Abstract
Fibrosis and apoptosis are juxtaposed in pulmonary disorders such as asthma and the interstitial diseases, and transforming growth factor (TGF)-β1 has been implicated in the pathogenesis of these responses. However, the in vivo effector functions of TGF-β1 in the lung and its roles in the pathogenesis of these responses are not completely understood. In addition, the relationships between apoptosis and other TGF-β1–induced responses have not been defined. To address these issues, we targeted bioactive TGF-β1 to the murine lung using a novel externally regulatable, triple transgenic system. TGF-β1 produced a transient wave of epithelial apoptosis that was followed by mononuclear-rich inflammation, tissue fibrosis, myofibroblast and myocyte hyperplasia, and septal rupture with honeycombing. Studies of these mice highlighted the reversibility of this fibrotic response. They also demonstrated that a null mutation of early growth response gene (Egr)-1 or caspase inhibition blocked TGF-β1–induced apoptosis. Interestingly, both interventions markedly ameliorated TGF-β1–induced fibrosis and alveolar remodeling. These studies illustrate the complex effects of TGF-β1 in vivo and define the critical role of Egr-1 in the TGF-β1 phenotype. They also demonstrate that Egr-1–mediated apoptosis is a prerequisite for TGF-β1–induced fibrosis and remodeling.
Keywords: asthma, pulmonary fibrosis, fibrosis reversibility, airway remodeling
Introduction
Fibrosis is an important cause of morbidity and mortality in a variety of lung diseases. This is nicely illustrated in asthma, which is characterized by chronic inflammation and subepithelial/airway fibrosis (1). It is also readily apparent in the interstitial lung diseases (ILDs) including idiopathic pulmonary fibrosis (IPF), scleroderma, and bleomycin lung where pulmonary fibrosis is a dreaded and, in many cases, fatal disease endpoint (2). In these disorders fibrosis often coexists with structural cell apoptosis (3–7). Surprisingly, the mechanisms of the fibrotic and apoptotic responses in these lung disorders are poorly understood. In addition, the relationship(s) between fibrosis and apoptosis has only been superficially investigated. As a result, therapies that control tissue fibrosis are not readily available and the usefulness of apoptosis-based therapies as antifibrotic interventions has not been adequately evaluated.
The “type II cytokine hypothesis of fibrosis” suggests that fibrosis occurs in chronic inflammatory disorders when cytokine balance shifts in a Th2 cell (type II) direction (8). In accord with this hypothesis, IL-13 is believed to be the key Th2 cell and fibrogenic effector in asthma (9, 10). IL-13 is also dysregulated in, and plays a critical role in, the pathogenesis of many ILDs including IPF, scleroderma, radiation-induced pulmonary fibrosis, and bleomycin lung (11–15). Studies from our laboratory and others demonstrated that IL-13 mediates its fibrotic effects via its ability to induce and activate TGF-β1 (16), and that TGF-β1 is expressed in an exaggerated fashion and contributes to the pathogenesis of asthma, the ILDs, and other fibrotic disorders (16–23). Surprisingly, the degree to which TGF-β1 can account for the varied pathologies in these disorders and the mechanism and reversibility of TGF-β1–induced fibrosis are poorly understood. In addition, our knowledge of TGF-β effector pathways is limited and does not explain how TGF-β1 stimulates fibrosis while simultaneously inducing structural cell apoptosis and tissue injury, and inhibiting epithelialization and wound healing (23–27). Lastly, because TGF-β1–induced fibrosis and apoptosis are considered distinct tissue responses, the role of apoptosis in TGF-β1–induced fibrosis has not been defined.
To characterize the in vivo effector functions of TGF-β1 and the mechanisms that mediate these responses, we targeted biologically active TGF-β1 to the human lung using the Clara cell 10-kD (CC10) protein promoter. To bypass the fetal lethality that is seen when TGF-β1 is expressed during lung development (28), we used a novel triple transgenic system recently described by our laboratory (29). In these transgenic animals, TGF-β1 produced a striking pulmonary phenotype characterized by a transient wave of epithelial apoptosis that is followed by mononuclear cell-rich tissue inflammation, subepithelial and parenchymal fibrosis, myofibroblast and myocyte hyperplasia, and eventually, alveolar septal rupture and tissue honeycombing. Studies of these mice highlighted the reversibility of TGF-β1–induced fibrosis. They also demonstrated that an intimate relationship exists between apoptosis and fibrosis because apoptosis was required for fibrosis to be generated. Lastly, they highlight the importance of the early growth response gene (Egr)-1 transcription factor in mediating the apoptotic and fibrotic responses induced by TGF-β1.
Materials and Methods
Generation of the CC10-rtTA-tTS-TGF-β1 Mice.
In virtually all inducible overexpression transgenic systems, there is a low level of transgenic protein production in the absence of the inducing stimulus. This leak might be a problem in studies of the effects of fetal lethal transgenes like TGF-β1 and studies of the reversibility of transgene-induced tissue alterations. To address these limitations, we developed a novel, triple transgenic system that allows fetal lethal transgenes like TGF-β1 to be expressed (see Fig. 1). The first construct, CC10-rtTA-hGH, contains the CC10 promoter, the reverse tetracycline transactivator (rtTA; provided by M. Gossen and H. Bujard, Universität Heidelberg, Heidelberg, Germany) and hGH intronic, nuclear localization, and polyadenylation sequences. The second construct, tet-O-CMV-hTGF-β1-SV40, contains a polymeric tetracycline operator (tet-O), minimal CMV promoter, a modified TGF-β1 cDNA (provided by R. Derynck, University of California, San Francisco, San Francisco, CA), and SV40 intronic, polyadenylation, and nuclear localization signals. In the TGF-β1 cDNA construct, the cysteine codons at positions 223 and 225 were replaced with serine codons. This mutation prevents latency-associated protein binding and gives rise to biologically active TGF-β1. The third construct, CC10-tTS-hGH, has the CC10 promoter driving the expression of the tetracycline-controlled transcriptional silencer (tTS). In this system, the CC10 promoter constitutively drives the expression of rtTA and tTS in a lung-specific fashion. In the absence of dox, tTS binds to and actively suppresses the expression of the tet-O–regulated TGF-β1 transgene. In the presence of dox, tTS is released allowing the activating, dox binding rtTA to bind to the tet-O and activate transgene expression. To generate these mice, the three constructs were generated, purified, and pooled. Simultaneously pronuclear microinjection and characterization of the progeny were undertaken as described previously by our laboratory (30). All founder and progeny animals were on a C57BL/6 background.
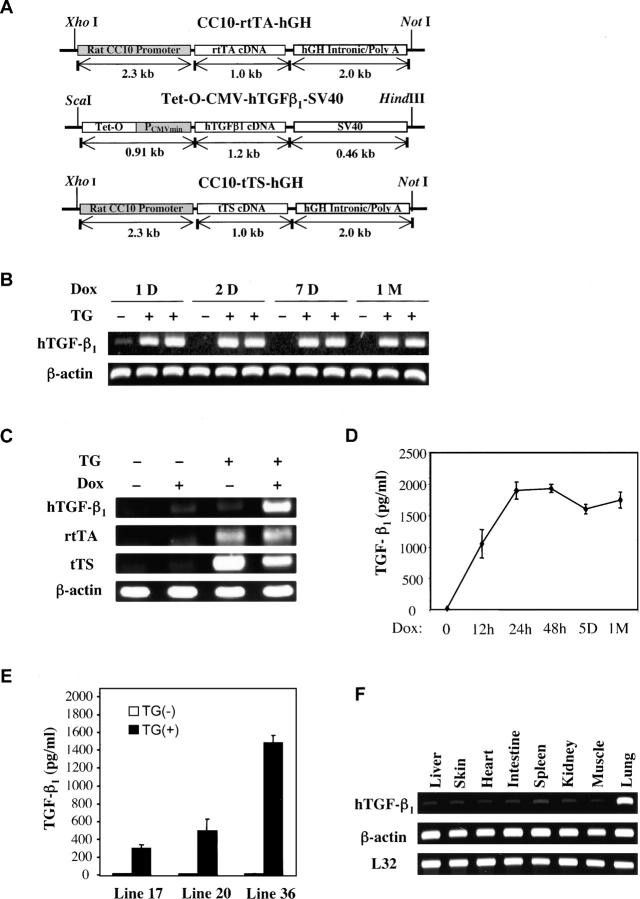
Figure 1.
