Abstract
Alveolar macrophages (AMs) express the class A scavenger receptor macrophage receptor with collagenous structure (MARCO), but its role in vivo in lung defense against bacteria and environmental particles has not been studied. We used MARCO-deficient mice to directly test the in vivo role of AM MARCO in innate defense against pneumococcal infection and environmental particles. In a murine model of pneumococcal pneumonia, MARCO−/− mice displayed an impaired ability to clear bacteria from the lungs, increased pulmonary inflammation and cytokine release, and diminished survival. In vitro binding of Streptococcus pneumoniae and in vivo uptake of unopsonized particles by MARCO−/− AMs were dramatically impaired. MARCO−/− mice treated with the “inert” environmental particle TiO2 showed enhanced inflammation and chemokine expression, indicating that MARCO-mediated clearance of inert particles by AMs prevents inflammatory responses otherwise initiated by other lung cells. Our findings point to an important role of MARCO in mounting an efficient and appropriately regulated innate immune response against inhaled particles and airborne pathogens.
Keywords: macrophage, phagocytosis, environment, innate immunity
Introduction
Lung macrophages are both sentinels and the first line of defense against infection or injury from inhaled agents. The repertoire of receptors expressed on the alveolar macrophage (AM) surface is critical for binding, uptake, and response to microbes and inhaled unopsonized environmental particles. One family of receptors likely to be important for normal host defense of the lung are the class A macrophage scavenger receptors (SRAs). This designation describes a group of pattern recognition receptors composed of three members as follows: SRA-I/II, macrophage receptor with collagenous structure (MARCO; reference 1), and the recently identified SRCL (scavenger receptor with C-type lectin; reference 2). All are multifunctional trimeric glycoproteins expressed primarily by macrophages.
The class A scavenger receptors (especially the relatively well-studied SRA-I/II) have been implicated to varying degrees in systemic innate immunity against infection (3–10). Other data also suggest the potential involvement of MARCO in antibacterial defense mechanisms. For example, constitutive MARCO is up-regulated in liver and spleen macrophages after bacterial infection (11–13). Moreover, MARCO can bind LPS or LTA (14), as well as intact bacteria (Escherichia coli and Staphylococcus aureus; references 15, 16). MARCO is also expressed on dendritic cells (17) and may play a role in adaptive immunity. We have previously identified MARCO as a major receptor on AMs for in vitro binding of unopsonized environmental particles and certain microorganisms (15, 18), but its actual functional importance for lung host defense in vivo has not been investigated. Use of mice with genetic deletion of MARCO allowed specific analysis of its in vivo role in defense of the lung against two commonly encountered challenges, pneumococcal bacteria and inert environmental dusts.
Materials and Methods
Animals.
8–12-wk-old male mice genetically deficient in MARCO (MARCO−/−) were used in all experiments. Age- and sex-matched wild-type (C57BL/6 MARCO+/+) mice purchased from Charles River Laboratories were used as controls. MARCO−/− mice were developed using targeted homologous recombination (unpublished data), and were backcrossed for at least eight generations to the C57BL/6 background. All animals were housed in sterile microisolator cages and had no evidence of spontaneous infection. Approval before all experimentation was obtained from the institutional animal use review committee.
Reagents and Particles.
Titanium dioxide (TiO2) particles (median diameter ∼1 μm) were provided by J. Brain (Harvard School of Public Health, Boston, MA). Particles were suspended in BSS− (124 mM NaCl, 5.8 mM KCl, 10 mM dextrose, and 20 mM Hepes) as stock solutions and sonicated ∼30 s before use. All reagents not otherwise specified were obtained from Sigma-Aldrich.
Cell Isolation and Flow Cytometric Assay of Particulate Binding.
Mice were killed by i.p. pentobarbital injection. AMs obtained by repeated lung lavage with BSS− were centrifuged at 150 g and resuspended in BSS+ Ca (0.3 mM) and Mg (1 mM). AMs (2 × 105 in 100 μl BSS+) were preincubated with 2.5 μg/ml cytochalasin D for 5 min on ice in a 1-ml microfuge tube. After the addition of probe-sonicated particles, the tubes were rotated at 37°C for 30 min, placed on ice, and analyzed by flow cytometry. Flow cytometry was performed as described previously (15). AM uptake of particles was measured using the increase in the mean right angle scatter (RAS) caused by these granular materials (15).
