Abstract
The signaling lymphocytic activation molecule (SLAM)/CD150 family includes a family of chromosome 1–encoded cell surface molecules with costimulatory functions mediated in part by the adaptor protein SH2D1A (SLAM-associated protein, SAP). Deficiency in SH2D1A protects mice from an experimental model of lupus, including the development of hypergammaglobulinemia, autoantibodies including anti–double stranded DNA, and renal disease. This protection did not reflect grossly defective T or B cell function per se because SH2D1A-deficient mice were susceptible to experimental autoimmune encephalomyelitis, a T cell–dependent disease, and they were capable of mounting normal T-independent antigen-specific immunoglobulin responses. Instead, T-dependent antibody responses were impaired in SH2D1A-deficient mice, reflecting defective germinal center formation. These findings demonstrate a specific role for the SLAM–SH2D1A system in the regulation of T-dependent humoral immune responses, implicating members of the CD150–SH2D1A family as targets in the pathogenesis and therapy of antibody-mediated autoimmune and allergic diseases.
Keywords: autoimmunity, mice, knockout, lupus, T lymphocytes
Introduction
The signaling lymphocytic activation molecule (SLAM)/CD150 family includes several CD2-like cell surface glycoproteins encoded on chromosome 1 of both mouse and human, including at least CD84, CD150/SLAM, CD229 (Ly-9), CD244 (2B4), and NTB-A (Ly-108). Collectively, these have been proposed to function in the immune synapse between T cells and antigen-presenting or other immune cells, perhaps via homotypic interactions (1). In human and murine cells, CD84, CD150, CD229, CD244, and NTB-A/Ly-108 bind to and use at least two adaptor proteins: the X-linked SLAM-associated protein SH2D1A, which is predominantly expressed in T, NK, and some B cells, and the SLAM-associated protein–like adaptor EAT2, which appears to account for CD150 family activity in other hematopoietic cell lineages. In general, the expression of each of these receptor and adaptor molecules increases during lymphocyte activation (2), suggesting that they modulate lymphocyte effector functions. Indeed, recent studies indicate that CD244 plays an inhibitory role during the cytotoxic responses of murine NK cells (3), whereas CD150 modulates IL-4 production by murine T cells as well as the activation of murine macrophages (4), and SH2D1A-deficient mice demonstrate increased IFN-γ production with defective IL-4 production by T cells as well as dysgammaglobulinemia (5–7). In addition, at least during infection with lymphocytic choriomeningitis virus, SH2D1A-deficient mice are unable to maintain long-term antiviral antibody responses in a T cell–dependent fashion, reflecting defective memory B cell and plasma cell formation, as well as defective germinal center formation (7). Thus, the CD150–SH2D1A system likely modulates T-dependent immune responses, but the biological context(s) in which it predominantly functions remains incompletely defined.
In humoral autoimmune diseases like lupus, hyperactivated B cells spontaneously elaborate pathogenic, high titer, high affinity autoantibodies, such as anti–double stranded (ds)DNA, which mediates immune complex end-organ diseases, such as glomerulonephritis (8). This process critically requires T cell help to promote class switching, and presumably also to promote affinity maturation via a germinal center reaction or similar T–B cell collaboration. Several target systems have been identified that likely participate in and/or promote this process, including CD40-CD154, types I and II IFNs, and BLys/BAFF, but several murine and human studies suggest that these systems are insufficient to explain fully the pathogenesis of lupus autoantibodies, and/or that these systems are not the most proximal immune aberrations during the development of the disease (8).
During ongoing studies to identify novel target genes in lupus, we found that CD150-related molecules, as well as SH2D1A, are up-regulated in the circulating lymphocytes of lupus-prone, but not nonautoimmune, mice. Therefore, we reasoned that SH2D1A-deficient mice could provide an initial insight as to the importance of this system in the pathogenesis of humoral autoimmunity. SH2D1A-deficient animals were resistant to experimentally induced lupus, but interestingly, these effects could not be attributed to gross defects in T or B cells. Instead, SH2D1A deficiency resulted in impaired humoral T-dependent humoral immunity associated with defective germinal center responses. Thus, the CD150–SH2D1A system appears to play a specific role in the elaboration of T-dependent humoral autoimmunity, implicating molecules within this family as both pathogenic and therapeutic targets in humorally mediated immune diseases in general.