Constructs involved in CC10-rtTA-tTS-TGF-β1 mice and regulation of transgenic TGF-β1. (A) The constructs that were used to make the transgenic mice are illustrated. (B–E) Transgene− and transgene+ mice were randomized to normal or dox water for the noted intervals (in B and D: h, hours; D, days; M, month) or for 7 d (C and E). Whole lung TGF-β1 mRNA (B and C) and BAL TGF-β1 protein (D and E) were assessed by RT-PCR and ELISA, respectively. The lung specificity of transgene targeting was assessed in F where RT-PCR was used to quantitate the levels of TGF-β1 mRNA in the lungs and other organs from transgene+ mice on dox for 1 wk. The values in D and E are the means ± SEM of evaluations in a minimum of five animals. The experiments in B, C, and F are representative of four similar experiments.
Breeding to Egr-1 Null Mutant (Egr-1−/−) Mice.
CC10-rtTA-tTS-TGF-β1 animals were bred with wild-type and Egr-1 null mutant (Egr-1−/−) animals as described previously by our laboratories (31). As a result of these crosses, CC10-rtTA-tTS-TGF-β1 animals with Egr-1+/+ and Egr-1−/− loci were generated. The phenotypes of these mice were compared as described below.
Dox Water Administration.
6–8-wk-old transgene+ mice and transgene− littermate controls were randomized to normal water or water containing 0.5 mg/ml of dox as described previously (29, 30). Transgene expression and phenotypic alterations were evaluated at intervals thereafter.
Bronchoalveolar Lavage (BAL) and Quantification of TGF-β1 Levels.
Mice were killed and BAL was undertaken as described previously (29, 30). The levels of TGF-β1 were determined by ELISA (R&D Systems) according to the manufacturer's instructions.
TdT-mediated dUTP Nick-End Labeling (TUNEL) Evaluations.
End labeling of exposed 3′ OH ends of DNA fragments was undertaken with the TUNEL in situ cell death detection kit AP (Roche Diagnostics) as described by the manufacturer. After staining, 20 fields of alveoli were randomly chosen and 2,000 nuclei were counted. The labeled cells were expressed as a percentage of total nuclei.
Type II Alveolar Epithelial Cell Isolation and Apoptosis Evaluation.
Type II cells were isolated from wild-type and transgenic mice using the methods developed by Rice et al. (32). After anesthesia, the trachea was cannulated with 20-gauge tubing, the lungs were filled with 2 ml dispase (Roche Diagnostics), followed by 0.5 ml of 1% low melting point agarose. The agarose was allowed to harden under crushed ice. The lungs were then placed in 2 ml dispase for 1 h at room temperature, and transferred to DMEM with 25 mM Hepes with 0.01% DNase I (Sigma-Aldrich). After teasing apart the digested tissue, the resulting cell suspension was sequentially filtered through nylon mesh filters and collected after centrifugation for 8 min at 130 g. Contaminating cells were removed by incubating the cell suspension in 100-mm tissue culture plates coated with a mixture of anti-CD16/CD32 and anti-CD45 monoclonal antibodies (BD Biosciences) overnight at 4°C, and washing the nonadherent cell population. In accord with the literature, the resulting cells were >97% type II cells and >97% viable as demonstrated by histochemical and immunohistochemical evaluations and trypan blue dye exclusion, respectively (32). These cells were resuspended in 1× binding buffer at 106 cells/ml for subsequent FACS® analysis. Annexin V and propidium iodine staining were undertaken with the annexin V FITC apoptosis detection kit (BD Biosciences). Analysis was undertaken by flow cytometry (Becton Dickinson).
Histologic Analysis.
The lungs were removed en bloc, inflated at 25 cm pressure with neutral buffered 10% formalin, fixed in 10% formalin, embedded in paraffin, sectioned, and stained. Hematoxylin and eosin and Mallory's trichrome stains were performed in the Research Histology Laboratory of the Department of Pathology at Yale University School of Medicine.
Collagen Assay.
Collagen content was determined biochemically using standard hydroxyproline assays or by quantifying total soluble collagen using the Sircol Collagen Assay kit (Biocolor) according to the manufacturer's instructions. The data is expressed as the collagen content of the entire right lung.
mRNA Analysis.
mRNA levels were assessed using RT-PCR assays and ribonuclease protection assays as described by our laboratories (29, 30). In these assays, total cellular RNA from lungs or other mouse tissues were obtained using trizol reagent (GIBCO BRL) according to the manufacturer's instructions.
Immunoblot Analysis.
Transgene− and transgene+ mice were randomized to normal water or dox at 6 wk of age and maintained on this regimen for 2 d. Lung lysates were then prepared and Western analysis was undertaken with antibodies that reacted selectively with caspase 3, caspase 7, caspase 8, poly(ADP)ribose polymerase (PARP), β-tubulin (Santa Cruz Biotechnology, Inc.), BID (Cell Signaling Technology), cleavage site–specific BID (Biosource International), αII-spectrin (Chemicon), or inhibitor of caspase-activated DNase (ICAD; Chemicon) as described previously (30).
Immunohistochemistry (IHC).
IHC was undertaken to localize α-smooth muscle actin (DakoCytomation), pro–SP-C (Chemicon), and α-smooth muscle myosin heavy chain (Biomedical Technology). These assays were undertaken as described previously by our laboratories (33).
Morphometric Analysis.
Alveolar remodeling was estimated from the mean cord length of the airspace. This measurement is similar to the mean linear intercept, a standard measure of air space size, but has the advantage that it is independent of alveolar septal thickness. These evaluations were performed as described previously by our laboratory (30). In selected experiments, inflammation was also evaluated. In these evaluations peribronchial cells were quantitated within a 4,000 μm2 area of the bronchovascular bundle as described previously (34).
Pharmacologic Interventions.
6–8-wk-old transgene− and transgene+ mice were randomized to the desired agent or the appropriate vehicle control. 2 d later, they were randomized to normal or dox water and maintained on this regimen for 2 wk. At the end of this interval, the animals were killed and pulmonary phenotype was assessed as described below. In experiments in which caspase-mediated apoptosis was being evaluated, Z-VAD-fmk was administered at a dose of 3 mg/kg/day via an i.p. route (Calbiochem). In experiments in which the selective inhibition of caspases 3 and 7 was being evaluated, caspase 3/7 inhibitor 1 (5-[(s)-(-)-2-(methoxymethyl)pyrrolidine]sulfonylisatin; 4 mg/kg/day, i.p.; Calbiochem) was used. In all cases, comparisons to appropriate vehicle controls were undertaken.
Statistical Analysis.
Values are expressed as means ± SEM. As appropriate, groups were compared by ANOVA with Scheffe's procedure posthoc analysis, with the Student's two-tailed unpaired t test or with nonparametric assessments (Wilcoxon's Rank Sum, Mann-Whitney U Test) using the software for the Macintosh (Stat View; Abacus Concepts, Inc.).
Results
Generation of TGF-β1 Transgenic Mice.
The constructs that were required to generate these mice were prepared and microinjected. Three triple transgene+ founder mice were identified and lines were established from each by breeding with wild-type C57BL/6 animals. In the transgene+ progeny from these matings, all three transgenic constructs were uniformly present and appropriate transgene regulation was appreciated. In the absence of dox, these mice did not express significant transgenic TGF-β1 in lung RNA RT-PCR (35 cycles) assays or ELISA evaluations of BAL fluids (Fig. 1, B–D). They did, however, manifest significant inducibility with transgenic TGF-β1 being noted after 12 h and steady-state levels of BAL TGF-β1 between 250 and 1,6000 pg/ml being appreciated after 1 wk of dox administration (Fig. 1, B–E, and not depicted). These levels were maintained for the duration (up to 3 mo) of dox administration and returned to undetectable levels within 48 h of the removal of dox from the animals' drinking water (not depicted). In all cases TGF-β1 was successfully targeted to the lung because TGF-β1 mRNA could not be appreciated in a variety of other tissues from these animals (Fig. 1 F).
Phenotypic Analysis of Transgenic Mice.
To define the effects of TGF-β1, 6-wk-old triple transgene positive (hereafter referred to as transgene+) animals and their transgene− littermate controls were randomized to normal water or dox water as described previously by our laboratory (30). At intervals thereafter, animals were killed and their lungs were analyzed. Qualitatively similar, dose-dependent phenotypes were appreciated in these mice with early apoptosis followed by inflammation, fibrosis, α-smooth muscle actin+ cell accumulation, and alveolar remodeling. Each feature is described below.
TGF-β1–induced Apoptosis.