Particle Effects In Vivo.
Mice were lightly anesthetized with halothane, and solutions of particles in pyrogen-free saline were administered via intranasal insufflation (50 μL). Pilot experiments in normal wild-type mice showed minimal response to 5 mg/ml of the inert particle TiO2. These concentrations were used in subsequent experiments comparing responses of MARCO-deficient mice. Mice were killed 4 h later. Bronchoalveolar lavage (BAL) was performed. Total cell yield was determined by hemocytometer and differential by microscopic evaluation of cytocentrifuge preparations stained with modified Wright-Giemsa. Aliquots of cell-free BAL fluid were stored at −70°C for the subsequent ELISA assay of TNF-α (R&D Systems). In some experiments, whole lung tissue was harvested without lavage for RNA extractions using the TRIzol reagent (GIBCO BRL). Expression of a panel of cytokine mRNAs (lymphotoxin, RANTES, eotaxin, MIP-1b, MIP-1a, MIP-2, IP-10, MCP-1, and TCA-3) was evaluated using a ribonuclease protection assay kit (mCK5; BD Biosciences) following the manufacturer's protocol. Blots were visualized using an InstantImager™ Phosphoimager (Packard Instrument, Co.), and densitometric analysis was performed using the dedicated Imager software package.
Bacteria.
Streptococcus pneumoniae serotype 3 was obtained from the American Type Culture Collection and cultured overnight on 5% sheep blood–supplemented agar Petri dishes (VWR). A stock suspension was prepared, aliquoted in small volumes, and kept at −80°C. For each experiment, an aliquot was grown overnight on a blood agar plate and resuspended in sterile isotonic saline. Bacterial concentration of the obtained suspension was estimated by OD600 measurements, and the appropriate dilution was prepared in saline to be administered intranasally to mice. The true concentration was determined by culture.
Mouse Model of Pneumococcal Pneumonia.
A well-characterized mouse model of pneumococcal pneumonia was used in this analysis (19). Pneumonia was induced by intranasal instillation of 25 μl of a bacterial suspension containing ∼105 CFU of S. pneumoniae type 3 of mice under short-term anesthesia with halothane. 4 or 24 h after infection, mice were killed with an i.p. injection of a lethal dose of sodium pentobarbital (FatalPlus; Vortech Pharmaceuticals). Separate experiments were performed for either BAL and lung harvest for histology, or total lung bacteria counts (CFU). At 4 or 24 h after infection, whole lungs were harvested and homogenized in 1 ml of sterile isotonic saline with a tissue homogenizer (Omni International). Serial 10-fold dilutions in sterile saline were made from these homogenates, and 100 μl volumes were plated onto sheep-blood agar plates and incubated at 37°C. CFUs were counted after 18–20 h. In some experiments, to assess survival, mice were instilled intranasally with a single 25-μl suspension of S. pneumoniae (∼2 × 105 CFU). Animal survival was monitored twice a day over a period of 12 d.
BAL.
Animals were killed 4 or 24 h after bacterial challenge. BAL was performed in situ with a 20-gauge catheter inserted into the proximal trachea, flushing the lower airways five times with 0.8 ml PBS. The fluid retrieved from the first flushing was kept for ELISA assays. The BAL cells were separated from the BAL fluid by centrifugation, resuspended in PBS, and counted, and a fraction was cytospun on microscopic slides for staining with Diff-Quick (Baxter Scientific Products) and for subsequent differential counts.
Histopathology.
Parallel infection experiments were performed to harvest the lungs for histopathology. Age- and sex-matched MARCO−/− and MARCO+/+ control animals were killed 24 h after inoculation. Lungs were harvested and kept in 10% formalin. Organs were examined histologically by hematoxylin and eosin staining. To quantitate AM number in situ, nonspecific esterase staining of cryostat sections of normal lung from wild-type or MARCO−/− mice was performed. Random fields of alveolar parenchyma were analyzed (10 per sample, three mice per group). An index of esterase-positive macrophages (numerator) and alveolar tissue points (denominator) were calculated using a grid projection system, and the data were expressed as a ratio.
Preparation of FITC-labeled Pneumococci.
