Abstract
Thymic stromal lymphopoietin (TSLP) signals via a receptor comprising the interleukin (IL)-7 receptor α chain and a distinctive subunit, TSLP receptor (TSLPR), which is most related to the common cytokine receptor γ chain, γc. We have generated TSLPR knockout (KO) mice and found that although these mice had normal lymphocyte numbers, γc/TSLPR double KO mice had a greater lymphoid defect than γc KO mice. This indicates that TSLP contributes to lymphoid development and accounts for some of the residual lymphoid development in γc KO mice and presumably in patients with X-linked severe combined immunodeficiency. Injection of TSLP into γc KO mice induced the expansion of T and B cells. Moreover, sublethally irradiated TSLPR KO mice showed weaker recovery of lymphocyte populations than wild-type (WT) littermates, even when neutralizing anti–IL-7 antibodies were injected. Interestingly, TSLP preferentially stimulated the proliferation and survival of CD4+ single positive thymocytes and peripheral T cells in vitro. Additionally, CD4+ T cells from TSLPR KO mice expanded less efficiently than WT CD4+ T cells in irradiated hosts, and TSLP preferentially expanded CD4+ T cells both in vitro and in vivo. Thus, as compared with other known cytokines, TSLP is distinctive in exhibiting a lineage preference for the expansion and survival of CD4+ T cells.
Keywords: thymocyte development, IL-7, SCID, CD8 T cells, knockout mice
Introduction
Cytokines regulate many aspects of the immune system, including hematopoiesis, inflammation, and both innate and acquired immunity (1). IL-7 plays a critical nonredundant role in the development of T and B lymphocytes in mice (2, 3) and of T cells in humans (4). IL-7 KO mice display a marked reduction in thymic cellularity, but maintain a normal CD4:CD8 ratio. Their B cell development is also defective, with a block in the BM at the transition point to pre–B cells (between Hardy classification B and C fraction cells and fraction D cells; reference 3). An even more severe B cell phenotype is apparent in IL-7Rα KO mice, where the block is at the earlier transition from pre-pro–B cells to pro–B cells (Hardy classification fraction B; reference 2). Like IL-7 KO mice, IL-7Rα KO mice exhibit severely reduced thymic cellularity and defective T cell maturation (2, 3).
Thymic stromal lymphopoietin (TSLP) is a cytokine that was originally identified as a growth factor in the supernatant of the Z210R.1 thymic stromal cell line that could support the development of immature NAG8/7 B cells to the B220+/IgM+ stage (5). Its biological actions were later found to overlap with those of IL-7. Both cytokines promote the maturation of B lymphocytes, with TSLP apparently supporting growth to more mature stages than IL-7 (6). TSLP has also been shown to be a regulator of dendritic cell–mediated control of Th2 cell–biased human allergic responses (7). Like IL-7, the TSLP receptor complex contains IL-7Rα (8, 9). In addition, the TSLP receptor also contains a specific subunit, TSLPR, whereas the IL-7 receptor complex includes the common cytokine receptor γ chain, γc (10, 11), which is also shared by the receptors for IL-2, IL-4, IL-9, IL-15, and IL-21 (12). Both IL-7 and TSLP activate Stat5a and Stat5b (13, 14). TSLPR is expressed in many tissues, including liver, brain, testis, BM, and thymus.
To study the biological role of murine TSLP in vivo, we generated TSLPR KO mice. These mice exhibited normal T and B cell development and cellularity. However, mice deficient in both TSLPR and γc displayed more severe lymphoid defects than γc KO mice. Consistent with a role for TSLP in lymphoid development/expansion, sublethally irradiated TSLPR KO mice showed weaker recovery of lymphocytes than WT controls. In addition, daily injection of TSLP into γc KO mice increased both thymic and splenic cellularities by enhancing the expansion of both T and B cells, with the accumulation of CD4+ T cells in the periphery. Moreover, TSLP could enhance TCR-induced expansion of purified CD4+ single positive (SP) thymocytes and of splenic CD4+ T cells. Finally, carboxyfluorescein succinimidyl ester (CFSE)-based experiments showed TSLP-dependent expansion of CD4+ T cells both in vitro and in vivo. These studies reveal an unexpected effect of murine TSLP in the preferential expansion of CD4+ T cells.
Materials and Methods
Generation of TSLPR−/− Mice.
TSLPR genomic DNA was obtained from a P1 clone prepared from 129sv mice (Genome Research). The targeting construct was designed to delete all exons of the TSLPR gene and replace them with the neomycin resistance gene (Neo; see Fig. 1). Embryonic stem (ES) cells were electroporated with 50 μg of linearized targeting construct. Positive and negative selection of transfected cells was conducted using 350 μg/ml G418 (Life Technologies) and 2 μM gancyclovir (Sigma-Aldrich), respectively. Of 800 ES clones screened, those that had undergone homologous recombination were identified by Southern blotting, using probes located 5 and 3′ to the targeting construct. Mice were mated once with WT C57BL/6. The resulting C57Bl6/129 mice were genotyped using three PCR primers: primer A, 5′-AACCTCTCCCACAAGAAGTCCAGAAGT-3′; Neo primer, 5′-ATCGCCTTCTATCGCCTTCTT-3′; and primer B, 5′-AGACTTTACCTGATTCCTGCCTTG-3′.
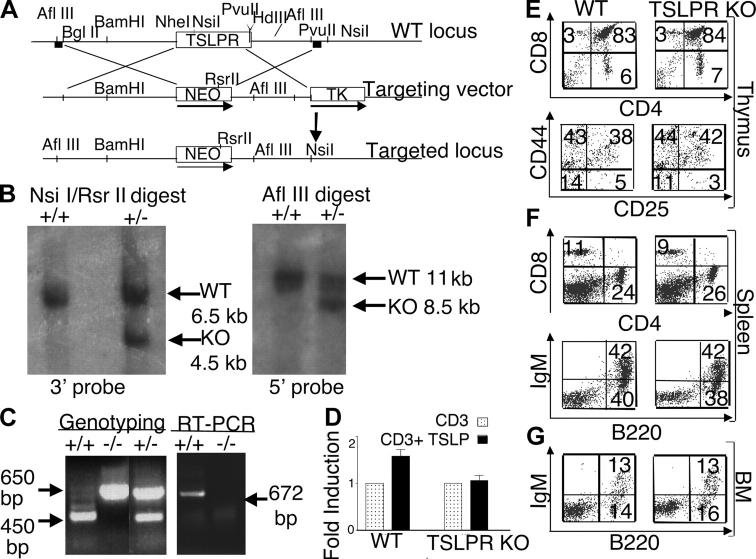
Figure 1.
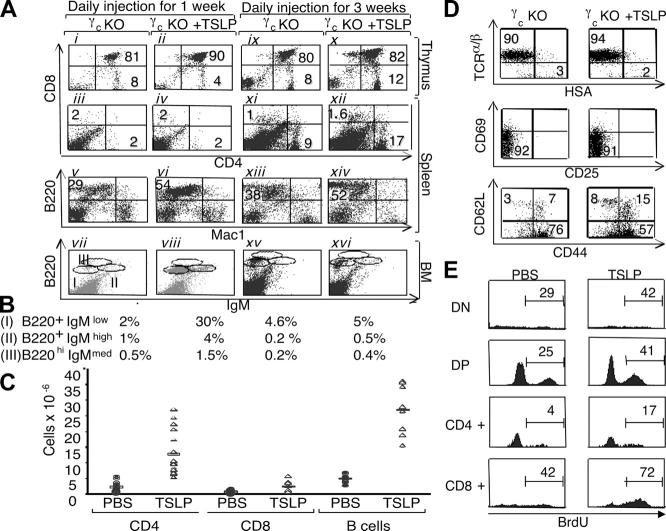
Normal immunological development in TSLPR KO mice. (A) Schematic of the TSLPR targeting strategy. The 6-kb BglII to Nhe I 5′ and 3-kb Pvu II 3′ flanking regions of the TSLPR gene were cloned 5′ and 3′ to the Neo gene. The targeting vector was linearized, electroporated into ES cells, and transfected clones were screened using 5 and 3′ probes (squares). (B) Southern hybridization of the ES clones. The restriction enzymes and fragment sizes are indicated (+/+, WT; +/−, heterozygote). (C) Genotyping of the mice was performed as described in Materials and Methods (left). RT-PCR was performed using TSLPR internal primers to detect the TSLPR mRNA transcript thymus (right). White lines indicate that intervening lanes have been spliced out. (D) TSLP significantly increased anti-CD3ɛ–induced proliferation of WT splenocytes (P < 0.001), but not of TSLPR KO splenocytes. Results are expressed as mean fold induction ± SEM (n = 14). (E–G) Flow cytometric analysis showing no difference in the CD4/CD8 profile of the total thymus (E, top), the CD25/CD44 profile of DN thymocytes (E, bottom), or in the spleen (F) and BM (G) of WT and TSLPR-deficient mice. Antibodies for the CD4+ and CD8+ surface markers were used to evaluate the T cell populations in the thymus (E) and spleen (F, top), whereas antibodies for B220+ and surface IgM+ were used to evaluate B cells populations in the spleen (F, bottom) and BM (G).
