Abstract
Cadherins are integral membrane proteins expressed in tissue-restricted patterns that mediate homophilic intercellular adhesion. During development, they orchestrate tissue morphogenesis and, in the adult, they determine tissue integrity and architecture. The synovial lining is a condensation of fibroblast-like synoviocytes (FLS) and macrophages one to three cells thick. These cells are embedded within the extracellular matrix, but the structure is neither an epithelium nor an endothelium. Previously, the basis for organization of the synovium into a tissue was unknown. Here, we cloned cadherin-11 from human rheumatoid arthritis (RA)-derived FLS. We developed L cell transfectants expressing cadherin-11, cadherin-11 fusion proteins, and anti–cadherin-11 mAb. Cadherin-11 was found to be expressed mainly in the synovial lining by immunohistologic staining of human synovium. FLS adhered to cadherin-11–Fc, and transfection of cadherin-11 conferred the formation of tissue-like sheets and lining-like structures upon fibroblasts in vitro. These findings support a key role for cadherin-11 in the specific adhesion of FLS and in synovial tissue organization and behavior in health and RA.
Keywords: synovium, synoviocyte, rheumatoid arthritis, cadherin, cell adhesion
Introduction
During development, the differential adhesive properties of various cell populations facilitate the cell rearrangement required for tissue morphogenesis. This cell sorting is directed in part by cadherins such as E-cadherin in epithelia and N-cadherin in the nervous system. Postnatally, cadherins play a major role in maintaining tissue integrity and architecture (1, 2). Cadherins are transmembrane glycoproteins expressed in restricted patterns that mediate homophilic adhesion between cells. In many tissues, adherens junctions are formed by cadherins linked through intracellular catenins to the actin cytoskeleton. These multiprotein complexes can activate intracellular signaling pathways, influence cytoskeletal organization, and orchestrate multicellular arrangements (1, 2). These properties of cadherins are likely to contribute to their role in tumor progression (2). Indeed, changes in cadherin expression have been associated with cell transformation and tumor metastasis (3–5).
The physiological role of the synovium is to balance cartilage remodeling and provide lubricant and nourishment for the synovial fluid that bathes the avascular cartilage surfaces of diarthrodial joints (6). The synovial membrane lining is composed of two major cell types: type A, macrophage-like synoviocyte, and type B, fibroblast-like synoviocyte (FLS). These synoviocytes form cell to cell contacts as well as attachments to an ordered extracellular matrix (ECM). However, the lining lacks a classical basement membrane, and the cellular contacts lack tight junctions and desmosomes (6, 7). Therefore, the lining does not possess the architecture typical of epithelium or endothelium.
The synovium displays marked changes in rheumatoid arthritis (RA) where the lining undergoes striking hyperplasia and the underlying loose connective tissue becomes massively infiltrated with leukocytes. These changes are associated with activation and condensation of the mesenchymal synovial cells that produce large amounts of matrix-degrading metalloproteinases, inflammatory cytokines, and lipid mediators of inflammation. This mass of cells extends and attaches onto cartilage and becomes locally invasive, damaging cartilage and eroding adjacent bone, resulting in permanent joint destruction.
Surprisingly, despite the central role of the synovial membrane in diarthrodial joint physiology in health and in inflammatory arthritis, little is known about the molecular basis for the organization of this tissue (6, 7). We hypothesized that cadherins might mediate homophilic adhesion between synoviocytes and explain their organization into a tissue. Here, we describe the identification and cloning of a cadherin expressed in the lining of normal synovium, synovium in osteoarthritis, and the hyperplastic rheumatoid synovium. This cadherin mediates adhesion of FLS and confers features of synovial tissue formation upon fibroblasts in vitro.
Materials and Methods
Isolation and Culture of FLS.
Synovial tissues from RA patients (American College of Rheumatology criteria; reference 8) were discarded tissue from synovectomy or joint replacement procedures, obtained with approval of the Brigham and Women's Hospital Institutional Review Board. Synoviocyte cell suspensions were prepared from synovial tissues by mincing; treatment with 1 mg/ml collagenase (type 1; Worthington Biochemicals), 0.15 mg/ml DNase I (Sigma-Aldrich), and 2 mM CaCl2 in HBS (20 mM Hepes, 137 mM NaCl, and 3 mM KCl, pH 7.4); and rocking at 37°C for 1 h. The cell suspension was passed through a 40-mesh sieve and cultured in DMEM, 10% FBS (Hyclone), 2 mM l-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, and 50 μM 2-mercaptoethanol at 37°C, 10% CO2. After the third passage, cells appeared fibroblast-like and were negative with anti-CD68 mAb, suggesting they were composed mainly of FLS as described previously (9).
Northern Blots.
Messenger RNA was isolated as described previously (10) from FLS after five passages, 16E6.A5 epithelial cells, and Jurkat T leukemia cells. 5 μg per sample were subjected to electrophoresis through 1.5% agarose/6% formaldehyde gels and transferred onto a Hybond nylon membrane (Amersham Biosciences). This membrane was probed serially with a 32P random prime–labeled 385-bp fragment of candidate synovial cadherin followed by control GAPDH cDNA (CLONTECH Laboratories, Inc.) at 43°C for 16 h. The membrane was washed at a final stringency of 0.1 × SSC with 2% (wt/vol) SDS at 56°C and autoradiographed on Kodak MS film at −70°C.
