Abstract
Substantial evidence indicates that mitochondria are a major checkpoint in several pathways leading to neuronal cell death, but discerning critical propagation stages from downstream consequences has been difficult. The mitochondrial permeability transition (mPT) may be critical in stroke-related injury. To address this hypothesis, identify potential therapeutics, and screen for new uses for established drugs with known toxicity, 1,040 FDA-approved drugs and other bioactive compounds were tested as potential mPT inhibitors. We report the identification of 28 structurally related drugs, including tricyclic antidepressants and antipsychotics, capable of delaying the mPT. Clinically achievable doses of one drug in this general structural class that inhibits mPT, promethazine, were protective in both in vitro and mouse models of stroke. Specifically, promethazine protected primary neuronal cultures subjected to oxygen-glucose deprivation and reduced infarct size and neurological impairment in mice subjected to middle cerebral artery occlusion/reperfusion. These results, in conjunction with new insights provided to older studies, (a) suggest a class of safe, tolerable drugs for stroke and neurodegeneration; (b) provide new tools for understanding mitochondrial roles in neuronal cell death; (c) demonstrate the clinical/experimental value of screening collections of bioactive compounds enriched in clinically available agents; and (d) provide discovery-based evidence that mPT is an essential, causative event in stroke-related injury.
Keywords: caspases, cell death, apoptosis, antidepressants, antipsychotics
Introduction
Evidence from multiple systems has established mitochondria as a critical checkpoint in cell death (1–9). In particular, both caspase-dependent and caspase-independent cell death cascades in the central nervous system (CNS) appear to require mitochondrial release of protein factors such as cytochrome c, AIF, and SMAC/Diablo (10–14). The basic mechanisms underlying the release of these factors remains unclear (15, 16). Induction of the mitochondrial permeability transition (mPT) has been one proposed mechanism underlying this release.
Induction of an mPT has been linked to cytotoxicity after pathological insults such as viral-induced cytotoxicity (17, 18), cardiac ischemia-reperfusion injury (8, 19), stroke and excitotoxicity (6, 7, 20–22), trauma (23), and hypoglycemia (24). mPT, which has been defined primarily using mitochondria isolated from heart and liver, is the opening of pores in the inner mitochondrial membrane, resulting in free diffusion of solutes <1.5 kD, destroying the proton gradient, generating reactive oxygen species, and releasing accumulated Ca2+ (25–28) as well as cytochrome c, AIF, and SMAC/DIABLO (12, 29–32). These findings link mPT to the release of the direct mediators of downstream caspase-dependent and -independent cell death pathway. A comparison of observations in isolated liver and brain mitochondria suggest that “mPT-like” events do occur in brain mitochondria, even if the characteristics of the brain mPT differ in detail from that which occurs, for example, in liver mitochondria (6, 20, 24, 29).
The specific case of cell death resulting from stroke is associated with pathophysiological changes/conditions that, acting in concert, are predicted, largely on the basis of experiments in isolated liver mitochondria, to favor induction of the mPT. These factors include high Ca2+, increases in reactive species, free inorganic phosphate, and activation of upstream protein factors, such as bid and bax (33). Most (20, 29, 34–37), but notably not all (5, 38), studies in cultured cells isolated CNS mitochondria, and experimental models of acute neurological injury (e.g., ischemia/reperfusion) are consistent with the existence of an event analogous with the mPT in the CNS.
However, it remains unclear whether mPT is on the causative pathway of cell death, or whether it is a downstream effect related to overall cellular collapse, which includes, for example, oxidative damage to components of the oxidative phosphorylation system (39, 40). Although neuroprotection mediated by cyclosporine A (CsA) was initially cited as evidence for causal involvement of mPT in ischemic injury (20, 21), this is now appreciated to be problematic as CsA also affects calcineurin, the blockade of which itself has been shown to be neuroprotective (41). Similar “lack of specificity” arguments hold for minocycline and tauroursodeoxycholic acid. Minocycline is a second generation tetracycline antibiotic known to be protective in models of stroke (42, 43), spinal cord injury (44, 45), and neonatal hypoxia-reperfusion injury (46). Although our recent work links minocycline to prevention of mPT-mediated release of mitochondrially sequestered protein factors that facilitate both caspase-dependent and -independent cell death pathways (29, 47), other actions of minocycline have been identified (42, 43), and the use of minocycline to build a case for mPT involvement awaits a more mechanistic analysis of the actions of minocycline. Tauroursodeoxycholic acid is an endogenous bile acid that protects against stroke, but it is known to modulate activities in three major pathways involved in ischemic damage, including mPT, activity of bcl-2 family members, and signal transduction pathways (48). Likewise, support for an obligate role for tbid activation in ischemia-reperfusion injury now exists (49), but evidence exists that tbid facilitates both mPT induction (33) and mPT-independent release of mitochondrially sequestered pro-apoptogenic factors (16, 50). Similar to the clear evidence for an obligate role of tbid activation, there is also evidence of the required Ca2+ deregulation, but there is at least one line of experimentation using cultured cerebellar granule cells that argues against an early role for mPT induction. Studies of excitotoxic injury in these cells often point to the critical event being a delayed, mitochondrially mediated deregulation of cytoplasmic calcium as the critical failure event at the level of individual cells (5). The mechanisms that follow kainite exposure seem to differ from those that follow NMDA exposure (51) and neither seems to involve mPT as a critical decision point check (51, 52). It is worth noting that these studies are conceptually difficult to extend in vivo as these cells are comparatively resistant to ischemia.
The best direct test of the hypothesis that mPT lies on the causative pathway of clinically relevant cell death comes from the studies of N-Met-Val-CysA, a nonimmunosuppressive analogue of CsA reputed not to interact with calcineurin. This compound reduces infarct size in a rat model of transient focal ischemia (6). This data is strengthened somewhat by evidence that the effects of CsA on protection against infarction may extend temporally in time from the effects of the calcineurin inhibitor FK506 (53). However, the universal acceptance of mPT involvement in stroke remains limited, in part because of the reliance on data from a single drug (54), and the limited availability and characterization of its analogue. Furthermore, CsA is not viewed as a long-term medical option, as the blood brain must be opened (e.g., by needle puncture) for any therapeutic efficacy (53).
Thus, there is a need to show that other characterized agents can modulate mPT induction and protect against cerebral infarction, both to answer this central mechanistic question in the pathogenesis of stroke-related neuropathology and to help reduce its clinical effects. The work presented here addresses these issues.
Given the potential clinical impact of drugs that inhibit mPT, we screened a collection of 1,040 bioactive compounds for their ability to delay mPT induction in isolated rat liver mitochondria. This collection was primarily composed of clinically approved drugs that could readily be moved to late-stage preclinical trials and then into clinical trials (55, 56). The remainder of the collection largely consisted of some analogues of these drugs, and other known bioactive agents (including natural products, toxins, and controlled substances). Thus, in contrast with large, high, and ultra-high throughput screens of combinatorial libraries, this screen emphasized a much smaller compound set focused on known, bioactive compounds, most of which were FDA approved and, thus, are more likely to be minimally toxic and potentially rapidly available for clinical usage. Screening of this collection was blind and was designed to identify compounds that act independently of antioxidant activity or ability to chelate Ca2+ approaches that have been taken previously by others.
The data obtained (a) provide discovery-based validation of the mPT as lying on the causative pathway of stroke-related neurologic injury, (b) identify potential therapeutics that are already FDA approved, and (c) demonstrate the potential utility of coupling mechanism-based screening with libraries enriched in well-characterized, clinically available agents.
Materials and Methods
Chemicals.
The drug collection screened was the National Institute of Neurological Disorders and Stroke Custom Collection from MicroSource Discovery Systems, Inc. Ultra-pure sucrose was obtained from ICN Biomedicals. All other compounds, of the highest purity available, were obtained from Sigma-Aldrich. All substrates used in mitochondrial respiration experiments were dissolved in respiration buffer (see Fig. 2) and brought to neutral pH.
Figure 2.