Primers A and B amplify a 250-bp segment of the endogenous TSLPR gene. The Neo primer and primer B identify the targeted gene and give a 650-bp product. The probes for Southern blots were generated using the Taq Gold kit (Applied Biosystems) under the following conditions: 94°C for 2 min, 25 cycles of 94°C for 30 s, 58°C for 45 s, and 45°C for 45 s, and then 72°C for 7 min before cooling to 4°C. Genotyping PCR reactions were performed similarly, but 33 cycles instead of 25 were performed using Ready-TO-Go PCR beads (Amersham Biosciences). To determine TSLPR mRNA expression, total RNA was isolated from the thymus using TRIzol (Promega). 1 μg RNA per RT-PCR reaction was amplified using internal upstream (5′-GCGAGGGCGGGGCTGCTGGAG-3′) and downstream (5′-CCTGGCTGGCGGGGCTGTGGC-3′) primers and an RNA PCR kit (Applied Biosystems). For Southern blots, genomic DNA was digested as indicated in Fig. 1 and the probe described above was used. Southern blot hybridization and washing were performed using QuickHyb (Stratagene). TSLPR−/− mice generated from two different ES clones showed no apparent differences. All experiments were performed under protocols approved by the National Institutes of Health Animal Use and Care Committee and followed the National Institutes of Health guidelines, Using Animals in Intramural Research. Unless indicated otherwise, all mice analyzed were 6–8-wk-old littermates and generally sex matched. No sex-related differences were observed related to the studies performed.
To generate γc/TSLPR double KO (DKO) mice, TSLPR KO females were mated to γc KO males on a C57BL/6 background (backcrossed for >15 generations). As γc is on the X chromosome, TSLPR+/− γc +/− female and TSLPR+/− γc −/Y male F1 progeny were then mated. γc/TSLPR DKO and γc KO littermate progeny were then analyzed.
Injection of IL-7 and TSLP.
2-wk-old WT or γc KO mice received daily i.p. injections with 0.1 ml PBS alone or containing 0.5 μg murine IL-7 or TSLP for 1 and 3 wk so that mice were 3 or 5 wk old at the time of analysis.
Treatment with IL-7–neutralizing mAb.
8–10-wk-old WT and TSLPR KO females were irradiated with 600 cGy of whole body irradiation. Mice were injected three times a week for 4 wk with 1 mg of a control mAb or M25 anti–IL-7–neutralizing mAb (15).
Flow Cytometric Analyses.
Single cell suspensions were prepared from the thymus, spleen, and BM. Cells were washed with FACS® buffer (PBS, pH 7.4, 0.5% BSA, 0.02% sodium azide). 106 cells were treated with Fc-block (BD Biosciences) for 15 min, and then incubated with the indicated fluorochrome-conjugated antibodies (all from BD Biosciences) for 20 min. Cells were then washed twice with FACS® buffer and analyzed. To examine cell death, a PE-conjugated annexin V kit (BD Biosciences) was used according to the manufacturer's instructions.
Measurement of IgM Levels.
Sera from 3-wk-old γc KO and γc/TSLPR DKO mouse littermates were analyzed for resting IgM levels using a sandwich ELISA. In brief, 100 μl (2 μg/ml in coating buffer: 0.15 M sodium carbonate, 0.35 M sodium bicarbonate, pH 9.5, 0.02% sodium azide) anti–mouse IgM capture antibody (BD Biosciences) was used to coat 96-well plates (EIA plates; Costar) overnight at 4°C. Wells were then coated with blocking buffer (PBS supplemented with 10% FBS) for 1 h at room temperature. Sera were diluted at 1:1,000 in blocking buffer and incubated in the wells overnight at 4°C. Wells were washed in PBS containing 0.1% Tween and then incubated with a 1:2,000 dilution of secondary horseradish peroxidase–conjugated anti-IgM (BD Biosciences) for 1 h at room temperature. The assay was ended by adding the substrate mixture (BD Biosciences) and measuring absorbance at 450 nm.
Proliferation and Survival Assays.
To isolate CD4+ and CD8+ SP T cells, thymocytes were first incubated with anti-CD8+ or anti-CD4+ paramagnetic beads, respectively, and the bound cells were removed by passing the samples through the autoMACS system (Miltenyi Biotec). The nonbound cells (negative eluted fraction) were then treated with CD4+ and CD8+ paramagnetic beads to separate CD4+ and CD8+ T cells, respectively, from the double negative (DN) cells. Mature splenic CD4+ and CD8+ T cells were isolated using their respective paramagnetic beads. Fresh thymocytes and splenocytes were cultured for 48 h in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, and antibiotics, in 96-well flat-bottom plates (2 × 105 cells/well) that were coated or not with 2 μg/ml anti-CD3ɛ (BD Biosciences). Cells were additionally incubated with 100 ng/ml IL-7 or TSLP as indicated. Wells were pulsed with 1 μCi [3H]thymidine (6.7 Ci/mmol; NEN Life Science Products) for the last 9 h of culture. Proliferation was also examined using 5 μM CFSE labeling (Sigma-Aldrich) for 10 min at 37°C. For in vivo proliferation, 0.8 mg 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) was injected 16 and 10 h before the mice were killed. Levels of BrdU were determined using PE-conjugated antibodies (16). To examine the survival of thymocytes, cells were isolated and cultured as described above for 1 wk, and the number of live cells was determined by trypan blue exclusion and annexin V/7-AAD staining.
Adoptive Transfer of T Cells.
C57BL/6 γc KO mice were irradiated with 600 cGy of whole body irradiation. 8 h later, the mice were injected with a single cell suspension of 8 × 106 splenic CD4+ or CD8+ T cells from WT or TSLPR KO mice that were labeled with CFSE for 10 min at 37°C. Spleens were analyzed on days 3 and 7 by flow cytometry.
Online Supplemental Material.
Fig. S1 shows the effect of TSLP on lymphopoiesis in WT mice. The flow cytometric analysis of the thymus and spleen 1 and 3 wk after injection of WT mice with PBS, IL-7, or TSLP is shown. Thymocytes were stained with anti-CD4 versus anti-CD8. Splenocytes were stained with anti-CD4 versus anti-CD8 and anti-B220 versus anti-IgM. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20031975/DC1.
Results
TSLPR KO Mice Exhibit Normal Lymphohematopoietic Development.
To generate TSLPR KO mice, we used a targeting vector designed to replace the TSLPR coding region with the neomycin resistance cassette (Fig. 1 A). 4 clones out of 800 showed homologous recombination as determined using both 3′ and 5′ probes (Fig. 1 B and not depicted). Two clones were used to generate chimeric mice, and heterozygous offspring corresponding to each clone were intercrossed to generate TSLPR KO mice as confirmed by PCR genotyping and TSLPR mRNA expression (Fig. 1 C). The progeny mice exhibited normal Mendelian ratios. 6–8-wk-old TSLPR KO mice were indistinguishable from WT littermates in viability, growth, development, and fertility. The inactivation of TSLP signaling was confirmed by the defective response of TSLPR KO splenocytes compared with WT splenocytes to treatment with anti-CD3 plus TSLP versus anti-CD3 alone (Fig. 1 D).