S. pneumoniae were labeled with FITC (Molecular Probes). Bacteria were grown overnight on sheep blood agar plates, washed twice, and resuspended in PBS. Bacterial concentration was set to ∼109 CFU/ml as determined by OD600. Bacteria were heat killed at 95°C for 10 min, centrifuged, and resuspended in 1 ml of labeling buffer (0.1 M NaHCO3, pH 9.2). 0.25 mg of newly prepared FITC in DMSO was added per milliliter of suspension, and bacteria were incubated under constant stirring in the dark at room temperature for 1 h. Bacteria were washed three times and dialyzed overnight against PBS. Cell number was determined using a hemocytometer under a fluorescence microscope. The final concentration was set to 109 bacteria/ml.
Phagocytosis Assay.
Binding and phagocytosis of FITC-labeled pneumococci by AMs were quantified using a flow cytometric assay. BAL of MARCO−/− and control mice was performed in PBS, and cells were washed and resuspended in BSS+. Aliquots of 0.5 ml (BAL cell count adjusted to 0.5 × 106 cells/ml, >95% macrophages, as judged by differential staining) were added to 1 ml conical microcentrifuge tubes together with FITC-labeled pneumococci at a bacteria/AM ratio of 50:1, incubated for 1 or 2 h at 37°C with continuous gentle rotation. Alternatively, AMs were preincubated with 10 μM cytochalasin D (Sigma-Aldrich) for 15 min at room temperature before the addition of bacteria. At the end of incubation, samples were washed once with cold BSS+ to remove unbound bacteria and analyzed for mean fluorescence intensity (MFI) of the macrophage population by flow cytometry. The rate of phagocytosed bacteria was calculated as the difference in fluorescence of untreated and cytochalasin D–treated AMs.
Quantitation of BALF Cytokine Content by ELISA.
MIP-2 and TNF-α were quantitated in BALF of infected MARCO−/− and control mice using commercially available ELISA kits (R&D Systems) following manufacturer's instructions.
Statistical Analysis.
Data were analyzed using the StatView software (Abacus Concepts). Differences in survival were determined by χ2 analysis. Kaplan-Meier curves were used to show survival over time, and differences between curves were analyzed using a log-rank test. For other measurements, differences between groups were examined by analysis of variance with correction for multiple groups. Data are presented as mean ± SEM. Results were considered significant when P < 0.05.
Results and Discussion
First, we sought to investigate whether the absence of MARCO receptor results in impaired bacterial clearance from the lungs of infected mice. Mice were challenged by intranasal administration of ∼105 CFU S. pneumonia, a relatively low inoculum likely to be cleared by normal AMs with minimal inflammation. Viable bacteria were quantitated in lung tissue homogenates obtained after 4 h. Wild-type MARCO+/+ mice showed efficient clearance of the bacteria, with only ∼8% of the inoculum being recovered on average (Fig. 1 A). In contrast, >50% of the inoculated bacteria were still alive and recovered from the lungs of MARCO−/− mice (Fig. 1 A). Similar results were seen at 24 h after intranasal administration (Fig. 1 B).
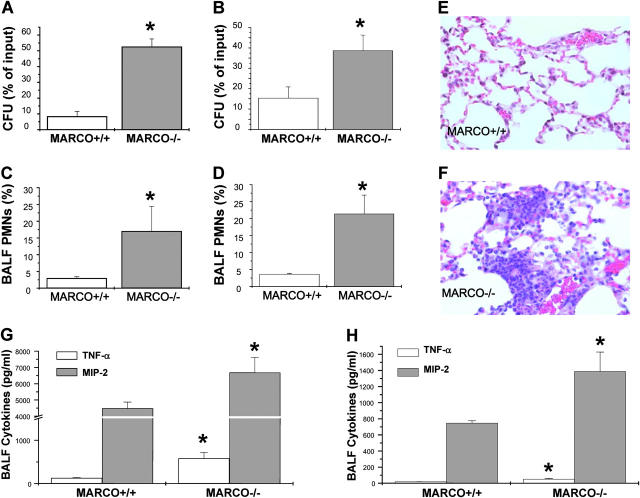
Figure 1.
