Abstract
The propensity of naive CD4 T cells to become T helper (Th) type 2 cells correlates with susceptibility to infection by the protozoal parasite Leishmania major. Using genetic linkage analysis, we earlier identified Dice1 as a Th2 cell bias-controlling quantitative trait locus on chromosome 16. Using interval-specific congenic mapping, we now resolve Dice1 into two independent genetic loci, Dice1.1 and Dice1.2, which control Il4 expression from naive Th cells and thereby indirectly control Th2 cell bias. Interestingly, only one of the two congenic intervals containing Dice1.1 and Dice1.2, respectively, also contained an L. major response locus, indicating that L. major responsiveness can be insensitive to determinants that influence Th2 cell bias by controlling naive T cell Il4 expression. These results lay the groundwork for identifying the Dice1.1 and Dice1.2 genes controlling naive T cell Il4 expression and L. major responses, and for testing whether these control other Th2 cell–dependent processes such as worm expulsion, allergic asthma, and dermatitis.
Keywords: early IL-4, naive T cell, BALB/C, Dice, IL-4
Introduction
Mature Th cells have been classified into two main lineages based upon their cytokine gene expression pattern: Th2, which is permissive for IL-4 and IL-13 but not IFN-γ, and Th1 with the reciprocal pattern. Th1 cells are essential for the control of intracellular pathogens such as Leishmania major (1), whereas Th2 cells play a key role in the control of large extracellular pathogens such as helminths (2). When inappropriately generated, Th2 cells can cause allergic diseases such as asthma (3) or impede protection from L. major and other intracellular pathogens (1).
IL-4, the signature cytokine of Th2 cells, is itself a critical determinant of Th2 cell development (4). On naive CD4 T cells (the common progenitor of Th1 and Th2 cells), IL-4 engages the IL-4R to activate STAT6 that in turn up-regulates the zinc finger transcription factor GATA3, a master regulator of Th2 cell development (5, 6). The initial in vivo source of the IL-4 necessary to instruct Th2 cell development has been controversial, with reports of contributions from mast cells, basophils, eosinophils, and NK T cells (7, 8). Although it is difficult to exclude that in specific contexts each of these cell types may play instructive roles for Th2 cell development, it is clear that in the absence of inflammatory stimuli such as IL-12 and IFN-γ, naive Th cells can suffice to instruct their own Th2 cell development by an autocrine IL-4 pathway (9–11). The initial production of IL-4 in this pathway occurs even in the presence of blocking anti–IL-4R antibody or from STAT6 KO naive Th cells. Despite its critical role in the initiation of Th2 cell development, the TCR-dependent, IL-4R/STAT6-independent pathway regulating the production of IL-4 from naive Th cells is not well understood.
The ability of naive Th cells to express IL-4 rapidly (hereafter, nIL-4) and Th2 cell bias are linked phenotypic traits subject to a wide degree of genetic variation. Among common inbred mouse strains, these traits range from the high nIL-4/high Th2 cell bias phenotypes of BALB/c to the low nIL-4/low Th2 cell bias phenotypes of C57BL/6 and B10.D2 (9–11). Susceptibility to L. major infection correlates with the high nIL-4/high Th2 cell bias phenotypes of BALB/c, consistent with the known antagonistic role of Th2 cell responses in the IFN-γ–dependent activation of macrophages required by the host to control intracellular parasite replication (12, 13).
To gain a better understanding of the genetic determinants controlling susceptibility to Th2 cell–dependent diseases, we earlier performed linkage analysis on a [(BALB/c × C57BL/6)F1 × C57BL/6] backcross cohort to identify loci regulating Th2 cell bias (9). 63 mice were genotyped for the segregation of simple sequence length polymorphism (SSLP) markers distributed across the genome at an average density of 10 cM. These data were correlated with phenotypic measurements of Th2 cell bias by determining the quantitative level of IL-4 secreted into the media of CD4-enriched splenocyte cultures activated by TCR ligation. In this culture system, parental BALB/c cells secrete ∼50-fold more IL-4 than C57BL/6 or B10.D2, and F1 animals display an intermediate phenotype, demonstrating codominant inheritance of this trait (9). The phenotype distribution profile of the backcross cohort indicated that Th2 cell bias was a multigenic trait (9). A quantitative trait locus (QTL) designated Dice1, accounting for 27% of the trait variance, was detected on centromeric chromosome 16 (9).
Here, we describe the use of interval-specific congenic mapping to identify the Th2 cell bias-controlling locus predicted by the Dice1 QTL. Instead of one, we discovered two loci, Dice1.1 and Dice1.2, which control Th2 cell bias. In each case, the proximal target of this genetic control is likely to be in the nIL-4 pathway, as both loci also control nIL-4 levels. Despite sharing in common the ability to control nIL-4 and Th2 cell bias, we found that only one locus (Dice1.2) could control susceptibility to L. major infection. We discuss the implications of these findings for the role of Th2 cell bias in determining L. major susceptibility.
Materials and Methods
Mice.
All mice were bred and maintained in specific pathogen-free or modified specific pathogen-free (for L. major infections) vivaria in the Health Sciences Complex at the University of Washington according to protocols approved by the University of Washington Animal Care and Use Committee. BALB/cAnN mice were obtained from Taconic Farms and B10.D2/OsnJ mice were obtained from The Jackson Laboratory. BALB. D2c16 was obtained at the eighth backcross to BALB/cAnN (provided by A. Beebe and B. Coffman, DNAX Research Institute, Palo Alto, CA). Before the generation of the C.16D2 congenic panel, BALB.D2c16 was maintained through at least two more rounds of serial backcrossing to BALB/cAnN coupled with genotypic selection at each generation for retention of B10.D2 chromosome 16.