After as little as 12 h of TGF-β1 induction, a TUNEL+ cell death response could be readily appreciated in lungs from transgene+ but not transgene− animals (Fig. 2 A). The TUNEL+ cells were largely epithelial cells as evidenced by their histologic location and double labeling experiments (Fig. 2 A and not depicted). Small numbers of TUNEL+ macrophages could also be appreciated. Propidium iodine and annexin V staining confirmed these findings. They also defined the nature of this death response by demonstrating that TGF-β1 increased the levels of apoptosis and combined apoptosis and necrosis in whole lung cells and isolated type II alveolar epithelial cells (Fig. 2 B). This TUNEL+ response peaked after 48 h of dox administration and disappeared despite continuous dox administration. It was no longer detectable after 4–5 d of dox administration and did not reappear during the 2-mo study interval (Fig. 2 C). During the apoptotic phase, enhanced levels of mRNA encoding caspases 3, 7, 8, and 11, and impressive levels of caspase 7 and caspase 8 and lesser levels of caspase 3 activation, were appreciated (Fig. 2, D and E, and not depicted). Western analysis also revealed impressive cleavage of caspase targets including PARP, ICAD, and αII spectrin, which were cleaved to 85-, 20-, and 110–115-kD fragments, respectively (Fig. 2 F). Control antisera did not reveal similar patterns (not depicted). Thus, TGF-β1 induces an early and transient wave of epithelial apoptosis that is repressed and/or eliminated with continuing TGF-β1 elaboration.
Figure 2.
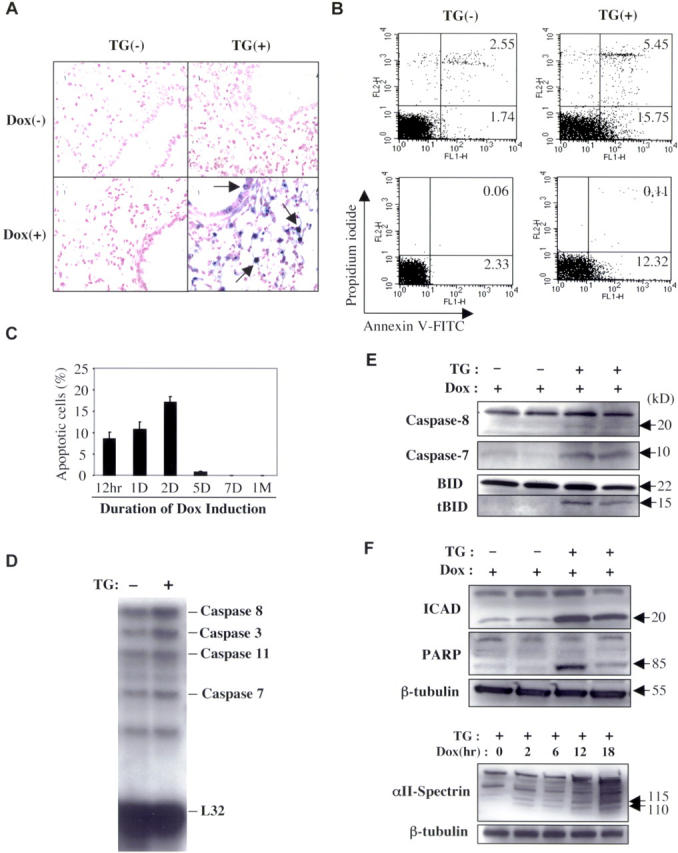
TGF-β1–induced apoptosis. Transgene− and transgene+ mice were randomized to normal water (Dox (−)) or dox water (Dox (+)). Lungs were obtained 48 h (A and B) or at intervals (C) thereafter and TUNEL (A and C) or propidium iodide/annexin V staining and FACS® (B) evaluations were undertaken. A is shown at a magnification of 40. (B) The top histograms illustrate the results with whole lung cells and the bottom histograms show the results with isolated alveolar type II cells. Using whole lung RNA or lung lysates from animals 48 h after randomization, ribonuclease protection was used to assess the expression of caspases (D), and Western evaluations were undertaken to evaluate caspase activation and the levels of total and cleaved BID (E, tBID). Western evaluations of the cleavage of PARP and ICAD and the kinetics of cleavage of αII-spectrin are shown in F. (C) The values represent the mean ± SEM of evaluations in five animals. (A and E–F) A representative of a minimum of five experiments is shown.
TGF-β1–induced Inflammation.
Dox administration caused a prominent inflammatory response in lungs from transgene+ animals but not their littermate controls. This effect could be seen after as little as 2 d of transgene activation and increased in intensity over the ensuing 10 d. In BAL, it manifests as an increase in total cell and macrophage recovery (Fig. 3 A and not depicted). In lung tissues, it was largely due to an increase in macrophages (Fig. 3 B, open arrows). However, a modest increase in eosinophils could also be appreciated (Fig. 3 B, closed arrows).
Figure 3.
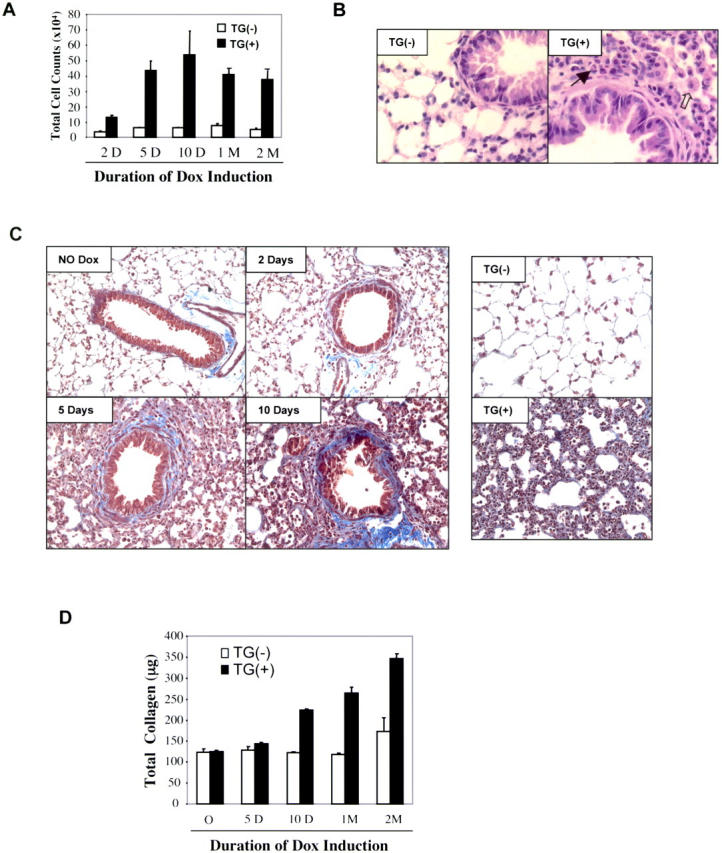
TGF-β1–induced inflammation and fibrosis. Transgene− and transgene+ mice were randomized to normal or dox water. BAL was undertaken and tissues were obtained at intervals thereafter. (A) Total BAL cell recovery is illustrated. (B) We compare the histology (hematoxylin and eosin staining, a magnification of 40) of transgene− and transgene+ mice on dox water for 1 mo. Accumulating macrophages (open arrow) and eosinophils (closed arrow) are appreciated. (C) Trichrome staining (a magnification of 20) was used to compare the collagen in lungs from transgene− and transgene+ mice on normal water and transgene+ mice on dox for up to 10 d (left) and 1 mo (right). (D) Hydroxyproline assays were used to assess collagen content. Transgene+ mice on normal water could not be differentiated from transgene− mice on normal or dox water in these assays. (A and D) The values represent the mean ± SEM of evaluations in a minimum of five animals. (B and C) A representative of a minimum of five experiments is shown.
TGF-β1–induced Fibrosis.
The induction of TGF-β1 in transgene+ mice caused a readily apparent airway and parenchymal fibrotic response when compared with transgene− mice on normal or dox water and transgene+ mice on normal water. On trichrome stains, this response could be appreciated after as little as 2 d of dox administration, predominately in the subepithelial and adventitial regions of the airway (Fig. 3 C). With longer periods of dox administration, this fibrotic response could be appreciated in the alveolar parenchyma (Fig. 3 C). In accord with these findings, lung collagen content continued to increase over the 2-mo period of dox administration (Fig. 3 D).
TGF-β1–induced Alveolar Remodeling.