Thymus, spleen, and BM were similar in size and cell number, and no differences in histology were observed between TSLPR KO mice and WT littermates (not depicted). Analysis of TSLPR KO thymuses showed that the DN, double positive (DP), and CD4+ and CD8+ SP T cells were present in normal distributions (Fig. 1 E). B220 versus anti-CD3 staining in the spleen was normal (not depicted), as was the CD4:CD8 T cell ratio (Fig. 1 F, top). Moreover, levels of both B220+ IgM+ immature cells and B220+ IgM− pre– and pro–B cells (both percent and absolute numbers) were similar in TSLPR KO and WT mice in both spleen (Fig. 1 F, bottom) and BM (Fig. 1 G). Surface levels of CD3ɛ, TCR-β, TCR-γδ, CD4, CD5, CD8, CD11c, CD21, CD23, CD43, CD44, CD62L, B220, IgM, IgD, DX5, Gr1, TER119, γc, HSA, and BP-1, as well as IL-7Rα, were comparable to those found in WT mice (not depicted). Thus, although TSLP has been reported to affect fetal lymphoid development (17), we found no overt defects in adult lymphopoiesis in TSLPR KO mice. It is possible that TSLP serves a redundant role and that other cytokines, especially the closely related IL-7, might compensate for the absence of TSLP signal. No difference was observed between WT and TSLPR KO mice in the number of colony forming units (CFU-B and CFU-GM assays) from BM cultures in vitro (not depicted). Although TSLPR is expressed in various tissues, including brain, liver, and lung (8), suggesting a possible role of TSLP in the physiological functions of these organs, histopathological analysis of these organs in TSLPR KO mice revealed no obvious abnormalities (not depicted).
TSLP and IL-7 Exhibit Overlapping Actions In Vivo.
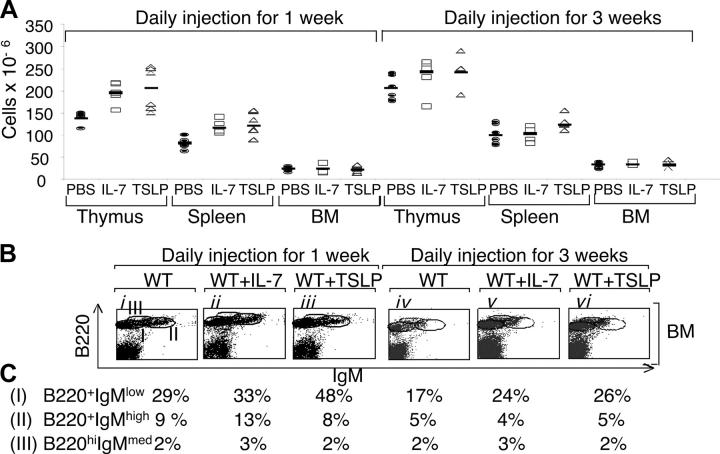
Because TSLP and IL-7 share IL-7Rα as a receptor component, we compared the actions of these cytokines. Both IL-7 and TSLP similarly increased thymic and splenic cellularity in WT mice after 1 wk of daily injections (Fig. 2 A, left), with CD4 and CD8 T cell populations present in normal ratios (Fig. S1 A, ii and iii vs. i, and v and vi vs. iv, available at http://www.jem.org/cgi/content/full/jem.20031975/DC1). Thus, thymic and splenic T cell populations were all increased. In the spleen, TSLP and IL-7 had similar abilities to increase B cells (Fig. S1 A, viii and ix vs. vii), whereas TSLP was more potent in expanding immature B220+ IgMlow cells in the BM (Fig. 2 B, ii and iii vs. i; see percentages for the different fractions of B cells in Fig. 2 C). After 3 wk of treatment of WT mice with TSLP or IL-7, the increase in thymic and splenic cellularity was no longer statistically significant (Fig. 2 A, right) and the CD4+:CD8+ T cell ratio remained constant (Fig. S1 B, ii and iii vs. i, and v and vi vs. iv), but the increase in B cells was still evident in the spleen (Fig. S1 B, viii and ix vs. vii) and BM (Fig. 2 B, v and vi vs. iv; see percentages for the different fractions of B cells in Fig. 2 C). The consistent absolute decrease in B cells in WT animals between 1 and 3 wk of treatment presumably, at least in part, reflects an age-dependent regulatory mechanism of B cell expansion, as a decrease was even seen in the untreated control animals (Fig. 2 C). These results indicate that both TSLP and IL-7 can promote lymphocyte expansion. The normal levels of lymphocytes in TSLPR KO mice are presumably, in part or entirely, explained by the continued action of IL-7.
Figure 2.
TSLP can expand lymphocyte populations in WT mice. (A) The absolute number of thymocytes, splenocytes, and BM cells in WT mice injected daily with PBS, IL-7, or TSLP for 1 and 3 wk. 1 wk of treatment with TSLP and IL-7 increased thymic cellularity (means ± SEM of 204 ± 46 × 106 and 195 ± 22 × 106 [P = 0.01 and 0.001, respectively] vs. 139 ± 13 × 106 for the PBS-treated mice). 1 wk of treatment with TSLP and IL-7 also increased splenic cellularity (means ± SEM of 126 ± 23 × 106 and 118 ± 13 × 106 [P = 0.004 and 0.002, respectively] vs. 80 ± 12 × 106 for the PBS-treated mice). No significant difference was observed in the BM. After 3 wk of treatment with TSLP or IL-7, the changes in cellularity of thymus, spleen, and BM were not significant. (B) Flow cytometric analysis of the BM 1 and 3 wk after injection of WT mice with PBS, IL-7, or TSLP. (C) Percentages of populations shown in B.
TSLP Signaling Is Required for Efficient Recovery of Lymphocyte Populations after Sublethal Irradiation.
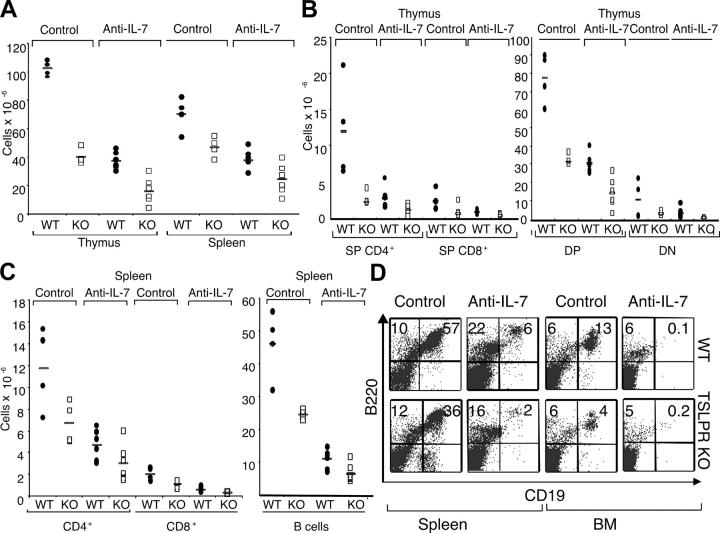
To further examine the role of TSLP in lymphopoiesis, WT and TSLPR KO mice were sublethally irradiated and their ability to recover cellular populations was evaluated. In these experiments, we included either a control mAb or neutralizing mAb to IL-7. Irradiated WT animals treated with the control mAb recovered most of their thymic and splenic cellularities within 4 wk, whereas TSLPR KO mice did not (Fig. 3 A). Similarly, CD4+ and CD8+ T cell subpopulations in the thymus (Fig. 3 B) and spleen (Fig. 3 C) were lower in TSLPR KO mice than in WT littermates. B cell recovery in TSLPR KO mice was also less efficient than in WT mice (Fig. 3 C), and flow cytometric analysis of B cells showed that B220+ CD19+ B cells were greatly diminished in the spleens in TSLPR KO mice (Fig. 3 D). These results establish a critical role for TSLP in mediating optimal T and B cell lymphopoiesis in mice.
