Abstract
Recent reports have indicated that the cysteine protease activity of Der p 1 may play a significant role in its ability to elicit IgE antibody responses, mainly through cleavage of membrane CD23 on B cells and interleukin (IL)-4 synthesis and secretion from mast cells and basophils. Here we demonstrate for the first time that Der p 1 also cleaves the α subunit of the IL-2 receptor (IL-2R or CD25) from the surface of human peripheral blood T cells and, as a result, these cells show markedly diminished proliferation and interferon γ secretion in response to potent stimulation by anti-CD3 antibody. Given that the IL-2R is pivotal for the propagation of Th1 cells, its cleavage by Der p 1 may consequently bias the immune response towards Th2 cells, thereby creating an allergic microenvironment.
We (1, 2) and others (3) have recently demonstrated that Der p 1, a major allergen of the house dust mite Dermatophagoides pteronyssinus, proteolytically cleaves CD23 (the low affinity receptor for IgE, also known as FcεRII) from the surface of cultured human B cells. Given that CD23 is involved in the negative feedback regulation of in vivo IgE synthesis (4–6), its cleavage by Der p 1 could potentially disrupt the IgE regulatory mechanism, thereby leading to excessive IgE synthesis.
Der p 1 was also shown in vitro to induce mast cell and basophil degranulation, and stimulate IL-4 synthesis and secretion in the absence of IgE (7). These actions of Der p 1, which were due to its proteolytic activity, are particularly significant, since mast cells and basophils can support IgE synthesis by secreting IL-4 and IL-13 and provide contact-mediated help through CD40L (8, 9). Therefore, the protease activity of Der p 1 not only subverts an important mechanism that controls IgE synthesis, namely the negative feedback regulation by membrane CD23, it apparently also provides a cytokine environment conducive for IgE synthesis.
These observations raise the question of whether the enzymatic activity of Der p 1 also perturbs human T cells with respect to favoring the emergence of a Th2 background, which would create an allergic microenvironment. In this paper, we demonstrate that Der p 1 cleaves CD25, the 55-kD α subunit of the IL-2 receptor (the high-affinity form of which consists of α, β, and γ subunits), from the surface of peripheral blood T cells and, as a result, these cells show markedly diminished proliferation and IFN-γ secretion in response to potent stimulation by anti-CD3 antibody. Given that the IL-2 receptor is pivotal for the propagation of Th1 cells (10), its cleavage by Der p 1 may consequently bias the immune response towards Th2 cells, which instead depend on the IL-4 receptor for their propagation.
Materials and Methods
Antibody Reagents.
The following PE- or FITC-labeled mouse monoclonal antibodies to human cell surface markers were purchased: anti-CD25 (ACT-1), anti-CD4 (MT310), anti-CD45RO (UCHL1), anti-CD2 (MT910), anti-CD3 (UCHT-1), anti-CD8 (DK25; Dako, Buckinghamshire, UK) and anti-CD69 (TP1.55.3; Immunotech, Luton, UK). Anti-CD3 antibody was purified on protein G from the supernatant of a hybridoma cell line (OKT3; ECACC, Porton Down, UK).
Der p 1 Preparation.
Der p 1 was purified from lyophilized house dust mite culture supernatant (SmithKline Beecham Pharmaceuticals, Worthing, UK) by affinity chromatography using monoclonal anti–Der p 1 antibody (4C1; Indoor Biotechnologies, Clwyd, UK) as previously described (11). The affinity-purified Der p 1 was then passed through a soybean trypsin inhibitor column to remove traces of serine protease activity. The purity of the preparation was assessed by silver stain SDS-PAGE (12% gel) analysis and its identity was confirmed by NH2-terminal sequencing on an automatic amino acid sequencer (Applied Biosystems, Foster City, CA); the sequence obtained (TNACSINGNA) matched the previously published sequence of Der p 1 (12).
CD25 Cleavage Assays.