After prolonged periods of dox administration, alveolar remodeling could be readily appreciated in lungs from transgene+ but not transgene− mice. This response was patchy in nature and associated with alveolar septal thickening and areas of septal rupture. In many areas it had the appearance of honeycomb lung (Fig. 4 A). This honeycombing caused increases in chord length (the morphometric quantification of the distance between alveolar walls) that could be appreciated after 7 d of dox and were most prominent with longer periods of TGF-β1 elaboration (Fig. 4 B).
Figure 4.
TGF-β1–induced alveolar remodeling. Transgene− and transgene+ mice were randomized to normal or dox water. Their lungs were obtained and fixed to pressure at intervals thereafter. (A) We compare (trichrome) lungs from transgene− and transgene+ mice on dox for 1 mo (a magnification of 20). (B) Morphometric assessments of chord length are compared. Transgene− mice on dox water and transgene+ mice on normal water could not be differentiated from transgene− mice on normal water in these assays. (A) A representative of a minimum of five experiments is shown. (B) The values represent the mean ± SEM of evaluations in a minimum of five animals.
TGF-β1–induced α-smooth Muscle Actin Accumulation.
To determine if TGF-β1 caused alterations in myocytes and or myofibroblasts, α-smooth muscle actin IHC was undertaken. When compared with lungs from transgene− mice on normal or dox water and transgene+ mice on normal water, 10 d of dox administration caused a prominent increase in α-smooth muscle actin+ cells in lungs from transgene+ animals (Fig. 5). Many of these cells were also α-smooth muscle myosin heavy chain+ and were located in smooth muscle bundles (Fig. 5). However, many were not in smooth muscle bundles and did not stain with myosin heavy chain (Fig. 5). Thus, the increase in smooth muscle actin staining cells is due, at least in part, to an increase in myocytes and myofibroblasts (Fig. 5).
Figure 5.
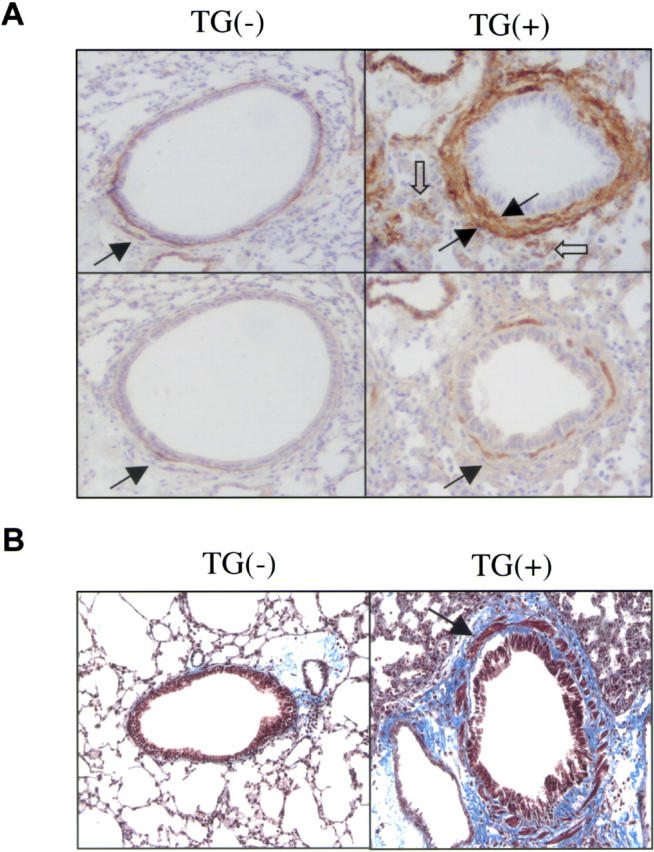
TGF-β1–induced myocyte and myofibroblast alterations. Transgene− and transgene+ mice were randomized to normal or dox water and lungs were evaluated 1 mo later. (A) On the top, IHC assessments of α-smooth muscle actin were undertaken. The closed arrows highlight peribronchial positive cells and the open arrows highlight positive cells a distance from the airway. On the bottom, IHC assessments of smooth muscle myosin heavy chain are compared. The arrows highlight the staining cells in the smooth muscle bundle. All photos are at a magnification of 15. (B) Trichrome staining is used to compare airways from transgene− and transgene+ mice on dox for 1 mo (shown at a magnification of 15). The arrow highlights the enlarged smooth muscle bundle. Transgene− mice on dox water and transgene+ mice on normal water could not be differentiated from transgene− mice on normal water in these assays. (A and B) A representative of a minimum of five experiments is shown.
Role of Egr-1.
We hypothesized that Egr-1 might play an important role in the pathogenesis of the TGF-β1 phenotype. To test this hypothesis, we first evaluated the expression of Egr-1 in our transgenic system. As noted in Fig. 6 A, Egr-1 was potently induced in lungs from dox-treated transgene+ animals but not transgene− controls. Interestingly, Egr-2 and Egr-3 were also induced, whereas the Egr-1 binding proteins NAB-1 and NAB-2 were not altered. The role of Egr-1 was then defined by breeding Egr-1−/− mice (31) and the TGF-β1 transgenic animals. This allowed us to compare the phenotypes induced by TGF-β1 in mice with wild-type (Egr-1+/+) and null mutant (Egr-1−/−) Egr-1 loci. The lungs from wild-type mice and transgene−/Egr-1−/− mice on normal or dox water were virtually identical. In contrast, the TGF-β1 phenotype was markedly altered in Egr-1−/− animals. In the absence of Egr-1, the early wave of apoptosis that was induced by TGF-β1 was no longer appreciated. This difference was most prominent after 48 h of dox, where 21.3 ± 3% of the nuclei in the lungs of transgene+/Egr-1+/+ mice were TUNEL+ versus no detectable staining in transgene+/Egr-1−/− animals (P < 0.001; Fig. 6 B). In the absence of Egr-1, TGF-β1–induced fibrosis was also markedly ameliorated. This was seen in trichrome evaluations (Fig. 6 C) and measurements of collagen content (Fig. 6 D; *, P < 0.01). Egr-1 deficiency also diminished TGF-β1–induced alveolar remodeling, which was readily apparent in histologic and with chord length evaluations (Fig. 6 E and not depicted; *, P < 0.01). Interestingly, TGF-β1–induced BAL inflammation was not altered but parenchymal inflammatory cell accumulation was modestly decreased in the absence of Egr-1 (Fig. 6, F and G). These studies demonstrate that Egr-1 is a critical mediator of key aspects of the in vivo TGF-β1 phenotype.
Figure 6.
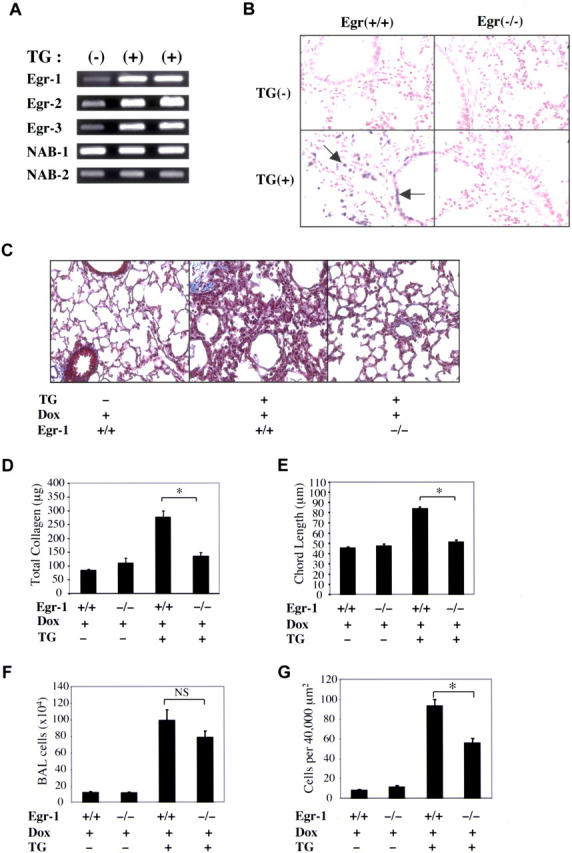
Regulation of Egr-1 and its role in TGF-β1–induced apoptosis, fibrosis, remodeling, and inflammation. Transgene− and transgene+ mice with wild-type (+/+) and null mutant (−/−) Egr-1 loci were randomized to normal or dox water. After 48 h of dox, the levels of mRNA encoding Egr transcription factors and binding proteins (A) and the levels of apoptosis (B) were evaluated with RT-PCR and TUNEL evaluations, respectively. After 10 d of dox administration, collagen content was evaluated with trichrome evaluations (C, shown at a magnification of 10) and Sircol assays (D) and inflammation was evaluated using BAL (F) and quantitative morphometry (G). Alveolar remodeling (chord length) was evaluated after 1 mo of dox (E, *, P < 0.01). (A–C) A representative of a minimum of five experiments is shown. (D–G) The values represent the mean ± SEM of evaluations in a minimum of five animals.