(A and B) Pulmonary bacterial burden after infection with S. pneumoniae. MARCO+/+ and MARCO−/− (n = 7–9) were intranasally infected with 105 CFU of S. pneumoniae. Lung bacterial load was determined at 4 h (A) and 24 h (B) after infection. Data shown are representative of at least three separate experiments, and presented as mean ± SEM. *, P < 0.05 for −/− versus +/+. (C and D) Lung inflammation in response to S. pneumoniae infection. PMN recruitment into BALF of infected mice, harvested 4 h (C) and 24 h (D) after infection, presented as percentage of total cells in BALF. (E and F) Representative lung histology sections from infected mice that showed mostly normal histology in MARCO+/+ mice (E) and increased focal PMN infiltrates in MARCO−/− mice (F). (G and H) Infected mice (n = 4) BALF TNF-α and MIP-2 at 4 h (G) and 24 h (H) after infection as measured by ELISA. Data are presented as mean ± SEM. *, P < 0.05 for −/− versus +/+.
BALF obtained 4 h after infection from MARCO−/− mice contained substantially more neutrophils compared with +/+ controls (Fig. 1, C and D, 17 ± 7.40 vs. 2.45 ± 0.46%), a finding corroborated by histologic analysis of infected lung tissue (Fig. 1, E and F). BAL fluid from infected MARCO−/− mice also contained higher amounts of TNF-α and MIP-2 (0.58 ± 0.14 and 6.68 ± 0.94 pg/ml, respectively) than samples from control mice (Fig. 1 G, 0.13 ± 0.01 and 4.48 ± 0.39 pg/ml, respectively). A similar profile was observed 24 h after infection, though the overall levels of cytokines were lower (Fig. 1 H). Morphometric comparison of the tissue density of macrophages showed no significant differences between wild-type and MARCO−/− (1.027 ± 0.034 vs. 1.077 ± 0.028; mean ± SEM; P = 0.29 +/+ vs. −/−), a finding consistent with equal numbers of AMs recovered in BAL from normal MARCO+/+ and MARCO−/− mice (unpublished data). These data exclude simple deficiency in numbers of AMs as the mechanism for the differences observed in responses to bacteria (or particles) in the MARCO−/− mice.
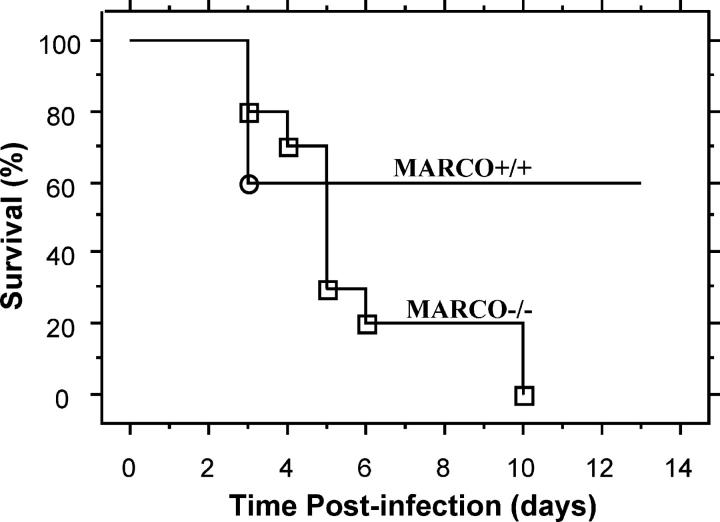
A failure to eliminate S. pneumoniae reaching the bronchoalveolar space may lead to increased mortality from severe pneumonia or disseminated infection. To test this postulate, we investigated survival over time of MARCO+/+ and MARCO−/− mice inoculated with 2 × 105 CFU of S. pneumoniae (a slightly higher dose required to detect mortality in the wild-type mice). Although mortality in the wild-type group was 40%, the total mortality in the MARCO−/− mice was 100% by day 10 (Fig. 2).
Figure 2.
Absence of MARCO increases mortality after S. pneumoniae administration to mice. MARCO+/+ and MARCO−/− mice were intranasally inoculated with 2.2 × 105 CFU of S. pneumoniae, and survival was monitored over a period of 13 d. Data are expressed as percentage of mice alive at each time point. Kaplan-Meier survival plots are shown for 10 animals per group. Mantel-Cox log-rank test showed that P = 0.0342. One experiment representative of two is shown.