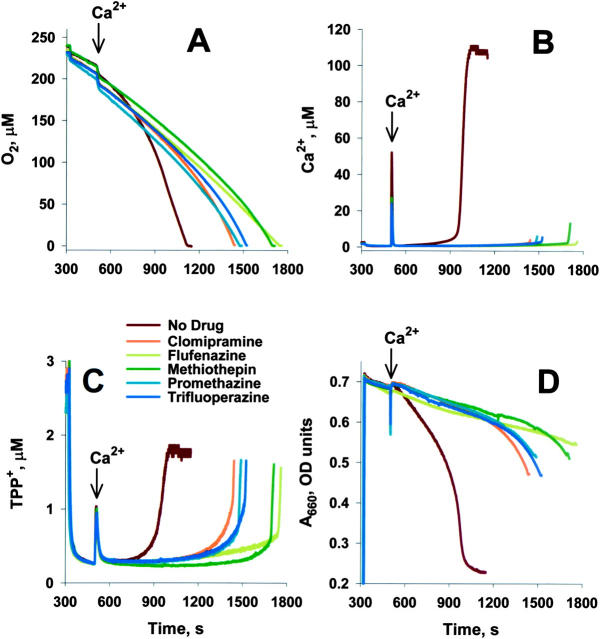
Heterocyclics delay mPT induction in isolated liver mitochondria without impairing mitochondrial physiology. TPP+ was titrated into buffer to enable construction of standard curves (0–300 s). Succinate-energized mitochondria were added to buffer (with 10 μM of drugs, where noted) within 10–20 s of the graph's origin (300 s). 100 μM Ca2+ was added where noted, and the curves were standardized at this point. (A) Oxygen consumption was measured by Clark electrode. Oxygen concentration in buffer decreases as signal decreases. (B) Ca2+ transport measured by Ca2+-selective electrode. Ca2+ in buffer increases as signal increases. (C) ΔΨ measured using a TPP+ electrode. ΔΨ decreases as signal increases. TPP+ uptake was determined by comparison with standard curve after correction for shift in electrode signal induced by drugs. Mitochondria appear to take up equal levels of TPP+ in the presence and absence of drug and restore TPP+ and Ca2+ levels after recovering from Ca2+ pulse, suggesting that these drugs have minimal or no direct effects on ΔΨ. Changes in ΔΨ consistent with the slight increases in respiration (equivalent to ∼5% of maximally uncoupled mitochondria) observed with some drugs is likely below the limit of detection. (D) Swelling (absorbance) monitored by a light emitting diode at A660. Mitochondrial are more swollen when signal decreases. Assay in 300 mM sucrose, 2.5 mM K-PO4, 3 mM Hepes, pH 7.2, and 5 mM succinate. Experiments were stopped after oxygen was consumed.
Mitochondrial Isolation.
Liver mitochondria were isolated from ∼4-mo-old male Fischer 344 × Brown Norway F1 rats by differential centrifugation using sucrose-based buffers (29). Animal protocols were approved by the Institutional Animal Care and Use Committees of Weill Medical College of Cornell University.
Library Screening and Dose Response Analysis.
Assays were run in 265 mM sucrose, 2.6 mM Hepes, pH 7.35, and 2.1 mM K-PO4, pH 7.35. Incubations included 10 mM α-ketoglutarate, 5 mM glutamate/malate, or 5 mM succinate and CaCl2 (10 μM for α-ketoglutarate and 20 μM for glutamate/malate and succinate) to energize mitochondria (57). Drugs were screened at 10 μM. Final mitochondrial concentration was 0.75 mg protein/ml. Swelling was monitored by following changes in absorbance at 540 nm and 660 nm (A540, A660) for 1.5 h using a SpectraMax 250 Plate Reader (Molecular Devices; reference 57). For secondary dose response analysis, 2 out of 27 plates and ∼3% of remaining data were excluded as technical outliers by visual inspection. Thus, the dose response data on these agents is based on 7–9 replicates (Fig. S1 and Supplemental Materials and Methods, available at http://www.jem.org/cgi/content/full/jem.20032053/DC1).
Simultaneous Measurement of ΔΨ, Oxygen, Swelling, and Ca2+ Transport.
Simultaneous measurement of ΔΨ, oxygen consumption, swelling, and Ca2+ transport was accomplished using a four-channel respiration system (29, 58). Buffers and additions are described in the figure legends. Oxygen uptake, membrane potential, and Ca2+ was measured using Clark, TPP+, and Ca2+-sensitive electrodes, respectively. Absorbance (A660) was measured using a diode. All experiments were performed in triplicate. Representative plots are shown in the paper.
Phospholipase A2 (PLA2) and Calmodulin Inhibition.
The ability of the heterocyclics to inhibit PLA2 was determined based on a modification of the method of Meshulam et al. (59). The ability of the heterocyclics to inhibit calmodulin function was assessed by examining the effect of these agents on calmodulin-dependent calcineurin activity (Upstate Biotechnology). Detailed methodologies are presented in the supplemental material.
Neurotoxicity Studies on Cultured Primary Cerebrocortical Neurons.
Neurotoxicity studies were conducted as described previously (60). Cerebral cortex of mouse embryos at day 15 (E15) were freed from meninges and separated from the olfactory bulb and hippocampus. Trypsinized cells were suspended in medium (neurobasal medium with 2% [vol/vol] B27 supplement/2 mM glutamine/100 U/ml penicillin) and streptomycin (GIBCO BRL) and seeded at a density of 2 × 104/cm2 on polylysin-coated dishes. Cells were used for experiments on day 7 of culture. Cell death was evaluated by the lactate dehydrogenase (LDH) release assay.
Stroke Studies in Mice.
Middle cerebral artery occlusion/reperfusion studies were conducted as described previously (60, 61). All animal experiments were conducted in accordance with the National Institutes of Health and institutional guidelines. Male C57/B6 (Charles River Laboratories) mice weighing 18–29 g were housed in standard temperature (22 ± 1°C) and in a light-controlled (light on 07:00–21:00) environment with ad libitum access to food and water. Weight of animals undergoing stroke were restricted to <22 g. Animals undergoing stroke were randomized to two groups: untreated (i.p. injection with 0.9% saline) and treated (i.p. injection with promethazine solution). Weight of animals undergoing sham operations was liberalized to 18–29 g.
Surgery and Occlusion.
Immediately before surgery, each mouse was anesthetized with 2% isoflurane (70% N2O/30% O2). During surgery, isoflurane was lowered to 1%. A midline incision was made in the neck and the right common carotid artery was dissected. The right MCA was occluded with a 7-0 nylon filament after permanent ligation of the external carotid artery and temporary ligation of the common carotid artery (61, 62). Right MCA occlusion was defined as a ≥90% drop of peak velocity measured by the laser Doppler flowmetry drop (63). The occluding filament was removed after 120 min. Body temperature was monitored and regulated using a heating pad. Sham operations consisted of the same duration and concentration of anesthesia, midline incision, doppler probe insertion over right temporal bone, and dissection of common carotid artery, but no manipulation of the common carotid artery or external carotid artery. The surgeon was blinded to treatment group. All mice were killed at 24 h after perfusion. Experiments were in accordance with protocols approved by the Harvard Medical School Animal Care Committee.
Drug Treatment.
Both treated and control groups received two i.p. injections: the first was delivered 1 h before ischemia, and the second was delivered 12 h later. Each i.p. dose was 10 mg/kg (in 0.5 ml) of promethazine dissolved in 0.9% NaCl or an equal volume of buffer alone (64).
Determination of Infarct Volume.
After 24 h of reperfusion, the mice were killed. The brains were quickly removed and chilled for 2 min. The cerebellum was removed. Coronal sections (2-mm thick; n = 6) were cut in a craniotome. Each slice was immersed in a saline solution containing 20 mg/cc of 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich) at 37°C for 30 min. After staining, each slice was scanned by an HP scanjet 4200C. The unstained areas in each hemisphere were quantified, and the ischemic area per slice = left (normal side) − right (ischemic side) (42, 62, 65). The infarct volume was calculated by summing up the infarcted areas in the six slices and calculating the volume for a cylinder (area × height).
Neuro Exam Scores.
Scores follow Bederson et al. (66). 0, no neurological deficits; 1, failure to extend the left forepaw; 2, circling to contralateral side; and 3, loss of ability to walk or righting reflex. The examiner was blinded to treatment group.
Online Supplemental Material.
Online supplemental material includes the following. Details on the library screening methods, primary results, and the validation of the methods used (Figs. S1 and S2). Discussion of preliminary structure–activity relationship (SAR) analysis. Graphic comparisons between inhibition of PLA2 activity and calmodulin activity and protection against mPT activation (Fig. S3). Methods for these supplemental tests and additional literature citations for activity of related agents against spinal cord injury, sepsis and cardiac, liver and kidney damage (e.g., from ischemia-reperfusion). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20032053/DC1.
Results
Library Screen.
Given the potential clinical impact of drugs that inhibit mPT, we screened a library of 1,040 bioactive compounds for their ability to delay mPT induction in isolated rat liver mitochondria (Fig. 1, Fig. S1, and Supplemental Materials and Methods). Of the 23 compounds showing “moderate” protection in the initial screen, 2 were known mPT inhibitors: tamoxifen and trifluoperazine (both “positive controls”).