Role of Apoptosis in TGF-β1–induced Fibrosis.
Our studies with Egr-1 null mutant mice demonstrated that an intervention that diminished TGF-β1–induced apoptosis also decreased the ability of TGF-β1 to induce fibrosis and alveolar remodeling. This led to the hypothesis that there is an intimate relationship between the apoptosis and the fibrotic and remodeling phenotypes with the apoptosis being required to allow the latter phenotypes to be seen. To address this hypothesis, experiments were undertaken in which apoptosis was blocked via an Egr-1–independent mechanism and the effects of this intervention on fibrosis and alveolar remodeling were assessed. In these experiments, transgene+ and transgene− mice were randomized to receive Z-VAD-fmk (3 mg/kg/day, i.p.) or vehicle control starting 24 h before the dox. Z-VAD-fmk was a potent inhibitor of TGF-β1–induced apoptosis. In lungs from transgene+ mice on dox for 48 h, 17.8 ± 2.5% of the cells in the vehicle control–treated lungs were TUNEL+ versus no detectable staining in the Z-VAD-fmk–treated lungs (P < 0.01). Interestingly, Z-VAD-fmk inhibition of apoptosis was also associated with a marked decrease in tissue fibrosis on trichrome (Fig. 7 A) and total lung collagen (Fig. 7 B; *, P < 0.01) evaluations. Z-VAD-fmk treatment also ameliorated TGF-β1–induced alveolar remodeling (Fig. 7 C; *, P < 0.01). Similar results were noted in mice treated with a selective caspase 3/7 inhibitor (not depicted). Interestingly, Z-VAD-fmk did not alter TGF-β1–induced fibrosis when it was administered 5 d after the initiation of dox (a time point when TUNEL+ cells are not longer appreciated; Fig. 6 D). Thus, in accord with what was noted with Egr-1 null mutant animals, interventions that blocked apoptosis simultaneously decreased fibrosis and alveolar destruction. When viewed in combination, these studies support the hypothesis that TGF-β1–induced apoptosis is a critical early event in the pathogenesis of TGF-β1–induced fibrosis and alveolar destruction.
Figure 7.
Role of apoptosis in TGF-β1–induced fibrosis and remodeling. Transgene− and transgene+ mice were randomized to normal or dox water. After 10 d of dox, collagen content was evaluated with trichrome evaluations (A) and Sircol assays (B), and alveolar remodeling was assessed with chord length determinations (C). (A–C) Z-VAD-fmk or its vehicle control (Z-VAD-fmk (−)) was administered starting the day before the dox. (D) Z-VAD-fmk or its control was administered 5 d after the initiation of dox (*, P < 0.01) and collagen accumulation was assessed. (A) A representative of a minimum of five experiments is shown. (B–D) The values represent the mean ± SEM of evaluations in a minimum of five animals.
Reversibility of TGF-β1–induced Fibrosis.
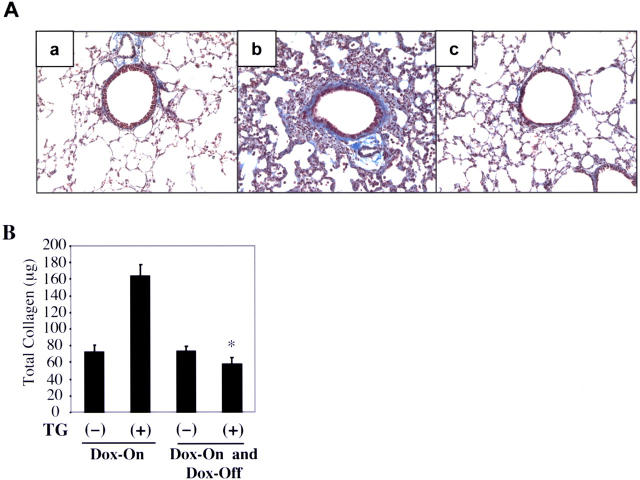
The reversibility of tissue fibrosis has critical consequences for the natural history of fibrotic disorders. Unfortunately, the reversibility of pulmonary fibrosis has not been adequately assessed and what is known engenders controversy (35–37). A unique feature of our transgenic system is the clarity with which it allows the reversibility of tissue phenotypes to be evaluated. To characterize the reversibility of TGF-β1–induced fibrosis, transgene+ and transgene− mice were randomized to normal or dox water for 10 d. At the end of this interval, one group was analyzed while the other was placed on normal water and evaluated at intervals thereafter. As noted above, dox caused an impressive increase in tissue fibrosis in transgene+ mice. Interestingly, after 1 mo on normal water, this parenchymal fibrotic response had almost completely resolved. This was readily apparent in histologic and collagen content evaluations (Fig. 8). These studies demonstrate that the parenchymal fibrotic response that is induced by TGF-β1 is reversible over time.
Figure 8.
Reversibility of TGF-β1–induced fibrosis. Transgene− and transgene+ mice were given normal or dox water for 10 d. A subgroup of animals were then killed and evaluated, and the others were placed on normal water for 30 d. (A) Trichrome evaluations were used to compare transgene− mice on normal water (a), transgene+ mice on dox for 10 d (b), and transgene+ mice on dox for 10 d and on normal water for 30 d (c; shown at a magnification of 15). Collagen content under these conditions is evaluated in B. (A) A representative of a minimum of five experiments is shown. (B) The values represent the mean ± SEM of evaluations in a minimum of five animals (*, P < 0.01 vs. transgene+ on dox for 10 d only).
Discussion
TGF-β1 family proteins are multifunctional cytokines that have been implicated in the pathogenesis of diverse biologic processes including cell growth and survival, cell and tissue differentiation, development, inflammation, immunity, hematopoiesis, and tissue remodeling and repair. On superficial analysis, TGF-β1 can be accurately described as a healing molecule that manifests impressive antiinflammatory and fibrotic effects. On closer analysis, it is clear that this perspective is only partially correct and that the effector profile of TGF-β1 can appear confusing and even contradictory (23, 25, 38, 39). This is impressively noted in the setting of inflammation where TGF-β1 has important antiinflammatory and immunosuppressive effects in some settings and proinflammatory effects in others (38, 40, 41). This is also seen in oncogenesis where TGF-β1 exerts growth inhibitory effects on tumor cells while enhancing tumor cell migration and invasion (39). Particularly relevant to the present proposal, are studies that demonstrate that, in the proper setting, TGF-β1 is essential for wound healing, stimulates matrix molecule deposition and angiogenesis, and is an essential mediator of the pathologic scaring in fibrotic disorders (16, 22, 38, 42, 43). On the other hand, TGF-β1 can also induce tissue injury (44) and cellular apoptosis, decrease epithelialization, and inhibit wound healing (23–27). The complexity of these responses can be attributed to a number of items including the state of activation and differentiation of the target cell, the presence of other stimuli in the local microenvironment, and the ability of TGF-β1 to exert its seemingly antagonistic effects via different effector pathways (23, 25). The “contradictory” nature of these responses also reflects an inadequate understanding of the mechanisms that TGF-β1 uses to induce its complex tissue phenotypes. This is due, in part, to the lack of experimental systems in which the acute and chronic effects of TGF-β1 can be characterized and their interrelationships can be investigated in vivo. It is also due to the assumption that each phenotype is a distinct endpoint in its own right. The possibility that TGF-β1 may induce one phenotype only if it induces an earlier “different” phenotype has not been considered. As a result, the relationships between endpoints such as apoptosis and fibrosis have not been investigated. To address these deficiencies, we generated and used a novel triple transgenic system that bypasses the fatal lethal effects of TGF-β1 (28). With this system, we demonstrated that transgenic TGF-β1 induces a complex pulmonary phenotype with a transient wave of epithelial apoptosis followed by inflammation, airway and parenchymal fibrosis, myocyte and myofibroblast hyperplasia, and alveolar remodeling. Experiments with these mice defined target genes that mediate these complex responses by highlighting the central role that Egr-1 plays in the TGF-β1 response. They also illustrate the intimate relationship between apoptosis and fibrosis because three different interventions that blocked apoptosis also ameliorated the TGF-β1–induced fibrotic response. This suggests that TGF-β1 simultaneously induces injury (apoptosis) and stimulates fibrosis, and that the injury is required for the fibrosis to occur.
