Abstract
Two groups have shown that, as in macaques infected with simian immunodeficiency virus (SIV), intestinal CD4+ T cells are selectively and rapidly depleted in the intestine of HIV-infected patients. Depletion of intestinal CD4+ T cells occurred at all stages of infection regardless of highly active antiretroviral therapy (HAART). Here we discuss the important implications of these papers for our understanding of HIV pathogenesis, treatment, and vaccine design.
It has been over 6 yr since we first described the rapid and marked depletion of intestinal CD4+ T cells in SIV-infected macaques (1). We demonstrated that intestinal CD4+ T cells were profoundly depleted in the lamina propria of the intestine of macaques within 14–21 d of SIV infection, a time when the numbers of CD4+ T cells in the blood or LNs (peripheral lymphoid tissues) were essentially unchanged. Earlier studies had indicated that CD4+ T cells were decreased in the intestine of HIV-infected patients in chronic infection (2), but the major significance of the macaque studies was the selectivity and speed of the depletion. The fact that these cells were being eliminated within days rather than months of infection challenged the prevailing views that SIV and HIV were “slow” or “smoldering” infections that did not induce immunodeficiency for several months to years after infection. In fact, the differences were so profound in the intestine within 14 d of SIV infection that it suggested the viral replication kinetics were much closer to those of other, more rapid viral infections rather than a “lentiviral” infection (HIV and SIV are classified as “lentiviruses,” which translates literally as “slow” viruses).
Subsequently, we showed that this depletion was selective for CD4+ T cells having an activated memory phenotype (3), a cell type which is abundant in the intestine but rare in peripheral lymphoid tissues. Thus, we proposed that only cells which were highly activated and thus transcribing DNA could support high levels of replication sufficient for “lytic” levels of viral replication. Conceivably, this alone could explain the selective destruction of intestinal CD4+ T cells. Intestinal lymphocytes and those in other mucosal surfaces (such as lung, vagina) are frequently exposed to environmental antigens; thus, a much higher percentage of mucosal lymphocytes are continually in a state of “activation.” However, the discovery of HIV coreceptors, and soon afterwards the discovery that activated memory cells selectively express CCR5, the major coreceptor for HIV and SIV, provided an additional (yet complementary) explanation for this selective CD4+ T cell depletion. Subsequently, we demonstrated that the intestinal CD4+ T cell depletion was selective for CD4+ T cells co-expressing CCR5, and this combined with the activated state of these cells provided a likely explanation for the selective loss of intestinal CD4+ T cells in early infection. We also demonstrated that this rapid depletion occurs in all mucosal sites examined, including the vagina and lung (4, 5). These findings have now been verified in the intestine of HIV-infected humans at virtually all stages of infection (6, 7). Although this (once again) validates the SIV/macaque model of AIDS, the major significance of these findings is that it supports a simple hypothesis to explain much of the pathogenesis of HIV infection—that most HIV replication occurs in the intestine and that disease progression may correlate with turnover of specific cell subsets in mucosal tissues.
Changing Views of HIV Pathogenesis.
The findings presented in this issue, that the intestine is the earliest site of CD4+ T cell depletion in HIV-infected patients and confirmation that this depletion occurs within the first few weeks of exposure (the earliest that an infected patient was examined; reference 6), perfectly parallel the macaque studies. Two papers in this issue (6, 7) demonstrate that effector memory (CCR5+) CD4+ T cells are rapidly eliminated in HIV-infected patients, regardless of the stage of infection. If we extrapolate other findings from macaques, this means that the major site of early viral replication in HIV-infected patients is the intestine and other mucosal sites, mainly because the vast majority of susceptible cells reside in mucosal tissues.
