Abstract
To determine the correlates of immune recovery from active human CMV (HCMV) disease, we compared the antigenic repertoire, diversity, magnitude, and differentiation of HCMV-specific CD8+ T cells in HIV-HCMV coinfected subjects with no, cured, or active HCMV disease and in healthy HIV-negative HCMV-positive controls. ELISPOT–IFN-γ assays using peptide pools spanning the pp65 and immediate early 1 (IE1) HCMV proteins showed that HCMV-specific CD8+ T cells had a significantly broader antigenic repertoire and greater diversity in HIV-positive patients controlling HCMV replication than in those with active HCMV disease, but the magnitude of the CD8 T cell response did not differ between the different groups. HCMV-specific T cells mainly were focused against IE1 during the short-term recovery from retinitis, and switched toward pp65 during long-term recovery. HCMV-specific T cells displaying an “early” (CD8+CD27+CD28+) and “intermediate” (CD8+CD27−CD28+) differentiation phenotype were increased significantly during long-term recovery compared with other HIV-positive patients and were nearly undetectable during active HCMV disease. HCMV-specific T cells with a “late” (CD8+CD27−28−) differentiation phenotype predominated in all cases. Therefore, restoration of immune protection against HCMV after active HCMV disease in immunodeficient individuals is associated with enlarged repertoire and diversity, and with early differentiation of virus-specific CD8+ T cells, thus defining immune correlates of protection against diseases caused by persistent viruses.
Human cytomegalovirus (HCMV) is a common pathogen that infects more than half of the adult human population, but generally causes only minor symptoms in immunocompetent individuals (1). After initial exposure, healthy individuals carry the virus as a latent infection for life. Evidences for a major role of T cells in the immune control of this persistent infection are provided by the uncontrolled viral replication and HCMV end-organ diseases observed in immunocompromised individuals with severely impaired T cell functions, which make the virus a major cause of morbidity and mortality in this population.
Most HIV-infected patients are coinfected with HCMV. In this population, retinitis—the most frequent manifestation of severe uncontrolled HCMV reactivation—generally occurs in the late stages of the T helper cell deficiency. Although sensitive to anti-HCMV therapy, retinitis usually relapses after discontinuation of treatment if the CD4 T cell defects persist (2, 3). The introduction of highly active antiretroviral therapy (HAART) has changed the frequency of HCMV end-organ diseases in HIV-infected patients dramatically and its outcome (4, 5). The immune reconstitution obtained with HAART allows secondary anti-HCMV prophylaxis to be discontinued safely after at least 3 mo of successful HAART (4–6); this suggests that a protective immunity to HCMV has been restored, but correlates of immune protection against active HCMV (AHCMV) disease are still unknown.
HCMV-specific HLA class I–restricted T cell responses are known to be essential for successful resolution of the infection and maintenance of long-term control of HCMV replication (7–10). Two HCMV proteins serve as key target antigens for HCMV-specific T cells: a late matrix protein (pp65) that is abundant throughout HCMV infection and an immediate early 1 (IE1) antigen protein that is indispensable for viral replication (1, 11).
The initial studies of antigen-specific CD8+ T cells reported that IE1 was the main target for HCMV-specific T cells (12), whereas subsequent studies found that they predominantly recognized pp65 (13–16). Experiments with MHC class I tetramer/peptide complexes for direct ex vivo measurement of the frequency of antigen-specific T cells suggested that large numbers of specific CD8+ T cells that focus against a limited set of immunodominant peptides from pp65 (13, 17–20) and IE1 (21, 22) helped to control HCMV replication. However, the latter studies may have been biased by the selection of only a few epitopes for incorporation in tetramers. Recent reports revealed that cytokine-producing T cells that are specific for HCMV recognize a far broader repertoire of epitopes in healthy individuals (23, 24) than previously believed, such as many epitopes from other viral proteins (e.g., the pp28 and pp50 structural proteins, gH and gB early/late proteins antigens, and HCMV-encoded immunomodulators; reference 24). However, none of these studies identified immune correlates of protection against HCMV replication and of recovery after an acute HCMV event.
Immune control of viral infections requires large numbers and an adequate repertoire of virus-specific MHC class I–restricted T cells and their appropriate differentiation to permit rapid effector function and survival potential. Studies of human virus–specific T cells showed that the expression of different combinations of the CD27 and CD28 costimulatory molecules on virus-specific CD8+ T cells correlates with diverse differentiation and functional characteristics (25–28).
A linear differentiation model was proposed for antigen-experienced T cells with the CD27+CD28+ “early” and CD27−CD28+ “intermediate” stages expressing low or intermediate levels of perforin, respectively, and a CD27−CD28− “late” stage supposed to represent fully differentiated effector T cells (28) because of their high perforin content, ability to produce large amount of IFN-γ after stimulation, and direct ex vivo mediation of cytotoxicity (25–30). A general consensus (25–31) is that most HCMV-specific CD8+ T cells in HCMV-infected individuals who do not have AHCMV disease are CD27−CD28−CD57+CCR7−, and this phenotype is believed to confer immune control. Nonetheless, no study has addressed this question in immunosuppressed patients who have HCMV end-organ disease or after recovery from acute HCMV events, and the differentiation stage of HCMV-specific T cells associated with restoration of immune control has not been analyzed. The impact of CD4 T helper cell defects, known to be key in the expansion of memory T cells specific for viruses, is unknown.
The goal of this study was to characterize the HCMV-specific CD8+ responses associated with restoration of a protective immunity after an acute HCMV event in patients who were coinfected with HCMV and HIV, and to determine whether protective immunity against HCMV depends on the antigenic repertoire, the diversity or magnitude of specific CD8+ responses, or is associated with a particular profile of CD8+ T cell differentiation (or any combination of these elements).
We studied a group of HIV-HCMV–coinfected patients who had suffered from an HCMV-retinitis when their CD4 counts decreased to <50/mm3, in whom we previously demonstrated that CD4 T cell reconstitution >100/mm3 under continuous HAART allowed discontinuation of the HCMV prophylaxis and permanent control of the HCMV disease (6). In this group, HCMV-specific CD8 responses were studied <2 yr (short-term recovery from retinitis; STRR) and >5 yr after stable recovery (long-term recovery from retinitis; LTRR). They were compared with responses observed in HIV-negative HCMV-infected healthy controls and in two groups of HIV-HCMV–coinfected patients—healthy (long-term nonprogressors; LTNPs) or suffering from AHCMV disease. Analyzing the HCMV-specific CD8+ T cells that produced IFN-γ after stimulation with synthetic 15-mer overlapping peptides spanning the two immunodominant proteins, pp65 and IE1, enabled us to test exhaustively each subject's responses with the entire sequence of each protein without any predefined HLA-restriction, and to define the magnitude, antigenic repertoire, and the diversity of the HCMV-specific responses, as well as the differentiation stage of the HCMV-specific CD8 cells involved.
RESULTS
Antigenic repertoire and diversity of the CD8+ T cell-mediated HCMV-specific responses
We first identified the various antigenic regions (i.e., the antigenic repertoire) targeted by the HCMV-specific T cells that produced IFN-γ in the ELISPOT assay by using pools of 15-mer peptides covering all of the pp65 and IE1 sequences.
Overall, 10 of the 11 available pp65 peptide pools (91%) and all 10 of the available IE1 peptide pools were recognized at least once. CD8 cell depletion experiments showed that the IFN-γ production induced by these pp65 and IE1 15-mers was mediated primarily by CD8 T cells (unpublished data). The pools were organized in a matrix to identify the individual targeted peptides recognized by the 11 HIV-negative subjects. 20 individual peptides were recognized in 17 pools, with a mean of 1.2 peptides (range: 1–4) recognized per pool. Considering the known HLA class I restriction of previously described HCMV epitopes, this 15-mer overlapping peptide pool organization reflected the actual epitope repertoire recognized by the HCMV-specific T cells because all of the pp65 and IE1 nonamers previously described were recognized (unpublished data).