In in vitro studies, TGF-β1 induces apoptosis in a variety of cells including epithelial cells, T cells, and tumor cells (45–47). This apoptosis and TGF-β–mediated growth inhibition can be correlated with the tumor suppressive effects of TGF-β1 (45). Apoptosis is also believed to play an important role in normal wound healing because TUNEL staining cells localize in granulation tissue beneath the advancing epithelial edge of dermal wounds (45), and phagocytic cells produce TGF-β after ingesting apoptotic bodies (48). However, the role of TGF-β1 in this apoptotic response has not been defined. In addition, little else is known about the importance of apoptosis in the generation of other TGF-β responses. To define the role of apoptosis in the pathogenesis of the TGF-β1 tissue phenotype, we compared the kinetics of induction of the different TGF-β1 responses and the phenotypes induced by transgenic TGF-β1 when apoptosis was inhibited or proceeding normally. These studies demonstrated that a transient wave of TGF-β1–induced apoptosis proceeds TGF-β1–induced tissue fibrosis, and that three different interventions that block this apoptosis (Egr-1 null mutation, Z-VAD-fmk treatment, and selective caspase 3/7 inhibition) markedly ameliorated this fibrotic response. This demonstrates, for the first time, that apoptosis precedes and is an essential prerequisite for TGF-β1–induced fibrosis. These findings have impressive implications. First, they suggest that the apoptosis at the advancing edge of wounds is required for a normal healing response to occur. Second, they suggest that genetic or acquired alterations in the intensity and/or kinetics of TGF-β1–induced apoptosis can contribute to the severity, rate of progression, and/or reversibility of fibrotic responses. Lastly, because TGF-β1, apoptosis, and fibrosis often coexist in diseases like asthma and the ILD (1–4, 6, 7, 17–21, 49), they suggest that interventions that alter apoptosis might be therapeutically useful in controlling tissue fibrosis in these disorders.
Our studies demonstrate that inhibition of apoptosis only ameliorates fibrosis when the therapy is applied during a critical temporal window early in the pathogenesis of a TGF-β1 tissue response. This finding has important implications regarding the potential usefulness of this sort of an intervention in preventing the progression of pathologic fibrosis. In diseases in which fibrosis is caused by a single insult with a single wave of TGF-β1, interventions that block early responses such as apoptosis would be expected to ameliorate fibrosis only when given early on or in a prophylactic fashion. In contrast, fibrosis can be caused by multiple temporally dissociated injuries, each of which causes its own wave of TGF-β1 elaboration. In this scenario, multiple initiation/apoptosis events occur and the clinical impression of disease progression is the result of the sum of each of these discrete injury and repair responses. In this setting, an intervention that blocks apoptosis can block disease initiation or the progression of ongoing disease. Interestingly, there is mounting evidence that multiple temporally discrete injuries contribute to the pathogenesis of diseases such as IPF, asthma, and wound healing. This is nicely illustrated in the exacerbations and remissions that characterize asthma and the ability of apoptotic cells to move with the leading edge of a healing wound (45). It is also seen in IPF, the histology of which is characterized by geographically discrete sites of injury that appear to be at different stages of evolution and the ready appreciation of apoptotic cells (5–7, 50). As a result, we believe apoptosis-based inhibitors can be effective in preventing fibrotic progression in these and other human disorders. Additional experimentation will be needed to test these assumptions.
Egr-1 is an 80–82-kD inducible zinc finger transcription factor that has also been identified as nerve growth factor–induced A, Krox-24, ZIF-268, ETR-103, and TIS-8 (51–53). It is the prototype of the Egr family that includes Egr-1, Egr-2, Egr-3, Egr-4, and the Wilms' tumor product. Members of this family have been implicated in commitments to proliferation, differentiation, and the activation of cell death pathways. Egr-1 can be induced, both acutely and chronically, at sites of injury and repair by a variety of stimuli including cytokines, oxidized lipids, angiotensin II, H2O2, and mechanical injury (51–55). It mediates its effects by regulating the transcription of a wide array of downstream genes involved in inflammation, matrix formation, thrombosis, and remodeling. Prominent targets include the A and B chains of PDGF, fibroblast growth factor 2, vascular endothelial growth factor, CD44, tissue factor, fibronectin, matrix metalloproteinases, plasminogen activator inhibitor 1, and urinary plasminogen activator (51–53). In accord with its ability to stimulate TNF, Fas, Fas L, PTEN (a proapoptotic Egr-1 target), and p53, Egr-1 is also a potent stimulator of cellular apoptosis in vitro (56, 57). Egr-1 can stimulate TGF-β1 production, be stimulated by TGF-β1, and inhibit TGF-βRII expression in vitro (58–61). Our studies demonstrate that TGF-β1 stimulates Egr-1 in vivo and that Egr-1 is a central mediator of TGF-β1–induced apoptosis, fibrosis, and alveolar remodeling in vivo. These observations suggest that therapeutic interventions that control Egr-1 activation or effector pathway initiation can be therapeutically useful in controlling pathologic TGF-β1 responses. This can be accomplished a variety of ways because Egr-1 is activated via a complex process that involves Egr-1 phosphorylation, Egr-1 specificity protein 1 binding, and competition between Egr-1 and specificity protein 1 for GC-rich cis elements in the promoters of target genes (62). These findings also have impressive implications for diseases like pulmonary emphysema, which is characterized by structural cell apoptosis, abnormal scarring, TGF-β1 induction, and Egr-1 activation (63–65). In these diseases, it is tempting to speculate that TGF-β1–induced Egr-1 activation is responsible for the apoptosis, fibrosis, and alveolar destruction in this disorder.
It is commonly stated that fibrosis in the lung is irreversible (35, 36). This perception is derived from the relentlessly progressive nature of diseases like IPF and from the progressive fibrosis that has been described after high dose virally mediated gene transfer with cytokines such as IL-1β and TGF-β (66, 67). In contrast, there is a substantial body of evidence in humans and modeling systems that fibrosis, in some settings, can regress over time. This has been most intensely studied in the liver where reversibility is seen in modeling systems and cirrhotic humans after adequate therapy for viral hepatitis (37, 68). Reversibility has also been reported in patients with isocyanate asthma after cessation of exposure (69) and in ILD modeling systems using low dose adenovirus-mediated TGF-β1 gene transfer (70). Our studies demonstrate that transgenic TGF-β1 causes a parenchymal fibrotic response that is largely reversible after the cessation of transgene expression. On superficial analysis, these results would appear to differ significantly from the findings that were obtained by Sime et al. (66) using high dose adenovirus gene transfer of TGF-β1. These differences could accurately reflect the existence of irreversible and reversible pulmonary fibrotic responses (68). Alternatively, the differences might be more technical than real because (a) the adenovirus system itself can cause significant lung injury; (b) the high dose adenovirus system engendered levels of BAL TGF-β1 as high as 90 ng/ml, which is ∼60-fold greater than the physiologic levels seen in our system; and (c) the adenovirus TGF-β1–treated mice were not observed for a significant interval after their peak level of tissue fibrosis, making it impossible to determine if resolution would have occurred with longer periods of evaluation. Regardless, it is clear from our studies that pulmonary fibrosis can resolve over time. Additional experimentation will be required to determine if this reversibility is still seen with higher, longer, or repeated doses of TGF-β1. Additional experimentation will also be required to test the exciting hypothesis suggested by our apoptosis studies: the degree of reversibility of a TGF-β1–induced fibrotic response in the lung is determined, at least in part, by the chronicity and/or severity of the preceding epithelial apoptosis.
Acknowledgments
The authors thank the investigators and institutions that provided the reagents that were used and Kathleen Bertier for her excellent secretarial and administrative assistance.
This work was supported by National Institutes of Health grants NL-64242, NL-56389, and NL-078744.
The authors have no conflicting financial interests.
Abbreviations used in this paper: BAL, bronchoalveolar lavage; Egr, early growth response gene; ICAD, inhibitor of caspase-activated DNase; IHC, immunohistochemistry; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; PARP, poly(ADP)ribose polymerase; rtTA, reverse tetracycline transactivator; tet-O, tetracycline operator; tTS, tetracycline-controlled transcriptional silencer; TUNEL, TdT-mediated dUTP nick-end labeling.
References
- 1.Elias, J.A., Z. Zhu, G. Chupp, and R.J. Homer. 1999. Airway remodeling in asthma. J. Clin. Invest. 104:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu, G. 1998. Interstitial lung disease: a clinical overview and general approach. Fishman's Pulmonary Diseases and Disorders. R.M. Senior, editor. Mc-Graw Hill, New York. 1037–1053.