Figure 1.
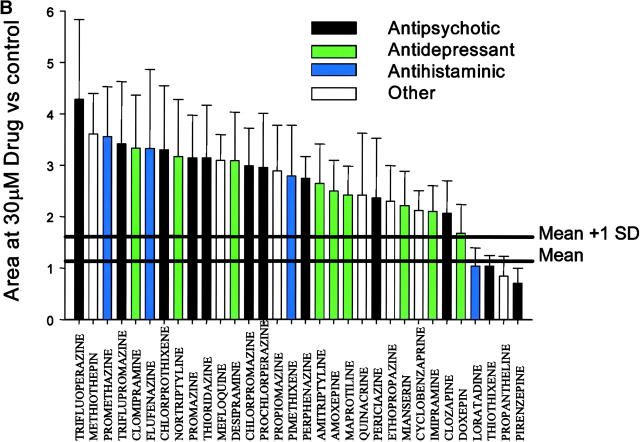
Heterocyclics mediate dose-dependent protection that is robust and not related to therapeutic class. (A) Drug structure, name, and therapeutic class of each heterocyclics-related structure in the initial compound library. Compounds ranked best to worst (from left to right and top to bottom) based on the data in B. As one measure of potential clinical utility, human use approval was examined using a list of U.S. FDA-approved drugs. Drugs currently in clinical use (23 out of 32) have no abbreviations after their name. Where abbreviations appear after the drug names, they refer to the following: not used in humans (NHU; 1 of 32); used in humans, current status and countries of acceptance uncertain (HU; 4 out of 32); and FDA-approved but believed discontinued (DSC; 4 out of 32). (B) Relative areas under the absorbance curve for the 32 heterocyclics studied at 30 μM. Bars are color coded by each drug's therapeutic class. Minimal protection was observed at concentrations ≤1 μM, and these data were pooled with the no-drug controls to serve as the control dataset. Total control dataset, n = 1207. Area relative to control, 1.14 ± 0.48 (mean ± SD). The mean coefficient of variation (CV) for individual experiments was 32%, and the mean CV within a specific challenge was 15%. Protection observed was statistically significant at P < 0.05 for 28 out of 32 agents (see Table I). Compounds were assayed in triplicate at seven concentrations (30, 10, 3, 1, 0.3, 0.1, and 0.03 μM) against three different challenges (50 μM Ca2+/2.5 mM K-PO4, 25 μM Ca2+/5 mM K-PO4, 25 μM Ca2+/2.5 mM K-PO4/100 μM tert-butyl hydroperoxide). Loss of absorbance (i.e., induction of PT) was followed as described in Materials and Methods, and areas under the curve were determined. The increased area under the curve is proportional to delay of PT induction. The area under the curve is expressed as a ratio to the area under the control curves on the same plate. For each drug, n = 7–9. Thick horizontal lines show mean and mean +1 SD.
Potency Studies of Heterocyclic, Tricyclic, and Phenothiazine-derived (HTPD) mPT Inhibitors.
Inspection of the structures of the 23 compounds that showed moderate protection revealed that trifluoperazine and 12 additional compounds were from a specific subclass of heterocyclics and their structural analogues, a major class of psychotropic drugs used clinically since the 1950's. These included tricyclic antidepressants and phenothiazine-derived antipsychotics. The compound library was reexamined to identify potential analogues that had been considered inactive. This was done both to determine if these were initially false negatives and to identify structurally related, but inactive compounds for SAR analysis (Supplemental Materials and Methods). Retrospective analysis showed that 32 compounds in the collection had a common chemical motif that includes or approximates the tricyclic/heterocyclic backbone (with either a six- or seven- member central ring; Fig. 1 A). Each of these 32 compounds was reassayed against three models of mPT induction and ranked according to the resistance it conferred to induction (Fig. 1 B). Challenges were combined to give a single score reflecting overall protection against mPT (Fig. 1 B and see Table I). Of the experimental set of 32 compounds, 28 gave statistically significant protection at concentrations ≤30 μM. Of these 28, 21 (75%) and 12 (43%) were protective at 10 and 3 μM, respectively. Analytical controls for dose and scoring method are provided as supplemental material (Fig. S2, A and B, available at http://www.jem.org/cgi/content/full/jem.20032053/DC1). Searches of the scientific literature and the US FDA-approved drug list indicate that 23 of these 32 agents are in clinical use, and 4 others are approved but no longer in active clinical use. Four others were approved, at least for clinical trials, in at least one country. Only one (methiothepin) appears not to have been used on humans (Fig. 1 A).
Table I.
Summary of Actions of HTPD Compounds
| Drug name | Drug class | PT | p-value | CAL | PLA2 | Cerebral IR | Cell |
|---|---|---|---|---|---|---|---|
| Trifluoperazine | Antipsychotic | 4.28 ± 1.56 | <0.0001 | 20 | ND | 74, 75 | 76, 78 |
| Methiothepin | Other | 3.61 ± 0.79 | <0.0001 | 78 | 54 | ||
| Promethazine | Antihistaminic | 3.56 ± 0.97 | <0.0001 | 84 | 97 | a | b |
| Triflupromazine | Antipsychotic | 3.41 ± 1.21 | <0.0001 | 70 | 75 | ||
| Clomipramine | Antidepressant | 3.33 ± 1.03 | <0.001 | 80 | 39 | 77, 79 | |
| Flufenazine | Antihistaminic | 3.32 ± 1.54 | <0.01 | 73 | 72 | ||
| Chlorprothixene | Antipsychotic | 3.30 ± 1.24 | <0.01 | 111 | 94 | ||
| Nortriptyline | Antidepressant | 3.16 ± 1.12 | <0.01 | 77 | 81 | c | 78, 80 |
| Promazine | Antipsychotic | 3.14 ± 0.83 | <0.01 | 74 | 38 | ||
| Thioridazine | Antipsychotic | 3.14 ± 1.03 | <0.0001 | 84 | 76 | ||
| Mefloquine | Other | 3.09 ± 0.50 | <0.0001 | 37 | 45 | ||
| Desipramine | Antidepressant | 3.08 ± 0.95 | <0.0001 | 52 | 54 | 79, 81 | 80, 82 |
| Chlorpromazine | Antipsychotic | 2.98 ± 0.74 | <0.001 | 82 | 69 | 75, 81, 82 | 83, 84 |
| Prochlorperazine | Antipsychotic | 2.96 ± 1.05 | <0.0001 | 66 | 40 | ||
| Propiomazine | Other | 2.88 ± 0.89 | <0.01 | 111 | 47 | ||
| Pimethixene | Antihistaminic | 2.79 ± 0.99 | 0.001 | 26 | 33 | ||
| Perphenazine | Antipsychotic | 2.74 ± 0.42 | <0.01 | 103 | 58 | ||
| Amitriptyline | Antidepressant | 2.64 ± 0.77 | <0.0001 | 72 | 80 | 78, 80 | |
| Amoxepine | Antidepressant | 2.50 ± 0.60 | <0.001 | 45 | 29 | ||
| Maprotiline | Antidepressant | 2.42 ± 0.56 | <0.001 | 50 | 10 | ||
| Quinacrine | Other | 2.42 ± 1.21 | <0.05 | 121 | 43 | 85–87 | 88, 89 |
| Periciazine | Antipsychotic | 2.36 ± 1.17 | <0.05 | 84 | 47 | ||
| Ethopropazine | Other | 2.30 ± 0.69 | <0.01 | 62 | 31 | ||
| Mianserin | Other | 2.21 ± 0.67 | <0.001 | 37 | 23 | 90, 92 | |
| Cyclobenzaprine | Other | 2.12 ± 0.38 | <0.0001 | 87 | 80 | ||
| Imipramine | Antidepressant | 2.10 ± 0.50 | <0.01 | ND | 79 | 78, 80 | |
| Clozapine | Antipsychotic | 2.07 ± 0.63 | <0.01 | 96 | 106 | 91, 93 | |
| Doxepin | Antidepressant | 1.68 ± 0.56 | <0.05 | 68 | 87 | 79, 81 | |
| Loratadine | Antihistaminic | 1.04 ± 0.35 | NS | 251 | 93 | None identified | |
| Thiothixene | Antipsychotic | 1.03 ± 0.21 | NS | 71 | 115 | ||
| Propantheline | Other | 0.84 ± 0.39 | NA | 87 | 76 | ||
| Pirenzepine | Other | 0.70 ± 0.30 | NA | 485 | 45 | ||
Drug class as in Fig. 1 B. PT presented as mean ± SD as fold protection over control. Calmodulin (Cal) and phospholipase A2 (PLA2) as percent control activity over untreated sample. Cerebral IR lists references for in vivo models are relevant for cerebral ischemia-reperfusion. Cell lists references for cell culture models are relevant for cerebral ischemia/reperfusion.