Producing Recombinant Congenic Strains.
BALB.D2c16 was backcrossed to BALB/cAnN and progeny were screened for recombination across the congenic interval using SSLP markers spanning chromosome 16 (see Online Supplemental Material section below for marker information). Nine animals were selected that together harbored a set of recombinant congenic intervals (1–9) comprising a tiling path across most of chromosome 16. Each of these was then backcrossed to BALB/cAnN. Nonrecombinant progeny from each backcross were identified by SSLP screening and intercrossed to produce homozygous recombinant congenic animals. These were identified by SSLP screening and then intercrossed to generate a pure-breeding stock that had been backcrossed to BALB/cAnN at least 11 times. The nine congenic lines are designated C.16D2/1, C.16D2/2, C.16D2/3, C.16D2/4, C.16D2/5, C.16D2/6, C.16D2/7, C.16D2/8, and C.16D2/9.
Th2 Cell Bias Assay.
CD4-enriched splenocytes were prepared by complement-mediated lysis of CD8+ T cells (3.155; American Type Culture Collection [ATCC]]), MHC class II+ B cells, dendritic cells, and macrophages (BP107; ATCC), and HSA+ B cells, γδ+ T cells, monocytes, granulocytes, and erythrocytes (J11d; ATCC). CD4 T cell purity, as determined by FACS analysis, was always >80%. In brief, total splenocytes (30 × 106/ml) were incubated at 37°C in a cocktail of purified antibodies and a combination of rabbit and guinea pig complement for 50 min. Live cells were recovered by centrifugation through Ficoll, washed, resuspended in cell culture medium (RPMI 1640 with 10% heat-inactivated fetal calf serum, 50 μM β-mercaptoethanol, 2 mM l-glutamine, and 100 U/ml penicillin and streptomycin), counted, and distributed (106 CD4 T cell equivalents/well, determined by FACS analysis) with 25 U/ml recombinant human IL-2 (Roche) into 24-well plates precoated with 1 ug/ml anti-TCRβ–specific antibody (H57.597; ATCC). On day 5, cells were harvested, washed extensively, counted, and redistributed (105/well) with 25 U/ml recombinant human IL-2 (Roche) into 96-well, flat-bottom plates precoated with 10 ug/ml anti-TCRβ–specific antibody (H57.597; ATCC). 2 d later, supernatants were harvested and IL-4 content was determined by ELISA (coating antibody, 11B11 [NIH Response modifier program]; detecting antibody, BVD6 [ATCC]).
nIL-4 Assay.
Total RNA was isolated (STAT60; Tel-Test) from CD4-enriched splenocytes (prepared and cultured as described above) after 16 h of stimulation. RNA was converted to cDNA with reverse transcriptase (Superscript II; Invitrogen) according to the manufacturer's instructions. Il4 and Hprt sequence content was then measured by quantitative real-time PCR using intron-spanning primers (Il4: 5′-CTTATCGATGAATCCAGGCATCG-3′ and 5′-CATCGGCATTTTGAACGAGGTCA-3′; Hprt: 5′-GAGGGTAGGCTGGCCTATAGGCT-3′ and 5′-GTTGGATACAGGCCAGACTTTGTTG-3′). Il4 expression was normalized internally to Hprt. These normalized values were then expressed relative to expression in NIH3T3 fibroblasts (ATCC) that do not express Il4 (unpublished data).
L. major Infection.
Cohorts of anesthetized mice were inoculated in both hind footpads with ∼4 × 105 metacyclic promastigotes of L. major (strain WHOM/IR/-/173), maintained as described previously (14). The size of footpad lesions was monitored weekly using metric calipers. Footpad thickness measurements are presented as the value measured on a given day (the group mean of six replicate measurements: three/foot/animal) minus the value measured just before infection. 5–6 wk after infection, animals were killed and footpad parasite burdens were determined. Footpads were washed in ethanol and rinsed and homogenized in 10 ml M199 media using a Tissu-Tearor (model 398; Biospec Products). Homogenates were serially diluted into 96-well microtiter plates and incubated in the dark at room temperature for 1 wk. Wells were monitored daily for motile promastigotes using inverted microscopy.
Statistical Analysis.
To compare results from different strains and from different experiments conducted over a period of time, we converted raw measurements into Index values (Itest) that normalized each mouse to the BALB/c and B10.D2 controls in a given experiment. This was computed as follows: Itest = (test − meanB10.D2)/(meanBALB/c − meanB10.D2). In this way, Itest values approaching 1 correspond to a BALB/c-like phenotype and those approaching 0 indicate a B10.D2-like phenotype. Before performing individual comparison tests of statistical significance, the statistical significance of each entire dataset was first assessed using the nonparametric Kruskal-Wallis test (nIL-4: P < 0.0001; Th2 cell bias: P < 0.0001). Multiple comparisons between control BALB/c and each C.16D2 congenic strain were then assessed using the nonparametric Mann Whitney test. Results of this analysis are shown in the legend to Fig. 3.
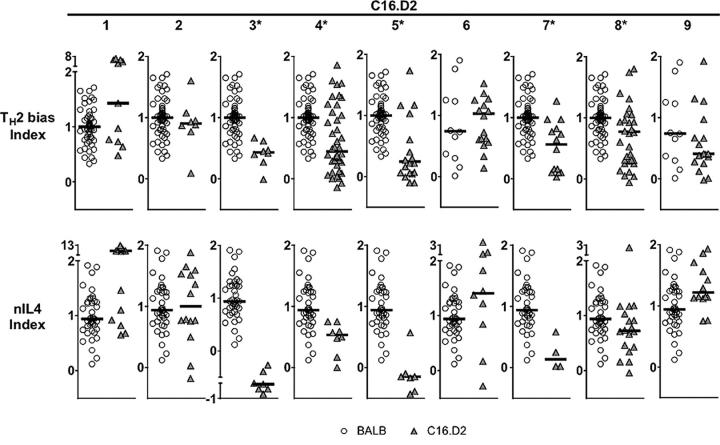
Figure 3.