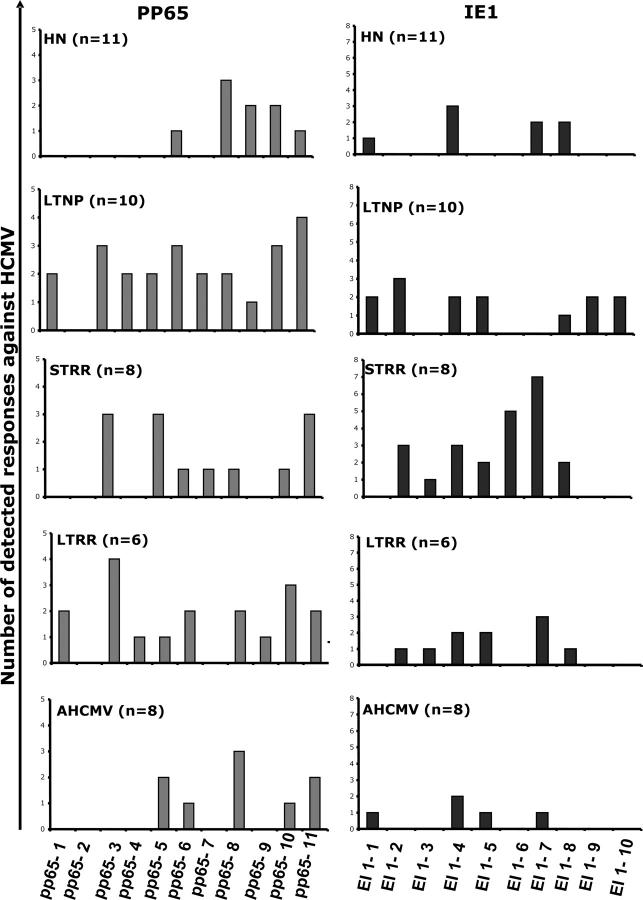
We then compared the antigenic repertoire of the pp65 and IE1 peptides recognized by CD8+ T cells from HIV-negative and HIV-positive subjects (Table I and Fig. 1). Only 5 distinct pp65 pools induced at least one response in the HIV-negative individuals: the pp65[251–310] and pp65[351–561] regions (pools 6 to 11; i.e., the COOH-terminal part of the protein). In contrast, PBMCs from HIV-positive subjects who had controlled HCMV replication (i.e, LTNPs, STRR, and LTRR subjects), recognized 10, 7, and 9 distinct pp65 pools, respectively, that covered the whole pp65 sequence. Similarly, the HIV-negative donors recognized only 4 IE1 pools, whereas LTNP, STRR, and LTRR subjects recognized 7, 7, and 6 distinct IE1 pools, respectively. Therefore, each distinct pp65 and IE1 pool was recognized by specific cells from a mean of 21% (range: 0–42%) and 20% (range: 0–42%), respectively, of HIV-positive subjects who had controlled HCMV replication, whereas a mean of only 7% (range: 0–27%) of the HIV-negative individuals recognized each distinct pp65 and IE1 pool. In addition, considering patients who had AHCMV disease, each distinct pp65 and IE1 pool was recognized by a mean of 10% (range: 0–37%) and 6% (range: 0–25%) of patients. Thus, the available HCMV antigenic repertoire engaged in CD8+ T cell responses against pp65 and IE1 was significantly broader among HIV-positive subjects who had controlled HCMV replication than in healthy HIV-negative individuals (pp65, P = 0.010; IE1, P = 0.010) and patients who did not control HCMV replication (pp65, P = 0.045; IE1, P = 0.006).
Table I.
Characteristics of study subjects
| Subjects | Age | Sex | HLA-I typing | CD4 count | Time from onset of episode of HCMV disease | HCMV viral loada |
|---|---|---|---|---|---|---|
| months | ||||||
| HN1b | 50 | M | A3/25 B18/35 | no disease | negative | |
| HN2 | 48 | M | A2 B18/15 | no disease | negative | |
| HN3 | 41 | M | A2/3 B7/18 | no disease | negative | |
| HN4 | 36 | F | A2/25 B18/44 | no disease | negative | |
| HN5 | 29 | M | A1/2 B8/57 | no disease | negative | |
| HN6 | 44 | M | A1/29 B8/44 | no disease | negative | |
| HN7 | 51 | M | A1/23 B44/61 | no disease | negative | |
| HN8 | 45 | M | A1/23 B8/44 | no disease | negative | |
| HN9 | 54 | M | A3/32 B18/47 | no disease | negative | |
| HN10 | 40 | M | A24/28 B51/61 | no disease | negative | |
| HN11 | 49 | M | A11/26 B12/40 | no disease | negative | |
| LTNP1 | 43 | M | 694 | no disease | negative | |
| LTNP2 | 47 | M | 807 | no disease | negative | |
| LTNP3 | 46 | M | 776 | no disease | negative | |
| LTNP4 | 49 | M | 1500 | no disease | negative | |
| LTNP5 | 41 | F | 1227 | no disease | negative | |
| LTNP6 | 64 | M | 640 | no disease | negative | |
| LTNP7 | 41 | M | 441 | no disease | negative | |
| LTNP8 | 64 | M | 679 | no disease | negative | |
| LTNP9 | 39 | M | 639 | no disease | negative | |
| LTNP10 | 54 | F | 773 | no disease | negative | |
| STRR1 | 57 | M | 194 | 17 | negative | |
| STRR2 | 41 | M | 490 | 20 | negative | |
| STRR3 | 56 | M | 425 | 20 | negative | |
| STRR4 | 41 | M | 366 | 20 | negative | |
| STRR5 | 56 | M | 241 | 17 | negative | |
| STRR6 | 55 | M | 348 | 22 | negative | |
| STRR7 | 49 | M | 194 | 17 | negative | |
| STRR8 | 50 | M | 490 | 20 | negative | |
| LTRR1 | 50 | M | 346 | >60 | negative | |
| LTRR2 | 47 | M | 483 | >60 | negative | |
| LTRR3 | 51 | M | 275 | >60 | negative | |
| LTRR4 | 45 | M | 361 | >60 | negative | |
| LTRR5 | 44 | M | 306 | >60 | negative | |
| LTRR6 | 55 | M | 344 | >60 | negative | |
| AHCMV1 | 43 | M | 5 | active retinitis | positive | |
| AHCMV2 | 36 | M | 5 | active retinitis | positive | |
| AHCMV3 | 48 | M | 352 | active retinitis | positive | |
| AHCMV4 | 33 | M | 17 | active colitis | positive | |
| AHCMV5 | 41 | M | 50 | active retinitis | positive | |
| AHCMV6 | 55 | M | 30 | active retinitis | positive | |
| AHCMV7b | 53 | M | 121 | active retinitis | positive | |
| AHCMV8b | 57 | F | 150 | active retinitis | positive |
Detection limit of 10 copies.
HIV-negative patient.
Figure 1.
Broader antigenic repertoire of HCMV-specific CD8+ T cell responses in HIV-positive subjects with controlled HCMV replication or recovering from AHCMV infection, compared with HIV-negative (HN) healthy individuals and patients who had uncontrolled HCMV replication. The number of distinct pp65 and IE1 pools of peptides detected by ELISPOT assay at least once, by group. Each bar represents the number of persons whose CD8 T cells recognize each individual HCMV-pp65 or IE1 peptide pool.
Taken together, these results suggest that HIV-infected individuals who control their HCMV infection, either spontaneously or after recovery of an AHCMV event, develop a broader repertoire of HCMV-specific CD8 T cells against pp65 and IE1 than healthy HIV-negative subjects and severely immunosuppressed patients who are unable to control HCMV.