PBLs were purified from 20 ml of heparinized whole blood on a discontinuous Histopaque density gradient (Sigma Chemical Company, Poole, UK). Cells (5 × 105) were suspended in serum-free AIM V medium (Gibco Life Technologies Ltd., Paisley, UK) in flat-bottomed, 24-well plates and were stimulated with PHA (5 μg/ml final concentration) for 3 d at 37°C in a humidified atmosphere of 5% CO2. CD25 cleavage was performed by incubating 105 cells with Der p 1 (preactivated with 5 mM cysteine) in a total volume of 200 μl AIM V medium for 1 h at 37°C. The cells were centrifuged and the supernatant was tested for soluble CD25 concentration by ELISA (R & D Systems, Abingdon, UK). The cells were then resuspended in PBS containing 0.5% BSA and 0.1% azide, stained with PE-labeled anti-CD25 antibody for 30 min at room temperature in the dark, and fixed with 5% formaldehyde. The expression of other T cell surface markers (i.e., CD2, CD3, CD4, CD8, CD45RO, and CD69) was monitored in the same way using the appropriate PE- or FITC-labeled antibodies. Cells were analyzed on a FACScan® (Becton Dickinson, Mountain View, CA) with a linear fluorescence setting of 660 volts. The fluorescence (FL1 or FL2) profile versus forward scatter was used to monitor the cells, and the amplification scale was altered according to the level of fluorescence. For each sample, 5,000 events were collected and then analyzed using the flowMATE program (DAKO, High Wycombe, UK).
Anti-CD3–Induced T Cell Proliferation Assay.
PBLs were prepared from heparinized whole blood of nonallergic volunteers as described above. Anti-CD3 antibody (0.2 μg/ml final concentration) was added in AIM V medium to round-bottomed, 96-well plates. After 1 h, 105 lymphocytes were added to each well and incubated overnight at 37°C in a humidified atmosphere of 5% CO2. On the next day (unless otherwise stated), Der p 1 (with or