Concordance between Th2 cell bias and nIL-4 traits among C.16D2 congenic strains. Shown are scatter plots of Th2 cell bias (top) and nIL-4 index values (bottom) of individual BALB/c (open circles) and C.16D2 congenic animals (gray triangles). Data shown are from experiments in which control BALB/c and B10.D2 differed significantly. For Th2 cell bias experiments involving C16.D2 strains 1–5, 7, and 8, P < 0.09; for C16.D2 strains 6 and 9, P < 0.2; and for all nIL-4 experiments, P ≤ 0.1. C16.D2 strains with phenotypes significantly lower than control BALB/c are indicated with an asterisk. Multiple comparison tests of significance were performed using the Mann-Whitney nonparametric test. P-values (Th2 cell bias, nIL-4) are as follows: C16D2/1 (0.5000, 0.500), C16D2/2 (0.7357, 0.8689), C16D2/3 (<0.0001, <0.0001), C16D2/4 (0.0002, 0.0008), C16D2/5 (0.0018, <0.0001), C16D2/6 (0.7884, 0.5000), C16D2/7 (0.0163, 0.003), C16D2/8 (0.0286, 0.0271), and C16D2/9 (0.1304, 0.5000).
Online Supplemental Material
Table S1 shows SSLP markers used to map chromosome 16. Shown are their names, positions on chromosome 16 (NCBI Mapviewer, build 33.1), forward and reverse primer sequences, and respective BALB/c and B10.D2 amplicon sizes (in basepairs). D16MIT markers are from MIT/Whitehead, whereas DA1.5, D1.3, D8.3, and D10.2 were generated by us. Table S1 is available at http://www.jem.org/cgi/content/full/jem.20040334/DC1.
Results
Producing the C.16D2 Congenic Panel.
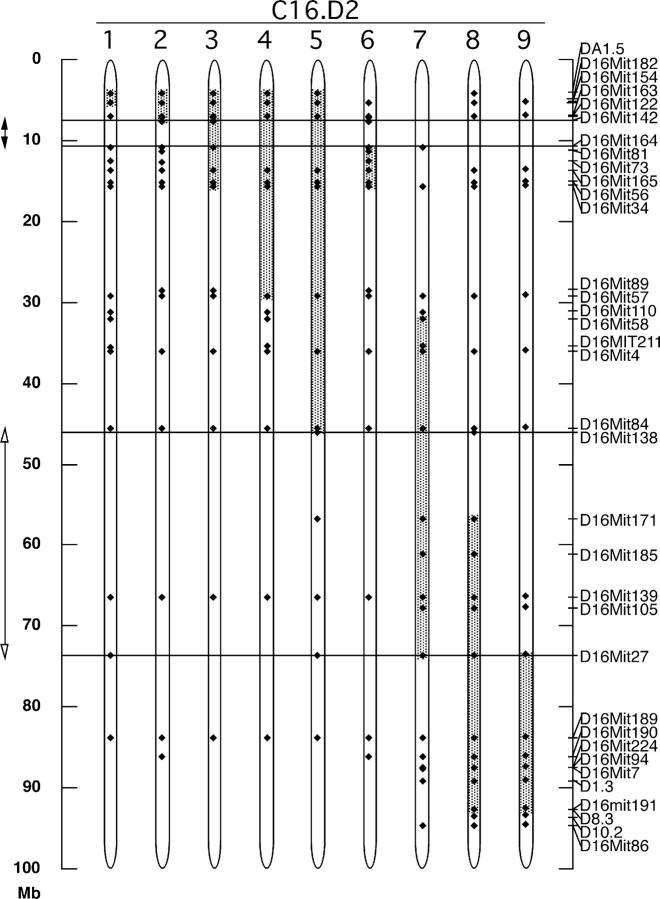
We adopted the classical strategy of Snell and Bunker (15) and constructed a panel of nine inbred BALB/c strains coisogenic with B10.D2 across discrete regions of chromosome 16, designated C.16D2/1-9. The C.16D2 congenic panel originated from BALB.D2c16, an animal that was produced from a founding (BALB/c × B10.D2)F1 backcrossed eight times to BALB/c and selected at each generation on the basis of resistance to L. major (16). It contained a B10.D2-derived congenic interval spanning the majority of chromosome 16. We propagated this strain by further backcrossing to BALB/c until at least N10, selecting progeny at each generation that continued to retain most of B10.D2 chromosome 16. Subsequently, recombinant progeny that had segregated only fragments of B10.D2 chromosome 16 were selected and independently backcrossed to BALB/c. Sister/brother progeny segregating the same recombinant intervals were then intercrossed to generate strains harboring homozygous congenic intervals. Collectively, these congenic strains represent an overlapping tiling path of B10.D2-derived genomic intervals that spans 92% of chromosome 16 (Fig. 1).
Figure 1.
Physical location of C.16D2 congenic intervals on chromosome 16. Shown for each C.16D2 strain (labeled at the top) are the portions of chromosome 16 inherited from B10.D2 (shaded) and BALB/c (unshaded). Locations where genotypes were determined are indicated with black diamonds. Genotyping markers used and their locations along chromosome 16 are indicated on the right axis. Double-headed arrows and horizontal lines indicate the inferred locations for Dice1.1 (closed arrowheads) and Dice1.2 (open arrowheads).