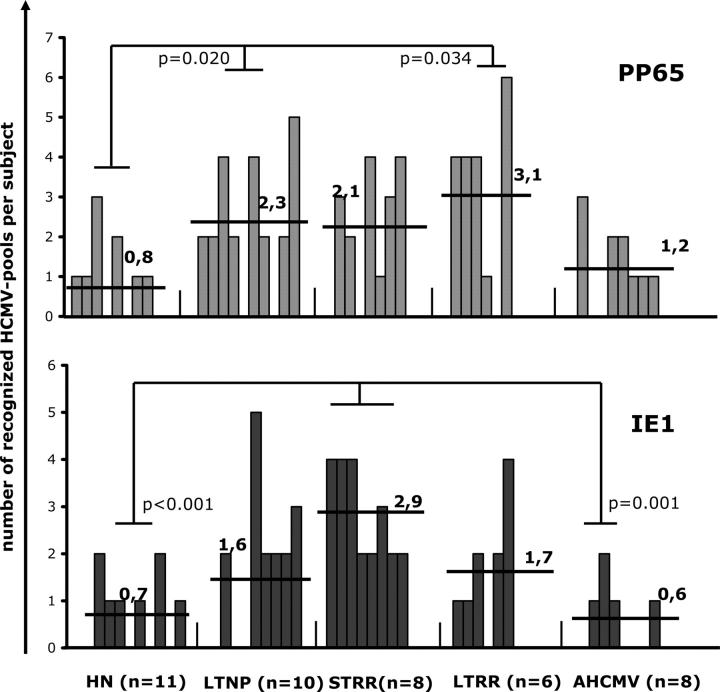
The diversity (number of peptide pools recognized per patient) of HCMV-specific CD8 T cell responses differs between HIV-negative and HIV-positive subjects
HIV-negative donors and patients who had AHCMV disease showed a limited diversity; they recognized a mean of only 0.8 and 1.2 pp65 pools per subject in both groups, respectively (range: 0–3 in both cases), and a mean of 0.7 and 0.6 IE1 pools, respectively (range: 0–2, in both cases). In contrast, HIV-infected patients who had controlled HCMV replication (LTNP, STRR, and LTRR) recognized a mean of 2.3 pp65 pools per subject (range: 0–5) and a mean of 2 IE1 pools per subject (range: 0–5). When compared with HIV-negative donors, patients who had controlled HCMV replication showed a broader diversity against pp65 (P = 0.012) and IE1 (P = 0.019), whereas the IE1 diversity (P = 0.025), but not the pp65 diversity (P = 0.122), was greater when compared with AHCMV retinitis.
Although the distribution of the HCMV peptides apparently was balanced equally between pp65 and IE1 proteins in all cases, the STRR subjects recognized significantly more IE1 pools (P < 0.001) than HIV-negative healthy subjects and AHCMV subjects (P = 0.001). In addition, LTNP (P = 0.020) and LTRR (P = 0.034) subjects recognized significantly more pp65 pools than HIV-negative healthy subjects (Fig. 2).
Figure 2.
Greater diversity of HCMV-specific CD8+ T cell responses in HIV-positive subjects with controlled HCMV replication or recovering from AHCMV infection, compared with HIV-negative (HN) healthy individuals and patients who had uncontrolled HCMV replication. The number of distinct HCMV-pp65 and -IE1 pools of peptides detected by ELISPOT assay per individual. Each bar represents one individual; the number in bold type is the mean number of recognized pools per individual.
These data suggest that immune control of HCMV replication in HIV-infected patients who are exposed to HCMV is associated with enlarged repertoire and diversity of the HCMV-specific CD8 cell responses. They also indicate that restoration of protective immunity to HCMV seems to focus on IE1 in the early period but switches toward pp65 in the late immune restoration period, when it is similar to the HCMV-specific immunity that is observed in LTNPs who never had HCMV retinitis.
Magnitude of HCMV-specific CD8+ response
The magnitude of HCMV-specific T cell responses was measured as the sum of the HCMV-specific T cells producing IFN-γ in response to the pp65 and the IE1 peptide pools. Immunodominant peptides (i.e., the peptide pool with the highest magnitude of response for each subject) were distributed equally between pp65 and IE1 (49% pp65 and 51% IE1 pools).
The mean magnitude of HCMV-specific T cell responses among HIV-negative subjects was 580 spot-forming cells (SFCs)/million PBMCs (range: 0–1,900) for pp65 and 471 SFCs/million PBMCs (range: 0–1,800) for IE1. Among subjects who had AHCMV disease, the mean magnitude of responses was 681 (range: 0–2480) and 973 SFCs/million PBMCs (range: 0–3,900) for pp65 and IE1, respectively. In line with the above results, magnitude of responses did not differ significantly between HIV-infected patients who had controlled HCMV replication (LTNP, STRR, and LTRR) as a whole (mean: 1817, range: 0–9,200 and 2,430 SFCs/million PBMCs, range: 0–7330 against pp65 and IE1, respectively) and patients who had AHCMV disease (pp65, P = 0.332; IE1, P = 0.115).
When analyzing each subgroup of HIV-positive patients who had controlled HCMV replication, we found that the LTNP and LTRR subjects did not differ from HIV-negative individuals in the magnitude of their CD8 cell responses to pp65 (LTNP, P = 0.214; LTRR, P = 0.097) and IE1 (LTNP, P = 0.702; LTRR, P = 0.059). In contrast, the STRR subjects had significantly higher frequencies of CD8 cells directed against IE1 (P < 0.001), but not against pp65 (P = 0.709), than HIV-negative donors (Fig. 3). Noteworthy in STRR responders to IE1, each individual IE1 peptide pool was recognized with a mean magnitude of 1699 SFCs/million PBMCs, (range: 975–2350) that was significantly higher than that observed in HIV-negative responders (662 SFCs/million PBMCs, range: 165–1800; P = 0.010); this suggested an actual increase in the magnitude of response against the HCMV-IE1 protein in this group. Thus, the immunodominant responses associated with immune protection appear to focus primarily against IE1 in the early period of recovery from acute HCMV events. Finally, STRR subjects showed significantly higher CD8 cell frequencies directed against IE1 than did patients who had AHCMV disease (P = 0.002; Fig. 3).
Figure 3.
Magnitude of HCMV-specific CD8+ T cell responses. The number of pp65 and IE1-specific IFNγ-producing T cells identified for each subject in ELISPOT assays. Each bar represents one subject. The numbers in bold type are the mean numbers of specific T cells per subject.
Therefore, our results suggest that although the diversity and the magnitude of IE1-specific CD8 T cells explain increased frequencies of IE1-specific CD8 T cells during the early recovery period after HCMV retinitis, the magnitude of the HCMV-specific response does not play a key role in the long-term control of HCMV replication in HIV-infected patients.
Differentiation of HCMV-specific CD8+ T cells
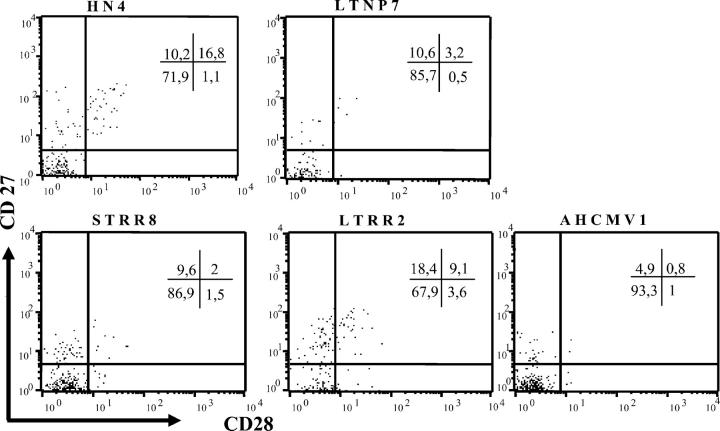
To study the differentiation characteristics of these CD8+ HCMV-specific T cells, we selected the dominant peptide pool recognized by each patient in the ELISPOT assay: overall 75% were IE1. For each pool, we used an intracellular cytokine detection assay to analyze the simultaneous production of IFN-γ and IL-2 in HCMV-specific CD8 cells. Only IFN-γ production was observed with a mean of 0.78% CD8+ cells producing IFN-γ; we were unable to detect any IL-2 production in those HCMV-specific T cells, even when tested from HIV-negative, LTNP, or LTRR subjects (unpublished data). Then we studied the coexpression of CD27 and CD28 on the cell surface of CD8+ T cells that produced IFN-γ against HCMV peptide pools, and quantified three subpopulations: CD27−CD28−, CD27−CD28+ and CD27+CD28+ (Fig. 4). Changes in subset repartition were observed among groups of patients for the CD27−28−, CD27−28+, and CD27+28+ cells, but not for the CD27+28− subset.
Figure 4.
Differentiation profiles of HCMV-specific CD8+ T cells. Flow-cytometry analysis of CD27 and CD28 coexpression gated on IFN-γ–producing CD8 T cells after stimulation with the dominant HCMV peptide pool as detected in the ELISPOT assay. Each display represents results from a representative donor from each group. Proportions of cells in each quadrant are represented in the labels.