This paper.
This paper and unpublished data.
Unpublished data.
NS, P > 0.05; NA, not applicable, mean < 1.
HTPD mPT Inhibitors Do Not Alter Basic Mitochondrial Physiology.
Mitochondrial physiological parameters were assessed to determine whether these heterocyclics and their structural analogues interfered directly with other mitochondrial functions, and whether such interference might underlie their inhibition of the mPT due to a nonspecific effect on aspects of mitochondrial function needed for the assay to measure mPT induction. Specifically, we tested three major physiological functions of mitochondria as follows: their ability to respire, their ability to retain a membrane potential (ΔΨ), and their ability to take up and retain exogenous calcium. Representative data under conditions favoring mPT induction are shown in Fig. 2. Compounds shown represent some of the structural and functional diversity present in the heterocyclic compound class (flufenazine and promethazine [antihistaminics]; methiothepin [serotonin modulator]; and clomipramine [antidepressant]). At 10 μM, these compounds protected against mPT induction (Fig. 2 D) without apparent effects on initial oxygen consumption before mPT induction (Fig. 2 A), Ca2+ transport (Fig. 2 B), or resting or recovered ΔΨ (Fig. 2 C). These results suggest that the protection against PT is not associated with any overt mitochondrial toxicity. The addition of the high Ca2+ dose induces mPT in the control sample; drug-treated samples are protected against MPT. Indeed, ΔΨ is maintained, swelling is prevented, and calcium is sequestered until the oxygen in the chamber has been consumed. The protective effects of these drugs at the level of purified mitochondria reflect a novel cellular site of action for these drugs, and the ability of these compounds to inhibit the mPT suggests that they represent potential inhibitors of some pathways of apoptosis and necrosis.
HTPD PT Inhibitors Do Not Mediate Induction by Inhibiting PLA2 or Calmodulin.
One heterocyclic/phenothiazine, trifluoperazine, has been identified as an mPT inhibitor and has been shown to be protective against ischemia-reperfusion injury in multiple models. Trifluoperazine can inhibit both PLA2 (67) and calmodulin (68). Both of these activities have been hypothesized to be protective against mPT. In particular, PLA2 inhibition has received substantial attention, despite some evidence arguing that it is not the mechanism by which trifluoperazine protects against mPT (67). Literature analysis suggested that other tricyclics and phenothiazines might also inhibit PLA2 or calmodulin activity. Therefore, we assayed the capacity of each tricyclic in the library to inhibit PLA2 or calmodulin at concentrations from 1–300 μM. The ability to inhibit either PLA2 or calmodulin did not correlate with the ability to inhibit mPT in the compound set tested (PLA2: r2 = 0.04 at 100 μM; calmodulin: r2 = 0.02 at 300 μM; see Table I and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20032053/DC1).
Physiological Studies of HTPD PT Inhibitors.
Because of the role of apoptosis in stroke-mediated pathology, the activity of a heterocyclic compound was next examined in two models of stroke: (a) oxygen-glucose deprivation (OGD) of cultured primary cerebrocortical neurons; and (b) in the mouse, middle cerebral artery (MCA) occlusion (MCAO)/reperfusion.
Promethazine was chosen as the compound representing this class for three reasons. First, of the most potent inhibitors of mPT, promethazine has one of the highest tolerated doses in humans; although trifluoperazine is the strongest mPT inhibitor, it is used in humans at doses 15-fold below those of promethazine. Furthermore, trifluoperazine has limited utility for long-term use in humans because of its toxicity in nonneural tissues. Although irrelevant for the short-term treatment required by stroke, this limitation would present complications for the treatment of chronic neurodegenerative diseases. Methiothepin, which is approximately equivalent to promethazine in potency, has not been used in humans. Second, promethazine is a well-tolerated drug with few adverse side effects, and, as opposed to the heterocyclics, promethazine has comparatively minor neurological side effects. We note that, from our experience with minocycline (29), the in vitro assay can overestimate the levels of drug necessary for protection in cell culture and in vivo. Therefore, concentrations used in the cell assay were chosen based on concentrations of promethazine shown in the literature to be bioactive. Doses chosen for the in vivo studies were also based on the literature. The dose used was one half those shown to be nontoxic in an analysis including cerebellar pathology. Doses were given at 12-h intervals as it is known promethazine has an 8-h half-life in mice. Third, promethazine does not appear to be a strong PLA2 or calmodulin inhibitor and, thus, promethazine can be used to demonstrate the independence of effects against mPT induction and action on calmodulin or PLA2.
Promethazine Protects Cultured Primary Neurons from OGD.
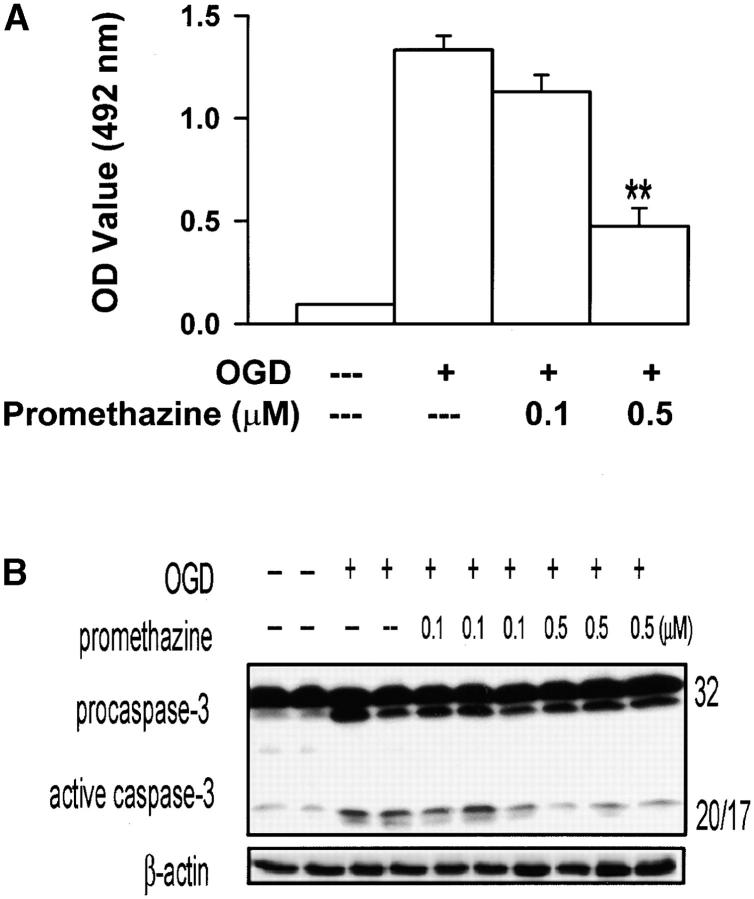
OGD of primary cerebrocortical neurons is a widely used cell culture model of stroke-related pathology. Promethazine inhibited OGD-mediated neuronal death, as determined by reduction of LDH release into the media (∼65% less release). Inhibition of neuronal death was observed at submicromolar concentrations of promethazine (Fig. 3, 0.1 μM, P = 0.05; 0.5 μM, P < 0.001). Caspase-3 activation plays a key role in hypoxia/ischemia-mediated injury. To gain insight into the mechanism of promethazine-mediated neuroprotection, we evaluated whether this compound could inhibit OGD-mediated caspase-3 activation. As expected from an mPT inhibitor, promethazine-treated neurons exposed to OGD showed reduced caspase-3 activation, supporting the hypothesis that the drug interferes with caspase-mediated cell death.
Figure 3.
Promethazine protects primary cerebrocortical neurons from OGD. LDH activity (A) and active caspase 3 (B) in the supernatant and lysates, respectively, of control and OGD challenged primary cerebrocortical neurons in the presence or absence of promethazine. *, P = 0.05; **, P < 0.001. Neurotoxicity studies were conducted as described previously (60).
Follow-up studies have indicated that the tricyclic antidepressant nortriptyline, which also inhibits mPT (Fig. 1), is also protective in this OGD model (unpublished data).
Promethazine Reduces Infarct Size and Neurological Impairment after MCAO-Reperfusion.