Th2 Cell Bias Phenotype of C.16D2 Congenic Strains.
To phenotypically screen the congenic panel for the Th2 cell bias trait we used a T cell restimulation assay similar to that used originally to identify the Dice1 QTL (9). In brief, this consisted of culturing CD4 T cell–enriched splenocytes with plate-bound anti-TCR antibody and IL-2 for 5 d, followed by recovery, washing, and restimulation at equivalent cell number with anti-TCR antibody and IL-2. 2 d later, IL-4 content in the media was measured by ELISA. To identify B10.D2-derived congenic intervals that shifted the phenotype of each BALB/c background congenic animal from BALB/c-like to B10.D2-like, we chose to compare the phenotypes of each congenic strain with the BALB/c parental strain. To compare results from multiple experiments performed over a period of time, we converted raw ELISA data (Fig. 2 A) into a Th2 cell bias index value that normalized IL-4 expression to the levels exhibited by the BALB/c and B10.D2 controls in a given experiment. Five of the nine C.16D2 congenic strains (C.16D2/3, 4, 5, 7, and 8) displayed significantly reduced Th2 cell bias compared with the high Th2 cell bias of the BALB/c control (Fig. 2 A and Fig. 3, top). By comparing the physical location of the chromosome 16 congenic intervals contained in the Dice + and Dice − C.16D2 strains we inferred the existence of at least two distinct loci controlling Th2 cell bias that we designated Dice1.1 and Dice1.2 (Fig. 1). The minimal Dice1.1 and Dice1.2 intervals are respectively, 3.2Mb (D16MIT142-D16MIT164) and 27.6Mb (D16MIT138-D16MIT27). Thus, by interval-specific congenic mapping we have revealed the Dice1 QTL to be genetically complex, comprising at least two independent loci, one of which (Dice1.1) is within the original 95% confidence interval for the location of Dice1. With these loci isolated in separate inbred strains segregating nonoverlapping congenic intervals, it was possible to test for the independent effects of each locus on other phenotypes.
Figure 2.
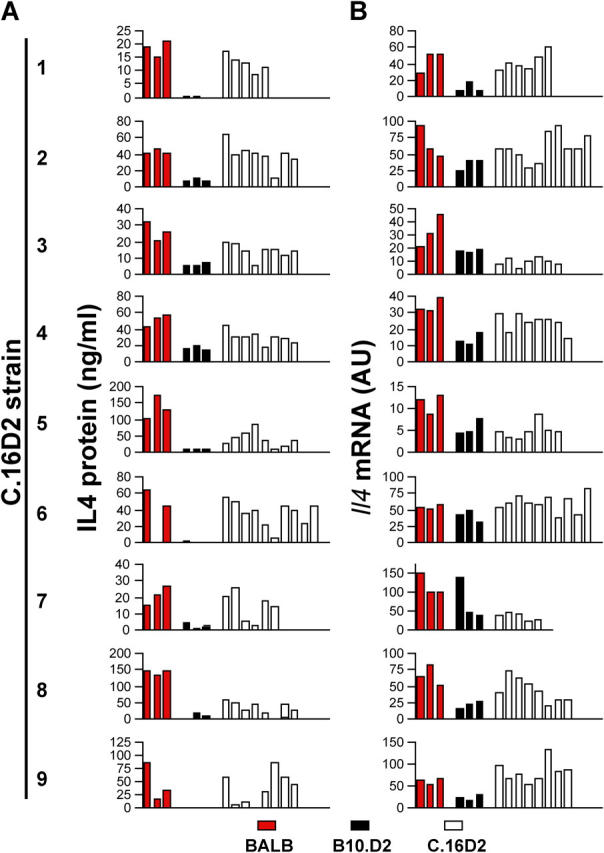
Representative raw Th2 cell bias and nIL-4 data. CD4-enriched splenocyte cultures stimulated with plate-bound anti-TCR and IL-2 were harvested (A) at 5 d for cells or (B) at 16 h for RNA. Cells harvested at 5 d were washed, counted, and restimulated at equivalent cell numbers for an additional 2 d, followed by analysis of IL-4 content in the media by ELISA. RNA analysis was by real-time RT-PCR with data normalized to HPRT and expressed relative to expression in NIH3T3 fibroblasts. Red, black, and open bars represent BALB/c, B10.D2, and C.16D2 strain mice (1–9), respectively. Where bars are not visible, value was below the limit of detection.
nIL-4 Phenotype of C.16D2 Congenic Strains.
In independent [(BALB/c × C57BL/6) × C57BL/6] backcross cohorts we noted similar phenotype distributions for the nIL-4 and the Th2 cell bias phenotypes (9). To test whether a common genetic pathway controlled both traits, we phenotyped the C.16D2 congenic strains for the nIL-4 trait by quantitative real-time RT-PCR measurement of Il4 mRNA abundance in 16-h stimulated, CD4 T cell–enriched splenocytes (Fig. 2 B). Five congenic strains (C.16D2/3, 4, 5, 7, and 8) displayed significant differences with the BALB/c control (Fig. 2 B and Fig. 3, bottom). Concordance in the phenotypes reported by the 16-h nIL-4 mRNA assay and the 7-d Th2 cell bias protein assay suggests that Dice1.1 and Dice1.2 control both traits. These results support the hypothesis that autocrine IL-4 production by naive CD4 T cells is a critical Th2 cell determinant and that Dice1.1 and Dice1.2 each act in a polymorphic pathway(s) leading to its production.