Figure 3.
TSLP is critical for optimal lymphopoiesis in the presence and absence of IL-7. WT and TSLPR KO mice were sublethally irradiated and injected three times a week for 4 wk with 1 mg of control or M25 anti–IL-7 mAbs. (A) TSLPR KO treated with control or anti–IL-7 mAbs displayed lower thymic cellularity (P < 0.001 for both mAbs) and splenic cellularity (P = 0.03 for anti–IL-7 and P = 0.02 for control mAb) when compared with WT littermates. (B) The absolute numbers of thymic subpopulations, except for DN cells, were decreased in TSLPR KO mice compared with WT mice (P < 0.05). (C) The absolute numbers of CD4+ and CD8+ T and B cells in the spleens of irradiated animals. Mice lacking TSLPR had fewer lymphocytes than WT mice, when experiments were performed with either control or anti–IL-7 mAbs (P < 0.05). (D) B220 versus CD19 flow cytometric analysis of the spleen and BM from WT (top) and TSLPR KO (bottom) mice.
We also examined reconstitution in mice in which a neutralizing mAb to IL-7 was injected (1 mg three times a week for 4 wk; reference 15). As expected, treatment with the anti–IL-7 mAb reduced lymphopoiesis in WT mice (Fig. 3 A). Importantly, however, recovery was even more impaired in TSLPR KO mice treated with anti–IL-7 mAb, including impaired thymic and splenic cellularity (Fig. 3 A), with CD4+ and CD8+ T cell subpopulations in the thymus (Fig. 3 B) and spleen (Fig. 3 C) being lower in TSLPR KO mice than in WT littermates. No significant differences in the percentages of DN1, DN2, DN3, and DN4 cells were observed (not depicted). B cell recovery in TSLPR KO mice was also less efficient than in WT mice in the presence of anti–IL-7 mAb (Fig. 3, C and D, BM and spleen). B220+ CD19+ B cells were nearly eliminated in the BM in both WT and TSLPR KO mice treated with anti–IL-7 (Fig. 3 D, far right panels). These results establish a critical role for TSLP in mediating optimal T and B cell lymphopoiesis in mice. Because recovery is less in the absence of signaling by TSLP plus IL-7 compared with the absence of only IL-7, our data indicate a critical IL-7–independent role for TSLP in recovering from lymphopenia.
γc/TSLPR DKO Mice Exhibit Greater Defects than γc KO Mice, and TSLP Can Increase Cellularity in γc KO Mice.
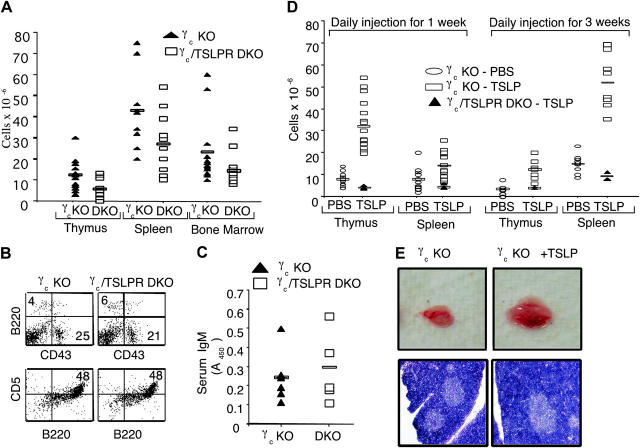
γc KO mice have defective T and B cell development. We hypothesized that a greater defect in γc/TSLPR DKO mice than in γc single KO mice would suggest a role for TSLP in contributing to the residual lymphoid development that is present in γc KO mice and in humans with XSCID, which results from mutations in γc (12). Indeed, the low thymic cellularity observed in γc KO mice (18, 19) was further diminished in γc/TSLPR DKO littermates (Fig. 4 A). However, CD4+ and CD8+ SP, CD4+ CD8+ DP, and CD4− CD8− DN T cells (DN1, DN2, DN3, and DN4) were all present in γc/TSLPR DKO thymus at ratios comparable to those in the thymus of γc KO mice (not depicted). Similarly, although total BM cellularity was greatly reduced in γc/TSLPR DKO mice, a similar distribution of B cells was present in the BM (Fig. 4 B) and spleen (not depicted) as were seen in γc KO mice. Specifically, B220 versus CD43 profiles were similar in both γc KO and the γc/TSLPR DKO mice (Fig. 4 B, top), as was expression of HSA, BP-1, and IgM (not depicted). Peritoneal CD5+ B1 cells were also present in similar numbers in both γc KO and the γc/TSLPR DKO mice (Fig. 4 B, bottom). Consistent with this, γc/TSLPR DKO mice did not have lower serum IgM levels than those found in γc KO mice (Fig. 4 C). Thus, TSLP does not appear to regulate numbers of B1 cells nor affect IgM production.
Figure 4.
Comparison of the lymphoid development in γc KO mice versus γc/TSLPR DKO mice. (A) γc/TSLPR DKO mice had lower thymic, spleen, and BM cellularities than γc KO mice (thymus: mean ± SEM of 12.3 ± 6.7 × 106 for γc KO mice vs. 5.7 ± 4.5 × 106 for γc/TSLPR DKO mice [P = 0.009]; spleen: mean ± SEM of 43 ± 17.7 × 106 for γc KO mice vs. 26.8 ± 13.7 × 106 for γc/TSLPR DKO mice [P = 0.02]; BM: mean ± SEM of 23.4 ± 15.5 × 106 for γc KO mice vs. 14.3 ± 7.6 × 106 for γc/TSLPR DKO mice [P = 0.047]). All mice were age matched and no sex-related differences were noted. (B) Flow cytometric analysis of BM (top, B220 vs. CD43) and peritoneal cavity lymphocytes (bottom, B220 vs. CD5). (C) Similar levels of IgM in the serum of γc KO and γc/TSLPR DKO mice. (D) Injection of 0.5 μg/day of TSLP (open rectangles) enhances lymphoid cellularity in γc KO mice. Treatment for 1 and 3 wk showed an enhanced cellularity in thymus (mean ± SEM of 7.8 ± 2.9 × 106 for PBS-injected mice vs. 32 ± 10 × 106 for TSLP-injected mice [P < 0.0001] after 1 wk of treatment and a mean ± SEM of 3.5 ± 2 × 106 for PBS-injected mice vs. 12.2 ± 4 × 106 for TSLP-injected mice [P = 0.001] after 3 wk) and spleen (mean ± SEM of 8 ± 4 × 106 for PBS-injected mice vs. 14 ± 5.4 × 106 for TSLP-injected mice [P = 0.003] after 1 wk of treatment and a mean ± SEM of 15 ± 4 × 106 for PBS-injected mice vs. 52 ± 12 × 106 for TSLP-injected mice [P < 0.0001] after 3 wk). No change was seen in the BM. All mice were age matched and no sex-related differences were noted. γc/TSLPR DKO mice did not respond to TSLP injections (closed triangles). (E) Photograph indicating thymic size in γc KO mice after wk of treatment with PBS or TSLP (top) and a histological analysis of these tissues by hematoxylin and eosin staining (bottom).
To further examine the actions of TSLP, we evaluated its effect on γc KO mice. Because of the sharing of IL-7Rα by the receptors for both IL-7 and TSLP, and the potential for overlapping actions of these cytokines, we hypothesized that γc KO mice that lack IL-7 signaling and have diminished thymocytes might exhibit hyperresponsiveness to TSLP. 2-wk-old γc KO mice received 0.5-μg daily i.p. injections of PBS or TSLP for 1 or 3 wk. Strikingly, after 1 wk, TSLP treatment increased thymic cellularity 5–10-fold in γc KO mice (Fig. 4 D, open rectangles vs. open ovals; P < 0.01), consistent with increased thymic size (Fig. 4 E, top). Histological analysis showed that the thymus from γc KO mice injected with PBS had a thin cortex, whereas those from TSLP-injected mice had a wider cortex (Fig. 4 E, bottom). As expected, TSLP had no effect in γc/TSLPR DKO mice (Fig. 4 D, closed triangles).