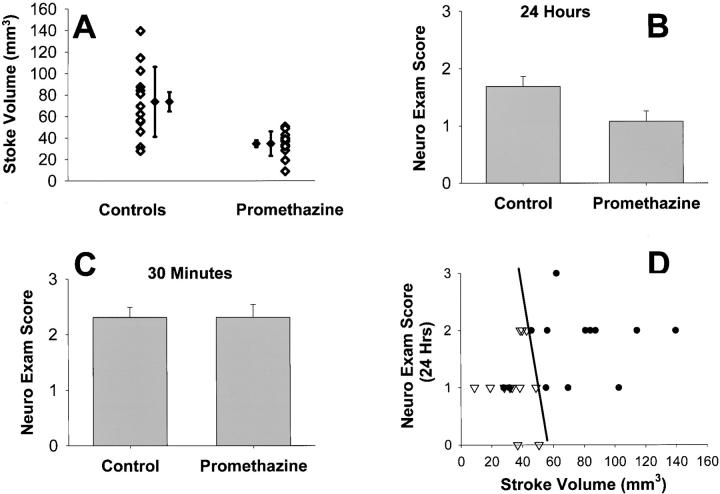
Because promethazine reduced OGD-mediated neuronal death, we evaluated whether it could also ameliorate ischemic damage in vivo, in which activation of the mPT and caspase-3 may play roles. After MCAO, promethazine-treated mice showed a 53% reduction in lesion size compared with saline-treated mice (Fig. 4 A, n = 13, P < 0.005). Both maximal infarct size and variability of the injury were reduced by 65%. Consistent with a reduction in ischemic damage, the neurologic impairment score of promethazine-treated mice was significantly improved 24 h after MCAO (Fig. 4 B, P < 0.05). Cerebral blood flow (measured by a laser-doppler flowmeter) and systemic blood pressure did not differ between the treated and control groups. As expected from studies of other neuroprotectants, the neurologic score was not different at 30 min after ischemia evaluation (Fig. 4 C and references 61, 62). Protection of both neural function and structure was consistent across all individual mice in the analysis (Fig. 4 D, diagonal line emphasizes that all individuals are protected).
Figure 4.
Promethazine protects mice from MCA occlusion/reperfusion. (A) Total stroke volume in C57/Bl6 mice untreated and treated. P < 0.005. Means shown with SD to emphasize reduced variability of stroke volume in drug treatment, and with standard error to show accuracy of population estimate. (B) Neuro exam assessed at 24 h after reperfusion of right MCA. Mean ± SEM, P < 0.05. (C) Neuro exam assessed at 30 min after occlusion of right MCA. Mean ± SEM, P = 1.0. (D) Stroke Volume versus Neurologic Exam. Diagonal line emphasizes that all treated individuals appear protected (left of line, 13 treated, 2 control; right of line, 11 control). (inverted triangles) Promethazine treated. (dots) Controls. n = 13 in each group. Middle cerebral artery occlusion/reperfusion studies were conducted as described previously (references 60, 61).
Follow-up studies have indicated that the tricyclic antidepressant nortriptyline, which also inhibits mPT (Fig. 1) is also protective in this MCAO model (unpublished data).
Discussion
Defining the Pharmacophore.
The heterocyclics and related compounds described here (HTPD) represent a series of structurally related inhibitors that may help better understand the mPT. SAR analysis, based on the final evidence that 28 of 32 HTPD compounds are protective versus 10 out of 1,008 nonheterocyclics suggests that the HTPD backbone's activity is highly significant within the intact screening set. Our data is most consistent with the active pharmacophore being the basic class structure, rather than a specific subclass as defined either clinically (e.g., antidepressant vs. antipsychotic), biochemically (e.g., ability to inhibit PLA2), or structurally (e.g., noncarbon atom containing rings vs. tricyclic). There is a wide range of potency for the heterocyclics (Fig. 2), but further SAR analysis is currently limited by a lack of inactive compounds having this basic structure in our current library. Of the group studied, four compounds are not true heterocyclics (one noncarbon atom in the “central” ring), suggesting the classical heterocyclic structure itself may not be mandatory. Mefloquine has only two rings, suggesting the three-ring backbone common in this class may be a reflection of the set of compounds tested rather than an obligate structural feature.
mPT in Stroke.
These data are in accord with and significantly extend findings of other groups who have provided evidence linking the mPT to excitotoxic and OGD injury in cultured neurons (7, 69, 70) and to damage subsequent to ischemia-reperfusion in intact animals (6). The Matsumoto et al. (6) and Khaspekov et al. (69) papers are of particular note, as they used the N-Met-Val analogue of cyclosporin A, which has been considered not to react with calcineurin. Retrospective literature analysis also indicates that several HTPD drugs had shown some protection against ischemia or against mPT or mPT-like phenomena, but that the existence of a structural class with a common biochemical target (mitochondria) was not previously recognized, and has not been followed up clinically, possibly due to the side effects of the specific agents tested. Specifically, our data allows us, for the first time, to pull together 15 yr of study of these agents to provide the first common linkage of the HTPD compounds that crosses previously recognized clinical, neurochemical target, and structural bounds, and to provide a probable mechanism for their actions in vivo. Thus, as shown in Table I, the in vivo and cell culture protection mediated by the HTPDs has been replicated already, by at least 22 labs, in at least 10 HTPD drugs in animal models of cerebral ischemia and related challenges and by at least 8 HTPD drugs in cell culture. We note that agents have been shown to work both before and after ischemia, and have been tested in mice, rats, and gerbils. Some isolated mitochondrial studies on HTPD agents other than trifluoperazine and tamoxifen has also been published. Histamine has been shown to cause swelling of liver mitochondria, and antihistamines to retard it (68), but this finding has not been examined further. Thioridazine has been suggested to inhibit apoptosis and delay mPT induction by acting to reduce mitochondrial oxidative stress (71). Thus, our data help explain and link a series of studies in different research areas by providing a common mechanism to explain the observations in these studies.
Our data also strengthen the case for mPT as a critical event in the causative pathway of stroke-mediated cell death, although some caveats still remain. Protection against mPT and in vivo protection against ischemia-reperfusion and related events/challenges were shown to be qualitatively correlated in at least 10 compounds (Table I). All of these compounds displayed activity in at least one study in the range of 3–20 mg/kg, consistent with relatively similar dose dependence in in vitro assays of mPT inhibition. In contrast, protection mediated by these agents does not correlate with their ability to inhibit PLA2 or calmodulin and cannot be attributed to either the clinical or structural class of the mPT inhibitor (Fig. 1, Table I, and Fig. S3). By providing direct evidence consistent with mechanistic involvement of mPT inhibition in the actions of these 10 compounds, removing potential alternative explanations (e.g., PLA2 inhibition, calmodulin inhibition, and neurochemical effects), and providing evidence that mPT inhibitors structurally distinct from CsA are neuroprotective, our data provide direct counter-arguments to each of the major concerns raised previously (see Introduction) against mPT playing a causative role in stroke-mediated damage. Remaining caveats include, for example, (a) the identification of these compounds as mPT inhibitors is still based on work in isolated liver mitochondria, the biochemically defined system, but not the target tissue; (b) there are systems, for example, the calcium overload model (72), that may not involve mPT as a primary mediator; and (c) it is always possible that a previously unrecognized system is the actual target (e.g., the TRMP7 channels recently recognized to contribute to anoxic cell death in some models; reference 73).
Potential Clinical Utility.
The results reported here may expand the potential clinical uses of HTPD drugs to protection against stroke, and set the stage for studies further expanding their utility to other disorders. Additional studies are required; however, our data suggest that these agents are potentially usable as long-term prophylactics (to reduce damage from an event that occurs, not to prevent the event) for individuals at risk for strokes and heart attacks (e.g., patients undergoing carotid endarterectomy or who have experienced a cardio- or cerebrovascular event). The heterocyclics may also be appropriate for acute management of stroke and heart attack as well as a component of the clinical management of neurodegenerative disease. Further retrospective analysis (see Online Supplemental Material) showed two HTPD compounds at delayed mPT are protective against spinal cord injury (trifluoperazine and chlorpromazine) and three against myocardial and kidney ischemia-reperfusion (trifluoperazine, chlorpromazine, and quinacrine). Chlorpromazine has also been shown to protect against sepsis and liver toxicity/ischemia.