Adhesion Assays.
Cells were labeled with 15 μg of calcein-AM (Molecular Probes) during release from culture flasks using 0.02% trypsin, 2 mM CaCl2 in HBS for 5 min at 37°C to minimize cadherin proteolysis. After adding two volumes of 0.04% soy bean trypsin inhibitor in HBS, they were washed twice and resuspended in HBS, 0.1% BSA, 50 mM glucose, and 1 mM CaCl2. Cells were allowed to settle for 10 min at 4°C onto cadherin–Fc-coated microtiter plates and, after incubation at 37°C for 40 min, the percentage of adherent cells was determined as described previously (11).
Immunohistochemical Analysis.
Synovial samples were snap-frozen in OCT compound (Sakura Finetek, Inc.). 5-μm sections were fixed in acetone at room temperature for 10 min, air dried, and stained with the avidin–biotin complex method (12). The sections were incubated with biotinylated antibodies or unconjugated antibodies followed by biotinylated horse anti–mouse Ig (Vector Laboratories). Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in PBS. Endogenous biotin was blocked by sequential incubations with avidin (Vector Laboratories) and biotin (Sigma-Aldrich). The sections were developed with 3-amino-9-ethylcarbazole (Sigma-Aldrich), postfixed with in 2% paraformaldehyde, and counterstained with hematoxylin (Fisher Scientific).
Immunofluorescence and Flow Cytometry.
For confocal immunofluorescence microscopy, cells were fixed in 2% paraformaldehyde in PBS for 15 min; permeabilized in 0.2% (wt/vol) saponin in 60-mM pipes, 25 mM Hepes, 10 mM EGTA, and 2 mM MgCl, pH 6.9, for 30 min; blocked; and incubated with primary antibodies for 1 h at room temperature followed by Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories) and Alexa 488–conjugated phalloidin (Molecular Probes) for 1 h at room temperature.
For flow cytometry, cultured cells were released essentially as described for adhesion assays and stained with primary antibodies in HBS, 1 mM CaCl2, 2% FBS for 1 h at 4°C followed by FITC-conjugated secondary antibody for 1 h at 4°C, and analyzed on a FACScan (Becton Dickinson). For multicolor flow cytometric analysis, freshly isolated RA synovial cells were disaggregated essentially as described before and resuspended in HBS, 2 mM Ca2+, 5% FBS, exposed to the following primary antibodies for 1 h at 4°C: IgG1-FITC, CD45-FITC, IgG1-biotin, or cadherin-11-5H6-biotin followed by Cychrome-conjugated streptavidin (BD Biosciences) for 1 h at 4°C and analyzed on a FACScan flow cytometer.
In Vitro Cadherin-induced Multicellular Organization.
L cells transfected with cadherin-11 or vector control were plated at 5 × 104 cells/ml (5 × 105 cells/75 cm2 surface area). Cellular organization was examined after 4 d by phase-contrast microscopy. For in vitro lining formation experiments, distinct areas of tissue culture flasks (Becton Dickinson) were coated with fibronectin (GIBCO BRL) by applying fibronectin drops (0.1–10 μg/ml) and incubation overnight at 4°C, followed by blocking of the entire surface with 1% BSA in HBS with 1 mM CaCl2 overnight at 4°C. Cells were released essentially as described for adhesion assays and, after washing twice, were plated at 5 × 104 cells/ml in serum- and ECM-free media (X-VIVO 15; Cambrex BioScience). Photographs were taken by phase microscopy after 2 d at 37°C.
Online Supplemental Material.
The online supplemental material includes descriptions of cloning of the synovial cadherin, generation of L cell transfectants, production and characterization of cadherin-11–Fc fusion protein, and anti–cadherin-11 monoclonal antibodies, including Fig. S1, and of other antibodies and cell lines used in this work. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20041545/DC1.
Results and Discussion
Cloning of the Synoviocyte Cadherin.