The CD27−CD28−CD8+ IFN-γ+ T cell subpopulation accounted for >72% of the HCMV-specific CD8+ T cells, and was the largest subpopulation for all groups of subjects tested. In patients who had uncontrolled HCMV replication (AHCMV), >90% of CD8+ T cells displayed this late differentiated stage. Similarly most HCMV-specific CD8+ T cells were CD57+ (unpublished data). The percentages of these cells were similar in LTRR subjects (72 ± 12%) and HIV-negative healthy individuals (76 ± 12%). When expressed as numbers of T cells per million PBMCs per subject (Table II), only the counts of HCMV-specific CD8+ CD27−CD28− T cells observed in patients who had AHCMV (P = 0.032) were significantly higher than in HIV-negative subjects, whereas those from LTNP (P = 0.286), STRR (P = 0.065), or LTRR (P = 0.095) subjects were not.
Table II.
Distinct pattern of differentiation of HCMV-specific CD8+ T cells associated with long-term immune recovery
| Frequencies of HCMV-specific T cells
|
Subsets of HCMV-specific CD8+ T cells producing IFNγa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects | HCMV pool tested | SFC/M PBMCb | CD8+IFN-γc | CD27−CD28− | CD27−CD28+ | CD27+CD28+ | |||
| % | % | N d | % | N d | % | N d | |||
| HN3 | IE1-4 | 440 | 54 | 90 | 214 | 2.6 | 6 | 3.7 | 9 |
| HN4 | IE1-8 | 1,300 | 79 | 71.9 | 738 | 1.1 | 11 | 16.8 | 173 |
| HN7 | IE1-8 | 500 | 71 | 63.4 | 225 | 7.3 | 26 | 19.5 | 69 |
| HN9 | IE1-4 | 500 | 63 | 67.3 | 212 | 3.6 | 11 | 25.4 | 80 |
| HN11 | IE1-1 | 1,800 | 93 | 88.6 | 1,483 | 1.5 | 25 | 5.3 | 89 |
| mean | 908 | 72 | 76.2 | 574 | 3.2 | 16 | 14.1 | 84 | |
| LTNP3 | pp65-5 | 1,240 | 30 | 73.5 | 273 | 4 | 15 | 2 | 7 |
| LTNP6 | IE1-1 | 1,600 | 71 | 95 | 1,080 | 1.2 | 14 | 2.5 | 28 |
| LTNP7 | IE1-4 | 1,650 | 79 | 88.1 | 1,148 | 1.1 | 14 | 4.5 | 58 |
| LTNP8 | IE1-2 | 1,330 | 78 | 85.7 | 889 | 0.5 | 5 | 3.2 | 33 |
| mean | 1,450 | 64 | 85.6 | 847 | 1.7 | 12 | 3 | 31 | |
| STRR1 | IE1-4 | 3,000 | 70 | 93.4 | 1,961 | 0.8 | 17 | 0.3 | 6 |
| STRR2 | IE1-2 | 3,000 | 50 | 78.2 | 1,173 | 1.7 | 26 | 0.7 | 11 |
| STRR3 | IE1-5 | 1,800 | 51 | 92.4 | 848 | 0.3 | 3 | 2.3 | 21 |
| STRR4 | IE1-7 | 3,000 | 66 | 92.5 | 1,831 | 1.3 | 26 | 0.7 | 14 |
| STRR5 | pp65-6 | 1,000 | 43 | 68 | 293 | 5.6 | 24 | 13 | 56 |
| STRR6 | pp65-5 | 910 | 30 | 78.6 | 215 | 9 | 25 | 9 | 25 |
| STRR7 | IE1-7 | 3,000 | 74 | 88 | 1,954 | 1.4 | 31 | 1.1 | 24 |
| STRR8 | IE1-7 | 3,000 | 72 | 86.9 | 1,877 | 1.5 | 32 | 2 | 43 |
| mean | 2,338 | 57 | 84.7 | 1,269 | 2.7 | 23 | 3.6 | 25 | |
| LTRR1 | pp65-9 | 3,000 | 69 | 61.3 | 1,269 | 1.7 | 35 | 8.8 | 182 |
| LTRR2 | IE1-7 | 3,000 | 95 | 67.9 | 1,935 | 3.6 | 102 | 9.1 | 260 |
| LTRR3 | pp65-6 | 3,000 | 75 | 88.6 | 1,993 | 2.6 | 58 | 6.1 | 137 |
| LTRR5 | IE-4 | 620 | 61 | 81.5 | 306 | 6.1 | 23 | 9.2 | 35 |
| LTRR6 | IE1-5 | 2,000 | 81 | 62.5 | 1,251 | 5.3 | 106 | 23.8 | 476 |
| mean | 2,324 | 76 | 72.4 | 1,351 | 3.9 | 65 | 11.4 | 218 | |
| AHCMV1 | pp65-8 | 3,000 | 85 | 91.2 | 2,325 | 1 | 26 | 0.8 | 20 |
| AHCMV3 | IE1-4 | 1,900 | 91 | 93.3 | 1,613 | 0.6 | 11 | 2.9 | 50 |
| AHCMV4 | IE-7 | 1,370 | 97 | 96.8 | 1,286 | 0.3 | 4 | 0.3 | 4 |
| AHCMV7 | IE1-5 | 2,330 | 80 | 90 | 1,677 | 0.6 | 11 | 0.8 | 15 |
| mean | 2,150 | 88 | 92.8 | 1,725 | 0.6 | 13 | 1.2 | 22 | |
Assessed by intracellular cytokine staining.
Assessed by ELISPOT assay.
%CD8 + IFN-γ+ = number of CD8+-producing IFN-γ/number of PBMCs producing IFN-γ as assessed by intracellular cytokine staining.
N = %CD27CD28 × (SFCs/M. PBMC × %CD8+IFN-γ+).
These results show that patients who are exposed to high levels of HCMV replication at advanced stages of CD4 immunodeficiency had maintained greater numbers of “late” differentiated HCMV-specific CD8+ T cells than patients who were recovering from retinitis (STRR, LTRR) and patients who never experienced AHCMV disease (LTNP, healthy HIV-negative donors).
In addition, the proportions of “early” differentiated HCMV-specific CD27+CD28+CD8+ T cells were significantly higher during the late recovery period (LTRR, 11 ± 7%) than among the other HIV-infected patients (LTNP, 3.0 ± 1.2%, P = 0.016; STRR, 3.6 ± 4.7%, P = 0.042; AHCMV, 1.2 ± 1.2%, P = 0.016), but were identical to the proportions observed in the HIV-negative group (14 ± 9%, P = 1.000). The patients who had AHCMV disease had the lowest percentages of CD27+CD28+CD8+ T cells. The mean numbers of CD27+CD28+CD8+ T cells in LTRR subjects were likewise significantly higher than in the other HIV-infected patients (LTNP, P = 0.032, STRR, P = 0.006, AHCMV, P = 0.032).
Furthermore, recovery of HCMV-specific immunity by LTRR subjects also was associated with the reinforcement of the “intermediate” differentiated subpopulation of CD27−CD28+CD8+ T cells. The mean number of these cells was significantly higher in these patients than in the other HIV-infected groups (LTNP: P = 0.016; STRR: P = 0.041; AHCMV: P = 0.032).
These results suggest that long-term immune restoration after several years of HAART was associated with a regeneration of new early and intermediate CD28+CD8+ T cells (i.e., differing only according to the presence of CD27), which had almost disappeared during the acute HCMV events.
DISCUSSION
Our findings show that restoration of an immune control after AHCMV disease in HIV-infected patients is associated with enlarged antigenic repertoire and diversity of HCMV-specific CD8+ responses, and with regeneration of “early” and “intermediate” differentiated CD27+/−CD28+CD8+ HCMV-specific T cells. In addition, focusing CD8+ T cell responses against the IE1-HCMV protein during the early recovery period also seems to be an essential feature of immune recovery.