We postulate that these data correspond well to the natural history of pneumococcal infection in a nonimmune host. Pneumococcal infection is relatively common, but far less common than might be predicted from high rates of nasopharyngeal colonization in normal adults and children (20) and from the frequency of normal nocturnal aspiration of upper airway secretions (21). Resident AMs are the most important initial cellular defense against inhaled pathogens (22, 23). Our data indicate that MARCO is a critical receptor for this early defense mechanism. Once established, pneumococcal infection causes neutrophil influx in response to unchecked bacterial proliferation and tissue injury. In the absence of specific antibody, the PMN influx is relatively ineffective in restricting progression of the pneumonia as evinced in the high mortality of untreated pneumococcal pneumonia in people (24) and in the increased mortality in MARCO−/− mice seen in survival studies.
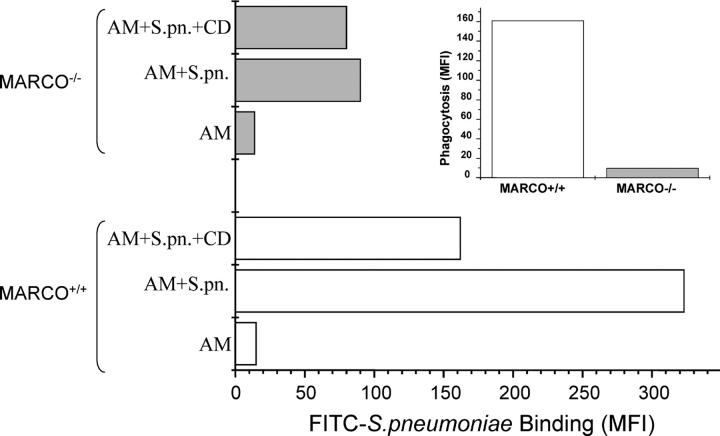
To more directly evaluate the contribution of MARCO receptor to AM binding and uptake of pneumococci, AMs from wild-type and MARCO−/− mice were incubated for 1 h with FITC-labeled S. pneumoniae in the absence or presence of cytochalasin D, and the samples were analyzed by flow cytometry. In the presence of cytochalasin D, only binding of fluorescent bacteria occurs because internalization is blocked (15, 25). Under these conditions, MARCO−/− AMs showed substantially diminished bacterial binding (Fig. 3, e.g., 80 vs. 162 MFI units). When both binding and internalization were intact (absence of cytochalasin D), AMs from wild-type mice exhibited almost a fourfold higher MFI value compared with the MARCO-deficient mice AMs (Fig. 3, 323 vs. 90 MFI units). Calculation of the fluorescence attributable to internalization of bacteria by AMs shows a substantially lower amount in MARCO−/− mice (Fig. 2, inset, 161 vs. 10 MFI units). These data are consistent with the marked defect in clearance of bacteria and the resulting increased inflammatory response observed in vivo.
Figure 3.
Binding and phagocytosis of FITC-labeled S. pneumoniae (S.pn.) by MARCO+/+ and MARCO−/− AMs. AMs were harvested from untreated mice, and phagocytosis was measured as described in Materials and Methods. Fluorescence was measured on untreated cells (AM) and cells incubated with labeled bacteria in the absence (AM + S.pn.) or presence of cytochalasin D (AM + S.pn. + CD) to block internalization. The deduced phagocytosis rate as expressed in MFI units is shown in the inset. Data shown are representative of two experiments.
Nevertheless, the persistence of some residual binding indicates the presence of other receptors capable of binding to S. pneumoniae. Interestingly, the absence of MARCO also reduced internalization of the lower numbers of AM-bound S. pneumoniae. The basis for this indirect implication of MARCO in the internalization process for bound bacteria is unclear and warrants further study.