L. major Susceptibility of C.16D2 Congenic Strains.
Given the known role that Th2 cell bias plays in the susceptibility of the BALB/c strain to infection by the protozoal parasite L. major (1), we hypothesized that the Dice1.1 + and Dice1.2 + C16.D2 strains would display reduced susceptibility compared with BALB/c. To test this, we injected parasites into the footpads of control and congenic mice and followed the infection course by measuring footpad thickness at weekly intervals and footpad parasite burdens at the end of the assay. Contrary to our expectation, the Dice1.1 + C.16D2/3 and C.16D2/5 strains shared an infection course with the susceptible Dice1.1 − C.16D2/6 strain and the BALB/c control (Fig. 4, A and C). By contrast, the Dice1.2 + C.16D2/8 strain exhibited an infection course shifted toward the resistant phenotype of the B10.D2 control (Fig. 4 C). The susceptible and resistant phenotypes of mice harboring Dice1.1 and Dice1.2 congenic intervals, respectively, was also reflected in footpad parasite burdens (Fig. 4, B and D). Thus, the Dice1.2 but not the Dice1.1 congenic interval contains an L. major response locus.
Figure 4.
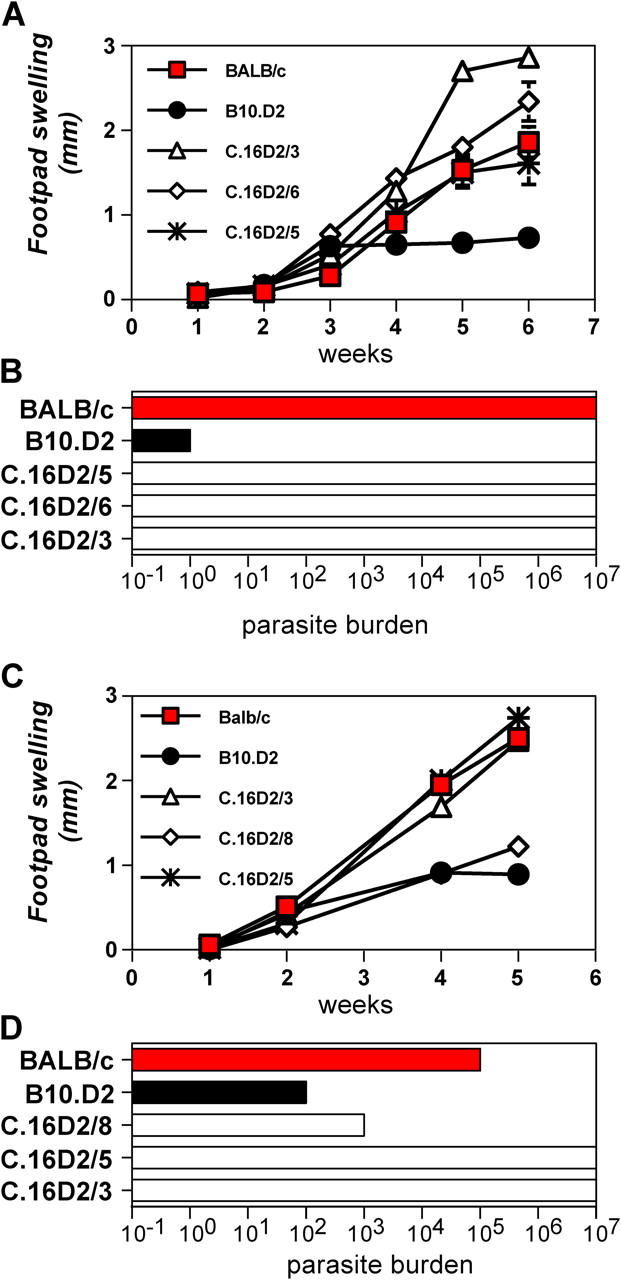
Response to L. major infection of mice harboring Dice1.1 and Dice1.2 congenic intervals. Groups of five to six mice were infected with metacyclic L. major parasites. The disease course was monitored by weekly measurement of footpad lesion size (A and C) and, at termination, footpad parasite burdens (B and D) in BALB/c (red fill), B10.D2 (black fill), and C16.D2 (white fill) strain mice. Error bars show SEM. Similar results for C.16D2/8 were obtained in three independent experiments.
Discussion
Interval-specific Congenic Mapping.
Although human genetic studies have succeeded in identifying many disease-linked QTL, rarely have these lead to the identification of susceptibility genes, and the majority languish in an unconfirmed state (17, 18). Most likely, this failure is due to the genetic complexity of disease susceptibility, involving multiple epistatic gene–gene and gene–environment effects that confound analysis. By modeling complex human diseases in mice, it becomes possible to take a reductionist approach to genetic dissection whereby individual susceptibility genes can be isolated on uniform genetic backgrounds and phenotypically analyzed in strictly controlled environments.