In addition to increased thymic cellularity, the fraction of DP cells increased so that this was the most expanded thymic population (Fig. 5 A, ii vs. i). γc KO mice injected with TSLP for 3 wk still showed higher total thymic cellularity than untreated mice (Fig. 4 D; P < 0.01), albeit less so than at 1 wk, with more CD4+ SP T cells present (Fig. 5 A, x vs. ix). TSLP treatment for 1 wk also increased the total number of splenocytes (Fig. 4 D; P < 0.01), mainly by increasing B cells (Fig. 5 A, vi vs. v). After 3 wk of TSLP, splenic cellularity further increased (Fig. 4 D; P < 0.01). As expected, this resulted in part from the expansion of B cells (Fig. 5 A, xiv vs. xiii). The increase in B cells in the spleen was also evident in the BM. Although the total BM cellularity in γc KO mice was not increased by TSLP (not depicted), TSLP dramatically increased the B cell subpopulations within 1 wk (Fig. 5 A, viii vs. vii), with immature B cells being the most affected, increasing from ∼2 to 30% of the total number of cells (see percent of B220+ IgMlow cells in Fig. 5 B). More mature stages also increased three- to fourfold. However, these changes in B cell populations were transient and at 3 wk of TSLP injection, these cells diminished to the BM B lymphocyte cellularity seen in the untreated mice (Fig. 5 A, xvi vs. xv, and B).
Figure 5.
TSLP can increase lymphoid subpopulations in γc-deficient mice. (A) Flow cytometric analysis of γc KO thymus, spleen, and BM 1 and 3 wk after injection of PBS or TSLP. (B) B cell populations in the BM from A (vii, viii, xv, and xvi). (C) TSLP injections induced an increase in CD4+ T cells (mean ± SEM of 2.36 ± 1.48 × 106 for control mice vs. 12.7 ± 6.7 × 106 for TSLP-treated mice [P < 0.0001], for a 5.4-fold increase), CD8+ T cells (mean ± SEM of 0.63 ± 0.43 × 106 for control mice vs. 2.4 ± 1.7 × 106 for TSLP-treated mice [P = 0.01], for a 3.8-fold increase), as well as B cells (mean ± SEM of 4.6 ± 1.2 × 106 for control mice vs. 27 ± 7 × 106 for TSLP-treated mice [P < 0.0001], for a 5.9-fold increase) in the spleen of γc KO mice 3 wk after injection. (D) Flow cytometric analysis of splenic CD4+ T cells in γc mice treated with PBS or TSLP for 3 wk. TSLP increased the absolute numbers of CD44high CD62Llow (mean ± SEM of 2.3 ± 1.2 × 106 for control γc mice vs. 11.3 ± 4.106 for TSLP-treated mice [P = 0.0004], for 4.9-fold increase), CD44high CD62Lhigh (mean ± SEM of 0.4 ± 0.2 × 106 for control γc mice vs. 3.2 ± 1.1 × 106 for TSLP-treated mice [P = 0.0003], for an eightfold increase), and CD44low CD62Lhigh (mean ± SEM of 0.08 ± 0.06 × 106 for control γc mice vs. 0.9 ± 0.16 × 106 for TSLP-treated mice [P < 0.0001], for an 11-fold increase). (E) γc KO mice were treated with PBS or TSLP for 1 wk and injected with BrdU 10 and 16 h before being killed. BrdU incorporation was measured by intracellular staining using PE-labeled BrdU of the thymocytes subpopulations. The number indicates the percent of BrdU+ cells within the gated region.
In addition to the increased B cells noted above, the increased splenic cellularity was also partially due to an increase in CD4+ T cells (Fig. 5 A, xii vs. xi, and C). Consistent with the age-dependent increase in CD4+ T cells that occurs in γc KO mice (19), control γc KO mice that were injected with PBS for 3 wk versus 1 wk showed an age-dependent increase in the percentage of CD4+ T cells (Fig. 5 A, xi vs. iii). Approximately 5.4-fold more CD4+ splenic T cells were found in TSLP-treated γc KO mice than in untreated mice (12.7 × 106 vs. 2.4 × 106), whereas the absolute and relative increase in mature splenic CD8+ T cell numbers was less marked in TSLP-treated versus control animals (3.8 fold, 2.4 × 106 vs. 0.63 × 106; Fig. 5 C). Almost all of the CD4+ T cells in γc KO mice were TCRhigh HSAlow and did not express the activation markers CD25 and CD69, and this was unaffected by TSLP treatment (Fig. 5 D, top and middle). As previously shown, CD4+ T cells in γc KO mice primarily have a CD62Llow CD44high memory phenotype (20). Interestingly, TSLP increased the number of CD62Lhigh CD44high cells, a population that has been identified as central memory cells (21), the most (Fig. 5 D, bottom).
Above, we observed an increase in the number of CD4+ T cells, particularly in the spleen but also in the thymus. Therefore, we examined the effect of TSLP on the proliferation of thymocytes in vivo. γc KO mice treated with PBS or TSLP for 1 wk were injected with BrdU 10 or 16 h before being killed. TSLP increased BrdU incorporation in all thymic subpopulations (Fig. 5 E). Interestingly, γc KO CD4+ SP thymocytes had the lowest basal level of BrdU incorporation but were the most responsive to TSLP treatment, increasing their proliferation approximately fourfold.
TSLP Cooperates with Anti-CD3 to Preferentially Expand CD4+ T Cells by Affecting Proliferation and Survival.
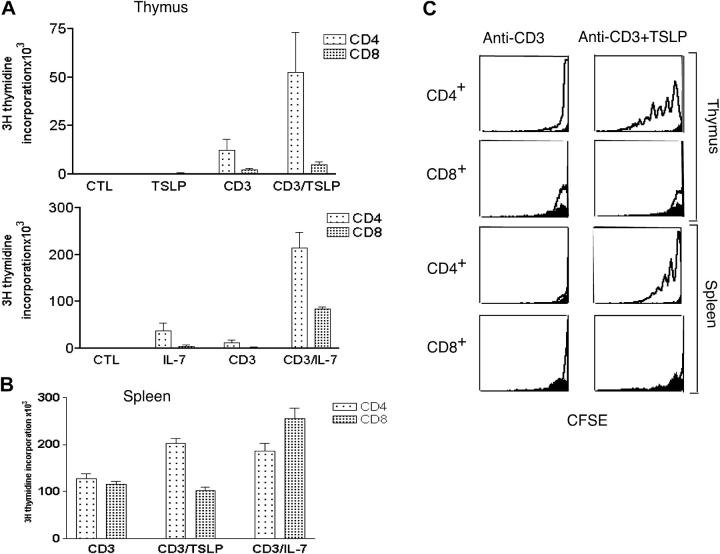
To clarify the mechanism by which TSLP promotes thymocyte expansion, we treated SP WT thymocytes with anti-CD3ɛ with or without IL-7 or TSLP in vitro (Fig. 6 A). TSLP alone had no significant effect, but when combined with anti-CD3ɛ, it markedly increased the proliferation of CD4+ SP thymocytes, whereas its effect on CD8+ SP thymocytes was much less (Fig. 6 A, top), suggesting that TSLP favors the expansion of CD4+ thymocytes. In contrast, IL-7 enhanced proliferation of both CD4+ and CD8+ SP thymocytes alone or in combination with anti-CD3ɛ (Fig. 6 A, bottom), and overall, IL-7 was more potent than TSLP even for CD4+ thymocytes (note the difference in scale in the top and bottom panels of Fig. 6 A). Correspondingly, TSLP preferentially enhanced the survival of CD4+ thymocytes, whereas IL-7 had similar effects on the viability of CD4+ and CD8+ thymocytes (Fig. 7 A). TSLP and IL-7 were equally potent in increasing the survival of DN and DP thymocytes (not depicted). Consistent with its effect on CD4+ SP thymocytes, TSLP treatment preferentially enhanced TCR-induced proliferation (Fig. 6 B) and survival (Fig. 7, B and C) of CD4+ splenic T cells compared with CD8+ T cells. We observed a dramatic dilution of CFSE in response to TSLP in both thymic and splenic CD4+ (Fig. 6 C). Correspondingly, the percent of viable cells was increased (Fig. 7, A and B) and the percent of annexin V+/7-AAD+ cells (Fig. 7 C) was decreased by TSLP.