- 3.Bucchieri, F., S.M. Puddicombe, J.L. Lordan, A. Richter, D. Buchanan, S.J. Wilson, J. Ward, G. Zummo, P.H. Howarth, R. Djukanovic, et al. 2002. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am. J. Respir. Cell Mol. Biol. 27:179–185. [DOI] [PubMed] [Google Scholar]
- 4.Trautmann, A., P. Schmid-Grendelmeier, K. Kruger, R. Crameri, M. Akdis, A. Akkaya, E.B. Brocker, K. Blaser, and C.A. Akdis. 2002. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J. Allergy Clin. Immunol. 109:329–337. [DOI] [PubMed] [Google Scholar]
- 5.Kuwano, K., H. Miyazaki, N. Hagimoto, M. Kawasaki, M. Fujita, R. Kunitake, Y. Kaneko, and N. Hara. 1999. The involvement of Fas-Fas ligand pathway in fibrosing lung diseases. Am. J. Respir. Cell Mol. Biol. 20:53–60. [DOI] [PubMed] [Google Scholar]
- 6.Kuwano, K., N. Hagimoto, M. Kawasaki, T. Yatomi, N. Nakamura, S. Nagata, T. Suda, R. Kunitake, T. Maeyama, H. Miyazaki, et al. 1999. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J. Clin. Invest. 104:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwano, K., R. Kunitake, M. Kawasaki, Y. Nomoto, N. Hagimoto, Y. Nakanishi, and N. Hara. 1996. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 154:477–483. [DOI] [PubMed] [Google Scholar]
- 8.Sime, P.J., and K.M. O'Reilly. 2001. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin. Immunol. 99:308–319. [DOI] [PubMed] [Google Scholar]
- 9.Wills-Karp, M., and M. Chiaramonte. 2003. Interleukin-13 in asthma. Curr. Opin. Pulm. Med. 9:21–27. [DOI] [PubMed] [Google Scholar]
- 10.Elias, J.A., C.G. Lee, T. Zheng, B. Ma, R.J. Homer, and Z. Zhu. 2003. New insights into the pathogenesis of asthma. J. Clin. Invest. 111:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belperio, J.A., M. Dy, M.D. Burdick, Y.Y. Xue, K. Li, J.A. Elias, and M.P. Keane. 2002. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 27:419–427. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, A., L. Armstrong, R. Gama, and A. Millar. 1998. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am. J. Respir. Cell Mol. Biol. 18:60–65. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa, M., M. Fujimoto, K. Kikuchi, and K. Takehara. 1997. Elevated serum levels of interleukin 4 (IL-4) IL-10, and IL-13 in patients with systemic sclerosis. J. Rheumatol. 24:328–332. [PubMed] [Google Scholar]
- 14.Majumdar, S., D. Li, T. Ansari, P. Pantelidis, C.M. Black, M. Gizycki, R.M. du Bois, and P.K. Jeffery. 1999. Different cytokine profiles in cryptogenic fibrosing alveolitis and fibrosing alveolitis associated with systemic sclerosis. Eur. Respir. J. 14:251–257. [DOI] [PubMed] [Google Scholar]
- 15.Wallace, W.A., E.A. Ramage, D. Lamb, and S.D. Howie. 1995. A type 2 (Th2-like) pattern of immune response predominates in the pulmonary interstitium of patients with cryptogenic fibrosing alveolitis (CFA). Clin. Exp. Immunol. 101:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, C.G., R.J. Homer, Z. Zhu, S. Lanone, X. Wang, V. Koteliansky, J.M. Shipley, P. Gotwals, P. Noble, Q. Chen, et al. 2001. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J. Exp. Med. 194:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno, I., Y. Nitta, K. Yamauchi, H. Hoshi, M. Honma, K. Woolley, P. O'Byrne, G. Tamura, M. Jordana, and K. Shirato. 1996. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am. J. Respir. Cell Mol. Biol. 15:404–409. [DOI] [PubMed] [Google Scholar]
- 18.Minshall, E.M., D.Y. Leung, R.J. Martin, Y.L. Song, L. Cameron, P. Ernst, and Q. Hamid. 1997. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol. 17:326–333. [DOI] [PubMed] [Google Scholar]
- 19.Tillie-Leblond, I., J. Pugin, C.H. Marquette, C. Lamblin, F. Saulnier, A. Brichet, B. Wallaert, A.B. Tonnel, and P. Gosset. 1999. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am. J. Respir. Crit. Care Med. 159:487–494. [DOI] [PubMed] [Google Scholar]
- 20.Khalil, N., R.N. O'Connor, K.C. Flanders, and H. Unruh. 1996. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am. J. Respir. Cell Mol. Biol. 14:131–138. [DOI] [PubMed] [Google Scholar]
- 21.Khalil, N., T.V. Parekh, R. O'Connor, N. Antman, W. Kepron, T. Yehaulaeshet, Y.D. Xu, and L.I. Gold. 2001. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 56:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, M., J. Lefaix, and S. Delanian. 2000. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 47:277–290. [DOI] [PubMed] [Google Scholar]
- 23.Roberts, A.B., E. Piek, E.P. Bottinger, G. Ashcroft, J.B. Mitchell, and K.C. Flanders. 2001. Is Smad3 a major player in signal transduction pathways leading to fibrogenesis? Chest. 120:43S–47S. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda, H., T. Motohiro, K. Nakai, K. Yamamichi, Y. Nakane, J. Fujisawa, and K. Hioki. 2001. Negative effect of transforming growth factor-beta-1 on intestinal anastomotic tissue regeneration. Eur. Surg. Res. 33:388–394. [DOI] [PubMed] [Google Scholar]
- 25.Flanders, K.C., C.D. Sullivan, M. Fujii, A. Sowers, M.A. Anzano, A. Arabshahi, C. Major, C. Deng, A. Russo, J.B. Mitchell, et al. 2002. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am. J. Pathol. 160:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan, T., A. Ghahary, J. Demare, L. Yang, T. Iwashina, P.G. Scott, and E.E. Tredget. 2002. Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-beta1 transgenic mouse. Wound Repair Regen. 10:177–187. [DOI] [PubMed] [Google Scholar]
- 27.Amendt, C., A. Mann, P. Schirmacher, and M. Blessing. 2002. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J. Cell Sci. 115:2189–2198. [DOI] [PubMed] [Google Scholar]
- 28.Zhou, L., C.R. Dey, S.E. Wert, and J.A. Whitsett. 1996. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev. Biol. 175:227–238. [DOI] [PubMed] [Google Scholar]
- 29.Zhu, Z., B. Ma, R.J. Homer, T. Zheng, and J.A. Elias. 2001. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J. Biol. Chem. 276:25222–25229. [DOI] [PubMed] [Google Scholar]
- 30.Zheng, T., Z. Zhu, Z. Wang, R.J. Homer, B. Ma, R.J. Riese, Jr., H.A. Chapman, Jr., S.D. Shapiro, and J.A. Elias. 2000. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Invest. 106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, S.L., Y. Sadovsky, A.H. Swirnoff, J.A. Polish, P. Goda, G. Gavrilina, and J. Milbrandt. 1996. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science. 273:1219–1221. [DOI] [PubMed] [Google Scholar]
- 32.Rice, W.R., J.J. Conkright, C.L. Na, M. Ikegami, J.M. Shannon, and T.E. Weaver. 2002. Maintenance of the mouse type II cell phenotype in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L256–L264. [DOI] [PubMed] [Google Scholar]
- 33.Tang, W., G.P. Geba, T. Zheng, P. Ray, R.J. Homer, C. Kuhn III, R.A. Flavell, and J.A. Elias. 1996. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J. Clin. Invest. 98:2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corry, D.B., K. Rishi, J. Kanellis, A. Kiss, L.Z. Song Lz, J. Xu, L. Feng, Z. Werb, and F. Kheradmand. 2002. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 3:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes, P.J. 1996. Pathophysiology of asthma. Br. J. Clin. Pharmacol. 42:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Kane, S. 2002. Wound remodelling and scarring. J. Wound Care. 11:296–299. [DOI] [PubMed] [Google Scholar]
- 37.Arthur, M.J. 2002. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 122:1525–1528. [DOI] [PubMed] [Google Scholar]