Materials and Methods
Mice.
SH2D1A-deficient (5) and WT mice of the 129 background, as well as C57BL/6, BALB/c, BXSB, and MRL/+ (The Jackson Laboratory) mice were maintained under specific pathogen-free conditions. All experiments were performed in compliance with the relevant laws and institutional guidelines of the Washington University School of Medicine.
CD150 and Ly-108 Quantification.
Peripheral blood leukocytes were isolated via Ficoll-Paque density centrifugation (Amersham Biosciences). RNA and cDNA were prepared and subjected to real-time PCR as described previously (9), using the following gene-specific primers: CD150, 5′-AGCAGTATCTCTAGGACCTTCAACCT and 5′-CCATGGACTCGATTCTGAGGA; Ly108, 5′-TGCTGTCAGCAATTTGTCAGTCT and 5′-CATTCCAGGGTGGATTAGTTAGAAC. Rela-tive mRNA abundance of each transcript was normalized against tubulin (10).
Experimental Lupus Induction and Assessment.
To induce lupus, 6–8-wk-old animals were injected with 0.5 ml pristane (2,6,10,14-tetramethylpentadecane; ICN Biomedicals; references 11 and 12). At the times indicated, assessments for serum immunoglobulins, autoantibodies, renal immune deposits, and glomerulonephritis were performed essentially as described previously (12–14).
Experimental Autoimmune Encephalomyelitis (EAE) Induction and Assessment.
EAE was induced using the MOG38-50 peptide, similar to a previously described protocol (15). In brief, on day 0 each animal received a subcutaneous injection of 300 μg peptide in 0.3 ml PBS emulsified in an equal volume of complete Freund's adjuvant (Pierce Chemical Co.) with 1.2 mg Mycobacterium tuberculosis H37RA (Difco), as well as an intraperitoneal injection of 200 ng pertussis toxin in 0.2 ml PBS. On day 2, the animals were given a second injection of 200 ng pertussis toxin in 0.2 ml PBS. Animals were then examined daily for signs of EAE and scored according to the following standard clinical scale: 0, no disease; 1, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 4, hind- and fore-limb paralysis; 5, moribund or dead.
Immunizations.
Naive animals were injected intraperitoneally with 50 μg TNP-CGG (T-dependent) or TNP-Ficoll (T-independent) in 0.5 ml PBS. Sera were collected weekly thereafter for the assessment of hapten-specific antibody by ELISA using TNP(3)-BSA and TNP(34)-BSA (Biosearch), and IgG isotype-specific antibodies (Southern Biotechnology Associates, Inc.). Germinal centers were identified on 5-μM OCT-embedded frozen spleen sections using FITC-conjugated peanut agglutinin (PNA) and visualization by fluorescence microscopy.
Online Supplemental Material.
Online materials include Fig. S1, which displays the real-time PCR data of CD150 family member expression in the peripheral blood leukocytes of lupus-prone mice, and Fig. S2, which displays the serum total Ig isotype data for pristane-treated SH2D1A WT and KO mice. Figs. S1 and S2 are available at http://www.jem.org/cgi/content/full/jem.20040526/DC1.
Results and Discussion
SH2D1A-deficient Mice Are Protected from Experimentally Induced Lupus.