To confirm and genetically resolve the Dice1 QTL controlling Th2 cell bias, a trait associated with susceptibility to allergic airway disease and L. major infection, we undertook the classical interval-specific congenic mapping approach of Snell and Bunker (15). This approach converts a complex multigenic trait into its component genes, each isolated on distinct congenic intervals, segregating in separate inbred strains that, once produced, are good substrates for straightforward positional, expression, and/or candidate gene cloning strategies. The main pitfall of the approach is that phenotypic expression of a targeted QTL, once isolated from other genes contributing to the measured trait, may no longer be sufficiently strong and penetrant to allow reliable detection. Second, even if scorable phenotypes are maintained, there is no guarantee that a particular QTL will contain only a single gene. Indeed, it is not uncommon (as was the case in this study) for QTL to resolve into multiple distinct loci (17). In this case, further genetic resolution will depend upon each component gene's ability to confer continued phenotypic detection. Fortunately, this dilution effect can be offset partially in the case of recessive and codominantly inherited traits by interrogating congenic intervals in their homozygous state. Despite its potential shortcomings, interval-specific congenic mapping is proving to be a powerful tool for the identification of component genes involved in complex multigenic traits. Prominent recent examples include susceptibility to allergic airway disease (asthma) and autoimmune disease (systemic lupus erythematosus; references 12 and 19). This study adds Dice1 to the growing list of QTL that have yielded to interval-specific congenic mapping, an example, in this case, of a locus controlling susceptibility in an experimental model of a human infectious disease, cutaneous leishmaniasis.
The Th2 cell bias phenotypes conferred by Dice1.1 and Dice1.2 were both incompletely penetrant, requiring the analysis of multiple mice for their reliable detection. This result is consistent with the expectation that each component gene involved in a multigenic trait will contribute only a portion of the overall phenotype. It will be interesting to compare the phenotypes of bicongenic mice harboring both Dice1.1 and Dice1.2 with either monocongenic. An additive/non-epistatic phenotype would suggest that Dice1.1 and Dice1.2 participate in parallel pathways, whereas a synergistic or epistatic phenotype would suggest participation in a linear pathway. Given that the original Dice1 QTL could only account for 27% of the variance in Th2 cell bias between BALB/c and C57BL/6 in our original backcross cohort, it is perhaps surprising that the phenotypes controlled by Dice1.1 and Dice1.2 are so robust. Most likely, this reflects the advantage of scoring a codominant trait within animals harboring homozygous congenic B10.D2 intervals on a genetically homogeneous BALB/c background in the absence of confounding epistatic “noise” present in the original backcross cohort, resulting from the random segregation of multiple interacting B10.D2 intervals.
Identifying Genes Involved in Th2 Cell–dependent Diseases by Screening Genetic Loci Involved in Th2 Cell Bias.
Susceptibility to many immunological diseases arises from variation in the type of Th cell response elicited by foreign agents. In particular, susceptibility to allergic airway disease and cutaneous leishmaniasis is associated with inappropriate Th2 cell–biased responses (1, 12, 13). We hypothesized that disease susceptibility genes could be identified by focusing on simpler cellular (as opposed to organismal) traits that control key disease susceptibility pathways. Thus, we set out to identify genes involved in controlling Th2 cell bias with the hope that these would lead to the identification of genes involved in Th2 cell–dependent diseases. Providing validation for this approach, a recent study of asthma susceptibility exploited the HBA congenic strain, produced on a BALB/c background with a chromosome 11 genomic interval inherited from DBA/2. Although the congenic interval in this strain was selected on the basis of its ability to regulate Th2 cell bias, subsequent positional cloning uncovered the controlling Tim gene family as having a role in asthma susceptibility (20). Similarly, our interval-specific congenic mapping of the Th2 cell bias regulatory Dice1 QTL led to identification of a novel response locus for L. major. Together, these two examples support the general strategy of identifying genes involved in genetically complex Th2 cell–dependent susceptibility phenotypes by screening loci involved in controlling a simpler cellular phenotype: Th2 cell bias. It remains to be seen whether the Tim gene family plays a role in L. major susceptibility or if Dice1.1 and Dice1.2 play a role in asthma susceptibility.
Candidate Genes.
The minimal Dice1.1 congenic interval spans 3.2 Mb, contains 35 genes (NCBI Mapviewer, build 33.1), and overlaps the 95% confidence interval for the location of the original Dice1 QTL (2.5 cM centered on D16MIT81; reference 9). Potential candidate genes in this interval include those encoding transcription factors CRHSP-24 and CIITA and the intracellular signaling protein SOCS1 (21–23). Of these, the transcription factor CIITA is perhaps the most interesting insofar as it has been shown to interfere directly with Il4 expression in vivo (22). However, despite significantly lower expression of Il4 in 16-h stimulated splenocytes from C57BL/6 compared with BALB/c, no difference was detected in the level of C2ta mRNA (unpublished data). We cannot exclude the possibilities that analysis of highly purified naive CD4 T cells would reveal differential expression for C2ta or that a structural polymorphism underlies Dice1.1. Comparative sequencing and further differential expression analysis studies of C2ta and other Dice1.1 candidate genes are ongoing.
The minimal Dice1.2 congenic interval is large, spans 27.6 Mb, and includes 145 genes (NCBI Mapviewer, build 33.1). Potential candidate genes in this interval include those encoding the HMG box transcription factor BBX, the E3 ubiquitin ligase CBL-B, and the pituitary-specific transcription factor PIT1 (24–26). Another Th2 cell bias-controlling QTL called Cypr1 (cytokine production 1) has been mapped to a region of chromosome 16 just centromeric of the Dice1.2 interval (27), suggesting the possible identity of the locus predicted by Cypr1 and Dice1.2. The founding mouse used to produce the C.16D2 panel arose in the course of a study that used serial backcross mapping to identify six L. major resistance QTL, one of which mapped to the distal part of chromosome 16 (16). Transmission/disequilibrium testing suggested that this QTL was dependent upon epistatic interactions for its phenotypic expression, possibly explaining why its association with resistance in that study fell short of statistical significance. Nonetheless, one of the two chromosome 16 SSLP markers (D16MIT5) with the lowest p-values for association with resistance in this earlier study occurs within the Dice1.2 congenic interval, suggesting the possibility of identity between the locus predicted by this QTL and the L. major response locus we have identified.