without preactivation with 5 mM cysteine) was added to the cell cultures and incubation continued for another 3 d. 16 h before termination of the culture, [6-3H]thymidine (specific activity 24.0 Ci/mmol; Amersham Life Science, Buckingham, UK) was added to each well at a final concentration of 4 μCi/ml. Cells were then transferred to Unifilter-96 plate GF/C and radioactivity was counted in scintillation fluid (Microscint O) using a top counter (both from Canberra Packard Limited, Pangbourne, UK). With some blood samples, parallel cultures were carried out for cytokine (IL-2, IL-4, and IFN-γ) measurements using Quantikine ELISA kits (R & D Systems).
To exclude cellular cytotoxicity of Der p 1, the number of apoptotic and necrotic cells were determined using the Annexin V/FITC kit (Boehringer Ingelheim Bioproducts Partnership, Heidelberg, Germany).
Results and Discussion
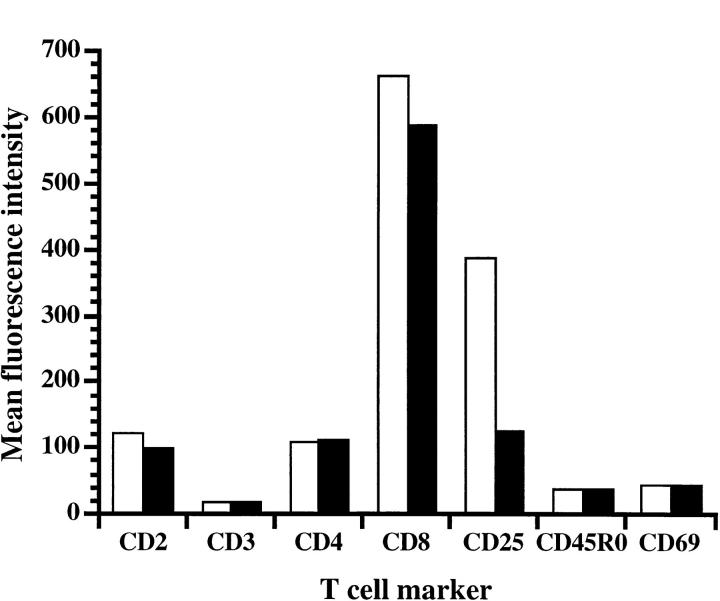
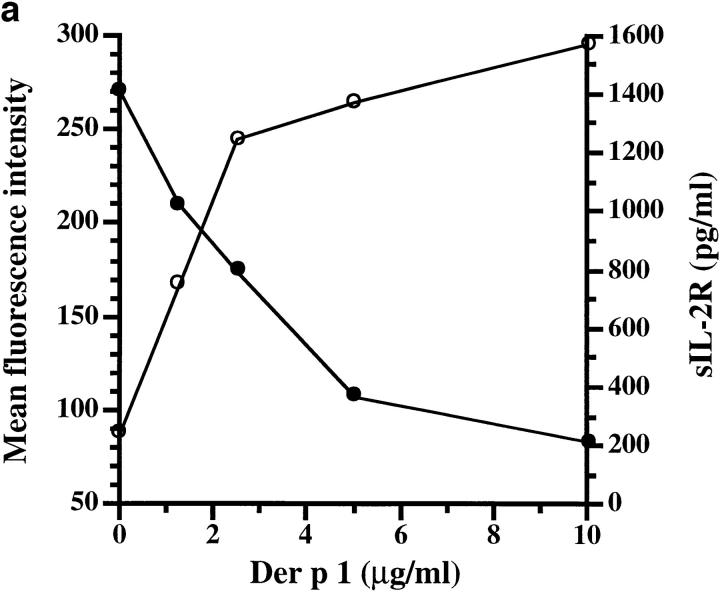
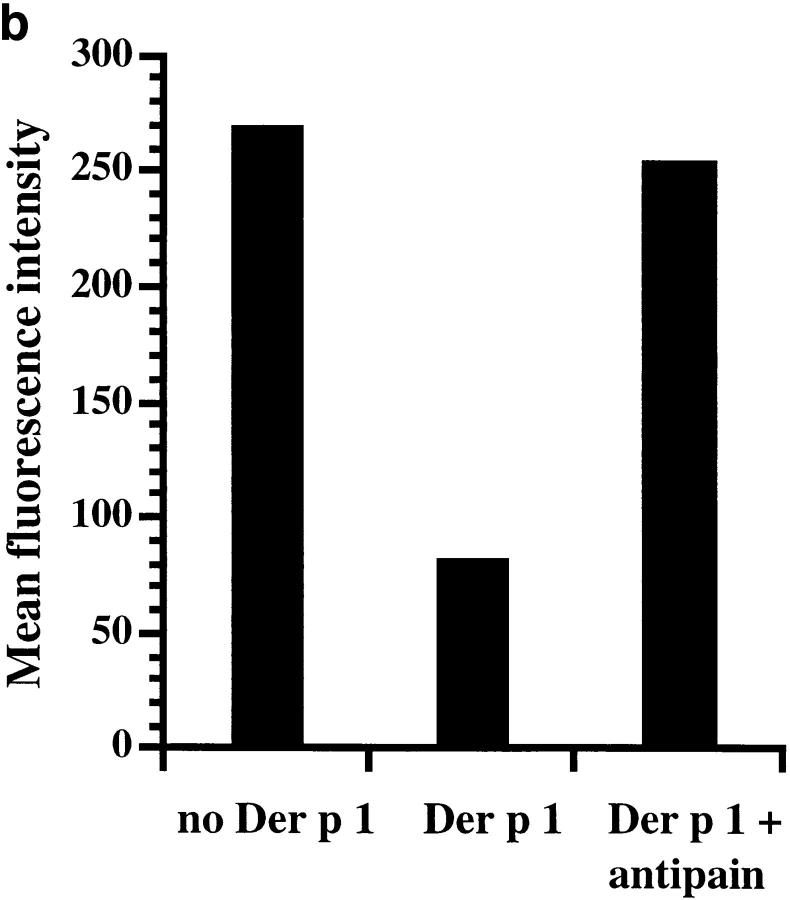
We have affinity purified Der p 1 from dust mite extract and confirmed its identity by NH2-terminal sequencing. The Der p 1 preparation was tested for its ability to proteolytically cleave functionally important molecules, including CD25, expressed on cultured human T cells. The data show that Der p 1 cleaves CD25, but not CD2, CD3, CD4, CD8, CD45RO, or CD69 (Fig. 1). The cleavage of CD25 by Der p 1 was associated with the release of soluble CD25 into the culture supernatant (Fig. 2 a). This indicates that Der p 1 causes limited digestion of CD25, since the CD25 fragment released was detectable with antibody reagents used for measuring spontaneously shed CD25. The cleavage of CD25 was inhibited by antipain, a low molecular weight protease inhibitor of microbial origin, thus confirming that the cleavage of CD25 was due to the proteolytic activity of Der p 1 (Fig. 2 b).