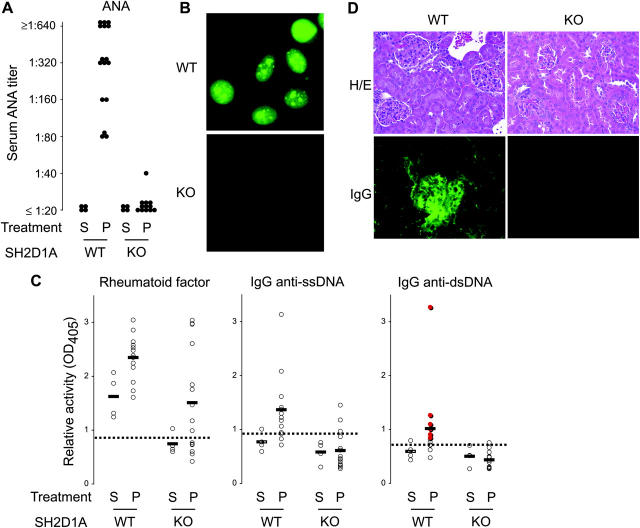
Using an ongoing microarray-based approach (9), we have found that CD150 family members are elevated in lymphocytes from lupus-prone (MRL/+, BXSB, and pristine-treated 129), but not nonautoimmune (BALB/c, C57BL/6, and saline-treated 129), mouse strains (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20040526/DC1, and unpublished data). Because many receptors of the CD150 family signal via SH2D1A (1), these findings prompted us to examine the role specifically of SH2D1A in lupus. Therefore, we tested the ability of SH2D1A-deficient (KO) mice to develop lupus using an experimentally induced model, pristane. Treated WT animals developed hypergammaglobulinemia of the IgG1 and IgG2a isotypes, which was evident as early as 6 wk after administration (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20040526/DC1, and unpublished data; P < 0.01 for both isotypes comparing pristane- to PBS-treated WT animals). In addition, pristane-treated WT animals developed relatively high titers (≥1:640) of antinuclear antibodies (ANAs), which were evident in 10% of the animals as early as 10 wk after pristane administration, and 100% of the animals by 5 mo (Fig. 1, A and B). They also developed both anti–single stranded (ss)DNA and anti-dsDNA specificities: 81% (13 out of 16) of pristane-treated WT animals were positive for both anti-ssDNA and anti-dsDNA IgG antibodies, 12 of which (92%) were positive by Crithidia lucillae immunofluorescence (P < 0.0001 comparing pristane-treated to PBS-treated WT animals for both specificities). Interestingly, PBS-treated WT animals developed perceptibly abnormal rheumatoid factor activity, at least when compared with unmanipulated BALB/c animals that were used as reference sera; nonetheless, this activity was also increased by pristane (P < 0.02 comparing pristane-treated to PBS-treated WT animals). Finally, pristane-treated WT animals reliably developed renal disease, as evidenced by renal immune deposits and a diffuse proliferative glomeulonephritis (Fig. 1 D and Table I). Thus, pristane-treated, but not PBS-treated, WT animals developed a clear lupus-like syndrome, including hypergammaglobulinemia, ANAs, anti–dsDNA antibodies, renal immune deposits, and glomerulonephritis.
Figure 1.
SH2D1A-deficient mice are protected from experimentally induced lupus. 6–8-wk-old SH2D1A-deficient (KO) or -sufficient (WT) mice were injected intraperitoneally with 0.5 ml pristane (P) or PBS (S, saline). (A and B) 5 mo later, sera were assayed and titered for the presence of ANAs (ANA). The representative ANA fluorescence pattern of a WT versus KO serum assayed at a 1:40 dilution is shown in B. (C) Rheumatoid factor and anti-ssDNA and anti-dsDNA titers were determined by ELISA. Specimens positive by Crithidia lucillae immunofluorescence are indicated in the anti-dsDNA graph in red. n = 4, 16, 4, and 18 for WT − P, WT − S, KO − P, and KO − S, respectively. Dotted lines indicate thresholds for positivity, as determined by three standard deviations above the mean of nonautoimmune BALB/c mice. (D) Representative renal histology (H/E) and glomerular IgG deposition (IgG) of WT versus KO animals 15 wk after treatment with pristane. Note the development of mesangila wall thickening and glomerular hypercellularity associated with IgG deposition in WT, but not KO, kidneys.
Table I.
Renal Disease in Pristane-treated SH2D1A Mice
| Genotype | Treatment | Timeexamineda | Igdepositsb | Glomerulardiseaseb |
|---|---|---|---|---|
| WT | Pristane | 15 | +++ | ++ |
| WT | Pristane | 15 | +++ | +++ |
| WT | Pristane | 15 | +++ | +++ |
| WT | Pristane | 15 | +++ | +++ |
| WT | Pristane | 15 | ++ | ++ |
| KO | Pristane | 15 | − | − |
| KO | Pristane | 15 | − | − |
| KO | Pristane | 15 | − | − |
| KO | Pristane | 15 | − | − |
| KO | Pristane | 15 | − | − |
| WT | PBS | 25 | − | − |
| WT | PBS | 25 | − | − |
| WT | Pristane | 25 | ++++ | +++ |
| WT | Pristane | 25 | ++++ | +++ |
| WT | Pristane | 25 | ++++ | +++ |
| KO | Pristane | 25 | − | − |
| KO | Pristane | 25 | − | − |
| KO | Pristane | 25 | − | − |
In contrast, KO mice were significantly protected from lupus. Although some pristane-treated KO animals developed hypergammaglobulinemia of the IgG1 isotype, the majority of animals failed to do so such that although the difference between pristane-treated WT and KO animals was mildly significant (P < 0.05), the difference between PBS- and pristane-treated KO animals was not (P = NS; Fig. S1). More impressively, SH2D1A was critical for the ability of pristane to induce hyper-IgG2a (P < 0.001 comparing pristane-treated WT and KO animals); however, there was a significant difference between IgG2a levels in PBS-treated WT and KO animals (P < 0.01), suggesting that SH2D1A might be required for the spontaneous (noninduced) generation of conventional IgG2a.
