 16:1–4. [DOI] [PubMed] [Google Scholar]
- 27.McDermott, M.R., and J. Bienenstock. 1979. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J. Immunol. 122:1892–1898. [PubMed] [Google Scholar]
- 28.Czerkinsky, C., S.J. Prince, S.M. Michalek, S. Jackson, M.W. Russell, Z. Moldoveanu, J.R. McGhee, and J. Mestecky. 1987. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc. Natl. Acad. Sci. USA. 84:2449–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]