Abstract
Regulatory T cells have been clearly implicated in the control of disease in murine models of autoimmunity. The paucity of data regarding the role of these lymphocytes in human autoimmune disease has prompted us to examine their function in patients with rheumatoid arthritis (RA). Regulatory (CD4+CD25+) T cells isolated from patients with active RA displayed an anergic phenotype upon stimulation with anti-CD3 and anti-CD28 antibodies, and suppressed the proliferation of effector T cells in vitro. However, they were unable to suppress proinflammatory cytokine secretion from activated T cells and monocytes, or to convey a suppressive phenotype to effector CD4+CD25− T cells. Treatment with antitumor necrosis factor α (TNFα; Infliximab) restored the capacity of regulatory T cells to inhibit cytokine production and to convey a suppressive phenotype to “conventional” T cells. Furthermore, anti-TNFα treatment led to a significant rise in the number of peripheral blood regulatory T cells in RA patients responding to this treatment, which correlated with a reduction in C reactive protein. These data are the first to demonstrate that regulatory T cells are functionally compromised in RA, and indicate that modulation of regulatory T cells by anti-TNFα therapy may be a further mechanism by which this disease is ameliorated.
Keywords: T lymphocytes, tolerane, autoimmune disease, TNFα, cytokines
Introduction
Studies in rodents have provided firm evidence for the existence of a naturally occurring population of CD4+CD25+ professional regulatory/suppressor T cells, which, upon in vitro TCR-mediated stimulation, suppress the proliferation of effector T cells (1, 2). These cells appear central to the control of T cell homeostasis and in the modulation of immune responses to autoantigens, cancer cells, pathogens, and alloantigens (1, 2). In the periphery of young mice not prone to autoimmune diseases, regulatory T cells constitute a stable 10% of CD4+ T cells. This proportion appears to be reduced in mice genetically prone to autoimmune disease such as diabetes (3, 4). Transfer of regulatory T cells is able to prevent a wide range of experimental autoimmune diseases, including diabetes, experimental autoimmune encephalomyelitis, and colitis (3–6). In addition, depletion of regulatory T cells has been shown to exacerbate various experimental autoimmune diseases, including collagen induced arthritis (7). In humans, an analogous population of CD4+CD25+ regulatory T cells has been identified in the peripheral blood (PB; 8–11) and in the thymus (12, 13). In comparison to the wealth of evidence from animal models, much less is known about human regulatory T cells and their potential to suppress autoimmune disease in patients. One recent analysis of human regulatory T cells demonstrated a reduction in their numbers in the PB of newly diagnosed type-1 diabetic patients, suggesting that a deficiency in this subset could be responsible for the breakdown of tolerance in human diabetes (14).
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that ultimately leads to the destruction of joint architecture. The pathogenic events that lead to the development of RA are not fully understood, although the pivotal role that proinflammatory cytokines (i.e., such as TNFα, IL-1β, IL-6, etc.) play in the induction and maintenance of this disease is well documented (15). Moreover, these cytokines are important in the homeostasis of T lymphocytes (16), including regulation of the CD4+CD25+ regulatory T cell subset. In RA, the CD4+CD25+ regulatory T cell subset represents ∼5–10% of the CD4+ T cell population, and this population has been shown to suppress the in vitro proliferation of autologous CD4+ T cells (17). Because proinflammatory cytokines are critical to the pathogenesis of RA, we postulated that regulatory T cells in active RA, although still capable of suppressing T cell proliferation, may be defective in controlling proinflammatory cytokine production. Therefore, we set out to investigate any changes in the function and/or number of regulatory T cells in the PB of patients with active RA and to determine if clinical response to anti-TNFα therapy results in changes in this cell population.
In this paper, we present evidence to suggest that regulatory T cells derived from patients with active RA are defective in their ability to suppress cytokine production and in their ability to convey a suppressive phenotype to CD4+ effector T cells. We go on to demonstrate that clinical response to anti-TNFα, but not conventional therapies, in RA patients is correlated with an increased number of PB regulatory T cells, suggesting a possible additional mechanism by which blockade of TNFα results in amelioration of RA.
Materials and Methods
Study Population.
27 patients with active RA, fulfilling the revised classification criteria of the American College of Rheumatology for RA, were evaluated before and after anti-TNFα therapy (Infliximab was given at a dose of 3 mg/kg i.v. at weeks 0, 2, 6, and at every 8 wk in combination with stable doses of methotrexate 7.5–15 mg/wk orally) and stable nonsteroidal antiinflammatory drugs. Patients taking prednisolone were excluded from the study because steroids are known to affect lymphocyte function and CD25 expression (18). Only patients with a disease activity score of >5.1 were treated with anti-TNFα therapy. Response was defined as a drop in the disease activity score of >1.2. Patients responding to conventional dosages of methotrexate (15-25 mg/wk) were also included in this analysis.
Antibodies.
The following Abs were used: FITC-conjugated anti-CD4 (RPAT4), Cy-Chrome–conjugated anti-CD25 (M-A251), and PE-conjugated anti-CD3 (UCHT1). Staining of MACS®-sorted T cells included the following: PE-conjugated, FITC-conjugated anti-CD3 (UCHT1); anti-CD4 (RPAT4); anti-CD25 (4E3); anti-CD28 (CD28.2); and anti–CTLA-4 (BNI3; all obtained from BD Biosciences). For T cell activation the stimulatory Abs, anti-CD3 (OKT3) and anti-CD28 (CD28.2; obtained from BD Biosciences) were used as indicated.
Cell Isolation.