Figure 6.
TSLP preferentially expands CD4+ T cells. In A and B, cells were treated with medium, 100 ng/ml IL-7, or 100 ng/ml TSLP, and/or 2 μg/ml anti-CD3ɛ antibodies. (A) In vitro proliferation of purified CD4+ and CD8+ SP thymocytes from WT mice. Results are expressed as mean ± SEM for four experiments. TSLP increased anti-CD3ɛ–induced proliferation of CD4+ SP cells (P = 0.02), but did not significantly affect CD8+ SP expansion to anti-CD3ɛ (P = 0.07). IL-7 significantly enhanced anti-CD3ɛ–induced expansion of both CD4+ and CD8+ SP thymocytes (P < 0.0001 for both). (B) In vitro proliferation of purified CD4+ and CD8+ splenocytes (prepared by positive selection) treated as described above. TSLP significantly increased anti-CD3ɛ–induced proliferation of mature CD4+ T cells (P = 0.0002), but not of CD8+ T cells (P = 0.1). IL-7 significantly enhanced anti-CD3ɛ–induced expansion of both CD4+ and CD8+ mature T cells (P = 0.008 and 0.0005, respectively). (C) CD4+ thymocytes and splenic T cells were labeled with CFSE and cultured for 1 wk with anti-CD3ɛ with or without TSLP, and cells were analyzed by flow cytometry. As evaluated by decreased CFSE staining, TSLP increased anti-CD3ɛ–induced proliferation of CD4+ but not CD8+ T cells. WT corresponds to the “open” curve, whereas TSLPR KO mice are shown in solid black.
Figure 7.
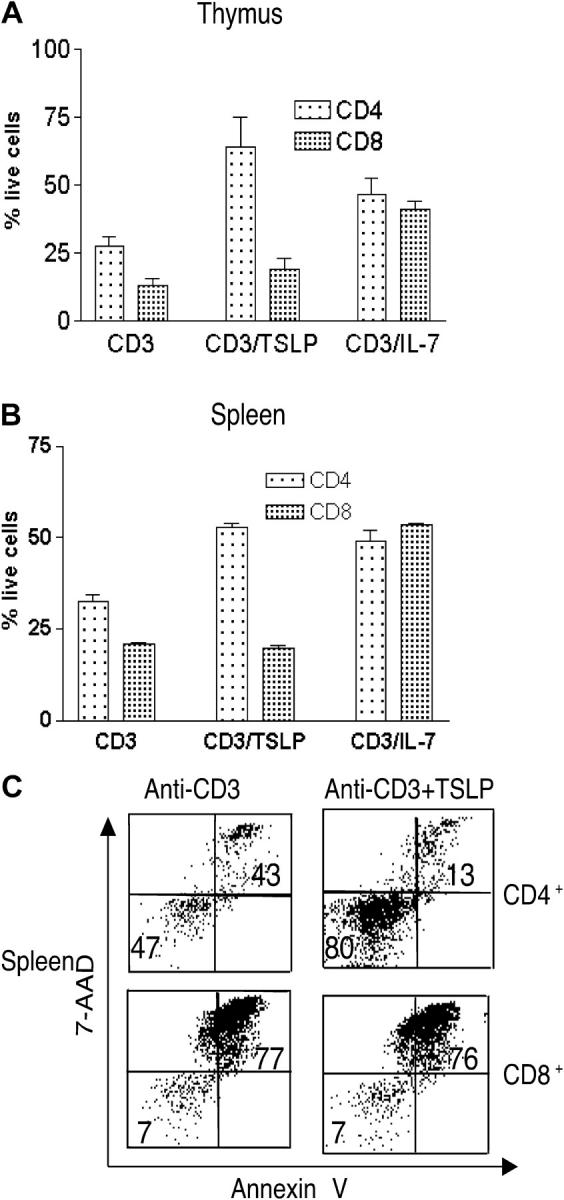
TSLP promotes survival of CD4+ T cells. (A) In vitro survival of purified CD4+ and CD8+ SP thymocytes from WT mice. The percent of viable cells (mean ± SEM for four experiments) was determined after 1 wk by trypan blue exclusion. TSLP increased anti-CD3ɛ–induced survival of CD4+ cells (P = 0.02), but did not significantly affect CD8+ survival (P = 0.24). IL-7 significantly enhanced anti-CD3ɛ–induced survival of both CD4+ and CD8+ thymocytes (P = 0.03 and P < 0.0001, respectively). (B) In vitro survival assay of purified CD4+ and CD8+ splenic T cells from WT mice. The percent of viable cells was determined by trypan blue exclusion. Results are expressed as mean ± SEM for five experiments. TSLP significantly increased anti-CD3ɛ–induced survival of CD4+ (P < 0.0001) but not CD8+ T cells (P = 0.21). IL-7 significantly enhanced anti-CD3ɛ–induced survival of both CD4+ and CD8+ T cells (P < 0.001 and P < 0.0001, respectively). (C) Purified CD4+ and CD8+ splenocytes cultured as indicated were stained with annexin V and 7-AAD.
TSLP Is Required for Efficient Expansion of Murine CD4+ T Cells In Vivo.
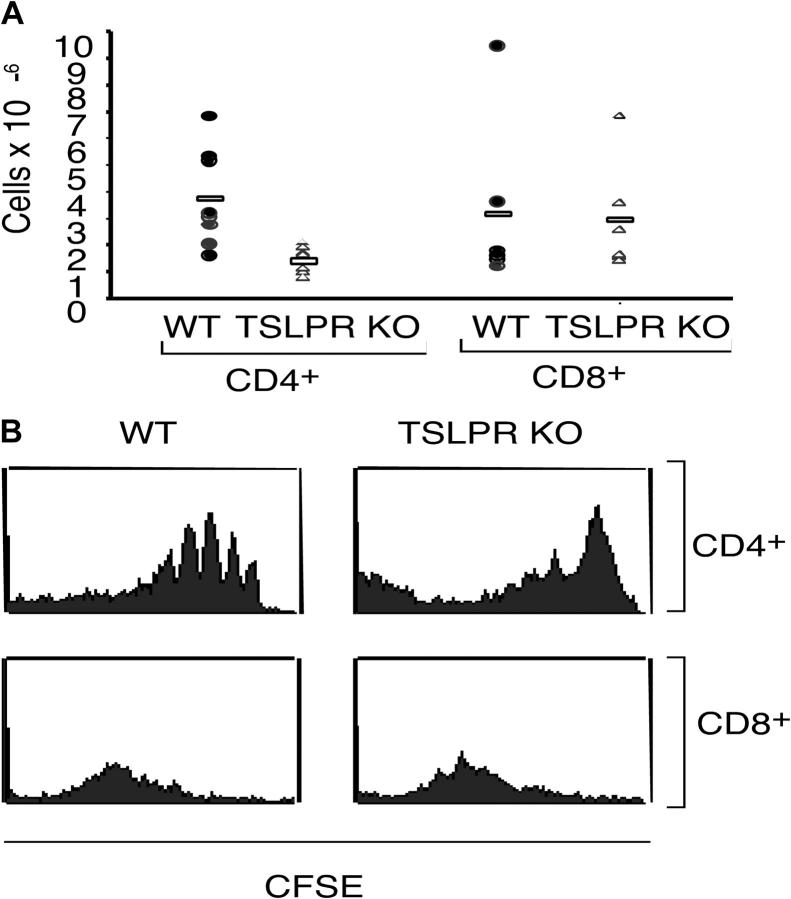
Compared with CD4+ T cells from WT mice, CD4+ T cells from TSLPR KO mice exhibited less expansion during the 7-d period after injection into sublethally irradiated recipient γc KO mice (Fig. 8 A). This indicates that TSLP signaling is critical for an efficient expansion of CD4+ lymphocytes. In contrast, CD8+ T cells from both WT and TSLPR KO expanded to a similar degree (Fig. 8 A). CFSE staining on day 3 showed the typical four to five divisions in WT CD4+ T cells (Fig. 8 B, top left); however, TSLPR KO CD4+ T cells persisted but had undergone fewer cell divisions (Fig. 8 B, top right). In contrast, CD8+ T cells from WT and TSLPR KO mice were similar in their CFSE profiles. Taken together, our results indicate that TSLP preferentially enhances both the expansion and survival of CD4+ T cells both in vitro and in vivo.