Although mPT involvement in chronic neurodegenerative disorders remains unknown and controversial, the common involvement of mitochondria in cell death pathways suggests that the HTPD drugs might be considered, in future experiments, as potential candidates for long-term use in individuals with neurodegenerative diseases that involve cell death in the nervous system, including amyotrophic lateral sclerosis, and Parkinson's, Alzheimer's, and Huntington's diseases. Applicability in Huntington's disease is supported by data showing that 5–10 mg/kg promethazine protects against striatal lesions in Lewis rats induced by 3-NP (unpublished data). Applicability in Parkinson's disease is supported by data showing that promethazine (best at 10 mg/kg) protects against striatal dopamine depletion and neuron loss in the substantia nigra pars compacta induced by MPTP (unpublished data). Both preclinical and clinical testing will be required to determine whether these drugs will be beneficial for these diseases.
These drugs appear to offer major advantages as clinical mitochondrial protective agents. First, 31 out of the 32 compounds tested here are clinically available, and 27 are clinically approved, suggesting the potential for rapid movement into preclinical and/or clinical trials. Some of these drugs have been used for five decades. Second, the active drugs appear from their clinical uses and side effects to cross the blood brain barrier (three possible exceptions being cyclobenzaprine, mefloquine, and quinacrine). Third, most are safe for long-term use, and many have only minor side effects (a notable exception being the phenothiazine trifluoperazine). Fourth, they are active in vitro and in vivo at concentrations close to or within those that are achievable in humans. The maximal therapeutic dose of promethazine in humans is 3 mg/kg, and several have LD50 in animals 5–10 times higher than promethazine with favorable safety profiles. Last, the many available classes of these agents with differing side effects and clinical actions suggest that a combination of agents, each one in a subclinical dose, can be used to achieve mPT inhibition without inducing unwanted effects. This work defines a structural family suitable for further pharmacological optimization, complementing the promise of available clinical therapies and novel experimental reagents with a longer range promise of even further optimized agents (e.g., for protection and for probing mitochondrial involvement in cell death in isolated systems, cultured cells, and in vivo). Although further preclinical study is warranted, these finding suggest that these clinically approved drugs will be candidates for human study in stroke and neurodegeneration.
Acknowledgments
The authors thank J. Moore for assistance with publication graphics.
This work was supported by grants from the Hereditary Disease Foundation (to B.S. Kristal), National Institutes of Health (NIH)/National Institute on Aging (to B.S. Kristal), NIH/National Institute of Neurological Disorders and Stroke (to R.M. Friedlander and A.M. Brown), the Huntington's Disease Society of America (to R.M. Friedlander), and Burke Medical Research Institute (to B.S. Kristal). No author has direct competing interests in the work presented. A patent application has been filed on aspects of this work by Weill Medical College, and some authors potentially have patent rights though university agreements.
Abbreviations used in this paper: CNS, central nervous system; CsA, cyclosporine A; HTPD, heterocyclic, tricyclic, and phenothiazine-derived; LDH, lactate dehydrogenase; MCA, middle cerebral artery; MCAO, MCA occlusion; mPT, mitochondrial permeability transition; OGD, oxygen-glucose deprivation; PLA2, phospholipase A2; SAR, structure–activity relationship.
References
- 1.Zamzami, N., S.A. Susin, P. Marchetti, T. Hirsch, I. Gomez-Monterrey, M. Castedo, and G. Kroemer. 1996. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchetti, P., M. Castedo, S.A. Susin, N. Zamzami, T. Hirsch, A. Macho, A. Haeffner, F. Hirsch, M. Geuskens, and G. Kroemer. 1996. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 184:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Susin, S.A., N. Zamzami, M. Castedo, E. Daugas, H.G. Wang, S. Geley, F. Fassy, J.C. Reed, and G. Kroemer. 1997. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/ CD95- and ceramide-induced apoptosis. J. Exp. Med. 186:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green, D.R., and J.C. Reed. 1998. Mitochondria and apoptosis. Science. 281:1309–1312. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls, D.G., and S.L. Budd. 2000. Mitochondria and neuronal survival. Physiol. Rev. 80:315–360. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto, S., H. Friberg, M. Ferrand-Drake, and T. Wieloch. 1999. Blockade of the mitochondrial permeability transition pore diminishes infarct size in the rat after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 19:736–741. [DOI] [PubMed] [Google Scholar]
- 7.Schinder, A.F., E.C. Olson, N.C. Spitzer, and M. Montal. 1996. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J. Neurosci. 16:6125–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchen, M.R., O. McGuinness, L.A. Brown, and M. Crompton. 1993. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc. Res. 27:1790–1794. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi, P., L. Scorrano, R. Colonna, V. Petronilli, and F. Di Lisa. 1999. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 264:687–701. [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh, M., C. Du, X. Wang, and E.M. Johnson, Jr. 2002. Exogenous smac induces competence and permits caspase activation in sympathetic neurons. J. Neurosci. 22:8018–8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troy, C.M., J.E. Friedman, and W.J. Friedman. 2002. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J. Biol. Chem. 277:34295–34302. [DOI] [PubMed] [Google Scholar]
- 12.Susin, S.A., N. Zamzami, M. Castedo, T. Hirsch, P. Marchetti, A. Macho, E. Daugas, M. Geuskens, and G. Kroemer. 1996. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 184:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Loo, G., X. Saelens, M. Van Gurp, M. MacFarlane, S.J. Martin, and P. Vandenabeele. 2002. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 9:1031–1042. [DOI] [PubMed] [Google Scholar]
- 14.Madesh, M., B. Antonsson, S.M. Srinivasula, E.S. Alnemri, and G. Hajnoczky. 2002. Rapid kinetics of tBid-induced cytochrome c and Smac/DIABLO release and mitochondrial depolarization. J. Biol. Chem. 277:5651–5659. [DOI] [PubMed] [Google Scholar]
- 15.Zamzami, N., and G. Kroemer. 2001. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell Biol. 2:67–71. [DOI] [PubMed] [Google Scholar]
- 16.Martinou, J.C., and D.R. Green. 2001. Breaking the mitochondrial barrier. Nat. Rev. Mol. Cell Biol. 2:63–67. [DOI] [PubMed] [Google Scholar]
- 17.Everett, H., M. Barry, S.F. Lee, X. Sun, K. Graham, J. Stone, R.C. Bleackley, and G. McFadden. 2000. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 191:1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacotot, E., L. Ravagnan, M. Loeffler, K.F. Ferri, H.L. Vieira, N. Zamzami, P. Costantini, S. Druillennec, J. Hoebeke, J.P. Briand, et al. 2000. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J. Exp. Med. 191:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiths, E.J., and A.P. Halestrap. 1995. Mitochondrial non-specific pores remain closed during cardiac ischemia, but open upon reperfusion. Biochem. J. 307:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieminen, A.L., T.G. Petrie, J.J. LeMasters, and W.R. Selman. 1996. Cyclosporin A delays mitochondrial depolarization induced by N-methyl-D-aspartate in cortical neurons: evidence of the mitochondrial permeability transition. Neuroscience. 75:993–997. [DOI] [PubMed] [Google Scholar]
- 21.Siesjo, B.K., and P. Siesjo. 1996. Mechanisms of secondary brain injury. Eur. J. Anaesthesiol. 13:247–268. [PubMed] [Google Scholar]