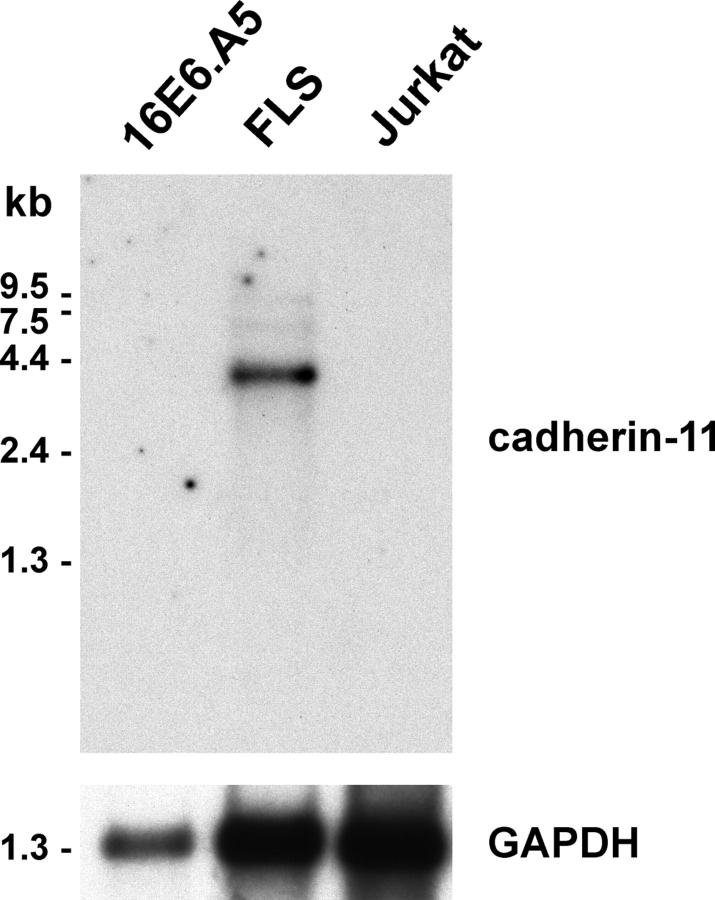
To determine if there is a cadherin that might function to mediate homophilic adhesion among synoviocytes, we performed PCR amplification of human synovial fibroblast cDNA using degenerate oligonucleotides based on the highly conserved cytoplasmic domains in human cadherins, as described in the online supplemental material (available at http://www.jem.org/cgi/content/full/jem.20041545/DC1). Six out of eight clones obtained matched the canonical cadherin-11 sequence. Northern blot analysis confirmed that the 4-kb cadherin-11 mRNA is present in total RNA of cultured FLS derived from RA synovial tissue, but not in RNA from epithelial cells (16E6.A5) or Jurkat T leukemia cells (Fig. 1). PCR amplification of the entire coding region of human cadherin-11 from FLS cDNA and nucleotide sequencing further confirmed the presence of bonafide cadherin-11 mRNA within cultured human FLS (see online supplemental text).
Figure 1.
Northern analysis of cadherin-11 expression. (A) Northern blot of mRNA (5 μg per lane) from the 16E6.A5 breast epithelial cell line, human RA-derived cultured FLS, and Jurkat T cells was hybridized with a 32P-labeled cadherin-11 (top) or control GAPDH probe (bottom).
Cadherin-11–Fc Supports Adhesion of Synoviocytes.
To test the adhesive capacity of human cadherin-11, we produced cadherin-11–Fc fusion protein and monoclonal anti–cadherin-11 antibodies (see online supplemental text and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20041545/DC1) and performed cell to substrate adhesion assays. Both cultured FLS derived from RA synovium, and cadherin-11–transfected L cells adhered to cadherin-11–Fc in a concentration-dependent fashion (Fig. 2 A). In a typical experiment, ∼30–40% of FLS adhered to cadherin-11–Fc. In contrast, only 5% of FLS adhered to control E-cadherin–Fc (Fig. 2 A). Similarly, when the cadherin-11 gene was transfected into L cells (L/cad-11), these cells bound efficiently to cadherin-11–Fc–coated plates, whereas empty vector-transfected L cells (L/vector) bound only at background levels (Fig. 2 B). The binding of cadherin-11 L cell transfectants to cadherin-11–Fc–coated wells was blocked 80% by anti–cadherin-11 mAbs cadherin-11–2G4 and cadherin-11–5H6 (Fig. 2 C). The cadherin-11–3H10 mAb caused partial inhibition of adhesion (unpublished data). In contrast, neither the isotype matched P3 mAb nor the cell-binding mouse anti–MHC class I mAb (36–7-5) blocked the adhesion of cadherin-11 L cell transfectants to cadherin-11–Fc. Together, these findings are in agreement with other reports of the ability of cadherin-11 to support homophilic cell aggregation (13–15) and demonstrate for the first time that cadherin-11 mediates homophilic adhesion of cultured human RA-derived FLS.
Figure 2.