KO animals were protected from the development of autoantibodies (Fig. 1, A–C), including ANA (P < 0.0001 comparing pristane-treated WT and KO animals) and anti-DNA (P < 0.001 comparing pristane-treated WT and KO animals for both anti-ssDNA and anti-dsDNA). Although some pristane-treated KO animals developed detectable anti-ssDNA IgG (3 out of 16 = 19%), these appeared to be of low affinity because the same sera failed to test positive in an anti-dsDNA ELISA or by Crithidia immunofluorescence. Interestingly, pristane significantly increased rheumatoid factor activity in KO animals (P < 0.01 comparing pristane-treated to PBS-treated KO animals), although their activities were modestly lower than their pristane-treated WT counterparts (P < 0.10 comparing pristane-treated WT and KO animals). Finally, KO animals were fully protected from the development of significant renal disease, failing to develop significant immune deposits or glomerular lesions (Fig. 1 D and Table I), in contrast to their WT counterparts (P < 0.0001). In addition, KO animals failed to develop significant proteinuria, in contrast to their WT counterparts (10 out of 12 WT vs. 0 out of 13 KO animals developed proteinuria >30 mg/ml; P < 0.0001). Thus, SH2D1A deficiency protects against murine lupus, as judged by hypergammaglobulinemia, ANAs, anti-DNA, renal immune deposits, and glomerulonephritis.
SH2D1A-deficient Mice Are Susceptible to EAE.
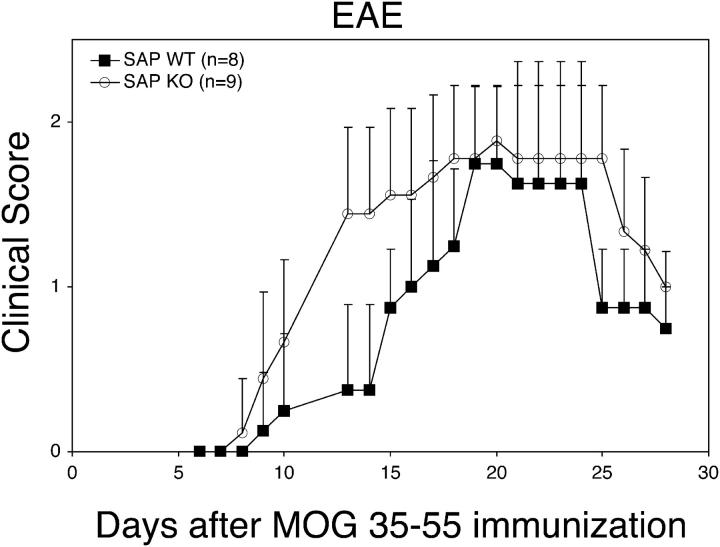
Because the pathogenesis of lupus heavily requires autoreactive Th cells (8), the KO animals may have been protected from lupus due to severely defective T cell function. To determine if an autoimmune-related Th cell effector function was defective in KO mice, we tested their ability to develop EAE in response to the MOG38-50 peptide, a Th1 cell–related, T cell–mediated disease (16). Surprisingly, KO animals were, if anything, more susceptible to EAE (Fig. 2, e.g., at days 13–15; P < 0.01). Thus, at least for this T-dependent autoimmune disease, SH2D1A is dispensable and is likely protective, suggesting that the protection of KO animals from experimental lupus cannot be explained by grossly defective T cell function. Indeed, KO T cells have been reported to produce increased amounts of IFN-γ, an effect that could exacerbate a Th1 cell–mediated cellular autoimmune syndrome (5, 6).
Figure 2.