Figure 8.
TSLP mediates efficient expansion of CD4+ T cells. CD4+ T cells were isolated from WT or TSLPR KO mice and labeled with CFSE before being injected into irradiated γc KO mice. (A) After 1 wk, TSLPR KO CD4+ T cells expanded less than CD4+ T cells from WT mice (P = 0.008). CD8+ T cells from WT or TSLPR mice expanded to a similar degree. (B) Examination of the CFSE dilution on day 3 by flow cytometry revealed that WT CD4+ T cells were expanding more rapidly than TSLPR KO CD4+ T cells. No differences were observed for CD8+ T cells.
Discussion
TSLP was discovered as a cytokine that could support the ability of pre–B cells to differentiate into more mature IgM+ B cells, whereas IL-7 promotes development only to an IgM− stage (5). Colonies emerging from murine B220+ IgM− BM cells develop into IgM+ cells after 7 d of culture in TSLP (22). In contrast, TSLP was previously shown to play only a minimal role in murine T lymphopoiesis by inducing proliferation of DN thymocytes, but only in an IL-1–dependent manner (22).
To clarify TSLP function, we now have studied TSLPR-deficient mice and looked at effects of TSLP in vivo and in vitro. Despite ubiquitous expression, TSLPR is not required for the physical development and fertility of mice. In addition, TSLPR KO mice exhibit normal myeloid, lymphoid, dendritic cell, and NK cell numbers, at least in part due to the continuous action of IL-7. To examine the role of TSLP in lymphopoiesis, TSLPR KO mice were crossed to γc KO mice. Inactivating TSLP signaling in γc KO mice further reduced the cellularity of the thymus, spleen, and BM, suggesting that TSLP can promote T and B cell expansion. Nevertheless, the existence of lymphocytes in these DKO mice indicates that other growth factors also contribute and that TSLP is not the only factor responsible for the lymphoid cells present in IL-7– and γc-deficient animals. Other cytokines, such as stem cell factor and Flt3 ligand, presumably play a role (23, 24). We also examined cellular recovery after sublethal irradiation of TSLPR versus WT mice. The defective cellular restoration in TSLPR KO mice suggests a role for TSLP in recovery from lymphopenia. Moreover, the defect was also more severe in TSLPR than WT mice injected with neutralizing antibodies to IL-7, indicating that TSLP has at least some actions that are independent of IL-7. As in our study, other investigators found no significant developmental abnormality in TSLPR KO mice (25). Interestingly, however, in Jak3/TSLPR DKO mice, they observed a decrease in B cells in the spleen but not in the BM, and saw no change in T cells. In contrast, in our analysis of γc/TSLPR DKO mice, total cellularity declined with marked decreases in both B and T cells, findings that are consistent with a role for TSLP in γc-independent expansion of both of these lineages.
TSLP promoted B cell maturation in γc mice to the B220+ IgM+ stage. It also enhanced B cell maturation in WT mice to an almost identical level as IL-7, similar to what was reported in neonatal WT mice injected with TSLP (22). Interestingly, B progenitor cells in the BM of γc KO mice expanded and matured when animals were injected daily with TSLP for 1 wk, but the effect was transient and no longer observed after 3 wk of TSLP. TSLP had less of an effect in WT mice. A recent report using mice deficient in IL-7, IL-7Rα, and γc on a TCR-β KO background indicated that TSLP promoted the expansion of fetal liver–derived pro–B cells but displayed no activity on adult BM-derived pro–B cells (17), which perhaps suggests that our findings in γc KO mice might reflect the changing potentials of the progenitor population in these young animals.
A distinctive action of IL-7 is its ability to increase survival of immature thymocytes and provide a proliferative signal in the pre–T cell stage after TCR-β rearrangement (26). IL-7 is also essential for γδ TCR generation (27). Although TSLP could not rescue γδ T cell development when it was injected into γc KO mice (not depicted), like IL-7, TSLP could promote the survival and proliferation of T cells as well as B cells from WT mice. Strikingly, TSLP preferentially increased TCR-mediated proliferation of CD4+ CD8− thymocytes and CD4+ peripheral T cells in vitro (Fig. 6), and the absence of TSLP signaling hinders the expansion of these cells in vivo in the adoptive transfer experiment (Fig. 8). The modest effect of TSLP on total thymocyte and splenocyte numbers in WT mice (Fig. 2) might be related to the presence of IL-7, which not only promotes CD8+ T cell homeostasis, but also increases CD4+ T cell survival (28). It is also possible that the T cell compartment is already “filled,” allowing less expansion. Alternatively, the cells might be less responsive if they have already received signals from γc-dependent cytokines, so that a potentially redundant signal from TSLP would have little effect. Interestingly, two other γc-dependent cytokines, IL-7 and IL-15, preferentially induce an expansion of CD8+ T cells rather than CD4+ T cells (29–31). Like IL-7 and IL-15, TSLP activates Stat5a and Stat5b (14, 32). However, Stat5a and Stat5b transgenic mice generated in our lab show an increase in CD8+ T cells (33, 34), suggesting that Stat5 by itself is unlikely to be mediating the preferential effect of TSLP in expanding CD4+ T cells.
Unlike IL-7, TSLP had no effect on the in vitro proliferation of thymocytes unless combined with TCR activation. Because the differentiation of DP thymocytes into SP cells is based on MHC specificity of their TCR signal and the strength of the TCR engagement (35), it is conceivable that TSLP provides a selective costimulatory signal that favors the CD4+ CD8− intermediate stage by enhancing the activation of these cells. Moreover, it is possible that TSLP competes with IL-7 for the IL-7Rα subunit, thus hindering the ability of IL-7 to promote CD8+ T cell expansion in WT mice. Interestingly, IL-7 has been suggested to be important as a survival factor for CD4+ memory T cells (28). Thus, TSLP and IL-7, both of which share IL-7Rα, appear to be important for CD4+ T cell homeostasis. In γc KO mice where IL-7 signaling is defective, the ability of TSLP to promote the expansion of CD4+ cells could be enhanced. Interestingly, a recent report showed that human TSLP indirectly promotes CD4+ T cell homeostasis through the activation of dendritic cells (36). Our results in the murine system with purified CD4+ and CD8+ SP thymocytes, as well as with splenic T cells, suggest a direct effect of TSLP on CD4+ T cells. Surprisingly, TSLP transgenic mice were reported to exhibit diminished lymphopoiesis and myelopoiesis, with decreased T and B cell precursors (37). These findings are different from ours, suggesting that TSLP may have complex roles in vivo, depending on the temporal and quantitative level of expression of TSLP.
In conclusion, TSLP is a type I cytokine that regulates the growth and function of lymphocytes. Although its role for B cell growth has been better appreciated, we now provide evidence that murine TSLP also plays a role in T cell expansion both in vitro and in vivo, particularly of CD4+ T cells, suggesting that murine TSLP may play a role in CD4+ T cell homeostasis. This preferential action of TSLP for CD4+ versus CD8+ T cells may also explain the relative increase in CD4+ T cells in γc KO mice, which might reflect the inactivation of signaling by IL-7 and IL-15 (which favor expansion of CD8+ T cells), coupled to the “unopposed” action of TSLP. Finally, it will be interesting to investigate whether TSLP may have usefulness in increasing CD4+ T cells in immunodeficient states.