- 22.Friberg, H., and T. Wieloch. 2002. Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 84:241–250. [DOI] [PubMed] [Google Scholar]
- 23.Scheff, S.W., and P.G. Sullivan. 1999. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J. Neurotrauma. 16:783–792. [DOI] [PubMed] [Google Scholar]
- 24.Friberg, H., M. Ferrand-Drake, F. Bengtsson, A.P. Halestrap, and T. Wieloch. 1998. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. 18:5151–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunter, T.E., K.K. Gunter, S.-S. Sheu, and C.E. Gavin. 1994. Mitochondrial calcium transport: physiological and pathological evidence. Am. J. Physiol. 267:C313–C339. [DOI] [PubMed] [Google Scholar]
- 26.Petronilli, V., P. Costantini, L. Scorrano, R. Colonna, S. Passamonti, and P. Bernardi. 1994. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J. Biol. Chem. 269:16638–16642. [PubMed] [Google Scholar]
- 27.Zoratti, M., and I. Szabo. 1995. The mitochondrial permeability transition. Biochim. Biophys. Acta. 1241:139–176. [DOI] [PubMed] [Google Scholar]
- 28.Zorov, D.B., C.R. Filburn, L.O. Klotz, J.L. Zweier, and S.J. Sollott. 2000. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192:1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu, S., I.G. Stavrovskaya, M. Drozda, B.Y. Kim, V. Ona, M. Li, S. Sarang, A.S. Liu, D.M. Hartley, D.C. Wu, et al. 2002. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 417:74–78. [DOI] [PubMed] [Google Scholar]
- 30.Petit, P.X., M. Goubern, P. Diolez, S.A. Susin, N. Zamzami, and G. Kroemer. 1998. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 426:111–116. [DOI] [PubMed] [Google Scholar]
- 31.Kantrow, S.P., and C.A. Piantadosi. 1997. Release of cytochrome c from liver mitochondria during permeability transition. Biochem. Biophys. Res. Commun. 232:669–671. [DOI] [PubMed] [Google Scholar]
- 32.Decaudin, D., M. Castedo, F. Nemati, A. Beurdeley-Thomas, G. De Pinieux, A. Caron, P. Pouillart, J. Wijdenes, D. Rouillard, G. Kroemer, and M.F. Poupon. 2002. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res. 62:1388–1393. [PubMed] [Google Scholar]
- 33.Zamzami, N., C. El Hamel, C. Maisse, C. Brenner, C. Munoz-Pinedo, A.S. Belzacq, P. Costantini, H. Vieira, M. Loeffler, G. Molle, and G. Kroemer. 2000. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 19:6342–6350. [DOI] [PubMed] [Google Scholar]
- 34.Friberg, H., C. Connern, A.P. Halestrap, and T. Wieloch. 1999. Differences in the activation of the mitochondrial permeability transition among brain regions in the rat correlate with selective vulnerability. J. Neurochem. 72:2488–2497. [DOI] [PubMed] [Google Scholar]
- 35.Kristal, B.S., and J.M. Dubinsky. 1997. Mitochondrial permeability transition in the central nervous system: induction by calcium cycling-dependent and -independent pathways. J. Neurochem. 69:524–538. [DOI] [PubMed] [Google Scholar]
- 36.Li, P.A., H. Uchino, E. Elmer, and B.K. Siesjo. 1997. Amelioration by cyclosporin A of brain damage following 5 or 10 min of ischemia in rats subjected to preischemic hyperglycemia. Brain Res. 753:133–140. [DOI] [PubMed] [Google Scholar]
- 37.Folbergrova, J., P.A. Li, H. Uchino, M.L. Smith, and B.K. Siesjo. 1997. Changes in the bioenergetic state of rat hippocampus during 2.5 min of ischemia, and prevention of cell damage by cyclosporin A in hyperglycemic subjects. Exp. Brain Res. 114:44–50. [DOI] [PubMed] [Google Scholar]
- 38.Andreyev, A., and G. Fiskum. 1999. Calcium induced release of mitochondrial cytochrome c by different mechanisms selective for brain versus liver. Cell Death Differ. 6:825–832. [DOI] [PubMed] [Google Scholar]
- 39.Zaidan, E., and N.R. Sims. 1997. Reduced activity of the pyruvate dehydrogenase complex but not cytochrome c oxidase is associated with neuronal loss in the striatum following short-term forebrain ischemia. Brain Res. 772:23–28. [DOI] [PubMed] [Google Scholar]
- 40.Sims, N.R. 1992. Energy metabolism and selective neuronal vulnerability following global cerebral ischemia. Neurochem. Res. 17:923–931. [DOI] [PubMed] [Google Scholar]
- 41.Drake, M., H. Friberg, F. Boris-Moller, K. Sakata, and T. Wieloch. 1996. The immunosuppressant FK506 ameliorates ischaemic damage in the rat brain. Acta. Physiol. Scand. 158:155–159. [DOI] [PubMed] [Google Scholar]
- 42.Yrjanheikki, J., T. Tikka, R. Keinanen, G. Goldsteins, P.H. Chan, and J. Koistinaho. 1999. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. USA. 96:13496–13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yrjanheikki, J., R. Keinanen, M. Pellikka, T. Hokfelt, and J. Koistinaho. 1998. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA. 95:15769–15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, S.M., T.Y. Yune, S.J. Kim, D.W. Park, Y.K. Lee, Y.C. Kim, Y.J. Oh, G.J. Markelonis, and T.H. Oh. 2003. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J. Neurotrauma. 20:1017–1027. [DOI] [PubMed] [Google Scholar]
- 45.Wells, J.E., R.J. Hurlbert, M.G. Fehlings, and V.W. Yong. 2003. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 126:1628–1637. [DOI] [PubMed] [Google Scholar]
- 46.Arvin, K.L., B.H. Han, Y. Du, S.Z. Lin, S.M. Paul, and D.M. Holtzman. 2002. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann. Neurol. 52:54–61. [DOI] [PubMed] [Google Scholar]
- 47.Wang, X., S. Zhu, M. Drozda, W. Zhang, I.G. Stavrovskaya, E. Cattaneo, R.J. Ferrante, B.S. Kristal, and R.M. Friedlander. 2003. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc. Natl. Acad. Sci. USA. 100:10483–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodrigues, C.M., S. Sola, Z. Nan, R.E. Castro, P.S. Ribeiro, W.C. Low, and C.J. Steer. 2003. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc. Natl. Acad. Sci. USA. 100:6087–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin, X.M., Y. Luo, G. Cao, L. Bai, W. Pei, D.K. Kuharsky, and J. Chen. 2002. Bid-mediated mitochondrial pathway is critical to ischemic neuronal apoptosis and focal cerebral ischemia. J. Biol. Chem. 277:42074–42081. [DOI] [PubMed] [Google Scholar]
- 50.Kuwana, T., M.R. Mackey, G. Perkins, M.H. Ellisman, M. Latterich, R. Schneiter, D.R. Green, and D.D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 111:331–342. [DOI] [PubMed] [Google Scholar]
- 51.Rego, A.C., M.W. Ward, and D.G. Nicholls. 2001. Mitochondria control ampa/kainate receptor-induced cytoplasmic calcium deregulation in rat cerebellar granule cells. J. Neurosci. 21:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholls, D.G., S.L. Budd, M.W. Ward, and R.F. Castilho. 1999. Excitotoxicity and mitochondria. Biochem. Soc. Symp. 66:55–67. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimoto, T., and B.K. Siesjo. 1999. Posttreatment with the immunosuppressant cyclosporin A in transient focal ischemia. Brain Res. 839:283–291. [DOI] [PubMed] [Google Scholar]
- 54.Hoyt, K.R., B.A. McLaughlin, D.S. Higgins, Jr., and I.J. Reynolds. 2000. Inhibition of glutamate-induced mitochondrial depolarization by tamoxifen in cultured neurons. J. Pharmacol. Exp. Ther. 293:480–486. [PubMed] [Google Scholar]
- 55.Heemskerk, J., A.J. Tobin, and L.J. Bain. 2002. Teaching old drugs new tricks. Trends Neurosci. 25:494–496. [DOI] [PubMed] [Google Scholar]
- 56.Heemskerk, J., A.J. Tobin, and B. Ravina. 2002. From chemical to drug: neurodegeneration drug screening and the ethics of clinical trials. Nat. Neurosci. Suppl 5:1027–1029. [DOI] [PubMed] [Google Scholar]
- 57.Kristal, B.S., and A.M. Brown. 1999. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J. Biol. Chem. 274:23169–23175. [DOI] [PubMed] [Google Scholar]
- 58.Zorova, L.D., B.F. Krasnikov, A.E. Kuzminova, I.A. Polyakova, E.N. Dobrov, and D.B. Zorov. 2000. Virus-induced permeability transition in mitochondria. FEBS Lett. 466:305–309. [DOI] [PubMed] [Google Scholar]
- 59.Meshulam, T., H. Herscovitz, D. Casavant, J. Bernardo, R. Roman, R.P. Haugland, G.S. Strohmeier, R.D. Diamond, and E.R. Simons. 1992. Flow cytometric kinetic measurements of neutrophil phospholipase A activation. J. Biol. Chem. 267:21465–21470. [PubMed] [Google Scholar]