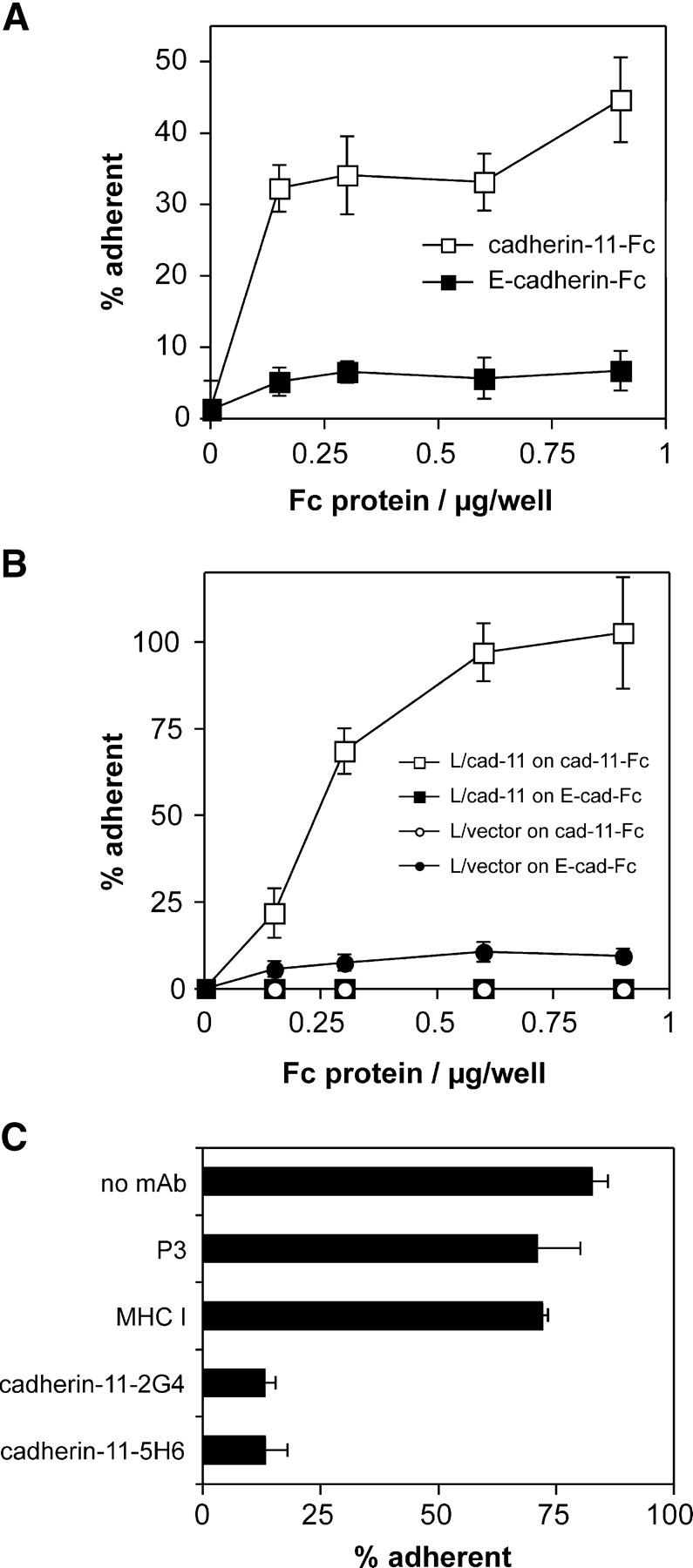
Cadherin-11–mediated adhesion of FLS and L cell transfectants. (A) Adhesion of cultured FLS derived from RA synovium to cadherin-11–Fc. Purified cadherin-11–Fc (open squares) or E-cadherin–Fc (closed squares) were immobilized on microtiter plate wells coated with polyclonal goat anti–human IgG antibody and blocked with BSA. The adhesion of RA-derived cultured FLS was determined in triplicate as described in Materials and Methods. (B) Specific adhesion of L cells to cadherin-11–Fc is conferred by cadherin-11 transfection. The assay was conducted as in A using control L cells (L/vector) or cadherin-11–transfected L cells (L/cad-11) and microtiter plates directly coated with the indicated concentrations of Fc proteins (cadherin-11–Fc or control E-cadherin–Fc). (C) Inhibition of adhesion of the L cell/cadherin-11 line to cadherin-11–Fc by anti–cadherin-11 antibodies. The assay was performed as in B but antibodies were preincubated with the cells for 10 min on ice. All the mAb were purified and used at 10 μg/ml and all mAb were mouse IgG1, except anti–mouse MHC-I (mouse IgG2a). The results are expressed as the mean percentage of cells that were adherent ±1 SD (n = 3).
Expression of Cadherin-11 in the Rheumatoid Synovium.
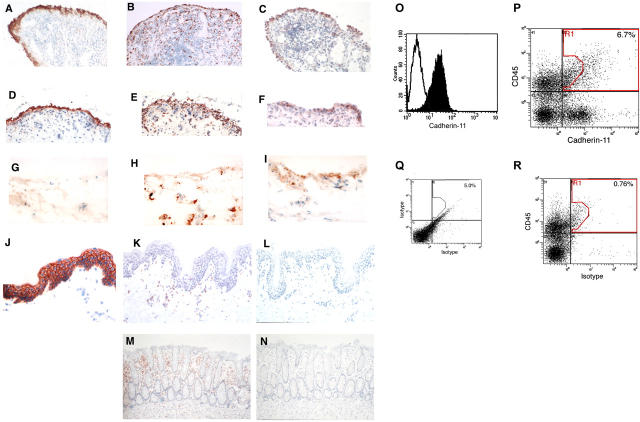
To determine if cadherin-11 protein was expressed in RA, we performed immunohistochemistry of frozen human synovial tissue sections from RA patients. First, we stained RA synovium with anti-DAF (CD55), a marker expressed by FLS in the synovial lining layer (Fig. 3 A). For comparison, we stained RA synovium with mAb against CD68, which is expressed by synovial macrophages. As expected, anti-CD68 labeled cells in both the lining and sublining regions (Fig. 3 B). The cadherin-11–3H10 mAb showed prominent staining of the lining in RA synovium (Fig. 3 C). In addition, rare, strongly cadherin-11–reactive cells were noted in the sublining region. A similar staining pattern was noted in synovia from patients with osteoarthritis (Fig. 3, D–F) and in normal synovium (Fig. 3, G–I), although reactivity was of weaker intensity. These findings contrast with results in skin and colonic tissues where no cadherin-11–reactive cells were seen, whereas the expected CD68 staining of tissue macrophages in all tissues and E-cadherin in skin was observed (Fig. 3, J–N). We found no evidence of E-cadherin expression in RA synovium (see online supplemental text). In addition, we find that cadherin-11 is expressed in normal mouse synovial tissue, but not in mouse skin (unpublished data).