The intestinal tract contains an abundance of both inductive (Peyer's patches, lymphoid follicles) and effector (diffuse lamina propria) lymphoid tissues. Inductive sites contain naïve, resting CD4+ (and CD8+) T cells which rapidly migrate to effector lymphoid tissues upon exposure to antigen (Fig. 1). Thus, most of the effector (CCR5+) CD4+ T cells reside in these effector lymphoid tissues, which are distributed throughout the length of the gastrointestinal tract. Thus, in uninfected patients a vast pool of these optimal viral target cells, i.e., those having a high state of activation and co-expressing CD4 and CCR5, are present in the intestine (8). Immediately after HIV exposure, very high levels of viral replication occur in the intestinal lamina propria, at least until these optimal target cells are destroyed (which in macaques usually occurs by 14 d of infection). Prior to this depletion, large numbers of SIV-infected cells are detectable in the lamina propria, but once these cells are eliminated most of the infected cells are limited to inductive lymphoid tissues (9), which is consistent with observations made in humans by Mehandru et al. (6). Subsequent infection of new cells and high levels of viral replication is likely dependent on the rates of turnover of these cells in mucosal tissues. Examining intestinal tissues of humans within 10 d of HIV infection is obviously difficult, so primary infection studies continue to rely on SIV-infected macaques. However, the data presented in this issue (6, 7) strongly support a major role for mucosal tissues in the pathogenesis of AIDS and suggest that HIV replication and tissue distribution is largely dependent on the availability and turnover of specific cells that are capable of being infected (CD4+CCR5+) and producing virus (those that are activated and transcribing DNA). Furthermore, the data suggest that much of our understanding of the pathogenesis of AIDS, which in turn influences vaccine design and therapeutic strategies, has been biased by reliance on examination of peripheral blood. We would argue that studies examining peripheral blood alone provide a very limited and in many cases misleading view of AIDS pathogenesis.
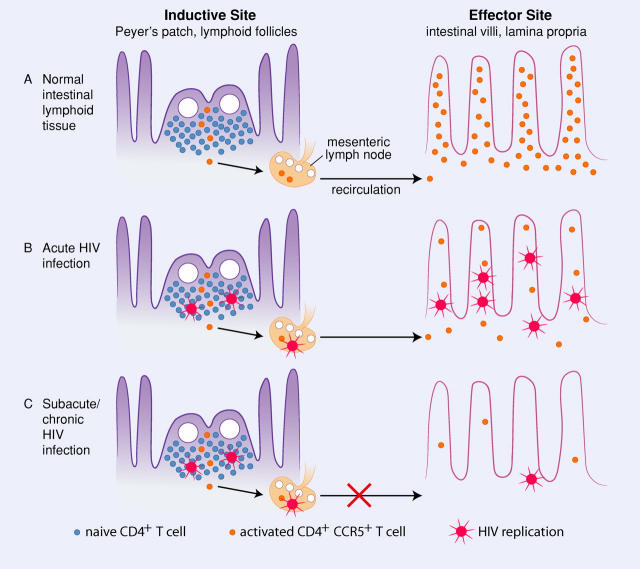
Figure 1.
Target cell distribution and viral replication in the intestinal tract. (A) In normal intestinal lymphoid tissue, naïve (CCR5−) CD4+ T cells mostly reside in inductive sites (Peyer's patches or organized lymphoid tissues; left), but these cells become activated and coexpress CCR5 as they are exposed to the variety of antigens routinely encountered in the intestinal tract. After activation, CD4+CCR5+ T cells rapidly recirculate and home to effector sites (lamina propria) where they reside in large numbers (right). (B) During acute HIV infection, activated CCR5+CD4+ T cells are rapidly eliminated from the lamina propria by HIV replication. This correlates with high levels of plasma viremia in primary infection, and loss of this subset may be a major contributor to the post-peak decline in viremia. (C) In subacute/chronic HIV infection, substantial loss of CCR5+CD4+ T cells in the lamina propria results in lower levels of virus in effector sites, but continual lymphocyte activation (from dietary antigens or intestinal microflora) in inductive sites maintains viral replication within intestinal organized lymphoid tissues. In chronic infection, the majority of infected cells may be destroyed before reaching effector sites, resulting in higher numbers of infected cells observed in organized lymphoid tissues. Intestinal lymphoid tissues with its inherent mechanisms for lymphocyte activation and homing, thus serves as a reservoir for both viral persistence and ongoing replication. As a result, intestinal effector CD4+CCR5+ T cells are never restored in HIV-infected patients, even in patients receiving highly active antiretroviral therapy.