- 60.Plesnila, N., S. Zinkel, D.A. Le, S. Amin-Hanjani, Y. Wu, J. Qiu, A. Chiarugi, S.S. Thomas, D.S. Kohane, S.J. Korsmeyer, and M.A. Moskowitz. 2001. BID mediates neuronal cell death after oxygen/glucose deprivation and focal cerebral ischemia. Proc. Natl. Acad. Sci. USA. 98:15318–15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedlander, R.M., V. Gagliardini, H. Hara, K.B. Fink, W. Li, G. MacDonald, M.C. Fishman, A.H. Greenberg, M.A. Moskowitz, and J. Yuan. 1997. Expression of a dominant negative mutant of interleukin-1 β converting enzyme in transgenic mice prevents neuronal cell death induced by trophic factor withdrawal and ischemic brain injury. J. Exp. Med. 185:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hara, H., R.M. Friedlander, V. Gagliardini, C. Ayata, K. Fink, Z. Huang, M. Shimizu-Sasamata, J. Yuan, and M.A. Moskowitz. 1997. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. USA. 94:2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang, Z., P.L. Huang, N. Panahian, T. Dalkara, M.C. Fishman, and M.A. Moskowitz. 1994. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 265:1883–1885. [DOI] [PubMed] [Google Scholar]
- 64.Galeotti, N., C. Ghelardini, and A. Bartolini. 2002. Antihistamine antinociception is mediated by Gi-protein activation. Neuroscience. 109:811–818. [DOI] [PubMed] [Google Scholar]
- 65.Swanson, R.A., M.T. Morton, G. Tsao-Wu, R.A. Savalos, C. Davidson, and F.R. Sharp. 1990. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 10:290–293. [DOI] [PubMed] [Google Scholar]
- 66.Bederson, J.B., L.H. Pitts, M. Tsuji, M.C. Nishimura, R.L. Davis, and H. Bartkowski. 1986. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 17:472–476. [DOI] [PubMed] [Google Scholar]
- 67.Broekemeier, K.M., and D.R. Pfeiffer. 1995. Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry. 34:16440–16449. [DOI] [PubMed] [Google Scholar]
- 68.Mehrotra, S., P.N. Viswanathan, and P. Kakkar. 1993. Influence of some biological response modifiers on swelling of rat liver mitochondria in vitro. Mol. Cell. Biochem. 124:101–106. [DOI] [PubMed] [Google Scholar]
- 69.Khaspekov, L., H. Friberg, A. Halestrap, I. Viktorov, and T. Wieloch. 1999. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. Eur. J. Neurosci. 11:3194–3198. [DOI] [PubMed] [Google Scholar]
- 70.Alano, C.C., G. Beutner, R.T. Dirksen, R.A. Gross, and S.S. Sheu. 2002. Mitochondrial permeability transition and calcium dynamics in striatal neurons upon intense NMDA receptor activation. J. Neurochem. 80:531–538. [DOI] [PubMed] [Google Scholar]
- 71.Rodrigues, T., A.C. Santos, A.A. Pigoso, F.E. Mingatto, S.A. Uyemura, and C. Curti. 2002. Thioridazine interacts with the membrane of mitochondria acquiring antioxidant activity toward apoptosis–potentially implicated mechanisms. Br. J. Pharmacol. 136:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nicholls, D.G., S. Vesce, L. Kirk, and S. Chalmers. 2003. Interactions between mitochondrial bioenergetics and cytoplasmic calcium in cultured cerebellar granule cells. Cell Calcium. 34:407–424. [DOI] [PubMed] [Google Scholar]
- 73.Aarts, M., K. Iihara, W.L. Wei, Z.G. Xiong, M. Arundine, W. Cerwinski, J.F. MacDonald, and M. Tymianski. 2003. A key role for TRPM7 channels in anoxic neuronal death. Cell. 115:863–877. [DOI] [PubMed] [Google Scholar]
- 74.Kuroda, S., A. Nakai, T. Kristian, and B.K. Siesjo. 1997. The calmodulin antagonist trifluoperazine in transient focal brain ischemia in rats. Anti-ischemic effect and therapeutic window. Stroke. 28:2539–2544. [DOI] [PubMed] [Google Scholar]
- 75.Zivin, J.A., A. Kochhar, and T. Saitoh. 1989. Phenothiazines reduce ischemic damage to the central nervous system. Brain Res. 482:189–193. [DOI] [PubMed] [Google Scholar]
- 76.Takano, H., H. Fukushi, Y. Morishima, and Y. Shirasaki. 2003. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain Res. 962:41–47. [DOI] [PubMed] [Google Scholar]
- 77.Nakata, N., H. Kato, and K. Kogure. 1992. Protective effects of serotonin reuptake inhibitors, citalopram and clomipramine, against hippocampal CA1 neuronal damage following transient ischemia in the gerbil. Brain Res. 590:48–52. [DOI] [PubMed] [Google Scholar]
- 78.McCaslin, P.P., X.Z. Yu, I.K. Ho, and T.G. Smith. 1992. Amitriptyline prevents N-methyl-D-aspartate (NMDA)-induced toxicity, does not prevent NMDA-induced elevations of extracellular glutamate, but augments kainate-induced elevations of glutamate. J. Neurochem. 59:401–405. [DOI] [PubMed] [Google Scholar]
- 79.Palmer, G.C., E.W. Harris, R. Ray, M.L. Stagnitto, and R.J. Schmiesing. 1992. Classification of compounds for prevention of NMDLA-induced seizures/mortality, or maximal electroshock and pentylenetetrazol seizures in mice and antagonism of MK801 binding in vitro. Arch. Int. Pharmacodyn. Ther. 317:16–34. [PubMed] [Google Scholar]
- 80.Li, Y.F., and Z.P. Luo. 2002. Desipramine antagonized corticosterone-induced apoptosis in cultured PC12 cells. Acta. Pharmacol. Sin. 23:311–314. [PubMed] [Google Scholar]
- 81.Gyertyan, I., G. Gigler, and A. Simo. 1999. The neuroprotective and hypothermic effect of GYKI-52466, a non-competitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-antagonist on histological and behavioural variables in the gerbil global ischemia model. Brain Res. Bull. 50:179–186. [DOI] [PubMed] [Google Scholar]
- 82.Katsuoka, M., and S.T. Ohnishi. 1989. Pharmacologic protection of perfused rat heart against global ischemia. Prostaglandins Leukot. Essent. Fatty Acids. 38:151–156. [DOI] [PubMed] [Google Scholar]
- 83.Peruche, B., and J. Krieglstein. 1991. Neuroblastoma cells for testing neuroprotective drug effects. J. Pharmacol. Methods. 26:139–148. [DOI] [PubMed] [Google Scholar]
- 84.Ray, S.D., L.M. Kamendulis, M.W. Gurule, R.D. Yorkin, and G.B. Corcoran. 1993. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J. 7:453–463. [DOI] [PubMed] [Google Scholar]
- 85.Phillis, J.W. 1996. Cerebroprotective action of the phospholipase inhibitor quinacrine in the ischemia/reperfused gerbil hippocampus. Life Sci. 58:97–101. [DOI] [PubMed] [Google Scholar]
- 86.Miettinen, S., F.R. Fusco, J. Yrjanheikki, R. Keinanen, T. Hirvonen, R. Roivainen, M. Narhi, T. Hokfelt, and J. Koistinaho. 1997. Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-D-aspartic acid-receptors and phospholipase A2. Proc. Natl. Acad. Sci. USA. 94:6500–6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Estevez, A.Y., and J.W. Phillis. 1997. The phospholipase A2 inhibitor, quinacrine, reduces infarct size in rats after transient middle cerebral artery occlusion. Brain Res. 752:203–208. [DOI] [PubMed] [Google Scholar]
- 88.Cazevieille, C., A. Muller, F. Meynier, N. Dutrait, and C. Bonne. 1994. Protection by prostaglandins from glutamate toxicity in cortical neurons. Neurochem. Int. 24:395–398. [DOI] [PubMed] [Google Scholar]
- 89.Moffat, M.P., and R.G. Tsushima. 1989. Functional and electrophysiological effects of quinacrine on the response of ventricular tissues to hypoxia and reoxygenation. Can. J. Physiol. Pharmacol. 67:929–935. [DOI] [PubMed] [Google Scholar]
- 90.Karasawa, Y., H. Araki, and S. Otomo. 1992. Effects of ketanserin and mianserin on delayed neuronal death induced by cerebral ischemia in Mongolian gerbils. Psychopharmacology (Berl.). 109:264–270. [DOI] [PubMed] [Google Scholar]
- 91.Fujimura, M., K. Hashimoto, and K. Yamagami. 2000. Effects of antipsychotic drugs on neurotoxicity, expression of fos-like protein and c-fos mRNA in the retrosplenial cortex after administration of dizocilpine. Eur. J. Pharmacol. 398:1–10. [DOI] [PubMed] [Google Scholar]
- 92.Olney, J.W., and N.B. Farber. 1994. Efficacy of clozapine compared with other antipsychotics in preventing NMDA-antagonist neurotoxicity. J. Clin. Psychiatry. 55:43–46. [PubMed] [Google Scholar]
- 93.Okamura, N., K. Hashimoto, N. Kanahara, E. Shimizu, C. Kumakiri, N. Komatsu, and M. Iyo. 2003. Protective effect of the antipsychotic drug zotepine on dizocilpine-induced neuropathological changes in rat retrosplenial cortex. Eur. J. Pharmacol. 461:93–98. [DOI] [PubMed] [Google Scholar]