Figure 3.
Cadherin-11 expression on FLS in the synovium. (A–N) Immunohistochemical analyses of frozen human tissue from RA synovium (A–C), osteoarthritis (OA) synovium (D–F), normal synovium (G–I), skin (J–L), and colon (M and N) stained with anti-CD55 (A and D), anti-CD68 (B, E, H, K, and M), anti–cadherin-11 (C, F, I, L, and N), IgG1 control (G), or anti–E-cadherin (J) are shown. Note the prominent cadherin-11 reactivity in the synovial lining that was similar to CD55 reactivity and the absence of detectable cadherin-11 reactivity in skin and colonic sections. Note also the distinct difference in reactivity pattern for the macrophage marker CD68. Magnification, 200. (O–R) Flow cytometry of FLS. Flow cytometry of passage three RA-derived FLS revealed that, after in vitro culture, essentially all express cadherin-11 (O), whereas nonfibroblast lineages do not propagate. Flow cytometric analyses of freshly disaggregated RA synovial cells, gated to exclude small particulate material by forward scatter, show separate subpopulations stained by anti–cadherin-11–biotin/streptavidin-Cychrome and the bone marrow lineage marker CD45-FITC (P), compared with IgG1 controls (Q) or to synovial cells stained with CD45-FITC and isotype control (R).
Next, we tested whether RA-derived FLS expressed cadherin-11 on their cell surfaces. In vitro–cultured, RA-derived FLS stained with anti–cadherin-11–3H10 mAb by flow cytometry (Fig. 3 O). Thus, all the data obtained, including molecular cloning, Northern analysis, and flow cytometry of cultured FLS, were consistent with cadherin-11 expression on FLS. To confirm that FLS cadherin-11 expression did not result from tissue culture artifact, we performed multicolor flow cytometry on disaggregated fresh ex vivo RA synovial tissue using anti–cadherin-11–5H6 mAb and anti-CD45 mAb (a lineage marker of bone marrow–derived cells). Cells expressing cadherin-11 predominantly lacked CD45, and cells expressing CD45 mainly lacked cadherin-11, although a small population (∼5%) of CD45-expressing synovial cells was stained by the mAb 5H6 (Fig. 3 P). Together, the immunohistology and the flow cytometry results indicated that within the inflamed rheumatoid synovium cadherin-11 expression is found predominantly on the FLS lineage.
Cadherin-11 Mediates Tissue Sheet and Lining Formation In Vitro.
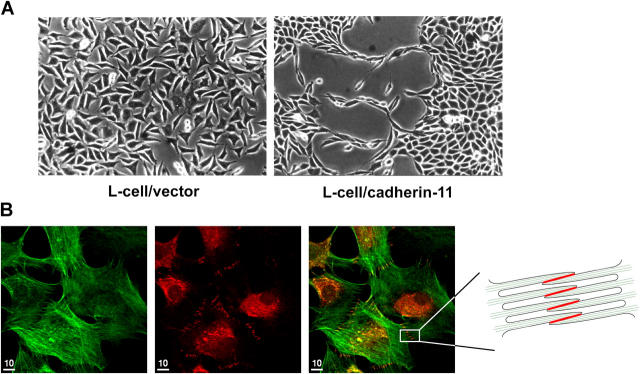
Because classical cadherins mediate homophilic adhesion that can result in cell sorting into aggregates and tissue morphogenesis, we examined the potential of cadherin-11 to mediate the association of cells into tissue-like sheets in vitro. L cells transfected with cadherin-11–containing vectors (L/cad-11) or with empty pCEP4 vectors (L/vector) were plated at equal numbers in tissue culture flasks. After 4 d of culture, cadherin-11–expressing L cells formed tight aggregates that grew as tissue-like sheets, whereas empty vector-containing L cells grew as random cells without specific cell–cell interactions (Fig. 4 A).
Figure 4.
Cadherin-11 mediates the formation of intercellular junctions, tissue sheet-like and lining-like cellular organization. (A) Cadherin-11 mediates tissue–sheet formation. Equal numbers of L cells were plated on culture dishes in conventional media. After 4 d, L cells transfected with vector alone were randomly organized without specific cell–cell interactions. In contrast, cadherin-11–transfected L cells formed extensive and intimate contacts along their surfaces and condensed together to form a continuous sheet of cells resembling a tissue-like structure. (B) Formation of cadherin-11 mediated intercellular junctions. FLS were plated at intermediate density and, after overnight culture, subjected to immunofluorescence using phalloidin to label F-actin (green, left) and anti–cadherin-11 3H10 (red, middle). A merged image is shown in at right. F-actin staining demonstrated numerous filopodia that interdigitated with those from neighboring cells. At the cell periphery, the anti–cadherin-11-3H10 mAb–labeled intercellular junctions resulted in a row of short parallel lines (middle, right, and schematic representation). Bars, 10 μm.