SH2D1A-deficient mice are susceptible to experimental autoimmune EAE. EAE was induced in 5–6-wk-old SH2D1A-deficient (KO) or -sufficient (WT) mice using the MOG38-50 peptide. Clinical score was determined daily on each animal. Data points indicate means ± standard deviations.
SH2D1A-deficient Mice Mount Normal T-independent, But Not T-dependent, Humoral Immune Responses.
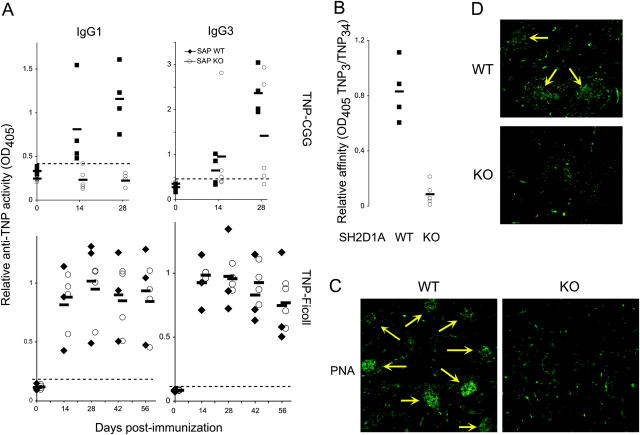
In contrast, lupus is hallmarked by uncontrolled humoral, not cellular, autoimmunity. Therefore, we turned to model immunization systems to understand further the role of SH2D1A in antibody production, and examined responses to two conventional antigens, the T-dependent TNP-CGG and the T-independent TNP-Ficoll (Fig. 3 A). KO animals were largely impaired in their ability to generate IgG1 antihapten antibodies in response to TNP-CGG (P < 0.001 comparing WT to KO at all time points tested), but only a minority (30–40%) of KO animals were able to develop IgG3 antihapten antibodies with activities that appeared comparable to WT animals, at least as judged by OD values (Fig. 3 A; P < 0.05 comparing WT to KO at 28 d). In contrast, KO animals were not at all impaired in their ability to generate antihapten antibodies of any IgG isotype in response to TNP-Ficoll immunization (Fig. 3 and unpublished data). Such findings, particularly with TNP-Ficoll, strongly suggest that intrinsic B cell functions are largely preserved in the absence of SH2D1A; rather, SH2D1A appeared to be required for T-dependent B cell antibody responses. Indeed, KO antibodies were of lower affinity than WT, at least as judged by the ratio of ELISA activities against TNP(3)-BSA and TNP(34)-BSA (Fig. 3 B). Thus, although KO animals could mount some detectable class-switched antibody to a T-dependent antigen, they did not appear to be capable of proper affinity maturation.
Figure 3.
Defective T-dependent, but not T-independent, humoral immunity in the absence of SH2D1A. 6-wk-old SH2D1A-deficient (KO) or -sufficient (WT) mice were immunized intraperitoneally with 50 μg TNP-CGG (T-dependent) or TNP-Ficoll (T-independent). (A) At the times indicated, sera were assessed for relative antihapten (TNP) antibody activity by ELISA using TNP(34)-BSA and IgG isotype-specific reagents. (B) Relative anti-TNP affinities of sera from day 35 TNP-CGG–immunized animals were evaluated by taking the ratio of activities obtained per serum against TNP(3)-BSA and TNP(34)-BSA (n = 5 in each group). (C and D) Germinal centers (yellow arrows) were identified in frozen spleen sections via staining with FITC-conjugated PNA on (C) day 28 TNP-CGG–immunized animals and (D) week 15 pristane-treated animals. Specimens shown in C and D are representative of at least five animals of each genotype. Data shown represent at least two independently performed trials.
Because affinity maturation in B cells is generally thought to arise in T-dependent germinal centers (17), we examined immunized SH2D1A-deficient mice for the presence of germinal centers (Fig. 3 C). As judged by (PNA) staining, KO animals were significantly impaired in the formation of germinal centers. Whereas WT spleens generally developed 10 ± 3 germinal centers per high powered field, KO animals developed only 0.1 ± 0.2 (P < 0.0001, n = 6 of each genotype tested). A similar difference was seen in pristane-treated animals (Fig. 3 D), where WT spleens generally contained 3 ± 0.3 PNA+ germinal centers per high powered field that were generally larger than their TNP-CGG–immunized counterparts, whereas KO spleens contained no (0 ± 0) detectable germinal centers (P < 0.0001, n = 4 of each genotype tested). Thus, SH2D1A deficiency is associated with defective T-dependent antibody response and germinal center formation. Interestingly, KO CD4+ T cells could generate levels of CXCR3, CXCR5, CCR5, ICOS, and CD154 comparable to their WT counterparts (unpublished data), suggesting that perhaps the homotypic interactions between the CD150 family members themselves, as has been proposed to mediate and/or promote lymphocyte collaboration (1), were responsible for this phenotype.