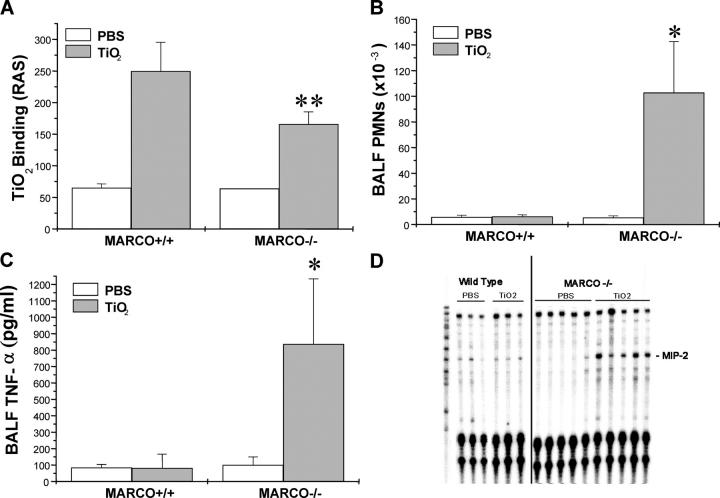
Next, we investigated in vivo responses to a prototypical “inert” particle (TiO2), so named because usually it is rapidly cleared by AM phagocytosis with minimal or no pulmonary inflammation or injury (26, 27). BAL analysis 4 h later showed diminished uptake of particles by AMs from the MARCO−/− mice (Fig. 4 A). TiO2 administration caused minimal PMN influx into BAL samples from wild-type control mice (Fig. 4 B). In contrast, MARCO−/− mice showed a dramatic increase in PMN trafficking into the lungs (102.8 ± 39.8 × 103 cells/ml), compared with the control mice (6.3 ± 1.2 × 103 cells/ml) and to vehicle (PBS)-induced recruitment in both groups (Fig. 4 B). Analysis of BAL fluid from TiO2-treated MARCO−/− mice also showed a marked increase of TNF-α (837 ± 397 pg/ml), as compared with 100 ± 50 pg/ml in response to PBS (Fig. 4 C). In wild-type mice, the level of BALF TNF-α induced by TiO2 (82 ± 85 pg/ml) was low and not different from the vehicle (PBS) control (84 ± 20 pg/ml). Analysis of cytokine mRNA expression using an RNase protection assay (Fig. 4 D) showed that TiO2 challenge induced a higher rate of MIP-2 expression in the lung tissue from MARCO−/− mice (relative increase after TiO2 vs. PBS challenge, MARCO−/− vs. MARCO+/+, 3.4 ± 1.4 vs. 0.6 ± 0.09).
Figure 4.
Lung inflammation in response to inhaled TiO2 particles. (A) MARCO+/+ and MARCO−/− AM binding of TiO2 as determined by right angle scatter (RAS) measurements of harvested BAL macrophages after intranasal instillation of particles to mice (n = 3 mice/group), **, P < 0.02 for TiO2 instilled MARCO−/− versus all other groups. (B) PMN recruitment into BALF of PBS and TiO2-treated mice, n > 9 mice/group. *, P < 0.05 for TiO2 instilled MARCO−/− mice versus all other groups. (C) BALF TNF-α as measured by ELISA, n > 3 mice/group. *, P < 0.05 for TiO2 instilled MARCO−/− mice versus all other groups. (D) Analysis of lung gene expression by RNase protection assay showing increased MIP-2 mRNA in lung samples from TiO2-treated MARCO−/− mice.
To test the possibility that MARCO−/− AMs show an altered cytokine response to TiO2, we measured TNF-α and MIP-2 release by AMs treated in vitro with TiO2. MARCO−/− and +/+ AMs showed similar levels of increased cytokine release in response to the positive control, LPS, and similarly minimal release in response to TiO2 (unpublished data). Also, AMs lavaged from MARCO−/− mice 2 h after instillation of TiO2 (before the large increase of PMNs seen at 4 h) also did not show any substantial increase in cytokine release after in vitro culture (unpublished data). These findings argue against an altered response in MARCO−/− AMs as the source of mediators seen after in vivo TiO2.
The enhanced inflammatory response seen with inert TiO2 in MARCO−/− mice is noteworthy. We postulate that the reduced binding of TiO2 by MARCO−/− AMs (as demonstrated in vivo; Fig. 4 A) allows increased interaction of TiO2 with other lung cells (i.e., epithelium) due to delayed uptake by the AM, which in turn leads to the increased MIP-2 expression and neutrophil influx observed. In contrast with its lack of proinflammatory effects on AMs, TiO2 has been shown to elicit release by lung epithelial cells of chemokines for neutrophils such as MIP-2 and other cytokines (28, 29).