Figure 1.
The proteolytic effect of Der p 1 on human T cell surface markers. Paired results represent the expression of markers in the absence (open bars) and presence (solid bars) of Der p 1 (5 μg/ml). Data presented are the means of duplicate experiments; SE was <5%.
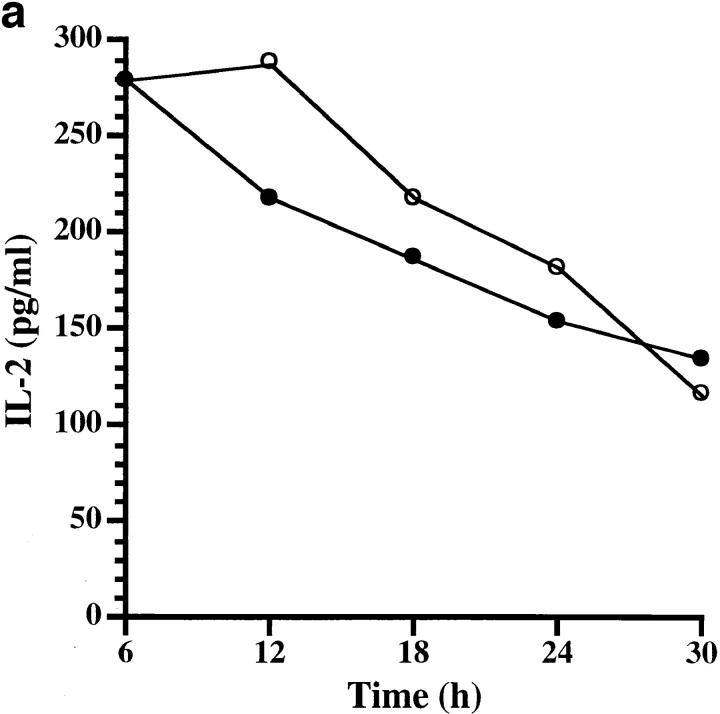
Figure 2.
(a) Der p 1–induced cleavage of membrane CD25 (filled circles) and concomitant release of soluble CD25 (open circles). (b) CD25 cleavage is blocked by earlier treatment of Der p 1 (5 μg/ml) with antipain (4 μM). Data presented in a and b are the means of duplicate experiments; SE was <5%.