- 38.Ling, E., and D.S. Robinson. 2002. Transforming growth factor-beta1: its anti-inflammatory and pro-fibrotic effects. Clin. Exp. Allergy. 32:175–178. [DOI] [PubMed] [Google Scholar]
- 39.Akhurst, R.J., and R. Derynck. 2001. TGF-beta signaling in cancer–a double-edged sword. Trends Cell Biol. 11:S44–S51. [DOI] [PubMed] [Google Scholar]
- 40.Fargeas, C., C.Y. Wu, T. Nakajima, D. Cox, T. Nutman, and G. Delespesse. 1992. Differential effect of transforming growth factor beta on the synthesis of Th1- and Th2-like lymphokines by human T lymphocytes. Eur. J. Immunol. 22:2173–2176. [DOI] [PubMed] [Google Scholar]
- 41.Letterio, J.J., and A.B. Roberts. 1998. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16:137–161. [DOI] [PubMed] [Google Scholar]
- 42.Nakao, A., M. Fujii, R. Matsumura, K. Kumano, Y. Saito, K. Miyazono, and I. Iwamoto. 1999. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest. 104:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalluri, R., and V.P. Sukhatme. 2000. Fibrosis and angiogenesis. Curr. Opin. Nephrol. Hypertens. 9:413–418. [DOI] [PubMed] [Google Scholar]
- 44.Pittet, J.F., M.J. Griffiths, T. Geiser, N. Kaminski, S.L. Dalton, X. Huang, L.A. Brown, P.J. Gotwals, V.E. Koteliansky, M.A. Matthay, et al. 2001. TGF-beta is a critical mediator of acute lung injury. J. Clin. Invest. 107:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster, N., and K. Krieglstein. 2002. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 307:1–14. [DOI] [PubMed] [Google Scholar]
- 46.Pelaia, G., G. Cuda, A. Vatrella, D. Fratto, R.D. Grembiale, P. Tagliaferri, R. Maselli, F.S. Costanzo, and S.A. Marsico. 2003. Effects of TGF-β and budesonide on MAPK activation and apoptosis in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 29:12–18. [DOI] [PubMed] [Google Scholar]
- 47.Jang, C.-W., C.-H. Chen, C.-C. Chen, J.-Y. Chen, Y.-S. Su, and R.-H. Chen. 2001. TGF-β induces apoptosis through Smad-mediated expression of DAP-kinase. Nat. Cell Biol. 4:51–58. [DOI] [PubMed] [Google Scholar]
- 48.Wang, L., J.M. Antonini, Y. Rojanasakul, V. Castranova, J.F. Scabilloni, and R.R. Mercer. 2003. Potential role of apoptotic macrophages in pulmonary inflammation and fibrosis. J. Cell. Physiol. 194:215–224. [DOI] [PubMed] [Google Scholar]
- 49.Hagimoto, N., K. Kuwano, I. Inoshima, M. Yoshimi, N. Nakamura, M. Fujita, T. Maeyama, and N. Hara. 2002. TGF-β 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J. Immunol. 168:6470–6478. [DOI] [PubMed] [Google Scholar]
- 50.Fellrath, J.M., and R.M. du Bois. 2003. Idiopathic pulmonary fibrosis/cryptogenic fibrosing alveolitis. Clin. Exp. Med. 3:65–83. [DOI] [PubMed] [Google Scholar]
- 51.Gashler, A., and V.P. Sukhatme. 1995. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog. Nucleic Acid Res. Mol. Biol. 50:191–224. [DOI] [PubMed] [Google Scholar]
- 52.Khachigian, L.M., V. Lindner, A.J. Williams, and T. Collins. 1996. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 271:1427–1431. [DOI] [PubMed] [Google Scholar]
- 53.Silverman, E.S., and T. Collins. 1999. Pathways of Egr-1-mediated gene transcription in vascular biology. Am. J. Pathol. 154:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCaffrey, T.A., C. Fu, B. Du, S. Eksinar, K.C. Kent, H. Bush, Jr., K. Kreiger, T. Rosengart, M.I. Cybulsky, E.S. Silverman, et al. 2000. High-level expression of Egr-1 and Egr-1-inducible genes in mouse and human atherosclerosis. J. Clin. Invest. 105:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okada, M., C.Y. Wang, D.W. Hwang, T. Sakaguchi, K.E. Olson, Y. Yoshikawa, K. Minamoto, S.P. Mazer, S.F. Yan, and D.J. Pinsky. 2002. Transcriptional control of cardiac allograft vasculopathy by early growth response gene-1 (Egr-1). Circ. Res. 91:135–142. [DOI] [PubMed] [Google Scholar]
- 56.Virolle, T., E.D. Adamson, V. Baron, D. Birle, D. Mercola, T. Mustelin, and I. de Belle. 2001. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat. Cell Biol. 3:1124–1128. [DOI] [PubMed] [Google Scholar]
- 57.Das, A., D. Chendil, S. Dey, M. Mohiuddin, J. Milbrandt, V.M. Rangnekar, and M.M. Ahmed. 2001. Ionizing radiation down-regulates p53 protein in primary Egr-1−/− mouse embryonic fibroblast cells causing enhanced resistance to apoptosis. J. Biol. Chem. 276:3279–3286. [DOI] [PubMed] [Google Scholar]
- 58.Liu, C., J. Yao, D. Mercola, and E. Adamson. 2000. The transcription factor EGR-1 directly transactivates the fibronectin gene and enhances attachment of human glioblastoma cell line U251. J. Biol. Chem. 275:20315–20323. [DOI] [PubMed] [Google Scholar]
- 59.Kane, S., M.A. Prentice, J.M. Mariano, F. Cuttitta, and S.B. Jakowlew. 2002. Differential induction of early response genes by adrenomedullin and transforming growth factor-beta1 in human lung cancer cells. Anticancer Res. 22:1433–1444. [PubMed] [Google Scholar]
- 60.Du, B., C. Fu, K.C. Kent, H. Bush, Jr., A.H. Schulick, K. Kreiger, T. Collins, and T.A. McCaffrey. 2000. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-beta type II receptor. J. Biol. Chem. 275:39039–39047. [DOI] [PubMed] [Google Scholar]
- 61.Ohba, M., M. Shibanuma, T. Kuroki, and K. Nose. 1994. Production of hydrogen peroxide by transforming growth factor-β 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J. Cell Biol. 126:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bahouth, S.W., M.J. Beauchamp, and K.N. Vu. 2002. Reciprocal regulation of beta(1)-adrenergic receptor gene transcription by Sp1 and early growth response gene 1: induction of EGR-1 inhibits the expression of the beta(1)-adrenergic receptor gene. Mol. Pharmacol. 61:379–390. [DOI] [PubMed] [Google Scholar]
- 63.Takizawa, H., M. Tanaka, K. Takami, T. Ohtoshi, K. Ito, M. Satoh, Y. Okada, F. Yamasawa, K. Nakahara, and A. Umeda. 2001. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am. J. Respir. Crit. Care Med. 163:1476–1483. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, W., S.D. Yan, A. Zhu, Y.S. Zou, M. Williams, G.C. Godman, B.M. Thomashow, M.E. Ginsburg, D.M. Stern, and S.F. Yan. 2000. Expression of Egr-1 in late stage emphysema. Am. J. Pathol. 157:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majo, J., H. Ghezzo, and M.G. Cosio. 2001. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur. Respir. J. 17:946–953. [DOI] [PubMed] [Google Scholar]
- 66.Sime, P.J., Z. Xing, F.L. Graham, K.G. Csaky, and J. Gauldie. 1997. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 100:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolb, M., P.J. Margetts, D.C. Anthony, F. Pitossi, and J. Gauldie. 2001. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Invest. 107:1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee, H.S., G.T. Huang, C.H. Chen, L.L. Chiou, C.C. Lee, P.M. Yang, D.S. Chen, and J.C. Sheu. 2001. Less reversal of liver fibrosis after prolonged carbon tetrachloride injection. Hepatogastroenterology. 48:1312–1315. [PubMed] [Google Scholar]
- 69.Saetta, M., P. Maestrelli, G. Turato, C.E. Mapp, G. Milani, F. Pivirotto, L.M. Fabbri, and A. Di Stefano. 1995. Airway wall remodeling after cessation of exposure to isocyanates in sensitized asthmatic subjects. Am. J. Respir. Crit. Care Med. 151:489–494. [DOI] [PubMed] [Google Scholar]
- 70.Warshamana, G.S., D.A. Pociask, K.J. Fisher, J.-Y. Liu, P.J. Sime, and A.R. Brody. 2002. Titration of non-replicating adenovirus as a vector for transducing active TGF-β1 gene expression causing inflammation and fibrogenesis in the lungs of C57BL/6 mice. Int. J. Exp. Pathol. 83:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]