The exhaustive and systematic analysis of the HCMV-pp65 and -IE1 CD8 cell recognition that we performed—combining 15-mer peptide pools and ELISPOT–IFN-γ assays—provided the same magnitude of anti-pp65 and anti-IE1 CD8 cell responses in healthy HIV-negative subjects as was described recently by using preselected HLA-matched optimal 9-mer epitopes (24). The repertoire of HCMV peptides available for CD8+ T cell recognition defined with our strategy also is consistent—considering HLA class-I haplotypes—with all HCMV epitopes described in the literature (10, 14, 23, 24, 32). Lack of response in two healthy HCMV carriers might suggest that HCMV-specific T cells can be directed against non-pp65 and non-IE1 proteins (24) or that this portion of the pp65 protein is poorly immunogenic. Although the HLA haplotypes of our HIV-negative donors are representative of a typical HLA class I Caucasian distribution, some MHC bias might explain the lack of response in HIV-negative subjects against the five N-proximal pp65 [1–260] pools, rather than a lack of immunogenicity of this region because it is recognized in HIV-infected subjects or published data (16, 20, 23, 24, 33).
Our study substantiates that the magnitude of the HCMV-specific responses does not play a key role in controlling HCMV replication, and is not sufficient to ensure protection against AHCMV disease. In contrast, antigenic repertoire and diversity of HCMV-specific CD8+ T lymphocytes that produce IFN-γ are key correlates of restoration of an immune control of HCMV and of protection against relapses of HCMV retinitis. The broad antigenic repertoire and diversity of HCMV-specific responses clearly differed from those that were observed in healthy HCMV-positive patients and in subjects who were unable to control HCMV replication.
One likely hypothesis is that a high level of HCMV replication in HIV-infected patients exposes the immune system to a broad repertoire of HCMV epitopes which can be recognized adequately by CD8 T cells only when CD4 help is present at sufficient levels. We previously reported in the RESTIMOP study (6), from which the STRR and LTRR subjects were drawn, that HAART initiation in patients who were recovering from retinitis was associated with restored CD4 counts and CD4 proliferative responses to HCMV. Similarly, a recent study pointed out the role of the CD4 help in primary HCMV infection (34). Our results and hypothesis also are consistent with murine models which showed that CD4 help ensures the maintenance and competitive fitness of virus-specific memory CD8 T cells (35, 36). Although characteristics of immune response to HCMV observed 17 mo to 60 mo or longer after an AHCMV disease might result from a major exposure to high HCMV viremia during such disease, HCMV remained undetectable in all recovering patients during the entire study period. Furthermore, it was shown that HCMV viremia was cleared 3 mo after initiation of HAART (37–39). Finally, severe uncontrolled HCMV reactivation generally occurs at late stages of the T helper cell deficiency when CD4 counts decrease to <75/mm3 (2), and secondary anti-HCMV prophylaxis can be discontinued safely only when CD4 counts are restored to >100/mm3 after introduction of HAART (4–6). Therefore, we propose that exposure to high HCMV viremia in the absence of help does not allow the characteristics of the immune responses that we observed after recovery of AHCMV disease, which require in addition a restored help. Thus, the strength of the antigenic pressure—defined as numbers of viral epitopes produced—and the available help determine the repertoire and the diversity, and thereby, the immune control of AHCMV replication and disease.
In addition, recovery from acute HCMV retinitis is associated with immunodominance of the IE1-HCMV antigen during the first 2 yr after retinitis, whereas pp65 might be more instrumental in the long term. A protective role of IE1-specific CD8 T cells was recognized recently in HIV-negative patients who had AHCMV disease (40). Changing patterns of CD8+ T cell immunodominance have been demonstrated in murine models of influenza virus infection and were shown to reflect the antigen density in antigen-presenting cells, the T cell repertoire available, and the differential antigen presentation in the course of the virus infection (41, 42). The dominant IE1 response that we observed might not reflect differences in amounts of IE1 and pp65 proteins because both are produced equally by cells replicating HCMV, but rather might reflect a different kinetics of production. IE1 is the first HCMV protein to be expressed during the virus cycle and can block apoptosis before other HCMV proteins that can block antigen processing and presentation selectively (1). Thus, presentation of IE1 might be favored in AHCMV replication and polarize the immune responses toward IE1, but might decrease progressively when HCMV replication is controlled durably, and thus, allows the balance between pp65 and IE1 to be restored.
According to the current consensus (25, 31, 43), we show that most CD8+ T cells that are engaged against HCMV have a CD27−CD28−CD57+ phenotype in healthy HIV-negative and HIV-positive individuals. CD57 and CD28 expression (mutually exclusive in CD8+ T cell populations; references 26, 44–47) is associated with terminal differentiation of antigen-experienced T cells that have undergone numerous cell divisions and are unable to proliferate (replicative senescence). These features might reflect the continuous stimulation of specific CD8+ T cells by HCMV antigens over years. The findings from patients who have AHCMV disease suggest that the predominant CD27−CD28−CD57+ differentiation profile, as well as the magnitude of those cells, is not sufficient to control HCMV replication if not associated with a broad repertoire of HCMV-specific cells in an earlier differentiation profile. The reinforcement of the HCMV-specific CD27−/+ CD28+CD8+ T cells, after recovery from retinitis to levels similar to those observed in healthy individuals, suggests that these cells, with a known higher proliferation ability (25, 31, 48), play a key protective role in ensuring stable control of HCMV replication. The regeneration of these subsets after HCMV retinitis and their long-term maintenance may depend on CD4 help, as suggested by studies demonstrating the need for CD4 help in maintaining effective cytotoxic T lymphocyte responses (49), and the importance of IL-2 production in virus-specific T cells in HIV-infected patients (50, 51). We were unable to detect any IL-2 production in those HCMV-specific T cells, even when tested from HIV-negative LTNP or LTRR subjects (unpublished data). Thus, recovery—with antiretroviral treatments—of an efficient CD4 help may to make it possible to restore these key subsets.
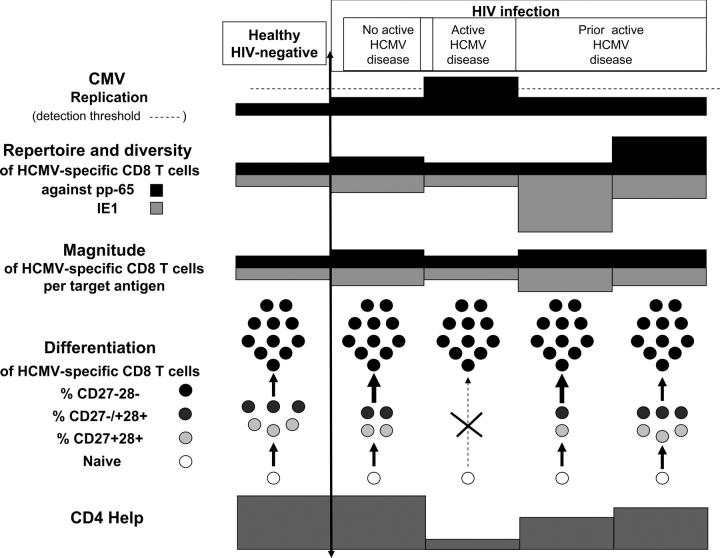
Finally, a scheme for immune control of HCMV can be drawn from these findings (Fig. 5). In healthy individuals with a normal CD4 help, a low level HCMV replication maintains a weak activation of HCMV-specific CD8+ T cells equally balanced against pp65 and IE1 antigens. Those HCMV-specific cells are composed of a mixed population of “early” and “intermediate” CD27+/−28+ cells that permanently give rise to “late” CD27−28+ effector cells, and thus, establish a quasi-equilibrium between controlled virus reactivation and host defenses. If HCMV replication increases slightly, as observed in concurrent HIV infection, the repertoire and diversity of HCMV-specific CD8 T cells broaden, as long as the CD4 help persists, with a mixed phenotype but an accelerated conversion toward “late” cells. If the CD4 help disappears, new early or intermediate HCMV-specific cells cannot regenerate and the repertoire cannot broaden; the remaining “late” HCMV-specific cells cannot control the outgrowth of the virus, even if in large numbers, and active disease occurs. Control of AHCMV disease takes place only when a strong CD4 help is restored and involves a broadening of the repertoire and diversity of the HCMV-specific CD8 T cells against IE1. If CD4 help is maintained over time, regeneration of “early” and “intermediate” CD27+/−28+ phenotypes occurs that will ensure long-term protection and a quasi-healthy equilibrium.