Acknowledgments
We thank Dr. Chengyu Liu for help in generating the TSLPR KO mice, Drs. Zu-Xi Yu and Allen Cheever for help in histopathological analyses, Drs. William E. Paul, Ron Germain, and B.J. Fowlkes for critical comments, Drs. Jian-Xin Lin and Panu Kovanen for valuable suggestions and discussions, and Ms. Julie Bollenbacher for assistance with animal experiments.
The online version of this article contains supplemental material.
Abbreviations used in this paper: BrdU, 5-bromo-2′-deoxyuridine; CFSE, carboxyfluorescein succinimidyl ester; DKO, double KO; DN, double negative; DP, double positive; ES, embryonic stem; SP, single positive; TSLP, thymic stromal lymphopoietin.
References
- 1.Leonard, W.J. 2003. Type I Cytokines and Interferons and Their Receptors. Fundamental Immunology. W.E. Paul, editor. Lippincott-Williams and Williams, Philadelphia. 701–747.
- 2.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Freeden-Jeffry, U., P. Vieira, L.A. Lucian, T. McNeil, S.E. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puel, A., S.F. Ziegler, R.H. Buckley, and W.J. Leonard. 1998. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 20:394–397. [DOI] [PubMed] [Google Scholar]
- 5.Friend, S.L., S. Hosier, A. Nelson, D. Foxworthe, D.E. Williams, and A. Farr. 1994. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 22:321–328. [PubMed] [Google Scholar]
- 6.Ray, R.J., C. Furlonger, D.E. Williams, and C.J. Paige. 1996. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur. J. Immunol. 26:10–16. [DOI] [PubMed] [Google Scholar]
- 7.Soumelis, V., P.A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, et al. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673–680. [DOI] [PubMed] [Google Scholar]
- 8.Pandey, A., K. Ozaki, H. Baumann, S.D. Levin, A. Puel, A.G. Farr, S.F. Ziegler, W.J. Leonard, and H.F. Lodish. 2000. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 1:59–64. [DOI] [PubMed] [Google Scholar]
- 9.Park, L.S., U. Martin, K. Garka, B. Gliniak, J.P. Di Santo, W. Muller, D.A. Largaespada, N.G. Copeland, N.A. Jenkins, A.G. Farr, et al. 2000. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noguchi, M., Y. Nakamura, S.M. Russell, S.F. Ziegler, M. Tsang, X. Cao, and W.J. Leonard. 1993. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 262:1877–1880. [DOI] [PubMed] [Google Scholar]
- 11.Kondo, M., T. Takeshita, M. Higuchi, M. Nakamura, T. Sudo, S. Nishikawa, and K. Sugamura. 1994. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 263:1453–1454. [DOI] [PubMed] [Google Scholar]
- 12.Leonard, W.J. 2001. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 1:200–208. [DOI] [PubMed] [Google Scholar]
- 13.Lin, J.X., T.S. Migone, M. Tsang, M. Friedmann, J.A. Weatherbee, L. Zhou, A. Yamauchi, E.T. Bloom, J. Mietz, S. John, et al. 1995. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 2:331–339. [DOI] [PubMed] [Google Scholar]
- 14.Isaksen, D.E., H. Baumann, P.A. Trobridge, A.G. Farr, S.D. Levin, and S.F. Ziegler. 1999. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J. Immunol. 163:5971–5977. [PubMed] [Google Scholar]
- 15.Bhatia, S.K., L.T. Tygrett, K.H. Grabstein, and T.J. Waldschmidt. 1995. The effect of in vivo IL-7 deprivation on T cell maturation. J. Exp. Med. 181:1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vosshenrich, C.A., A. Cumano, W. Muller, J.P. Di Santo, and P. Vieira. 2003. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat. Immunol. 4:773–779. [DOI] [PubMed] [Google Scholar]
- 18.DiSanto, J.P., W. Muller, D. Guy-Grand, A. Fischer, and K. Rajewsky. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA. 92:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao, X., E.W. Shores, J. Hu-Li, M.R. Anver, B.L. Kelsall, S.M. Russell, J. Drago, M. Noguchi, A. Grinberg, E.T. Bloom, et al. 1995. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 2:223–238. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima, H., E.W. Shores, M. Noguchi, and W.J. Leonard. 1997. The common cytokine receptor γ chain plays an essential role in regulating lymphoid homeostasis. J. Exp. Med. 185:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprent, J., and C.D. Surh. 2001. Generation and maintenance of memory T cells. Curr. Opin. Immunol. 13:248–254. [DOI] [PubMed] [Google Scholar]
- 22.Sims, J.E., D.E. Williams, P.J. Morrissey, K. Garka, D. Foxworthe, V. Price, S.L. Friend, A. Farr, M.A. Bedell, N.A. Jenkins, et al. 2000. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 192:671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waskow, C., S. Paul, C. Haller, M. Gassmann, and H. Rodewald. 2002. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 17:277–288. [DOI] [PubMed] [Google Scholar]
- 24.Mackarehtschian, K., J.D. Hardin, K.A. Moore, S. Boast, S.P. Goff, and I.R. Lemischka. 1995. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 3:147–161. [DOI] [PubMed] [Google Scholar]
- 25.Carpino, N., W.E. Thierfelder, M.S. Chang, C. Saris, S.J. Turner, S.F. Ziegler, and J.N. Ihle. 2004. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell. Biol. 24:2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotnik, A., and T.A. Moore. 1995. Cytokine production and requirements during T-cell development. Curr. Opin. Immunol. 7:206–213. [DOI] [PubMed] [Google Scholar]
- 27.Moore, T.A., U. von Freeden-Jeffry, R. Murray, and A. Zlotnik. 1996. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7−/− mice. J. Immunol. 157:2366–2373. [PubMed] [Google Scholar]
- 28.Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680–686. [DOI] [PubMed] [Google Scholar]
- 29.Kieper, W.C., J.T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C.D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15–independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schluns, K.S., and L. Lefrancois. 2003. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3:269–279. [DOI] [PubMed] [Google Scholar]
- 31.Lodolce, J.P., D.L. Boone, S. Chai, R.E. Swain, T. Dassopoulos, S. Trettin, and A. Ma. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 9:669–676. [DOI] [PubMed] [Google Scholar]
- 32.Isaksen, D.E., H. Baumann, B. Zhou, S. Nivollet, A.G. Farr, S.D. Levin, and S.F. Ziegler. 2002. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction. J. Immunol. 168:3288–3294. [DOI] [PubMed] [Google Scholar]
- 33.Kelly, J.A., R. Spolski, P.E. Kovanen, T. Suzuki, J. Bollenbacher, C.A. Pise-Masison, M.F. Radonovich, S. Lee, N.A. Jenkins, N.G. Copeland, et al. 2003. Stat5 synergizes with T cell receptor/antigen stimulation in the development of lymphoblastic lymphoma. J. Exp. Med. 198:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly, J., R. Spolski, K. Imada, J. Bollenbacher, S. Lee, and W.J. Leonard. 2003. A role for Stat5 in CD8+ T cell homeostasis. J. Immunol. 170:210–217. [DOI] [PubMed] [Google Scholar]
- 35.Germain, R.N. 2002. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2:309–322. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe, N., S. Hanabuchi, V. Soumelis, W. Yuan, S. Ho, R. de Waal Malefyt, and Y.J. Liu. 2004. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 5:426–434. [DOI] [PubMed] [Google Scholar]
- 37.Osborn, M.J., P.L. Ryan, N. Kirchhof, A. Panoskaltsis-Mortari, F. Mortari, and K.S. Tudor. 2004. Overexpression of murine TSLP impairs lymphopoiesis and myelopoiesis. Blood. 103:843–851. [DOI] [PubMed] [Google Scholar]