These studies using MARCO-deficient mice show an important function for this AM class A scavenger receptor in host defense against pneumococcal pneumonia and against pulmonary inflammation from otherwise inert environmental dusts. Because SRA (including MARCO) expression levels can increase or decrease in response to various stimuli (30), the possibility that pathologic or therapeutic changes in MARCO expression might impact lung host defense merits further investigation.
Acknowledgments
This work was supported by the National Institutes of Health (grant nos. ES 011008, 00002) and the Jere Mead Fellowship (to M. Arredouani). The authors have no conflicting financial interests.
References
- 1.Platt, N., R. Haworth, L. Darley, and S. Gordon. 2002. The many roles of the class A macrophage scavenger receptor. Int. Rev. Cytol. 212:1–40. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura, K., H. Funakoshi, F. Tokunaga, and T. Nakamura. 2001. Molecular cloning of a mouse scavenger receptor with C-type lectin (SRCL)(1), a novel member of the scavenger receptor family. Biochim. Biophys. Acta. 1522:53–58. [DOI] [PubMed] [Google Scholar]
- 3.Pearson, A.M. 1996. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 8:20–28. [DOI] [PubMed] [Google Scholar]
- 4.Hampton, R.Y., D.T. Golenbock, M. Penman, M. Krieger, and C.R. Raetz. 1991. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 352:342–344. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki, H., Y. Kurihara, M. Takeya, N. Kamada, M. Kataoka, K. Jishage, O. Ueda, H. Sakaguchi, T. Higashi, T. Suzuki, et al. 1997. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 386:292–296. [DOI] [PubMed] [Google Scholar]
- 6.Thomas, C.A., Y. Li, T. Kodama, H. Suzuki, S.C. Silverstein, and J. El Khoury. 2000. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J. Exp. Med. 191:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiser, L., M.P. De Winther, K. Makepeace, M. Hollinshead, P. Coull, J. Plested, T. Kodama, E.R. Moxon, and S. Gordon. 2002. The class A macrophage scavenger receptor is a major pattern recognition receptor for Neisseria meningitidis which is independent of lipopolysaccharide and not required for secretory responses. Infect. Immun. 70:5346–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunne, D.W., D. Resnick, J. Greenberg, M. Krieger, and K.A. Joiner. 1994. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc. Natl. Acad. Sci. USA. 91:1863–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haworth, R., N. Platt, S. Keshav, D. Hughes, E. Darley, H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1997. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 186:1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi, Y., C. Miyaji, H. Watanabe, H. Umezu, G. Hasegawa, T. Abo, M. Arakawa, N. Kamata, H. Suzuki, T. Kodama, and M. Naito. 2000. Role of macrophage scavenger receptor in endotoxin shock. J. Pathol. 192:263–272. [DOI] [PubMed] [Google Scholar]
- 11.van der Laan, L.J., M. Kangas, E.A. Dopp, E. Broug-Holub, O. Elomaa, K. Tryggvason, and G. Kraal. 1997. Macrophage scavenger receptor MARCO: in vitro and in vivo regulation and involvement in the anti-bacterial host defense. Immunol. Lett. 57:203–208. [DOI] [PubMed] [Google Scholar]
- 12.van der Laan, L.J., E.A. Dopp, R. Haworth, T. Pikkarainen, M. Kangas, O. Elomaa, C.D. Dijkstra, S. Gordon, K. Tryggvason, and G. Kraal. 1999. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J. Immunol. 162:939–947. [PubMed] [Google Scholar]
- 13.Ito, S., M. Naito, Y. Kobayashi, H. Takatsuka, S. Jiang, H. Usuda, H. Umezu, G. Hasegawa, M. Arakawa, L.D. Shultz, et al. 1999. Roles of a macrophage receptor with collagenous structure (MARCO) in host defense and heterogeneity of splenic marginal zone macrophages. Arch. Histol. Cytol. 62:83–95. [DOI] [PubMed] [Google Scholar]