The Role of the CD150–SH2D1A System in Humoral Immunity.
Interestingly, a recent study has suggested that SH2D1A is not required for early antigen-specific antibodies, but is required for the maintenance of long-term antibodies, at least in a virus infection model (7). In contrast, our data indicate that T-dependent antibody responses are impaired in KO mice, even at early time points (Fig. 3 A), whereas T-independent antibody responses are not affected and can actually be maintained for >8 wk after initial immunization (Fig. 3 A), which is clearly longer than the 14–30-d time points at which a difference in antiviral titers between WT and KO animals were observed (7). These observations could be reconciled by considering that much of the early (<21 d) antibody during viral infection reflects T-independent B cell activation, whereas longer-term (>21 d) antibody reflects the additional contribution of high titer, high affinity antibodies generated after T cell help and germinal center reaction (18). Thus, perhaps the early antiviral immunity seen in KO animals reflects T-independent activation, whereas the apparent defect in long-term antibody responses reflects an inability to generate T-dependent immunity in addition to the T-independent response, which alone would have a relatively low activity due to the lack of affinity maturation. Therefore, continued investigation into the role of SH2D1A in different antigen-specific antibody responses is warranted.
Although SH2D1A does not map to any known lupus susceptibility locus (19), the CD150 family are candidate genes for Sle1b, a chromosome 1 lupus susceptibility locus in the New Zealand murine lupus model, where it segregates with a higher penetrance of autoantibodies and hypergammaglobulinemia, and is associated with polymorphisms in and/or dysregulated expression of various CD150 family members, including Ly-108, CD229, CD48, and CD84 (20–22). Given the present findings, dysregulation of the CD150–SH2D1A system in lupus may therefore promote pathogenic T-dependent B cell reactions, such as the germinal center reaction, leading to heightened autoantibody production and subsequent immune complex disease. As such, it will be of significant interest to explore the role of SH2D1A and the CD150 molecules in other murine autoimmune models, particularly the New Zealand strains, as well as human lupus.
At the same time, these findings suggest that the CD150–SH2D1A system may play a more general role in several types of pathogenic antibody responses, such as those that might require high affinity, germinal center–dependent antibodies, including humoral autoimmune syndromes like myasthenia gravis, or other inappropriate humoral immune diseases like allergies. In this sense it is worth reiterating that SH2D1A deficiency does not seem to significantly impair gross intrinsic T or B cell function, but rather an effector function that specifically requires their cooperation/collaboration. Thus, the CD150–SH2D1A system may prove to be a uniquely specific and valuable target in the pathogenesis and therapy of humorally mediated inflammatory disorders.
Acknowledgments
We are grateful to John Russell and his laboratory for assistance with the EAE model.
This work was supported in part by the Siteman Cancer, Rheumatic Diseases, Diabetes Research and Training, and the Digestive Diseases Research Core Centers of the Washington University School of Medicine, as well as grants from the National Institutes of Health (AI01803 and AI057471) and the Lupus Research Institute. S.L. Peng is supported in part by an Arthritis Investigator Award from the Arthritis Foundation.
J.D. Hron and L. Caplan contributed equally to this work.