Figure 5.
Immune correlates of a stable recovery after AHCMV retinitis. A model based upon the experimental findings is proposed.
Such key associations between antigenic repertoire, diversity, differentiation of HCMV-specific CD8 T cells, and restoration of an immune control of AHCMV disease also might apply to other immune deficiencies that expose carriers to HCMV reactivation and disease. If so, our results may provide a model for defining immune correlates of long-term protection against other persistent viruses, such as HIV.
MATERIALS AND METHODS
Patients.
PBMC samples that were frozen and stored as described below were obtained from 38 HCMV-infected individuals. The HCMV infection was determined by serologic status. Samples from 11 HCMV-positive healthy HIV-negative blood bank donors for whom HLA class I haplotype was known come from a kind collaboration with E. Robinet (Etablissement Français du Sang, Besançon, France). A series of 30 HCMV-positive HIV-infected patients were classified into three groups according to clinical status. The first group was made up of 10 LTNPs from the French Asymptomatiques à Long Terme cohort reported elsewhere with the following characteristics: HIV diagnosis for >8 yr; normal CD4+ T lymphocytes >600/mm3 at inclusion; no antiretroviral treatment; and no HIV-related symptoms, especially no history of AHCMV disease and undetectable HCMV viremia (52). The second group consisted of 14 HAART-treated patients who were enrolled in the RESTIMOP clinical trial (6) after recovery from an episode of HCMV retinitis that had occurred before the HAART era and was cured under anti-HCMV therapy followed by HAART, and who discontinued secondary anti-HCMV prophylaxis when their CD4 counts had reached a threshold of 75/mm3. All remained without anti-HCMV secondary prophylaxis during follow-up without subsequent progression of retinitis during the study; they were monitored carefully by ophthalmologic examinations. Classification of these 14 patients into two subgroups was based on length of postretinitis HAART: a group with STRR (n = 8) who had been receiving HAART for <2 yr and a group with LTRR (n = 6) who had been receiving HAART for >5 yr. The third group contained 8 patients who had AHCMV disease: 6 HIV-infected individuals (2 naive of HAART and 4 with uncontrolled HIV replication despite HAART) and 2 non-HIV infected individuals who had severe CD4 immunodeficiency (one had Good's syndrome [AHCMV7] and one had an idiopathic CD4 lymphopenia [AHCMV8]). All patients in this group had an HCMV retinitis except one who had an HCMV colitis. All clinical samples were obtained according to ethical rules after agreement from institutional review boards. All subjects provided written informed consent. All patients except for the 8 in the third group had undetectable HCMV viral loads. HCMV PCR was performed with the HS ELOSA CMV kit (Lambdatech), which has a detection limit of 10 copies/ml. Table 2 summarizes the characteristics of the study patients.
PBMC preparation and storage.
PBMCs were obtained after separation with a standard Ficoll-Hypaque gradient (Pharmacia) separation on fresh blood samples and immediate cryopreservation in FCS (GIBCO BRL, Life Technology) containing 10% DMSO (Merck) in liquid nitrogen. Trypan blue exclusion after thawing showed a mean viability of 85%.
Peptides.
The synthetic peptides were 15 amino acid–long peptides overlapping by 10 amino acids and spanning the two HCMV proteins, pp65 and IE1, according to EPYTOP (53) and obtained from the INSERM “Action-Thematique-Concertée on antiviral immunity.” A total of 110 pp65 peptides were clustered in 11 pools of 10 contiguous peptides (pp65 pools 1–11), and 96 IE1 peptides were clustered in 8 pools of 10 contiguous peptides (IE1 pools 1–8) and in 2 pools of 8 contiguous peptides (IE1 pools 9 and 10). Each pool covered a sequence of 59 amino acids, except IE1 pools 9 and 10, which covered sequences of 49 amino acids and 50 amino acids, respectively.
ELISPOT assay.
The ELISPOT assay was adapted from a previously described method (54). In brief, 96-well nitrocellulose plates (Multiscreen Immobilon-p Filtration Plates, Millipore) were coated with human monoclonal anti–IFN-γ IgG1 (Mabtech). After three washings and blockage with 10% FCS, PBMCs were added at a concentration of 105 in triplicate wells for each experimental condition. Pools of overlapping 15-mer peptides spanning HCMV pp65 and HCMV IE1 (100 μl at a final concentration of 2 μg/ml), RPMI 1640+ (negative control), or PHA (GIBCO BRL) (control antigen) were added to the appropriate wells; the plates were incubated for 18 h at 37°C in a 5% CO2 atmosphere and then washed. A biotinylated detection antibody, anti–IFN-γ (Mabtech), was added and incubated at 37°C for 4 h and, after washings, followed by the addition of streptavidin-alkaline phosphatase (Amersham Biosciences), substrate, and incubation at room temperature. SFCs were counted using an automated ELISPOT reader (Carl Zeiss MicroImaging, Inc.). Results were expressed as the mean number of SFCs/106 PBMC (SFC/M) in each experimental condition after subtraction of the mean negative control values. All peptide pools that induced IFN-γ response >50 SFCs/M above background were considered putative CD8+ T cell epitopes. Saturated responses were counted arbitrarily as responses of 3,000 SFCs/M.
The cell origin of this IFN-γ production was assessed in 11 HIV-negative individuals by reevaluating positive responses against peptide pools on CD8+-depleted PBMCs after using CD8 magnetic beads (Dynal). Each pool was tested with total and CD8+-depleted PBMCs. A mean of 81% inhibition of recognition was obtained with CD8+-depleted PBMCs assessing the predominant CD8 cell involvement (unpublished data). In HIV-infected patients, the predominant CD8 cell origin of the HCMV-specific IFN-γ production was assessed by intracellular cytokine staining.
Cell surface and intracellular staining by flow cytometry.
The assay was adapted from a previously described method (32, 46, 53, 55, 56). 1 million PBMCs were incubated for 6 h in the presence of peptide pools detected as epitopes in the ELISPOT assay at the final concentration of 2 μg/ml, control antigen (PHA; GIBCO BRL), or RPMI 1640+ (negative control). Brefeldin A (Sigma-Aldrich) was added to the culture for the final 4 h. After washings, PBMCs were incubated for 15 min at room temperature with the following fluorescent-labeled conjugated monoclonal antibodies: anti-CD4–FITC, anti–CD8-PerCP, anti–CD27-PE or FITC, and anti–CD28-FITC or PE (all Becton Dickinson). After washings, cells were fixed with buffered formaldehyde acetone solution and permeabilized with 0.1% saponin, 50 mM D-glucose. Cells were incubated, stained with anti–IFN-γ-APC or anti–IL-2-PE (BD Biosciences), washed, and fixed in PBS 1X – 0.5% BSA. Flow cytometric analysis was performed immediately using a FACScalibur (Becton Dickinson). Data files contained 75,000 events positive for CD8-PerCP fluorescence per lymphocyte gate. The frequency of IFN-γ+ cells within each CD8+ lymphocyte gate was assessed with Cellquest software (Becton Dickinson); results were considered positive if >0.1% of the CD8+ cells within the CD8+ gate were IFN-γ–positive after the negative control values were subtracted. A mean of 68 ± 18% CD8 cells was found to be responsible for the IFN-γ production within lymphocytes.
Statistical analysis.
Exact nonparametric two-tailed tests from SPSS 12.0.1.0 for windows from SPSS Inc. were used. The Wilcoxon signed-rank test was used to compare the frequency of recognition of each pool, and the nonparametric Mann-Whitney test was used to analyze differences between the groups. P < 0.05 was considered to be statistically significant.
Acknowledgments
We would like to thank D. Olive (Centre Paoli Calmettes, Marseille, France) who coordinates this INSERM-ATC and his colleagues who prepared all peptide samples; Dr. E. Robinet (Etablissement Français du Sang, Besançon, France) who kindly provided the cell samples from healthy HCMV-infected blood bank donors to the INSERM-ATC; the study participants; the coinvestigators of the RESTIMOP-ANRS (M. Jouan, M. Saves, R. Tubiana, C. Aubron-Olivier, M. Mciri, B. Senechal, G. Chene, C, Tural, S. Lasry) Clinical Trial and of the ALT-ANRS Cohort (I. Theodorou, J.P. Clauvel, D. Sicard, H. Agut, P. Debré, C. Rouzioux). We also thank J.A. Cahn for help with manuscript preparation.