PBMCs were isolated by Ficoll-Paque Plus (Amersham Biosciences) gradient centrifugation. Cells were cultured in RPMI 1640 media supplemented with 2 nM l-glutamine, 5 mM Hepes, 100 U/μg/ml penicillin/streptomycin, 0.5 mM sodium pyruvate, 0.05 mM of nonessential amino acids (both obtained from Life Technologies), and 10% FCS (all obtained from BioWhittaker) in 96-well U-bottom plates (Nunc). Non-CD4+ T cells were stained using a biotin antibody cocktail (10 μl/107 total cells), incubated for 10 min at 4°C, magnetically labeled with antibiotin microbeads (20 μl/107 total cells), incubated for 15 min at 4°C, washed twice, and depleted over a MACS® LD column. CD4+CD25+ T cells were directly labeled with anti-CD25 microbeads (10 μl/107 CD4+ cells), incubated for 15 min at 4°C, and positively selected using MACS® MS columns according to manufacturer's instructions (Miltenyi Biotec). T cell subpopulations of CD4+CD25+ T cells were used immediately after isolation. All the plate-bound anti-CD3 cultures received 104 irradiated PBMCs. In separate experiments, cells were isolated using a MoFlo cell sorter after staining using FITC-conjugated anti-CD4 and Cy-Chrome–conjugated anti-CD25. The purities of the populations obtained using these two methods of cell isolation are shown in Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20040165/DC1.
Cytokine Detection.
For analysis of intracellular cytokine production T cells were stimulated with either 5 μg/ml of soluble anti-CD3/anti-CD28 or 1 μg/ml of plate-bound anti-CD3/irradiated PBMCs for 48 h. 2 μM monensin was added for the last 5 h. Staining of CD4 and CD25 surface expression was performed. Cells were washed, fixed, permeabilized, and stained for detection of intracellular cytokines using PE-conjugated anti-IFNγ, TNFα, or IL-10. Alternatively, supernatants were collected, before the addition of monensin, and IL-2, IL-4, IL-5, IL-10, IFNγ, and TNFα levels were measured by the cytometric bead array kit (CBA kit; BD Biosciences), according to the manufacturer's instructions.
Proliferation Assay.
To assess proliferation of different CD4+ subtypes, 105 cells sorted of MACS purified T cells were incubated in complete medium with 1 μg/ml of plate-bound anti-CD3, 1 μg/ml of plate-bound anti-CD3, and 5 μg/ml of soluble anti-CD28 or 5 μg/ml of soluble anti-CD3/anti-CD28 in 96-well U-bottom plates (Nunc). After 5 d of culture, [3H]Tdr was added for the remaining 8 h of culture. Proliferation was measured using a liquid scintillation counter.
Flow Cytometric Analysis.
Immunofluorescence staining was performed after washing the cells twice with PBS plus 0.5% human serum albumin. Cells were incubated for 20 min at 4°C with each mAb (5 μg/ml, 105 cells/test). After washing with cold PBS/human serum albumin, the indirectly labeled cells were incubated with FITC- and PE-conjugated second-step mAb for 20 min at 4°C, washed three times, and analyzed by flow cytometry (FACScalibur™, CELLQuest™ software; Becton Dickinson).
Statistical Analysis.
Analysis for statistically significant differences was performed with Student's t test. p-values <0.05 were considered significant.
Online Supplemental Material.
Fig. S1 shows the purity of the populations isolated using either a MoFlo cell sorter or a MACS® column as analyzed by FACS®. The clinical details of the patients studied are included in Table S1. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20040165/DC1.
Results
CD4+CD25+ T Cells Isolated from RA Patients Are Anergic and Suppress the Proliferation of Effector T Cells.
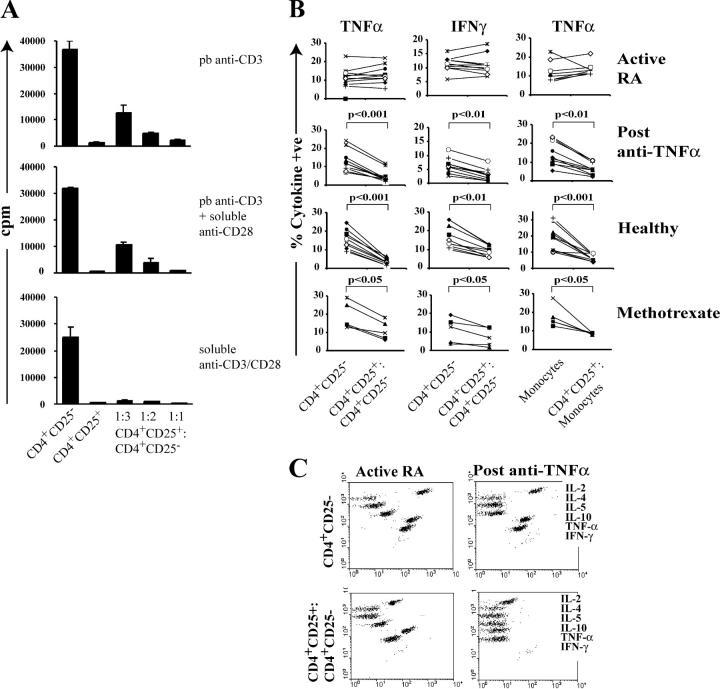
To test the hypothesis that CD4+CD25+ regulatory T cells isolated from active RA patients may be functionally defective compared with those isolated from healthy individuals, or from the same patients after anti-TNFα treatment, CD4+CD25+ T cells were purified by MACS® sorting (as described in Materials and Methods) and cultured alone or mixed with CD4+CD25− T cells. Three different stimuli were used to reveal any possible defect in the CD4+CD25+ T cell population in the PB of patients with RA. The results in Fig. 1 A show that CD4+CD25+ T cells isolated from active RA patients displayed an anergic phenotype and were able to inhibit the proliferation of effector CD4+CD25− T cells in a dose-dependent mode when different T cell receptor signal strengths were used. CD4+CD25+ T cells isolated from active RA patients were capable of suppressing the proliferation of responder T cells to a similar level as found in healthy individuals or in the same group of patients 3 mo after anti-TNFα treatment (unpublished data).