To examine the formation of cadherin-11–mediated intercellular junctions in human FLS, we plated cultured FLS at 50% confluence where cells are not crowded. After overnight culture, the cells were well spread and contained actin stress fibers (Fig. 4 B, left). Additionally, the FLS developed numerous filopodial processes with the greatest number of filopodia at sites of close cell–cell contact. Specifically, at sites of intimate cell–cell contact with numerous interdigitating filopodia, the anti–cadherin-11 antibody labeled a ladder-like series of lines corresponding to intercellular junctions (Fig. 4 B, middle, right, and schema). No cadherin-11 staining was seen at sites without cell–cell contact. A perinuclear granular cadherin staining demonstrated that FLS contained intracellular cadherin-11, consistent with similar findings for E-cadherin (Fig. 4 B and reference 16).
In vivo, the synovial lining exists as a condensed cell layer, one to three cells thick, overlying and imbedded in the ECM of the synovial sublining that also contains small blood vessels and scattered fibroblasts. The other side of the lining layer faces the synovial space (Fig. 5 A). The basis for the formation and organization of the synovial lining is unknown. To study this process, we set up a culture system in vitro to simulate synovial lining formation. To mimic the in vivo setting of a tissue layer adjacent to the joint cavity, we coated the ECM component fibronectin (representing synovial sublining ECM) onto discrete regions of a tissue culture flask. The surrounding plastic surface was blocked with BSA (representing the joint cavity) and FLS were added in serum- and ECM-free medium. After 2 d of culture, the FLS formed a lining-like structure at the fibronectin–BSA interface and formed visible interconnections with cells below the interface, in a distribution resembling that in the synovium (Fig. 5 B). To confirm that this phenomenon could be accounted for by cadherin-11 expression, we compared L cells expressing or lacking cadherin-11. When L cells were plated in the same culture system, they adhered randomly to the fibronectin-coated surface and did not form a lining layer (Fig. 5 C). Strikingly, after culture for 2 d, L cells expressing cadherin-11 formed a lining-like structure at the fibronectin–BSA interface similar to that obtained with FLS (Fig. 5 D). Although these studies do not attempt to incorporate all of the cellular elements of the synovial tissue, they support the hypothesis that cadherin-11 mediates intercellular adhesion that imparts morphologic characteristics of the tissue in vivo. These include the formation of tissue sheets and a lining-like layer at a matrix interface, analogous to the interface between the loose connective tissue matrix of the synovium and the joint space.
Figure 5.
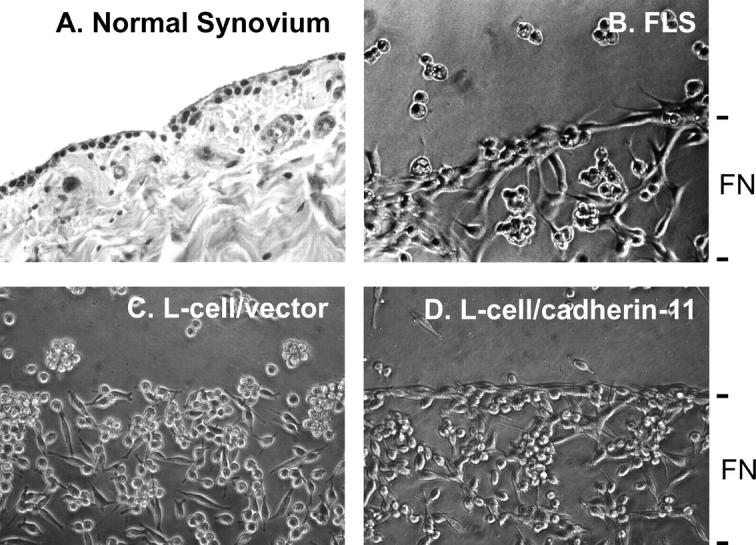
Cadherin-11 mediates lining-like formation by rheumatoid FLS and L cell transfectants. (A) Normal synovium stained with hematoxylin and eosin. The synovial lining is a thin one to three cell layer of cells at the surface of the sublining composed of the loose connective tissue, containing scattered fibroblasts, blood vessels, and ECM. (B) In vitro synovial lining formation. Discrete regions of tissue culture flasks were coated with fibronectin, followed by blocking of the entire surface with BSA. RA-derived human FLS were plated and cultured under serum- and ECM-free conditions. After 2 d, the FLS demonstrated organization into a thin, few-cell-thick, lining-like structure at the fibronectin–BSA interface (200×, phase-contrast). (C and D) L cells transfected with vector alone (control) or cadherin-11 were plated in the same culture system as in B. After 2 d, vector L cells were randomly distributed over the fibronectin-coated surface and did not form a lining layer. In contrast, cadherin-11 transfectant L cells formed a lining-like structure at the fibronectin–BSA interface similar to that of FLS in B (200×, phase-contrast).
Implications for Synovial Lining Formation and RA.
Based on our findings and the fact that cadherins are widely recognized to mediate tissue morphogenesis in development and contribute to tissue architecture in the adult (2), we propose that cadherin-11 may serve this function for the synovium. Cadherin-11 may allow the homophilic adhesion between FLS that is required for the process of cell rearrangement responsible for the organization of the synovial lining layer. Additional interactions including those between FLS and macrophage-like synoviocytes, possibly through VCAM-1 and integrin α4β1 (6, 9), are likely to contribute to the overall structure of the synovium in vivo.