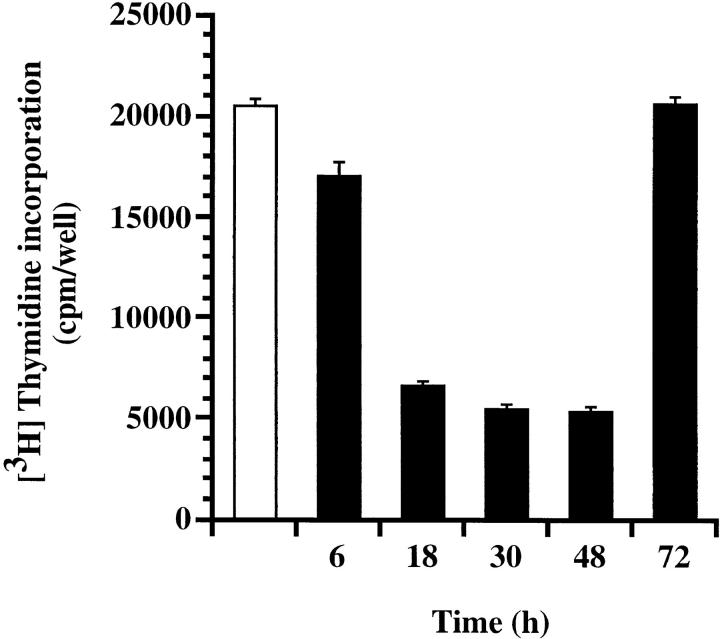
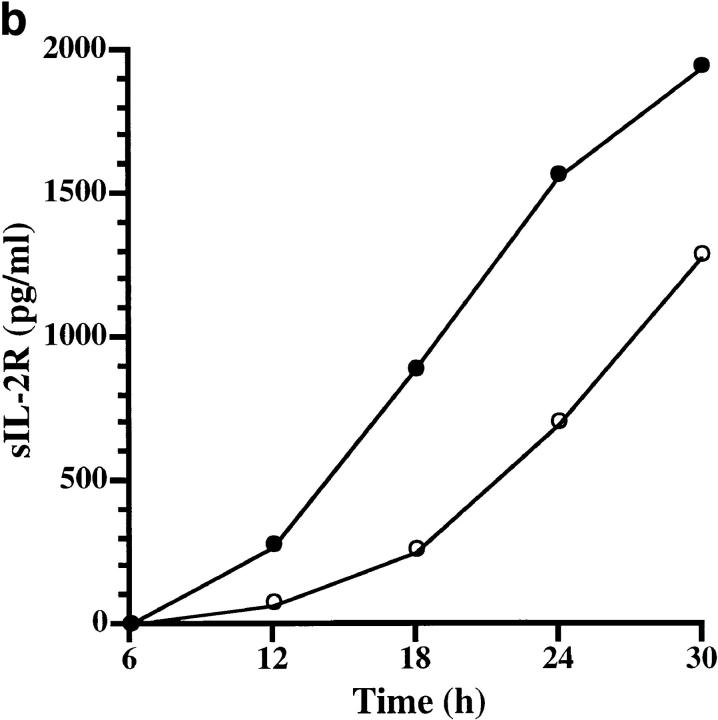
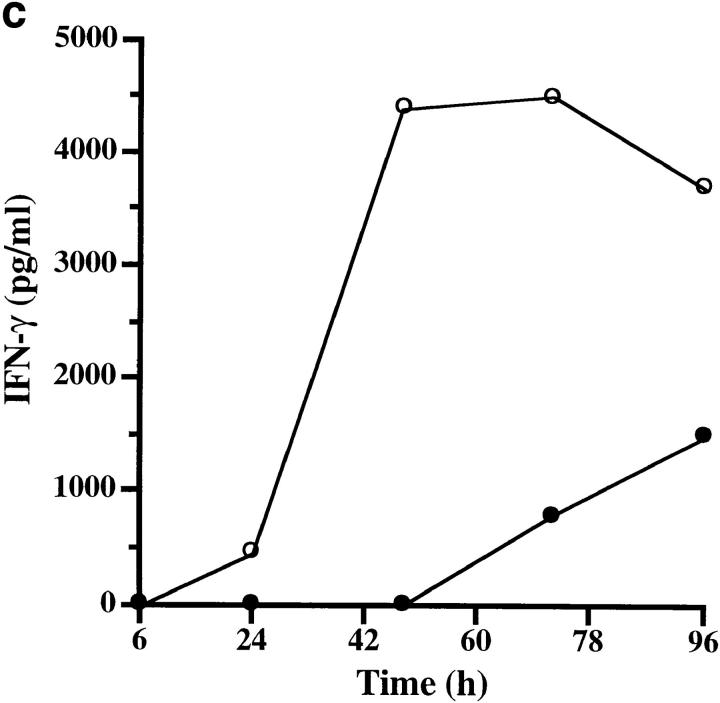
To assess the biological consequences of Der p 1–induced CD25 cleavage, we conducted an IL-2R–dependent T cell proliferation assay. This was carried out by stimulating human T cells with anti-CD3, which is known to induce T cell proliferation through IL-2 production and IL-2R expression (13). Der p 1–treated cultures showed up to 61% decrease in T cell proliferation, an effect that was due to the enzymatic activity of Der p 1 (Table 1). This action of Der p 1 was most effective within 18–48 h of culture initiation (Fig. 3), and appeared to coincide with the time course of CD25 expression (14). To further test the hypothesis that Der p 1–induced suppression of T cell proliferation is due to CD25 cleavage, we examined the kinetics of IL-2, IL-4, and IFN-γ production and soluble CD25 release during this window of Der p 1 action. We found that the early peak of IL-2 production, namely within 6–24 h of culture initiation, was not significantly affected by Der p 1 (Fig. 4 a), thereby indicating that the Der p 1–induced suppression of T cell proliferation was not due to decreased IL-2 production or its cleavage by Der p 1. On the other hand, Der p 1–treated cultures showed marked release of soluble CD25 as demonstrated by a shift in the soluble CD25 release curve (Fig. 4 b). Cleavage of CD25 by Der p 1 clearly renders the T cells unresponsive to the proliferative effect of IL-2, as manifested by a decrease of at least fourfold in IFN-γ production (Fig. 4 c). Taken together, our data suggest that the inhibition of T cell proliferation was indeed due to the cleavage of CD25, since it has been shown that T cell proliferation, apart from depending on the concentration of IL-2, depends on the level of IL-2R expression and the contact time between IL-2 and IL-2R (15). At the time points studied, there was very little IL-4 detectable in the T cell cultures (data not shown).