This work was supported by the INSERM Action Thématique Concertée (ATC) on Immunity to Infections, which provided the HCMV-IE1 and pp65 peptides, and by a gift from Mr. and Mrs. Lagadec.
The authors have no conflicting financial interests.
Abbreviations used: AHCMV, active human CMV; HAART, highly active antiretroviral therapy; HCMV, human CMV; IE1, immediate early 1; LTNP, long-term nonprogressor; LTRR, long-term recovery from retinitis; SFC, spot-forming cell; STRR, short-term recovery from retinitis.
References
- 1.Mocarski, E.S., and C.T. Courcelle. 2001. Cytomegalovirus and their replication. Virology. 4th ed. B. Fields, D.M. Knipe, and P. Howley, editors. Raven Press, New York. 2626–2673.
- 2.Pertel P., R. Hirschtick, J. Phair, J. Chmiel, L. Poggensee, and R. Murphy. 1992. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodefiency virus. J. Acquir. Immune Defic. Synd. 5:1069–1074. [PubMed] [Google Scholar]
- 3.Gallant J.E., R.D. Moore, D.D. Richman, J. Keruly, and R.E. Chaisson. 1992. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J. Infect. Dis. 166:1223–1227. [DOI] [PubMed] [Google Scholar]
- 4.Deayton J.R., A. Mocroft, P. Wilson, V.C. Emery, M.A. Johnson, and P.D. Griffiths. 1999. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS. 13:1203–1206. [DOI] [PubMed] [Google Scholar]
- 5.O'Sullivan C.E., W.L. Drew, D.J. McMullen, R. Miner, J.Y. Lee, R.A. Kaslow, J.G. Lazar, and M.S. Saag. 1999. Decrease of cytomegalovirus replication in human immunodeficiency virus infected-patients after treatment with highly active antiretroviral therapy. J. Infect. Dis. 180:847–849. [DOI] [PubMed] [Google Scholar]
- 6.Jouan M., M. Saves, R. Tubiana, G. Carcelain, N. Cassoux, C. Aubron-Olivier, A.M. Fillet, M. Nciri, B. Senechal, G. Chene, et al., RESTIMOP Study Team. 2001. Discontinuation of maintenance therapy for cytomegalovirus retinitis in HIV infected patients receiving highly active antiretroviral therapy. AIDS. 15:23–31. [DOI] [PubMed] [Google Scholar]
- 7.Zaia J.A., J.G. Sissons, S. Riddell, D.J. Diamond, M.R. Wills, A.J. Carmichael, M.P. Weekes, M. Gandhi, C. LaRosa, M. Villacres, et al. 2000. Status of cytomegalovirus prevention and treatment in 2000. Hematol. (Am. Soc. Hematol. Educ. Program). 339–355. [DOI] [PubMed]
- 8.Reddehase M.J., F. Weiland, K. Munch, S. Jonjic, A. Luske, and U.H. Koszinowski. 1985. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 55:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddel S.R., K.S. Watanabe, J.M. Goodrich, C.R. Li, M.E. Agha, and P.D. Greenberg. 1992. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T-cell clones. Science. 257:238–241. [DOI] [PubMed] [Google Scholar]
- 10.Weekes M.P., M.R. Wills, K. Mynard, A.J. Carmichael, and J.G. Sissons. 1999. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J. Virol. 73:2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan D.C. 2001. Cytomegalovirus in renal transplantation. J. Am. Soc. Nephrol. 12:848–855. [DOI] [PubMed] [Google Scholar]
- 12.Borysiewicz L.K., J.K. Hickling, S. Graham, J. Sinclair, M.P. Cranage, G.L. Smith, and J.G. Sissons. 1988. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J. Exp. Med. 168:919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provenzano M., S. Mocellin, M. Bettinotti, J. Preuss, V. Monsurro, F.M. Marincola, and D. Stroncek. 2002. Identification of immune dominant cytomegalovirus epitopes using quantitative real-time polymerase chain reactions to measure interferon-gamma production by peptide stimulated peripheral blood mononuclear cells. J. Immunother. 25:342–351. [DOI] [PubMed] [Google Scholar]
- 14.Wills M.R., A.J. Carmichael, K. Mynard, X. Jin, M.P. Weekes, B. Plachter, and J.G. Sissons. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mc Laughlin-Taylor E., H. Pande, S.J. Forman, B. Tanamachi, C.R. Li, J.A. Zaia, P.D. Greenberg, and S.R. Riddell. 1994. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J. Med. Virol. 43:103–110. [DOI] [PubMed] [Google Scholar]
- 16.Kern F., T. Bunde, N. Faulhaber, F. Kiecker, E. Khatamzas, I.M. Rudawski, A. Pruss, J.W. Gratama, R. Volkmer-Engert, R. Ewert, et al. 2002. Cytomegalovirus phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J. Infect. Dis. 185:1709–1716. [DOI] [PubMed] [Google Scholar]
- 17.Papanicolaou G.A., J.B. Latouche, C. Tan, J. Dupont, J. Stiles, E.G. Pamer, and M. Sadelain. 2003. Rapid expansion of cytomegalovirus-specific cytotoxic T lymphocytes by artificial antigen-presenting cells expressing a single HLA allele. Blood. 102:2498–2505. [DOI] [PubMed] [Google Scholar]
- 18.Diamond D.J., J. York, J.Y. Sun, C.L. Wright, and S.J. Forman. 1997. Development of candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus. Blood. 90:1751–1767. [PubMed] [Google Scholar]
- 19.Gratama J.W., J.W. van Esser, C.H. Lamers, C. Tournay, B. Lowenberg, R.L. Bolhuis, and J.J. Cornelissen. 2001. Tetramer-based quantification of cytomegalovirus-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 98:1358–1364. [DOI] [PubMed] [Google Scholar]
- 20.Masuoka M., T. Yoshimuta, M. Hamada, M. Okamoto, T. Fumimori, J. Honda, K. Oizumi, and K. Itoh. 2001. Identification of the HLA A24 peptide epitope within cytomegalovirus protein pp65 recognized by CMV-specific cytotoxic T lymphocytes. Viral Immunol. 14:369–377. [DOI] [PubMed] [Google Scholar]
- 21.Kern F., I.P. Surel, N. Faulhaber, C. Frommel, J. Schneider-Mergener, C. Schonemann, P. Reinke, and H.D. Volk. 1999. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prod'homme V., C. Retiere, B.M. Imbert-Marcille, M. Bonneville, and M.M. Hallet. 2003. Modulation of HLA-A*0201-restricted T cell response by natural polymorphism in the IE1 (315-324) epitope of human cytomegalovirus. J. Immunol. 170:2030–2036. [DOI] [PubMed] [Google Scholar]
- 23.Kondo E., Y. Akatsuka, K. Kuzushima, K. Tsujimura, S. Asakura, K. Tajima, Y. Kagami, Y. Kodera, M. Tanimoto, Y. Morishima, and T. Takahashi. 2004. Identification of novel CTL epitopes of CMV-pp65 presented by a variety of HLA alleles. Blood. 103:630–638. [DOI] [PubMed] [Google Scholar]
- 24.Elkington R., S. Walker, T. Crough, M. Menzies, J. Tellam, M. Bharadwaj, and R. Khanna. 2003. Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. J. Virol. 77:5526–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appay V., P.R. Dunbar, M. Callan, P. Klenerman, G.M. Gillespie, L. Papagno, G.S. Ogg, A. King, F. Lechner, C.A. Spina, et al. 2002. Memory CD8+T cells vary in differentiation phenotype in different persistent virus infections. Nature Med. 8:379–385. [DOI] [PubMed] [Google Scholar]
- 26.Weekes M.P., M.R. Wills, K. Mynard, R. Hicks, J.G. Sissons, and A.J. Carmichael. 1999. Large clonal expansions of human virus-specific memory T-lymphocytes in the CD57+ CD28-CD8+ T cell populations. Immunology. 98:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamman D., P.A. Baars, M.H. Rep, B. Hooibrink, S.R. Kerkhof-Garde, M.R. Klein, and R.A. van Lier. 1997. Phenotypic and functional separation of memory and effectors human CD8+ T cells. J. Exp. Med. 186:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Lier R.A.W., J. Borst, T.M. Vroom, H. Klein, P. Van Mourik, W.P. Zeijlemaker, and C.J. Melief. 1987. Tissue distribution and biochemical and functional properties of CD27, a novel T cell differentiation antigen. J. Immunol. 139:1589–1596. [PubMed] [Google Scholar]