Our data indicate that synovial fibroblasts, but not all fibroblasts, express cadherin-11 in vivo. Cadherin-11 is also expressed on another important cell type in close proximity to the diarthrodial joint, the osteoblast (15). Indeed, mice lacking functional cadherin-11 demonstrate bone abnormalities (17). Although the complete tissue distribution of cadherin-11 in adult humans is unknown, mRNA expression has been noted in placenta, brain, lung, and heart (14, 18). Thus, cadherin-11 is selectively but not uniquely expressed on FLS.
Unlike other cadherins, cadherin-11 is expressed predominantly on mesenchymal tissues (13, 19, 20). During development, the expression of cadherin-11 in the placenta and in embryonic tissues, such as developing limb buds and branchial arches, indicates a role in outgrowing or extending tissue (13, 19–22). These features suggest that cadherin-11 plays an important role in limb and joint development (4, 13, 20). Our findings raise the possibility that cadherin-11 also participates in synovial development. Future studies in cadherin-11–deficient mice should provide insight into this unexplored process.
It is important to consider that fundamental differences may exist between the functions of epithelial cadherins, such as E-cadherin, and mesenchymally expressed cadherins, such as cadherin-11. Unlike E-cadherin, cadherin-11 may be associated with a mesenchymal invasive tissue phenotype. Interestingly, although epithelial tissues normally do not express cadherin-11, expression has been noted on breast and prostate carcinomas that normally express E-cadherin. In these tumor cells, the anomalous expression of cadherin-11 at high levels has been correlated with invasive tumor behavior (2, 4, 5). Furthermore, cadherin-11 transfection is reported to increase the invasive and migratory properties of an E-cadherin expressing breast carcinoma cell line in vitro (23). Thus, it will be important to determine if cadherin-11 participates in the locally invasive behavior of the rheumatoid synovium.
In addition to their role in regulating cell to cell adhesion, cadherins also modulate other cell functions via intracellular signaling pathways (2). Thus, cadherin-11 likely plays a role in regulating the behavior of RA FLS that are capable of foci formation, anchorage-independent growth in soft agar, and invasive tumor-like behavior in SCID mice (24, 25). It is also intriguing that cell contact between fibroblasts mediated by cadherin-11 induces up-regulation of endothelial growth factor VEGF-D gene expression (26), and that in embryonic lung, only cadherin-11–expressing cells appear to produce VEGF-D (27). Therefore, it is possible that synovial cadherin-11 expression also contributes to the increased angiogenesis in RA (28).
Our identification of a synovial cadherin and its expression in normal and RA FLS offer a previously unrecognized opportunity to understand the structural basis for this tissue and the biology of the synovium. FLS of the synovial lining are of great interest in understanding both the normal physiology of the joint and how it is damaged in synovitis. In the normal synovium, FLS participate in the active process of synovial homeostasis via production of matrix components, matrix remodeling enzymes, and enzyme inhibitors. In pathological states such as RA, it remains unclear whether synovial lining hyperplasia results from underlying inflammation in the synovium or from primary abnormalities of FLS. Nevertheless, FLS are key producers of matrix-damaging enzymes (MMPs), cytokines (IL-6), growth factors (FGF), and angiogenic factors (VEGF) during inflammatory arthritis (29), and fibroblast-like cells are the major population in the invasive pannus that ultimately leads to joint destruction. Many of the processes that are key to rheumatoid synovitis, such as cellular condensation, tissue extension, and invasive behavior, have been linked to cadherin function. Therefore, the synovial cadherin may have a determining role in the mesenchymal tissue response to chronic inflammation. A new focus on the role of cadherin-11 in FLS biology will provide new insights into the aggressive behavior of the synovial tissue in conditions such as RA.
Acknowledgments
We wish to thank E. Gravelese for advice on synovial histology.
The authors received grant support from the National Institutes of Health, nos. AR48114 (to M.B. Brenner), AR02214 (to D.M. Lee), DK47677 (to A.K. Bhan), and DK43351 (to A.K. Bhan); the Eli and Edythe L. Broad Foundation, the Inflammatory Bowel Disease grant (to E. Mizoguchi), and the Arthritis Foundation (to H.P. Kiener).
Drs. Brenner and Valencia wish to disclose that Brigham and Women's Hospital is considering licensing patents involving cadherin-11 to commercial entities. The authors have no other potential conflicting financial interests.
References
- 1.Gumbiner, B.M. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 84:345–357. [DOI] [PubMed] [Google Scholar]
- 2.Wheelock, M.J., and K.R. Johnson. 2003. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 19:207–235. [DOI] [PubMed] [Google Scholar]
- 3.Perl, A.K., P. Wilgenbus, U. Dahl, H. Semb, and G. Christofori. 1998. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 392:190–193. [DOI] [PubMed] [Google Scholar]
- 4.Pishvaian, M.J., C.M. Feltes, P. Thompson, M.J. Bussemakers, J.A. Schalken, and S.W. Byers. 1999. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 59:947–952. [PubMed] [Google Scholar]
- 5.Tomita, K., A. van Bokhoven, G.J. van Leenders, E.T. Ruijter, C.F. Jansen, M.J. Bussemakers, and J.A. Schalken. 2000. Cadherin switching in human prostate cancer progression. Cancer Res. 60:3650–3654. [PubMed] [Google Scholar]
- 6.Lee, D.M., H.P. Kiener, and M.B. Brenner. 2005. Synoviocytes. Kelley's Textbook of Rheumatology, 7th ed. E.D. Harris, R.C. Budd, G.S. Firestein, M.C. Genovese, J.S. Sergent, S. Ruddy, and C.B. Sledge, editors. W.B. Saunders/Elsevier, Philadelphia, PA. In press.