Figure 1.
Regulatory T cells from the PB of patients with active RA can suppress proliferation but not proinflammatory cytokine production by effector T cells and monocytes. (A) PB was collected from patients with RA; MACS®-sorted CD4+CD25+ T cells and CD4+CD25− T cells were cultured either alone or mixed at 1:3, 1:2, 1:1 ratio (105 cells/well) and stimulated with 1 μg/ml of plate-bound anti-CD3, 1 μg/ml of plate-bound anti-CD3/5 μg/ml of soluble anti-CD28, and 5 μg/ml soluble anti-CD3/anti-CD28 for 48 h. Proliferation after 5 d was determined by 3[H]Tdr incorporation. Results are expressed as mean ± SEM of triplicate cultures. One out of five independent experiments is shown. (B) MACS®-sorted CD4+CD25+ and CD4+CD25− T cells isolated from 10 patients before and after anti-TNFα therapy (3 mo) with 5 patients responding to methotrexate therapy or 10 healthy controls were cultured alone or mixed at a 1:1 ratio (105 cells/well) and stimulated with 5 μg/ml of soluble anti-CD3/anti-CD28 for 48 h. Monocytes were stimulated with 10 ng/ml LPS for 48 h. 2 μM monensin was added for the last 5 h of culture. Staining of CD4 and CD25 surface expression was performed and, subsequently, the cells were washed, permeabilized, and stained with PE-conjugated anti-TNFα or anti-IFNγ. (C) Cytokines from the supernatants collected from the experiment (B) were measured by CBA. 1 out of 10 experiments is illustrated.
CD4+CD25+ Regulatory T Cells Isolated from RA Patients Do Not Suppress Cytokines Produced by Effector T Cells.
As proinflammatory cytokines play an essential role in the pathogenesis of RA, next we assessed whether CD4+ CD25+ regulatory T cells, isolated before and after anti-TNFα treatment, were able to suppress proinflammatory cytokine production by effector T cells. Similar to the suppressive effects that regulatory T cells have on the proliferation of effector T cells, the high levels of TNFα and IFNγ synthesized by the responder CD4+ T cells were suppressed after coculture with CD4+CD25+ T cells derived from healthy or anti-TNFα–treated patients (Fig. 1 B). However, regulatory T cells isolated from patients with active RA failed to inhibit the production of these cytokines by CD4+CD25− effector T cells. Similarly, TNFα production by monocytes, a key producer of this cytokine in RA, was also markedly inhibited by CD4+CD25+ T cells isolated from healthy individuals and patients with RA after treatment. No IL-10 production was detected in any of the samples analyzed (unpublished data). The response of CD4+CD25+ T cells isolated from patients responding to methotrexate treatment was included in this analysis. Although CD4+CD25+ T cells efficiently suppressed TNFα produced by monocytes, the ability to inhibit cytokines produced by effector T cells was less effective compared with CD4+CD25+ T cells isolated from anti-TNFα–treated patients (20–30% inhibition compared with 60–70% inhibition after anti-TNFα). To complement the results obtained by intracellular staining, cytokine production was also measured in the supernatants derived from the aforementioned experiments by CBA. In keeping with the results obtained from the intracellular staining, only regulatory T cells isolated from anti-TNFα–treated patients strongly suppressed TNFα and IFNγ released by effector CD4+CD25− T cells upon anti-CD3/CD28 stimulation in vitro. A reduction in IL-2 production was detected in both groups. Comparable results were obtained when plate-bound anti-CD3 and irradiated PBMCs were used instead of soluble stimuli (unpublished data).
CD4+CD25high T Cells from Active RA Patients Are Functionally Defective.
The results described thus far are based on the use of populations of CD4+CD25+ T cells purified with magnetic beads, containing mixtures of CD25high and CD25low cells. It has been shown previously that amongst CD4+ T cells expressing CD25, only those expressing high levels of CD25 display regulatory function (10). To dissect which subset of CD4+CD25+ T cells (high or low) was responsible for the suppressive effect on cytokines released by the effector T cells, CD4+ T cells were sorted according to their CD25 expression (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20040165/DC1). Results in Fig. 2 A demonstrate that the suppressive effect on cytokine production resides in the CD4+CD25high T cell fraction when isolated from anti-TNFα–treated patients. In contrast, the CD4+CD25high T cells from patients with active RA were unable to suppress TNFα and IFNγ produced by CD4+CD25− T cells. No differences were found in TNFα or IFNγ produced by CD4+CD25high T cells isolated from patients before or after anti-TNFα treatment. Cytokines were also measured in the culture supernatants by CBA, and the results in Fig. 2 B reproduce the aforementioned findings. In agreement with the suppression of proliferation results shown in Fig. 1 A, regulatory T cells irrespective of their provenance strongly suppressed the production of IL-2 released by autologous effector T cells. No consistent changes in IL-5, IL-4, and IL-10 were found in the different samples analyzed (unpublished data). Together, these results demonstrate that regulatory T cells isolated from patients with active RA are “compromised” at least in terms of their ability to suppress proinflammatory cytokine production by responder T cells as well as from monocytes.
Figure 2.

The regulatory defect resides within the CD4+CD25high T cell population. (A) The CD4+CD25−, CD4+CD25low, CD4+CD25high population were FACS®-sorted (MoFlo) from an active RA patient before treatment (white columns) and a responding RA patient (black columns) receiving anti-TNFα therapy using the indicated gates is depicted in Fig. S1. The CD4+CD25high and the CD4+CD25low T cells were cultured alone or with CD4+CD25− responder T cells (5 × 104 cells/well) and stimulated with 5 μg/ml of soluble anti-CD3/anti-CD28 for 48 h. 2 μM monensin was added for the last 5 h of culture. Cells were stained to detect intracellular TNFα and IFNγ in a similar fashion to those in Fig. 1 B. Mean ± SEM of three patients before and after anti-TNFα therapy is shown. (B) Cytokines from the supernatants collected from the experiments from the three patients before and after anti-TNFα treatment (A) were measured by CBA and shown separately in each of the panels.