References
- 1.Engel, P., M.J. Eck, and C. Terhorst. 2003. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 3:813–821. [DOI] [PubMed] [Google Scholar]
- 2.Cocks, B.G., C.C. Chang, J.M. Carballido, H. Yssel, J.E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature. 376:260–263. [DOI] [PubMed] [Google Scholar]
- 3.Lee, K.-M., M.E. McNerney, S.E. Stepp, P.A. Mathew, J.D. Schatzle, M. Bennett, and V. Kumar. 2004. 2B4 acts as a non–major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 199:1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, N., A. Satoskar, W. Faubion, D. Howie, S. Okamoto, S. Feske, C. Gullo, K. Clarke, M.R. Sosa, A.H. Sharpe, et al. 2004. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med. 199:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czar, M.J., E.N. Kersh, L.A. Mijares, G. Lanier, J. Lewis, G. Yap, A. Chen, A. Sher, C.S. Duckett, R. Ahmed, et al. 2001. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc. Natl. Acad. Sci. USA. 98:7449–7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu, C., K.B. Nguyen, G.C. Pien, N. Wang, C. Gullo, D. Howie, M.R. Sosa, M.J. Edwards, P. Borrow, A.R. Satoskar, et al. 2001. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat. Immunol. 2:410–414. [DOI] [PubMed] [Google Scholar]
- 7.Crotty, S., E.N. Kersh, J. Cannons, P.L. Schwartzberg, and R. Ahmed. 2003. SAP is required for generating long-term humoral immunity. Nature. 421:282–287. [DOI] [PubMed] [Google Scholar]
- 8.Shlomchik, M.J., J.E. Craft, and M.J. Mamula. 2001. From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 1:147–153. [DOI] [PubMed] [Google Scholar]
- 9.Lin, L., M. Spoor, A.J. Gerth, S.L. Brody, and S.L. Peng. 2004. Modulation of Th1 activation and inflammation by the NF-κB repressor Foxj1. Science. 303:1017–1020. [DOI] [PubMed] [Google Scholar]
- 10.Gerth, A.J., L. Lin, and S.L. Peng. 2003. T-bet regulates T-independent IgG2a class switching. Int. Immunol. 15:937–944. [DOI] [PubMed] [Google Scholar]
- 11.Satoh, M., and W.H. Reeves. 1994. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 180:2341–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin, L., A.J. Gerth, and S.L. Peng. 2004. Susceptibility of mast-cell deficient W/Wv mice to pristane-induced experimental lupus nephritis. Immunol. Lett. 91:91–95. [DOI] [PubMed] [Google Scholar]
- 13.Peng, S.L., M.P. Madaio, D.P. Hughes, I.N. Crispe, M.J. Owen, L. Wen, A.C. Hayday, and J. Craft. 1996. Murine lupus in the absence of αβ T cells. J. Immunol. 156:4041–4049. [PubMed] [Google Scholar]
- 14.Peng, S.L., M.P. Madaio, A.C. Hayday, and J. Craft. 1996. Propagation and regulation of systemic autoimmunity by γδ T cells. J. Immunol. 157:5689–5698. [PubMed] [Google Scholar]
- 15.Hilliard, B., E.B. Samoilova, T.S. Liu, A. Rostami, and Y. Chen. 1999. Experimental autoimmune encephalomyelitis in NF-κB-deficient mice:roles of NF-κB in the activation and differentiation of autoreactive T cells. J. Immunol. 163:2937–2943. [PubMed] [Google Scholar]
- 16.Hafler, D.A. 2004. Multiple sclerosis. J. Clin. Invest. 113:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manser, T. 2004. Textbook germinal centers? J. Immunol. 172:3369–3375. [DOI] [PubMed] [Google Scholar]
- 18.Zinkernagel, R.M., H.P. Pircher, P. Ohashi, S. Oehen, B. Odermatt, T. Mak, H. Arnheiter, K. Burki, and H. Hengartner. 1991. T and B cell tolerance and responses to viral antigens in transgenic mice: implications for the pathogenesis of autoimmune versus immunopathological disease. Immunol. Rev. 122:133–171. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, J.A., K.L. Moser, and J.B. Harley. 2002. The genetics of systemic lupus erythematosus: putting the pieces together. Genes Immun. 3:S71–S85. [DOI] [PubMed] [Google Scholar]
- 20.Morel, L., K.R. Blenman, B.P. Croker, and E.K. Wakeland. 2001. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc. Natl. Acad. Sci. USA. 98:1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harley, J.B., J. Trent, and D.L. Kastner. 2002. American College of Rheumatology Basic Research Conference: Genetics and genomics in rheumatic disease. Arthritis Rheum. 47:93–98. [DOI] [PubMed]
- 22.Jorgensen, T.N., M.R. Grubbels, and B.L. Kotzin. 2003. Links between type I interferons and the genetic basis of disease in mouse lupus. Autoimmunity. 36:491–502. [DOI] [PubMed] [Google Scholar]