- 7.Barland, P., A.B. Novikoff, and D. Hamerman. 1962. Electron microscopy of the human synovial membrane. J. Cell Biol. 14:207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnett, F.C., S.M. Edworthy, D.A. Bloch, D.J. McShane, J.F. Fries, N.S. Cooper, L.A. Healey, S.R. Kaplan, M.H. Liang, H.S. Luthra, et al. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315–324. [DOI] [PubMed] [Google Scholar]
- 9.Shang, X.Z., B.J. Lang, and A.C. Issekutz. 1998. Adhesion molecule mechanisms mediating monocyte migration through synovial fibroblast and endothelium barriers: role for CD11/CD18, very late antigen-4 (CD49d/CD29), very late antigen-5 (CD49e/CD29), and vascular cell adhesion molecule-1 (CD106). J. Immunol. 160:467–474. [PubMed] [Google Scholar]
- 10.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159. [DOI] [PubMed] [Google Scholar]
- 11.Higgins, J.M.G., D.A. Mandlebrot, S.K. Shaw, G.J. Russell, E.A. Murphy, Y.T. Chen, W.J. Nelson, C.M. Parker, and M.B. Brenner. 1998. Direct and regulated interaction of integrin αEβ7 with E-cadherin. J. Cell Biol. 140:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizoguchi, E., A. Mizoguchi, and A.K. Bhan. 1997. Role of cytokines in the early stages of chronic colitis in TCR alpha-mutant mice. Lab. Invest. 76:385–397. [PubMed] [Google Scholar]
- 13.Kimura, Y., H. Matsunami, T. Inoue, K. Shimamura, N. Uchida, T. Ueno, T. Miyazaki, and M. Takeichi. 1995. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev. Biol. 169:347–358. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi, J., S. Takeshita, T. Kashima, T. Imai, R. Machinami, and A. Kudo. 1999. Expression and function of the splice variant of the human cadherin-11 gene in subordination to intact cadherin-11. J. Bone Miner. Res. 14:764–775. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki, M., S. Takeshita, S. Kawai, R. Kikuno, A. Tsujimura, A. Kudo, and E. Amann. 1994. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J. Biol. Chem. 269:12092–12098. [PubMed] [Google Scholar]
- 16.Le, T.L., A.S. Yap, and J.L. Stow. 1999. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi, J., Y. Azuma, K. Hoshi, I. Kii, S. Takeshita, T. Ohta, H. Ozawa, M. Takeichi, O. Chisaka, and A. Kudo. 2001. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J. Bone Miner. Res. 16:1265–1271. [DOI] [PubMed] [Google Scholar]
- 18.Shibata, T., A. Ochiai, M. Gotoh, R. Machinami, and S. Hirohashi. 1996. Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett. 99:147–153. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann, I., and R. Balling. 1995. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev. Biol. 169:337–346. [DOI] [PubMed] [Google Scholar]
- 20.Simonneau, L., M. Kitagawa, S. Suzuki, and J.P. Thiery. 1995. Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes. Commun. 3:115–130. [DOI] [PubMed] [Google Scholar]
- 21.MacCalman, C.D., S. Getsios, and G.T. Chen. 1998. Type 2 cadherins in the human endometrium and placenta: their putative roles in human implantation and placentation. Am. J. Reprod. Immunol. 39:96–107. [DOI] [PubMed] [Google Scholar]
- 22.Horikawa, K., G. Radice, M. Takeichi, and O. Chisaka. 1999. Adhesive subdivisions intrinsic to the epithelial somites. Dev. Biol. 215:182–189. [DOI] [PubMed] [Google Scholar]
- 23.Nieman, M.T., R.S. Prudoff, K.R. Johnson, and M.J. Wheelock. 1999. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 147:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller-Ladner, U., J. Kriegsmann, B.N. Franklin, S. Matsumoto, T. Geiler, R.E. Gay, and S. Gay. 1996. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 25.Firestein, G.S. 1996. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 39:1781–1790. [DOI] [PubMed] [Google Scholar]
- 26.Orlandini, M., and S. Oliviero. 2001. In fibroblasts Vegf-D expression is induced by cell-cell contact mediated by cadherin-11. J. Biol. Chem. 276:6576–6581. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg, J.M., F.Y. Thompson, S.K. Brooks, J.M. Shannon, K. McCormick-Shannon, J.E. Cameron, B.P. Mallory, and A.L. Akeson. 2002. Mesenchymal expression of vascular endothelial growth factors D and A defines vascular patterning in developing lung. Dev. Dyn. 224:144–153. [DOI] [PubMed] [Google Scholar]
- 28.Feldmann, M., and R.N. Maini. 2001. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu. Rev. Immunol. 19:163–196. [DOI] [PubMed] [Google Scholar]
- 29.Ritchlin, C. 2000. Fibroblast biology. Effector signals released by the synovial fibroblast in arthritis. Arthritis Res. 2:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]