- 14.Sankala, M., A. Brannstrom, T. Schulthess, U. Bergmann, E. Morgunova, J. Engel, K. Tryggvason, and T. Pikkarainen. 2002. Characterization of recombinant soluble macrophage scavenger receptor MARCO. J. Biol. Chem. 277:33378–33385. [DOI] [PubMed] [Google Scholar]
- 15.Palecanda, A., J. Paulauskis, E. Al-Mutairi, A. Imrich, G. Qin, H. Suzuki, T. Kodama, K. Tryggvason, H. Koziel, and L. Kobzik. 1999. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 189:1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elomaa, O., M. Kangas, C. Sahlberg, J. Tuukkanen, R. Sormunen, A. Liakka, I. Thesleff, G. Kraal, and K. Tryggvason. 1995. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 80:603–609. [DOI] [PubMed] [Google Scholar]
- 17.Granucci, F., F. Petralia, M. Urbano, S. Citterio, F. Di Tota, L. Santambrogio, and P. Ricciardi-Castagnoli. 2003. The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood. 102:2940–2947. [DOI] [PubMed] [Google Scholar]
- 18.Palecanda, A., and L. Kobzik. 2001. Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr. Mol. Med. 1:589–595. [DOI] [PubMed] [Google Scholar]
- 19.Rijneveld, A.W., M. Levi, S. Florquin, P. Speelman, P. Carmeliet, and T. van Der Poll. 2002. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J. Immunol. 168:3507–3511. [DOI] [PubMed] [Google Scholar]
- 20.McCullers, J.A., and E.I. Tuomanen. 2001. Molecular pathogenesis of pneumococcal pneumonia. Front. Biosci. 6:D877–D889. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson, K., D.F. Eggli, and S.L. Maxwell. 1997. Quantitative aspiration during sleep in normal subjects. Chest. 111:1266–1272. [DOI] [PubMed] [Google Scholar]
- 22.Gordon, S.B., and R.C. Read. 2002. Macrophage defences against respiratory tract infections. Br. Med. Bull. 61:45–61. [DOI] [PubMed] [Google Scholar]
- 23.Dockrell, D.H., H.M. Marriott, L.R. Prince, V.C. Ridger, P.G. Ince, P.G. Hellewell, and M.K. Whyte. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380–5388. [DOI] [PubMed] [Google Scholar]
- 24.Marrie, T.J. 1999. Pneumococcal pneumonia: epidemiology and clinical features. Semin. Respir. Infect. 14:227–236. [PubMed] [Google Scholar]
- 25.Ogle, J.D., J.G. Noel, R.M. Sramkoski, C.K. Ogle, and J.W. Alexander. 1988. Phagocytosis of opsonized fluorescent microspheres by human neutrophils. A two-color flow cytometric method for the determination of attachment and ingestion. J. Immunol. Methods. 115:17–29. [DOI] [PubMed] [Google Scholar]
- 26.Lindenschmidt, R.C., K.E. Driscoll, M.A. Perkins, J.M. Higgins, J.K. Maurer, and K.A. Belfiore. 1990. The comparison of a fibrogenic and two nonfibrogenic dusts by bronchoalveolar lavage. Toxicol. Appl. Pharmacol. 102:268–281. [DOI] [PubMed] [Google Scholar]
- 27.Rehn, B., F. Seiler, S. Rehn, J. Bruch, and M. Maier. 2003. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol. Appl. Pharmacol. 189:84–95. [DOI] [PubMed] [Google Scholar]
- 28.Driscoll, K.E., D.G. Hassenbein, J. Carter, J. Poynter, T.N. Asquith, R.A. Grant, J. Whitten, M.P. Purdon, and R. Takigiku. 1993. Macrophage inflammatory proteins 1 and 2: expression by rat alveolar macrophages, fibroblasts, and epithelial cells and in rat lung after mineral dust exposure. Am. J. Respir. Cell Mol. Biol. 8:311–318. [DOI] [PubMed] [Google Scholar]
- 29.Tao, F., and L. Kobzik. 2002. Lung macrophage-epithelial cell interactions amplify particle-mediated cytokine release. Am. J. Respir. Cell Mol. Biol. 26:499–505. [DOI] [PubMed] [Google Scholar]
- 30.Peiser, L., S. Mukhopadhyay, and S. Gordon. 2002. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14:123–128. [DOI] [PubMed] [Google Scholar]