An understanding of the pathogenesis of HIV infection has remained elusive. The fact that the majority of patients are asymptomatic for months to years after infection, combined with our inability to detect low levels of virus in plasma, led some to propose concepts of viral “latency,” a period in which the virus purportedly lay dormant, waiting for some mysterious signal to begin replication and elimination of CD4+ T cells. These theories were largely debunked by seminal studies by Ho et al. (10), who used antiretroviral therapy to measure viral replication in HIV-infected patients. This study demonstrated that active, high level replication was ongoing in asymptomatic patients. However, it did not indicate where this replication was occurring. Moreover, since patients with CD4+ T cell depletion lacked sufficient numbers of HIV-infected cells in the blood to account for the CD4+ T cell depletion, others proposed “bystander apoptosis” and/or “bone marrow suppression” to explain the loss of CD4+ T cells. Apoptosis of uninfected CD4+ T cells mediated by chronic immune stimulation was proposed to explain the CD4+ T cell loss, but the findings in macaques, confirmed in HIV-infected patients in the papers in this issue (6, 7), both prove that there is nothing “chronic” about the intestinal CD4+ T cell loss. In fact, the rapid nature of the CD4+ T cell loss combined with the selective loss of activated CCR5+CD4+ T cells strongly supports the notion that depletion is selective for HIV-infected cells, presumably due to lytic levels of viral replication in cells that support it. It is also likely that profound and sustained deficits in mucosal immune responses are induced within days of HIV infection. This is supported by the observation of asymptomatic opportunistic infections in SIV-infected macaques beginning as early as 8 wk after infection (11).
Confirmation by Mehandru et al. (6) and Brenchley et al. (7) that the intestine is the major site of viral replication and CD4+ T cell depletion, essentially proves that examining events in the peripheral blood alone is inadequate for detecting key events in the pathogenesis of HIV infection. Moreover, these findings may provide a simplified explanation for HIV replication and pathogenesis. HIV replication may largely be governed by the presence and turnover of viral “fuel cells,” i.e., those cells that have the appropriate coreceptors for viral entry and the state of activation necessary to produce lytic levels of virus. If these cells are turning over in individual patients (either by enteritis, dietary allergies, or even a heightened state of physiologic inflammation), viral replication is likely to increase (12). If these cells are eliminated and the host does not make efforts to replenish them, viral replication slows. Unfortunately, stimulation of effector CD4+ T cells, which are necessary for immune surveillance and “help” for essentially all other arms of the immune system, is likely to exacerbate viral replication in infected patients. This poses unique challenges to both vaccine design and HIV treatment strategies.
Implications for Treatment.
Recently, Anton et al. (13) demonstrated that HIV could consistently be detected in the intestine of HIV patients, even those receiving HAART and with no detectable virus in plasma (13). Others have demonstrated that higher frequencies of virus-specific CTL are present in the intestine of HIV-infected patients compared with blood (14). Both papers in this issue (6, 7) demonstrate that mucosal effector CD4+ T cells are not restored, even in patients receiving HAART. Combined, these findings suggest that viral replication is ongoing in the intestinal tract, regardless of HAART therapy. The current trend in HIV therapy is to delay initiation of HAART until patients are either symptomatic, have high viremia, or demonstrate substantial CD4+ T cell loss in the blood. In our experience, intestinal CD4+ T cells may be at least partially restored in SIV-infected macaques receiving HAART, provided that treatment is initiated no later that 1 wk after infection (unpublished data). Although it is unlikely that such treatment regimens can be implemented in humans, these data suggests that intestinal CD4+ T cells are turning over and have the capacity to repopulate the intestine as long as this CD4+ T cell loss is stopped before the restoration capacity of the thymus is depleted. There is clearly an ongoing battle between HIV and the intestinal immune system, regardless of viral loads in plasma, and it seems logical that treatment should be initiated as soon as possible to limit the continual destruction of intestinal CD4+ T cells. Allowing continuous replication and CD4+ T cell loss in the intestine undoubtedly results in the eventual loss of important and irreplaceable CD4+ T cell clones and the continual erosion of the immune system, despite being “all quiet on the surface” (i.e., the peripheral blood).
Monitoring intestinal CD4+ T cells or viral loads in intestinal biopsies may also be useful for measuring responses to therapy. Although current therapies seem inadequate for clearing virus from the intestine, new therapeutic strategies may benefit from monitoring intestinal CD4+ T cells and/or viral loads in intestinal biopsies. Moreover, new therapies aimed at blocking chemokine receptor–mediated fusion may prove to be an effective adjunct therapy, particularly in the mucosal immune system where the vast majority of CD4+CCR5+ T cells reside.
Implications for Vaccines.