The Functions of Dice1.1 and Dice1.2.
The strength of the nIL-4 and Th2 cell bias phenotypes (as reflected in the magnitude of Mann-Whitney p-values) displayed excellent concordance in all nine C16.D2 strains. The strength of both phenotypes was uniformly higher for strains containing Dice1.1 as opposed to Dice1.2. Together with the known requirement for nIL-4 in the development of Th2 cell bias (9–11), this result strongly suggests that the primary targets of both Dice1.1 and Dice1.2 are in the nIL-4 pathway. Developmental acquisition of the Dice-controlled nIL-4 trait has been shown to be T cell lineage autonomous (9), implying that Dice1.1 and Dice1.2 also act directly within naive T cells rather than indirectly through some other interacting cell type. Previous studies have shown that the Th2 cell bias trait is reflected in the proportion of IL-4–expressing cells as opposed to the level of IL-4 expression per cell, indicating a stochastic element to the Th2 cell commitment process (9). Together, our evidence that Dice1.1 and Dice1.2 influence Th2 cell bias indirectly through effects on nIL-4 imply that the level of IL-4 secreted by naive CD4 T cells contributes to their likelihood of maintaining Il4 in a transcriptionally permissive state. Biochemical and proteomic analysis of Th cells from the C.16D2 congenic strains will allow elucidation of the signaling pathways and components proximal to the Dice1.1 and Dice1.2 gene products, and may facilitate the identification of the Dice1.1 and Dice1.2 genes.
The Role of Dice1.1 and Dice1.2 in L. major Resistance.
The decreased susceptibility of L. major–infected C.16D2/8 compared with control BALB/c indicates the existence of a disease response locus within the Dice1.2 congenic interval. Given the critical role of IL-4 regulation in L. major susceptibility (1), and given that Dice1.2 controls nIL-4 and Th2 cell bias, it is tempting to speculate that the same locus in the Dice1.2 congenic interval controls all three phenotypes. However, given that the nIL-4 and Th2 cell bias phenotypes of Dice1.1 are stronger than Dice1.2, how can we explain the inability of the Dice1.1 congenic strains to resist L. major infection? One possibility is that the magnitudes of the nIL-4 and Th2 cell bias phenotypes controlled by either Dice1.1 or Dice1.2 are insufficient to confer L. major resistance. Dice1.1 and Dice1.2 may act in parallel biochemical pathways, each with independent as well as common targets, L major susceptibility being a Dice1.2-specific target and Il4 a shared target. Alternatively, L. major resistance may require an additional gene cosegregating with Dice1.2. In this case, it should be possible to separate an L. major response locus (Lmr) from Dice1.2 itself. Such a locus would be predicted to behave like the recently described Lmr loci that use a Th1/Th2 cell–independent mechanism to control the differential responses to L. major of BALB/c and C57BL/6 (28). However, if a resistance locus in the Dice1.2 congenic interval operates in a Th1/Th2 cell–independent Lmr-like pathway, it would represent a novel member of this group because the known Lmr loci are located on chromosomes 9, 17, and X (28). Nevertheless, in support of this possibility is the similar abundance of Il4 mRNA recovered from draining popliteal lymph nodes 6 wk after L. major infection of resistant C.16D2/8 and susceptible BALB/c mice (unpublished data).
In summary, the genetic locus predicted by the Th2 cell bias–controlling Dice1 QTL is confirmed and resolved into two independent loci, Dice1.1 and Dice1.2. The additional ability of each locus to control Il4 expression from naive CD4 T cells, a known determinant of Th2 cell bias, suggests strongly that the proximal targets of both Dice1.1 and Dice1.2 occur in the naive T cell IL-4 pathway. In addition, Dice1.2 (or a tightly linked gene) controls the response to L. major infection, a paradigm for Th2 cell–dependent disease. The C.16D2 congenic panel will provide an opportunity to molecularly dissect the naive T cell IL-4 pathway and study the role of Th2 cell bias in the development of other Th2 cell–dependent processes such as worm expulsion, allergic asthma, and dermatitis.
Acknowledgments
We thank Robert Coffman and Amy Beebe for providing BALB.D2c16; William Paul, Jim Allison, and Richard Locksley for generously providing reagents; and Murali Krishna-Kaja and Mike Bevan for commenting on the manuscript. We also thank Yan Zhang and Duangkamol Chungsiriwat for superb technical assistance. M. Bix thanks D.D. and L.J. for support.
This work was supported by grants to M. Bix from the University of Washington Royalty Research Fund, the Marian Smith Foundation, the Burroughs Welcome Foundation, the Cancer Research Institute, and the National Institutes of Health (grant no. AI48636).
The authors have no conflicting financial interests.
A. Baguet and J. Epler contributed equally to this work.
Abbreviations used in this paper: QTL, quantitative trait locus/loci; SSLP, simple sequence length polymorphism.