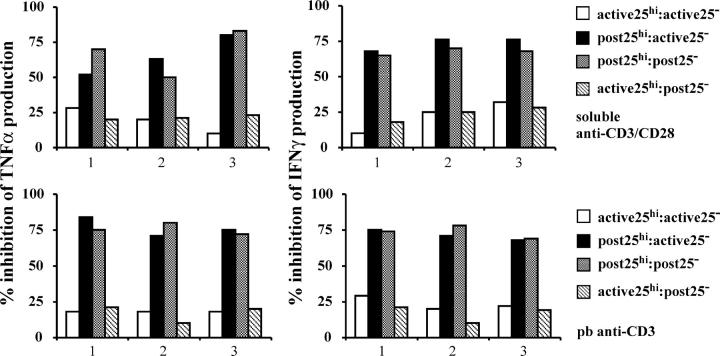
Next, we investigated the possibility that effector T cells are resistant to the suppressive effect exerted by the regulatory T cells in patients with RA. CD4+CD25− or CD4+CD25high T cells from patients before therapy were cultured with either CD4+CD25high T cells or with CD4+CD25− T cells isolated 3 mo after therapy. The results in Fig. 3 show that CD4+CD25high T cells from active RA patients were unable to suppress the production of cytokines released by effector T cells isolated after anti-TNFα treatment. In contrast, 3 mo after anti-TNFα therapy, CD4+CD25high T cells regained their ability to inhibit IFNγ and TNFα released by autologous effector T cells from active RA patients. The same conclusion could be drawn whether the cultures were stimulated with soluble anti-CD3/anti-CD28 (Fig. 3, top) or plate-bound anti-CD3 (Fig. 3, bottom). Therefore, our data demonstrate that CD4+CD25high T cells are functionally defective in RA patients and the lack of suppression is not due to effector T cell resistance.
Figure 3.
CD4+CD25high T cells from patients with active RA fail to suppress cytokines produced by CD4+CD25− T cells derived from the same patient either before or after anti-TNFα therapy. CD4+CD25high and CD4+CD25− T cells (5 × 104 cells/well) isolated from patients with active RA (n = 3) or from patients responding to anti-TNFα therapy (n = 3) were mixed and stimulated with 5 μg/ml of soluble anti-CD3/anti-CD28 (top) or with 1 mg/ml of plate-bound anti-CD3 (bottom). Supernatants were analyzed by CBA. The results indicate the percentage of inhibition of the cytokine production relative to the CD4+CD25− T cells stimulated alone. One out of two experiments is shown.
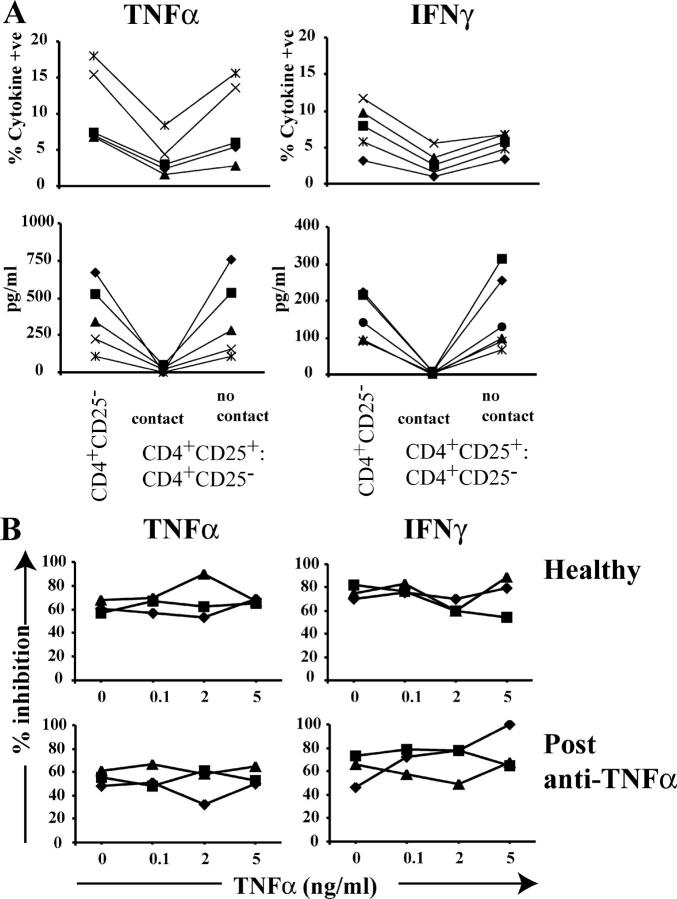
In the next experiment, we wished to establish whether the recovery in function mediated by anti-TNFα therapy is cell contact dependent and cytokine independent. In coculture experiments, freshly isolated CD4+CD25− T cells were separated in transwell chambers from CD4+CD25high T cells. Prevention of cell contact abolished their suppressive capacity (Fig. 4 A). Next, we investigated whether addition or neutralization of TNFα in vitro affects regulatory T cells. The results in Fig. 4 B demonstrate that the addition of TNFα to the coculture system did not alter the suppressive function of regulatory T cells isolated after anti-TNFα treatment. In vitro neutralization of TNFα (Infliximab) was not sufficient to restore the defect in CD4+CD25high T cell–mediated suppression (unpublished data).
Figure 4.