The fact that the intestine is the earliest target for viral infection and CD4+ T cell loss makes it clear that mucosal immunity will be essential for designing an effective AIDS vaccine. It is increasingly apparent that essentially all candidate vaccines that have shown any promise have, in one way or another, induced mucosal immune responses. Conversely, some candidate vaccines that induced relatively strong systemic immune responses have failed to provide adequate protection in macaque models (for review see reference 15). It should also be remembered that vaccines for other important diseases with systemic manifestations were elusive until the importance of the mucosal immune system was finally recognized. An effective vaccine for poliomyelitis, for example, was elusive until a mucosal delivery system was implemented, and this was considered previously to be a disease of the central nervous system.
Given the confirmation herein, that HIV infection is primarily a disease of the mucosal immune system (6, 7), it should now be clear that candidate vaccines should be tested for their ability to induce cell-mediated and antibody responses specifically in mucosal tissues, or at least for their ability to protect and preserve mucosal effector CD4+ T cells. Although this is a daunting challenge in terms of the design of clinical trials, it should also be appreciated that, since the loss of intestinal CD4+ T cells occurs so rapidly, evaluation or interpretation of at least this parameter alone could be obtained in weeks, rather than months or years. Thus, monitoring mucosal immune responses and/or preservation of intestinal CD4+ T cells could conceivably accelerate evaluation of vaccine candidates, particularly in nonhuman primate models. Regardless, the articles herein (6, 7) should now make it clear the intestine is the “launching pad” for HIV infection, replication, and persistence, and that stimulating mucosal immune responses will be key in the development of an effective AIDS vaccine.
References
- 1.Veazey, R.S., M. DeMaria, L.V. Chalifoux, D.E. Shvetz, D.R. Pauley, H.L. Knight, M. Rosenzweig, R.P. Johnson, R.C. Desrosiers, and A.A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 280:427–431. [DOI] [PubMed] [Google Scholar]
- 2.Schneider, T., H.-U. Jahn, W. Schmidt, E.-O. Reicken, M. Zeitz, and R. Ulrich. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut. 37:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey, R.S., I.C. Tham, K.G. Mansfield, M. DeMaria, A.E. Forand, D.E. Shvetz, L.V. Chalifoux, P.K. Sehgal, and A.A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vajdy, M., R. Veazey, I. Tham, C. deBakker, S. Westmoreland, M. Neutra, and A. Lackner. 2001. Early immunologic events in mucosal and systemic lymphoid tissues after intrarectal inoculation with simian immunodeficiency virus. J. Infect. Dis. 184:1007–1014. [DOI] [PubMed] [Google Scholar]
- 5.Veazey, R.S., P.A. Marx, and A.A. Lackner. 2003. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 187:769–776. [DOI] [PubMed] [Google Scholar]
- 6.Mehandru, S., M.A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley, J.M., T.W. Schacker, L.E. Ruff, D.A. Price, J.H. Taylor, G.J. Beilman, P.L. Nguyen, A. Khoruts, M. Larson, A.T. Haase, and D.C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anton, P.A., J. Elliott, M.A. Poles, I.M. McGowan, J. Matud, L.E. Hultin, K. Grovit-Ferbas, C.R. Mackay, I.S.Y. Chen, and J.V. Giorgi. 2000. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 14:1761–1765. [DOI] [PubMed] [Google Scholar]
- 9.Veazey, R.S., J.D. Lifson, I. Pandrea, J. Purcell, M. Piatak Jr., and A.A. Lackner. 2003. Simian immunodeficiency virus infection in neonatal macaques. J. Virol. 77:8783–8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, D.D., A.U. Neumann, A.S. Perelson, W. Chen, J.M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 373:123–126. [DOI] [PubMed] [Google Scholar]
- 11.Lackner, A.A., P. Vogel, R.A. Ramos, J.D. Kluge, and M. Marthas. 1994. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am. J. Pathol. 145:428–439. [PMC free article] [PubMed] [Google Scholar]
- 12.Veazey, R.S., P.A. Marx, and A.A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626–633. [DOI] [PubMed] [Google Scholar]
- 13.Anton, P.A., R.T. Mitsuyasu, S.G. Deeks, D.T. Scadden, B. Wagner, C. Huang, C. Macken, D.D. Richman, C. Christopherson, F. Borellini, et al. 2003. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. AIDS. 17:53–63. [DOI] [PubMed] [Google Scholar]
- 14.Shacklett, B.L., C.A. Cox, J.K. Sandberg, N.H. Stollman, M.A. Jacobson, and D.F. Nixon. 2003. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veazey, R., and A. Lackner. 2003. The mucosal immune system and HIV-1 infection. AIDS Rev. 5:245–252. [PubMed] [Google Scholar]