- 29.Gonzalo J.A., T. Delaney, J. Corcoran, A. Goodearl, J.C. Gutierrez-Ramos, and A.J. Coyle. 2001. Cutting edge: the related molecules CD28 and inductible costimulator deliver both unique and complementary signals required for optimal T cell activation. J. Immunol. 166:1–5. [DOI] [PubMed] [Google Scholar]
- 30.De Rosa S.C., L.A. Herzenberg, and M. Roederer. 2001. 11-color, 13-parameter flow cytometry: identification of human naïve T-cells by phenotype, function and T-cell receptor diversity. Nature Med. 7:245–249. [DOI] [PubMed] [Google Scholar]
- 31.Van Lier R.A.W., I.J. Ten Berge, and L.E. Gamadia. 2003. Human CD8+T-cell differentiation in response to viruses. Nat. Rev. Immunol. 3:931–938. [DOI] [PubMed] [Google Scholar]
- 32.Kern F., N. Faulhaber, C. Frommel, E. Khatamzas, S. Prosch, C. Schonemann, I. Kretzschmar, R. Volkmer-Engert, H.D. Volk, and P. Reinke. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 30:1676–1682. [DOI] [PubMed] [Google Scholar]
- 33.Solache A., C.L. Morgan, A.I. Dodi, C. Morte, I. Scott, C. Baboonian, B. Zal, J. Goldman, J.E. Grundy, and J.A. Madrigal. 1999. Identification of 3 HLA-A*0201 restricted cytotoxic T cell epitopes in the cytomegalovirus protein pp65 that are conserved between eight strains of virus. J. Immunol. 163:5512–5518. [PubMed] [Google Scholar]
- 34.Gamadia L.E., E.B. Remmerswaal, J.F. Weel, F. Bemelman, R.A. van Lier, and I.J. Ten Berge. 2003. Primary immune responses to human CMV: a critical role of IFN-γ-producing CD4+ T cells in protection against CMV disease. Blood. 101:2686–2692. [DOI] [PubMed] [Google Scholar]
- 35.Shedlock D.J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 300:337–339. [DOI] [PubMed] [Google Scholar]
- 36.Johansen P., P. Stamou, R.E. Tascon, D.B. Lowrie, and B. Stockinger. 2004. CD4 T cells guarantee optimal competitive fitness of CD8 memory T cells. Eur. J. Immunol. 34:91–97. [DOI] [PubMed] [Google Scholar]
- 37.Deayton J., A. Mocroft, P. Wilson, V.C. Emery, M.A. Johnson, and P.D. Griffiths. 1999. Loss of cytomegalovirus viremia following highly active antiretroviral therapy in the absence of specific anti-CMV therapy. AIDS. 13:1203–1206. [DOI] [PubMed] [Google Scholar]
- 38.O'Sullivan C.E., L. Drew, D.J. McCullen, R. Miner, J.Y. Lee, R.A. Kaslow, J.G. Lazar, and M.S. Saag. 1999. Decrease of cytomegalovirus replication in human immunodeficiency virus infected patients after treatment with highly active antiretroviral therapy. J. Infect. Dis. 180:847–849. [DOI] [PubMed] [Google Scholar]
- 39.Boivin G., and R.P. Leblanc. 2000. Clearance of cytomegalovirus viraemia after initiation of highly active antiretroviral therapy. J. Infect. Dis. 181:1216–1218. [DOI] [PubMed] [Google Scholar]
- 40.Bunde, T., A. Kirchner, B. Hoffmeister, D. Habedank, R. Hetzer, G. Cherepnev, S. Proesch, P. Reinke, H.D. Volk, H. Lehmkuhl, and F. Kern. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1–specific CD8 T cells. J. Exp. Med. 201:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daly K., P. Nguyen, D.L. Woodland, and M.A. Blackman. 1995. Immunodominance of major histocompatibility complex class-I restricted influenza virus epitopes can be influenced by the T cell receptor repertoire. J. Virol. 69:7416–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowe S.R., S.J. Turner, S.C. Miller, A.D. Roberts, R.A. Rappolo, P.C. Doherty, K.H. Ely, and D.L. Woodland. 2003. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infection. J. Exp. Med. 198:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champagne P., G.S. Ogg, A.S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G.P. Rizzardi, S. Fleury, M. Lipp, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111. [DOI] [PubMed] [Google Scholar]
- 44.Monteiro J., F. Batliwalla, H. Ostrer, and P.K. Gregersen. 1996. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply replicative history that distinct from their CD28+CD8+ counterparts. J. Immunol. 156:3587–3590. [PubMed] [Google Scholar]
- 45.Lloyd D.E., L. Yang, D.N. Tang, T. Bennett, W. Schober, and D.E. Lewis. 1997. Regulation of CD28 costimulation in humans CD8+ T cells. J. Immunol. 158:1551–1558. [PubMed] [Google Scholar]
- 46.Mollet L, B. Sadat-Sowti, J. Duntze, V. Leblond, F. Bergeron, V. Calvez, C. Katlama, P. Debré, and B. Autran. 1998. CD8hi+CD57+ T lymphocytes are enriched in antigen-specific T cells capable of down-modulating cytotoxic activity. Int. Immunol. 10:311–323. [DOI] [PubMed] [Google Scholar]
- 47.Brenchley J.M., N.J. Karandikar, M.R. Betts, D.R. Ambrozak, B.J. Hill, L.E. Crotty, J.P. Casazza, J. Kuruppu, S.A. Migueles, M. Connors, et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 101:2711–2720. [DOI] [PubMed] [Google Scholar]
- 48.Papagno L., C.A. Spina, A. Marchant, M. Salio, N. Rufer, S. Little, T. Dong, G. Chesney, A. Waters, P. Easterbrook, et al. 2004. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biology. 2:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalams S.A., and B.D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harari A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 103:966–971. [DOI] [PubMed] [Google Scholar]
- 51.Zhang D., P. Shankar, Z. Xu, B. Harnisch, G. Chen, C. Lange, S.J. Lee, H. Valdez, M.M. Lederman, and J. Lieberman. 2003. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood. 101:226–235. [DOI] [PubMed] [Google Scholar]
- 52.Candotti D., D. Costagliola, C. Joberty, O. Bonduelle, C. Rouzioux, B. Autran, and H. Agut. 1999. Status of long-term asymptomatic HIV-1 infection correlates with viral load but not with virus replication properties and cell tropism. French ALT Study Group. J. Med. Virol. 5:256–263. [PubMed] [Google Scholar]
- 53.Maecker H.T., H.S. Dunn, M.A. Suni, E. Khatamzas, C.J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T.M. Fu, E. Sinclair, et al. 2001. Use of overlapping peptide mixtures as antigen for cytokine flow cytometry. J. Immunol. Methods. 255:27–40. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y., E. Iglesias, A. Samri, G. Kamkamidze, T. Decoville, G. Carcelain, and B. Autran. 2003. A systematic comparison of methods to quantify HIV-1 specific CD8 T cells. J. Immunol Methods. 272:23–34. [DOI] [PubMed] [Google Scholar]
- 55.Kern F., I.P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, R. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Rabbruch, P. Walden, and H.D. Volk. 1998. T cell epitope mapping by flow cytometry. Nature Med. 4:975–978. [DOI] [PubMed] [Google Scholar]
- 56.Jacobson M.A., H.T. Maecker, P.L. Orr, R. D'Amico, M. Van Natta, X.D .Li, R.B. Pollard, and B.M. Bredt; Adult AIDS Clinical Trials Group and the Studies of Ocular Complications of AIDS Research Group. 2004. Results of cytomegalovirus-specific CD8+/interferon-γ cytokine flow cytometry assay correlate with clinical evidence of protective immunity in patients with AIDS with CMV retinitis. J. Infect. Dis. 189:1362–1373. [DOI] [PubMed] [Google Scholar]