The inhibitory effect of CD4+CD25high T cells requires cell contact and is not affected by the addition of TNFα in vitro. (A) CD4+CD25high and responder T cells derived from patients responding to anti-TNFα therapy were stimulated with 5 mg/ml of soluble anti-CD3/anti-CD28 either with or without the use of a transwell membrane to prevent cell contact. (top) 2 μM monensin was added for the last 5 h of culture, and cells were stained for detection of intracellular cytokine production. (bottom) Cytokines from the supernatants collected from the same experiment were measured by CBA. (B) CD4+CD25high and CD4+CD25− T cells isolated from healthy as well as responding patients were cultured 1:1 ratio, upon anti-CD3/anti-CD28 stimulation, in the presence of different concentrations of TNFα. The percentage of suppression of cytokine production was calculated in each individual. One out of two experiments is shown.
Depletion of CD4+CD25+ T Cells from PB Isolated from Anti-TNFα–treated Patients Results in an Increase in TNFα Levels and in an Inhibition of IL-10 Production.
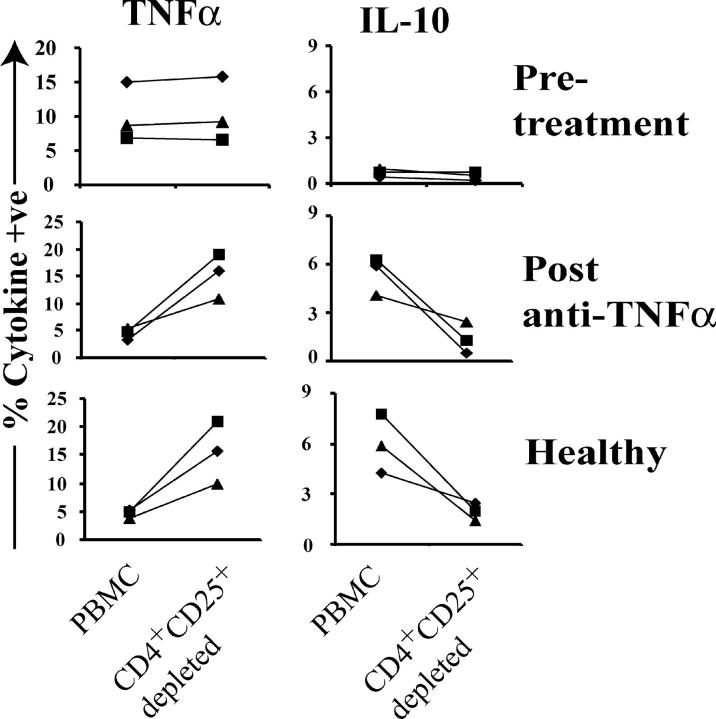
Next, we evaluated how CD4+CD25+ T cell depletion influences the cytokine profile of PB before and after anti-TNFα treatment. PB from patients with active RA synthesized high levels of TNFα, but no IL-10 (Fig. 5). After treatment, a reduced amount of TNFα and an increased number of IL-10–secreting cells were detected in PB. After depletion of CD4+CD25high T cells from PB derived from patients with active RA, there was no change in the percentage of cells producing TNFα or IL-10, whereas a fivefold increase in TNFα and a >10-fold reduction in IL-10 production occurred when regulatory T cells were depleted from anti-TNFα–treated RA patients. Analysis of PB from healthy individuals gave similar results to patients responding to anti-TNFα therapy. These results provide additional evidence that regulatory T cells from patients with active RA are ineffective in terms of suppressing TNFα release and that this function is restored after treatment.
Figure 5.
Effect of depletion of CD4+CD25+ T cells from PBMCs on TNFα and IL-10 production from the same patient before and after treatment. Total PBMCs or PBMCs depleted of CD25+ cells (by MACS® sorting) were stimulated with 5 μg/ml of soluble anti-CD3/anti-CD28 for 48 h. 2 μM monensin was added for the last 5 h of culture. Staining of CD4 and CD25 surface expression was performed. Cells were permeabilized and stained with PE-conjugated anti-TNFα or anti–IL-10. Results from three patients before and after therapy and three healthy individuals are shown.
CD4+CD25+ Regulatory T Cells from Active RA Patients Lack the Ability to Convey Suppressor Activity to Effector T Cells: Restoration after Anti-TNFα Treatment.
The generation of newly formed regulatory T cells may be a further mechanism by which regulatory T cells could control autoreactive T cells and the consequent inflammation. It has been shown recently that regulatory T cells, isolated from healthy individuals, convey a suppressive phenotype to “conventional” activated T cells (19). To explore this possibility in patients with RA, we tested the capacity of regulatory T cells, isolated before and after treatment, to convey a suppressive phenotype to CD4+CD25− T cells. CD4+CD25+ T cells were isolated as previously mentioned and cultured 1:1 with the CD4+CD25 T cell fraction in the presence of soluble anti-CD3/anti-CD28. After 3 d, CD4+CD25+ T cells were depleted, and the remaining negative fraction (CD4+CD25−) was cultured 1:1 with freshly isolated autologous CD4+CD25− T cells for an additional 5 d. In agreement with results reported from Jonuleit et al. (19), the resulting CD4+CD25− T cell fraction purified from healthy or anti-TNFα–treated patients suppressed the proliferation of freshly isolated autologous T cells (Fig. 6). In contrast, similarly prepared CD4+CD25− T cells from patients with active RA showed no suppressive activity on freshly isolated T cells, suggesting that regulatory T cells from these patients are unable to imprint a “suppressive” phenotype onto the CD4+CD25− T cells. As an additional control, we also depleted CD4+CD25+ T cells (which should represent the newly activated T cells) from the CD4+CD25− T cells cultured alone, and replated with freshly isolated CD4+CD25− T cells. No suppressive phenotype was acquired under these experimental conditions (unpublished data), confirming that this phenomenon is regulatory T cell dependent. These results demonstrate that regulatory T cells isolated from patients with active RA are unable to inhibit the production of proinflammatory cytokines and to induce the differentiation of additional populations of suppressor cells.