Table 1.
Inhibition of IL-2–mediated Human T Cell Proliferation by Der p 1 Requires Enzymatically Active Protease (i.e., Preactivated with Cysteine)
| CPM (mean ± SEM) | ||||
|---|---|---|---|---|
| Cell culture containing | With cysteine | Without cysteine* | ||
| no anti-CD3 | 429 ± 22 | 432 ± 26 | ||
| anti-CD3 | 68,322 ± 2,287 | 76,212 ± 1,076 | ||
| anti-CD3 + Der p 1 (0.15 μg/ml) | 63,272 ± 1,902 (7) | 74,824 ± 1,226 (2) | ||
| anti-CD3 + Der p 1 (0.6 μg/ml) | 55,884 ± 1,590 (18) | 72,104 ± 1,636 (5) | ||
| anti-CD3 + Der p 1 (2.5 μg/ml) | 35,835 ± 849 (47) | 67,652 ± 1,125 (11) | ||
| anti-CD3 + Der p 1 (10 μg/ml) | 26,615 ± 149 (61) | 64,593 ± 1,383 (15) | ||
Figures in parentheses represent percentage of inhibition.
Omission of cysteine was used in preference to Der p 1 inhibition by antipain, as the latter was found to interfere with the proliferation assay.
Figure 3.
Der p 1 suppresses human T cell proliferation mostly within 18–48 h of culture initiation. Results represent cultures with no Der p 1 (open bar) or those to which Der p 1 (10 μg/ml) was added at different time points (solid bars). The thymidine label was added 80 h after culture initiation. Data presented are the means of quadruplicate experiments; error bars represent SEM.
Figure 4.
Effect of Der p 1 (10 μg/ml; added 6 h after culture initiation) on the time course of Th1 cytokine expression and soluble CD25 release. Data represent the kinetics of IL-2 expression (a) soluble CD25 release (b), and IFN-γ expression (c) in the absence (open circles) and presence (filled circles) of Der p 1. Data presented are the means of duplicate experiments; SE was <5%.