References
- 1.Reiner, S., and R. Locksley. 1995. The regulation of immunity to Leishmania Major In Annual Review of Immunology. W.E. Paul, editor. Annual Reviews Inc., Palo Alto. 151–177. [DOI] [PubMed]
- 2.Finkelman, F.D., and J.F. Urban Jr. 2001. The other side of the coin: the protective role of the TH2 cytokines. J. Allergy Clin. Immunol. 107:772–780. [DOI] [PubMed] [Google Scholar]
- 3.Ray, A., and L. Cohn. 1999. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J. Clin. Invest. 104:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Gros, G., S.Z. Ben-Sasson, R. Seder, F.D. Finkelman, and W.E. Paul. 1990. Generation of interleukin 4 (IL-4)–producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4–producing cells. J. Exp. Med. 172:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan, M.H., and M.J. Grusby. 1998. Regulation of T helper cell differentiation by STAT molecules. J. Leukoc. Biol. 64:2–5. [DOI] [PubMed] [Google Scholar]
- 6.Zhou, M., and W. Ouyang. 2003. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol. Res. 28:25–37. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimoto, T., A. Bendelac, C. Watson, J. Hu-Li, and W.E. Paul. 1995. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 270:1845–1847. [DOI] [PubMed] [Google Scholar]
- 8.Sabin, E.A., and E.J. Pearce. 1995. Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J. Immunol. 155:4844–4853. [PubMed] [Google Scholar]
- 9.Bix, M., Z.E. Wang, B. Thiel, N.J. Schork, and R.M. Locksley. 1998. Genetic regulation of commitment to interleukin 4 production by a CD4+ T cell–intrinsic mechanism. J. Exp. Med. 188:2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagi, R., W. Suzuki, N. Seki, M. Kohyama, T. Inoue, T. Arai, and M. Kubo. 2002. The IL-4 production capability of different strains of naive CD4(+) T cells controls the direction of the T(h) cell response. Int. Immunol. 14:1–11. [DOI] [PubMed] [Google Scholar]
- 11.Noben-Trauth, N., J. Hu-Li, and W.E. Paul. 2002. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur. J. Immunol. 32:1428–1433. [DOI] [PubMed] [Google Scholar]
- 12.Ewart, S.L., D. Kuperman, E. Schadt, C. Tankersley, A. Grupe, D.M. Shubitowski, G. Peltz, and M. Wills-Karp. 2000. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am. J. Respir. Cell Mol. Biol. 23:537–545. [DOI] [PubMed] [Google Scholar]
- 13.Corry, D.B., H.G. Folkesson, M.L. Warnock, D.J. Erle, M.A. Matthay, J.P. Wiener-Kronish, and R.M. Locksley. 1996. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 183:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiner, S.L., S. Zheng, Z.-E. Wang, L. Stowring, and R.M. Locksley. 1994. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 179:447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snell, G.D., and H.P. Bunker. 1965. Histocompatibility genes of mice. V. Five new histocompatibility loci identified by congenic resistant lines on a C57b 10 background. Transplantation. 35:235–252. [DOI] [PubMed] [Google Scholar]
- 16.Beebe, A.M., S. Mauze, N.J. Schork, and R.L. Coffman. 1997. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity. 6:551–557. [DOI] [PubMed] [Google Scholar]
- 17.Rogner, U.C., and P. Avner. 2003. Congenic mice: cutting tools for complex immune disorders. Nat. Rev. Immunol. 3:243–252. [DOI] [PubMed] [Google Scholar]
- 18.Glazier, A.M., J.H. Nadeau, and T.J. Aitman. 2002. Finding genes that underlie complex traits. Science. 298:2345–2349. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen, C., N. Limaye, and E.K. Wakeland. 2002. Susceptibility genes in the pathogenesis of murine lupus. Arthritis Res 4:S255–S263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntire, J.J., S.E. Umetsu, O. Akbari, M. Potter, V.K. Kuchroo, G.S. Barsh, G.J. Freeman, D.T. Umetsu, and R.H. DeKruyff. 2001. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2:1109–1116. [DOI] [PubMed] [Google Scholar]
- 21.Groblewski, G.E., M. Yoshida, M.J. Bragado, S.A. Ernst, J. Leykam, and J.A. Williams. 1998. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J. Biol. Chem. 273:22738–22744. [DOI] [PubMed] [Google Scholar]
- 22.Sisk, T.J., T. Gourley, S. Roys, and C.H. Chang. 2000. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J. Immunol. 165:2511–2517. [DOI] [PubMed] [Google Scholar]
- 23.Diehl, S., J. Anguita, A. Hoffmeyer, T. Zapton, J.N. Ihle, E. Fikrig, and M. Rincon. 2000. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 13:805–815. [DOI] [PubMed] [Google Scholar]
- 24.Gordon, D.F., W.W. Woodmansee, J.N. Black, J.M. Dowding, J. Bendrick-Peart, W.M. Wood, and E.C. Ridgway. 2002. Domains of Pit-1 required for transcriptional synergy with GATA-2 on the TSH beta gene. Mol. Cell. Endocrinol. 196:53–66. [DOI] [PubMed] [Google Scholar]
- 25.Fang, N., D. Fang, H.Y. Wang, A. Altman, and Y.C. Liu. 2002. Regulation of immune responses by E3 ubiquitin-protein ligases. Curr. Dir. Autoimmun. 5:161–175. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki, Y., M. Furuno, T. Kasukawa, J. Adachi, H. Bono, S. Kondo, I. Nikaido, N. Osato, R. Saito, H. Suzuki, et al. 2002. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 420:563–573. [DOI] [PubMed] [Google Scholar]
- 27.Kosarova, M., H. Havelkova, M. Krulova, P. Demant, and M. Lipoldova. 1999. The production of two Th2 cytokines, interleukin-4 and interleukin-10, is controlled independently by locus Cypr1 and by loci Cypr2 and Cypr3, respectively. Immunogenetics. 49:134–141. [DOI] [PubMed] [Google Scholar]
- 28.Elso, C.M., L.J. Roberts, G.K. Smyth, R.J. Thomson, T.M. Baldwin, S.J. Foote, and E. Handman. 2004. Leishmaniasis host response loci (lmr1-3) modify disease severity through a Th1/Th2-independent pathway. Genes Immun. 5:93–100. [DOI] [PubMed] [Google Scholar]