Figure 6.
Only CD4+CD25+ regulatory T cells derived from patients treated with anti-TNFα, but not from patients with active RA, convey a suppressive phenotype to effector T cells. MACS®-sorted CD4+CD25+ T cells and CD4+CD25− T cells were cultured either alone or mixed at a 1:1 ratio (105 cells/well) and stimulated with 1 μg/ml of soluble anti-CD3 and 2 μg/ml anti-CD28. After 5 d of culture, CD4+CD25+ T cells were depleted and the remaining CD4+CD25− T cells were replated 1:1 with freshly isolated autologous CD4+CD25− T cells in the presence of anti-CD3 and anti-CD28 for an additional 3 d (third column). 3[H]Tdr was added for the final 8 hof culture (expressed as mean ± SEM of triplicate cultures). Results from three patients before and after therapy and three healthy individuals are shown.
The Frequency of CD4+CD25high T Cells Is Higher in Anti-TNFα–responding Patients Compared with the Same Patients before Treatment.
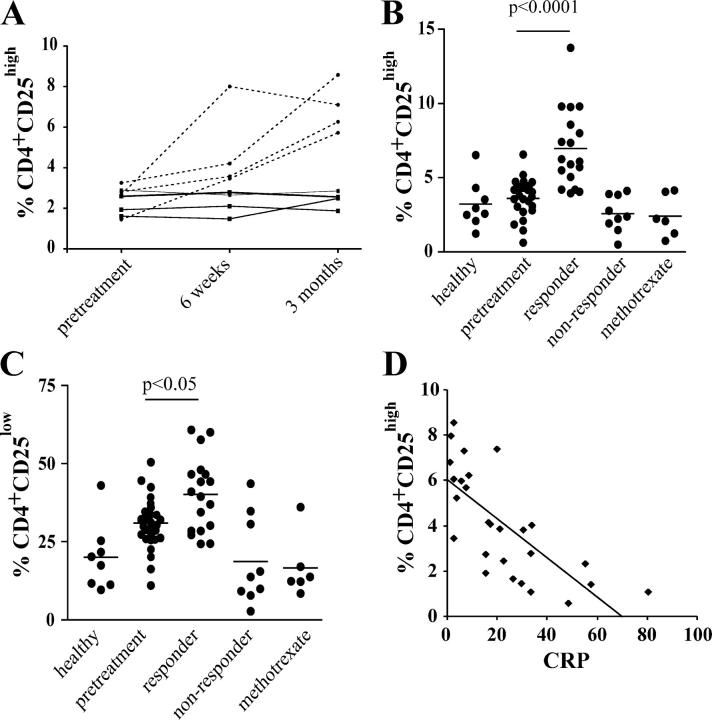
Finally, we monitored the numbers of regulatory T cells in patients with RA, before and after treatment (Table S1, available at http://www.jem.org/cgi/content/full/jem.20040165/DC1). The expression of CD25high/low on CD4+ T cells was initially examined in four responding and four nonresponding patients during the first 3 mo of treatment using FACS® analysis. Although the percentage of CD4+CD25high T cells increased over time in those patients who responded to anti-TNFα therapy, there was no change in the percentage of CD4+ CD25high T cells in the four nonresponding patients (Fig. 7 A). The percentage of this population continued to rise in three out of the four responding patients 6 wk after the first infusion of Infliximab and remained stable after 3 mo of treatment (unpublished data). The subsequent analysis in a larger cohort of patients, including those responding to methotrexate therapy, revealed that the percentage of both CD4+CD25high/low T cells was significantly increased in anti-TNFα–responding patients compared with the levels measured in those with active RA, methotrexate-treated patients, and healthy controls (Fig. 7, B and C). Active RA patients also showed an increased percentage of CD4+CD25low T cells, which likely reflects increased activation of CD4+ T cells, and does not represent any changes in the regulatory T cell population, in accordance with our (Fig. 2) and previously published data indicating that the regulatory CD4+ T subset is largely found within the CD25high subset (10). The methotrexate-treated individuals were matched to the responding anti-TNFα–treated patients with respect to their response to treatment. Consistent with previous results, no reduction in numbers of regulatory T cells was measured in patients with active RA compared with healthy individuals (17), suggesting that numerical deficiency is unlikely to be responsible for any break in immune tolerance in RA, as has been suggested in some mouse models of autoimmune disease (3, 4), and in patients with type 1 diabetes mellitus (14). No differences in CD4+CD25high/low expression were detected in patients with RA who failed to respond to anti-TNFα therapy compared with healthy controls. A significant correlation between the CRP, a measure of rheumatoid disease activity, and the percentage of CD4+CD25high T cells (but not CD4+CD25low) confirmed the relationship between a response to anti-TNFα therapy and an expansion in this cell population (Fig. 7 D). In agreement with previous papers, there was no significant change in the absolute numbers of CD4+ T cells in PBMCs before treatment and 3 mo after anti-TNFα treatment, indicating that the changes in percentages observed in CD4+CD25+ T cells reflected alterations in the absolute numbers of cells (20).
Figure 7.
Increase in CD4+CD25high T cells in the PB of RA patients responding to anti-TNFα therapy. (A) The percentage of CD4+ T cells expressing CD25high was followed in four anti-TNFα responder (dashed line) and four nonresponder (solid line) patients with RA before and after treatment. (B) Analysis of larger group of patients treated with anti-TNFα (n = 27), methotrexate alone (n = 6), and healthy controls (n = 8). CD4+CD25high and (C) CD4+CD25low T cells (as defined in Fig. S1 A). (D) Scatter plot showing a significant correlation between the C reactive protein and the percentage of CD4+CD25high T cells before and after treatment (P < 0.001, r2 = 0.5278).
Discussion
The data presented here provide new insight into the biology of regulatory T cells within the context of a human autoimmune disease. CD4+CD25high T cells isolated from patients with active RA, although still anergic, show compromised function as demonstrated by their inability to regulate proinflammatory cytokines released by effector T cells and monocytes. After Infliximab treatment, regulatory T cell–mediated suppression was restored to the level found in healthy individuals, whereas only a partial restoration was seen in regulatory T cells isolated from patients responding to methotrexate. Although it is well documented that Infliximab blocks both soluble and transmembrane TNFα, resulting in a strong inhibition of other proinflammatory cytokines, there is no unanimity about the effects of methotrexate on cytokine production in RA (21, 22). If proinflammatory cytokines are not efficiently suppressed in methotrexate-treated patients, this could have a deleterious effect on the function of regulatory T cells, thus explaining the different level of regulatory T cell–mediated inhibition in patients treated with these two therapies.
It is unclear why regulatory T cells isolated from RA patients are unable to suppress lymphocyte and monocyte cytokine production. One possibility is that the presence of TNFα as well as other proinflammatory cytokines may hinder the ability of regulatory T cells to prevent autoimmune disease. In a recent paper that examined the role of this cytokine with respect to regulatory T cells, TNFα impaired the ability of regulatory T cells to suppress disease in the NOD mouse model of diabetes (4). These authors suggested that endogenous levels of TNFα might act centrally in the thymus to mediate these effects on regulatory T cells. Pertinent to their hypothesis is the recent observation that CD4+CD25+ T cells derived from the thymus of healthy donors have an increased expression of TNFRII compared with CD4+CD25− T cells (12), which might result in an increased susceptibility of regulatory T cells to the actions of TNFα. However, our in vitro data do not lend support to a direct effect of TNFα on regulatory T cells because, after exposure to a gradient of TNFα concentration alone, regulatory T cells were still viable and functionally active. These results indicate that more complex mechanisms are likely to operate in vivo. That regulatory T cells isolated from patients affected by chronic inflammatory disorders show altered function is not only peculiar to RA. Indeed, it has been reported recently that regulatory T cells isolated from the PB of patients with multiple sclerosis are functionally defective in terms of their ability to suppress proliferation and cytokines production by activated T cells (23). This finding, together with our data, might shed some light on the role that regulatory T cells play in controlling the maintenance of peripheral tolerance and in the prevention of human autoimmunity.
It has been speculated previously that the spreading of suppression from CD4+CD25+ T cells to responder T cells is an important mechanism by which regulatory T cells exert their effect in the maintenance of peripheral tolerance (19). This is an attractive explanation for how such small numbers of cells could prevent autoimmune disease. Our in vitro data suggest that, unlike regulatory T cells from healthy individuals, those derived from active RA patients cannot convey a suppressive phenotype to activated T cells. To our knowledge, this is the first time that this phenomenon has been shown to be impaired in an autoimmune disease. This defect could have a significant impact on the regulation of RA pathology because it is likely that the generation of newly formed (and fully functional) suppressor T cells is critical to control autoimmunity.
In searching for the mechanism by which these regulatory T cells act, we found that the suppression of cytokine production by regulatory T cells after treatment was contact dependent, and soluble factor independent as neutralization of IL-10 and TGFβ did not alter their suppressive function (unpublished data). However, depletion of CD4+ CD25+ T cells from treated PB led to a reduction in the number of IL-10–secreting cells. This suggests that the production of IL-10 may be dependent on the presence of regulatory T cells, but that its release is by another population such as a subset of suppressor cells that do not express CD25. It has been suggested that CD4+CD25+ T cells may actively promote the differentiation of Tr1 cells that mediate their suppression through production of IL-10 (24). As IL-10 is widely known for its antiinflammatory properties, it is tantalizing to hypothesize that the increase in this cytokine, previously reported in anti-TNFα–treated patients (20, 25), is orchestrated by regulatory T cells whose function has been restored. Interestingly, we were unable to detect IL-10 in any of the T cell populations analyzed, suggesting that the production of IL-10 depends on the interaction of a variety of cell types (and/or their soluble products) present in whole PBMCs.
The other major finding described in our paper is the increased regulatory T cell number in RA patients treated with anti-TNFα, which significantly correlated with a reduction in CRP value. It is tempting to speculate that the increase in regulatory T cells in responding patients is important in the amelioration of disease and may help to differentiate between responder and nonresponder patients because the changes occur within 6 wk of initiation of therapy. It is conceivable that TNFα has a direct effect on regulatory T cell viability, such as the induction of apoptosis (26), which would explain the increased number of regulatory T cells after TNFα neutralization. As aforementioned, our in vitro data do not support this hypothesis. Moreover, we found no reduction in regulatory T cell numbers in patients with active RA compared with healthy donors, suggesting that neither apoptosis, nor any other direct effect on regulatory T cell numbers, are the primary mechanisms by which TNFα is acting. The lack of difference in CD25high regulatory T cell numbers found in active patients, compared with healthy individuals, needs more careful study, as the CD25high regulatory T cells derived from active RA could be “contaminated” by recently activated CD4+ T cells, which would mask the total number of regulatory T cells. Indeed, human CD4+CD25+ T cells are not a homogenous population and contain nonsuppressive fractions (10, 27). Of equal importance to the issues already raised is the possibility that an increase in regulatory T cells may have deleterious consequences, in particular an increased susceptibility to infections, which is a major issue in patients receiving anti-TNFα therapy (28).
Further studies are required to elucidate the mechanisms that underlie these observations in patients with RA, and to relate the clinical and immunological responses in patients receiving anti-TNFα therapy. Restoration of the function of regulatory T cells or transfer of fully competent regulatory T cells could be a useful therapeutic tool in the treatment of RA (29).
Acknowledgments
We thank Dr. L. Wedderburn for helpful comments on the paper.
This work was supported by the Arthritis Research Campaign and The Wellcome Trust.
The authors have no conflicting financial interests.
Abbreviations used in this paper: CBA, cytometric bead array; PB, peripheral blood; RA, rheumatoid arthritis.
References
- 1.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18–32. [DOI] [PubMed] [Google Scholar]
- 2.Bach, J.F., and J. Francois Bach. 2003. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 3:189–198. [DOI] [PubMed] [Google Scholar]
- 3.Salomon, B., D.J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J.A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12:431–440. [DOI] [PubMed] [Google Scholar]
- 4.Wu, A.J., H. Hua, S.H. Munson, and H.O. McDevitt. 2002. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc. Natl. Acad. Sci. USA. 99:12287–12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohm, A.P., P.A. Carpentier, H.A. Anger, and S.D. Miller. 2002. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169:4712–4716. [DOI] [PubMed] [Google Scholar]
- 6.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan, M.E., R.P. Sutmuller, H.J. Witteveen, L.M. van Duivenvoorde, E. Zanelli, C.J. Melief, A. Snijders, R. Offringa, R.R. de Vries, and R.E. Toes. 2003. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 48:1452–1460. [DOI] [PubMed] [Google Scholar]
- 8.Taams, L.S., J. Smith, M.H. Rustin, M. Salmon, L.W. Poulter, and A.N. Akbar. 2001. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122–1131. [DOI] [PubMed] [Google Scholar]
- 9.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baecher-Allan, C., J.A. Brown, G.J. Freeman, and D.A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245–1253. [DOI] [PubMed] [Google Scholar]
- 11.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annunziato, F., L. Cosmi, F. Liotta, E. Lazzeri, R. Manetti, V. Vanini, P. Romagnani, E. Maggi, and S. Romagnani. 2002. Phenotype, localization, and mechanism of suppression of CD4+CD25+ human thymocytes. J. Exp. Med. 196:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephens, L.A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247–1254. [DOI] [PubMed] [Google Scholar]
- 14.Kukreja, A., G. Cost, J. Marker, C. Zhang, Z. Sun, K. Lin-Su, S. Ten, M. Sanz, M. Exley, B. Wilson, et al. 2002. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 109:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann, M. 2002. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2:364–371. [DOI] [PubMed] [Google Scholar]
- 16.Van Parijs, L., and A.K. Abbas. 1998. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 280:243–248. [DOI] [PubMed] [Google Scholar]
- 17.Cao, D., V. Malmstrom, C. Baecher-Allan, D. Hafler, L. Klareskog, and C. Trollmo. 2003. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33:215–223. [DOI] [PubMed] [Google Scholar]
- 18.Lamas, M., E. Sanz, L. Martin-Parras, E. Espel, P. Sperisen, M. Collins, and A.G. Silva. 1993. Glucocorticoid hormones upregulate interleukin 2 receptor alpha gene expression. Cell. Immunol. 151:437–450. [DOI] [PubMed] [Google Scholar]
- 19.Jonuleit, H., E. Schmitt, H. Kakirman, M. Stassen, J. Knop, A.H. Enk, A. Tuettenberg, and G. Schuler. 2002. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuerwegh, A.J., J.F. Van Offel, W.J. Stevens, C.H. Bridts, and L.S. De Clerck. 2003. Influence of therapy with chimeric monoclonal tumour necrosis factor-alpha antibodies on intracellular cytokine profiles of T lymphocytes and monocytes in rheumatoid arthritis patients. Rheumatology (Oxford). 42:541–548. [DOI] [PubMed] [Google Scholar]
- 21.Swaak, A.J., H.G. van den Brink, and L.A. Aarden. 1997. Cytokine production in whole blood cell cultures of patients with rheumatoid arthritis. Ann. Rheum. Dis. 56:693–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudwaleit, M., Z. Yin, S. Siegert, M. Grolms, A. Radbruch, J. Braun, and J. Sieper. 2000. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann. Rheum. Dis. 59:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viglietta, V., C. Baecher-Allan, H.L. Weiner, and D.A. Hafler. 2004. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199:971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levings, M.K., R. Bacchetta, U. Schulz, and M.G. Roncarolo. 2002. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int. Arch. Allergy Immunol. 129:263–276. [DOI] [PubMed] [Google Scholar]
- 25.Ohshima, S., Y. Saeki, T. Mima, M. Sasai, K. Nishioka, H. Ishida, M. Shimizu, M. Suemura, R. McCloskey, and T. Kishimoto. 1999. Long-term follow-up of the changes in circulating cytokines, soluble cytokine receptors, and white blood cell subset counts in patients with rheumatoid arthritis (RA) after monoclonal anti-TNF alpha antibody therapy. J. Clin. Immunol. 19:305–313. [DOI] [PubMed] [Google Scholar]
- 26.Sarin, A., M. Conan-Cibotti, and P.A. Henkart. 1995. Cytotoxic effect of TNF and lymphotoxin on T lymphoblasts. J. Immunol. 155:3716–3718. [PubMed] [Google Scholar]
- 27.Levings, M.K., R. Sangregorio, C. Sartirana, A.L. Moschin, M. Battaglia, P.C. Orban, and M.G. Roncarolo. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor β, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 196:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bresnihan, B., and G. Cunnane. 2003. Infection complications associated with the use of biologic agents. Rheum. Dis. Clin. North Am. 29:185–202. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz, D.A., J.D. Gray, and S.G. Zheng. 2002. The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases. Arthritis Res. 4:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]