Given that the CD25 molecule is critical to the anti-CD3 model of T cell proliferation (13), our data show that Der p 1–induced CD25 cleavage has a significant downregulatory effect on this aspect of T cell function. These findings raise the question of the role of the enzymatic activity of Der p 1 in perturbing the homeostatic control of the human Th1-like/Th2-like subset distribution. The Th1 and Th2 cell populations each enhance the development of cells of the same subset while suppressing the propagation of those of the other subset. Within this cytokine driven process of polarization of T cell development, IL-2, along with IFN-γ, and IL-4 are considered to be autocrine growth factors for the Th1 and Th2 cells, respectively (10). Therefore, Der p 1–induced cleavage of CD25 is likely to lead to impaired development of cells of the Th1 subset and consequent augmentation of those of the Th2 subset. For instance, it has been shown that mite allergen–specific T cell clones exhibit Th2 cytokine profile (16) and require IL-4 for their optimal growth (17), which means that in an ongoing allergic response to Der p 1, such Th2 cells will not be sensitive to the enzymatic activity of Der p 1 compared to IL-2–dependent Th1 cells. Clearly, it would have been of interest to also monitor the effect of Der p 1 on the IL-4 receptor (CD124), but, due to low levels of expression of this receptor on peripheral blood T cells, we were unable to carry out this experiment.
A number of studies indicate that Th2 responses are involved in the pathogenesis of allergic diseases (18), mainly through the secretion of IL-4/IL-13 and IL-5, which are responsible for stimulating IgE synthesis and eosinophil function, respectively. It is also highly relevant in this connection that IL-2 and IL-2Rα knockout mice show enhanced synthesis of immunoglobulins including IgE (19), a finding which is consistent with uncontrolled Th2 cell propagation. Recent reports indicate that the protease activity of Der p 1 plays a significant role in its ability to elicit IgE responses, mainly through cleavage of membrane CD23 on B cells (1–3) and IL-4 synthesis and secretion from mast cells and basophils (7). The data described here suggest yet another mechanism by which Der p 1 uses its protease activity to bias the immune response in favor of IgE, this time by preferentially targeting a molecule (i.e., the IL-2Rα subunit) which plays a pivotal role in the development and propagation of Th1 cells, thus favoring the emergence and dominance of Th2 responses (10).
Acknowledgments
The authors wish to thank Alison Galvin for her help with flow cytometry.
Footnotes
O. Schulz is a recepient of a University of Nottingham/Peptide Therapeutics joint PhD studentship.
References
- 1.Schulz O, Laing P, Sewell HF, Shakib F. Der pI, a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23) Eur J Immunol. 1995;25:3191–3194. doi: 10.1002/eji.1830251131. [DOI] [PubMed] [Google Scholar]
- 2.Schulz O, Sutton BJ, Beavil RL, Shi J, Sewell HF, Gould HJ, Laing P, Shakib F. Cleavage of the low affinity receptor for human IgE (CD23) by a mite cysteine protease: nature of the cleaved fragment in relation to the structure and function of CD23. Eur J Immunol. 1997;27:584–588. doi: 10.1002/eji.1830270303. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt CRA, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flores-Romo L, Shields J, Humbert Y, Graber P, Aubry J-P, Gauchat J-F, Ayala G, Allet B, Chavez M, Bazin H, Capron M, Bonnefoy J-Y. Inhibition of an in vivo antigen-specific IgE response by antibodies to CD23. Science. 1993;261:1038–1041. doi: 10.1126/science.8351517. [DOI] [PubMed] [Google Scholar]
- 5.Yu P, Kosco-Vilbois M, Richards M, Kohler G, Lamers MC. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994;369:753–756. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]
- 6.Lamers MC, Yu P. Lessons from the study of IgE transgenic and CD23-deficient mice. Immunol Rev. 1995;148:71–95. doi: 10.1111/j.1600-065x.1995.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 7.Machado DC, Horton D, Harrop R, Peachell PT, Helm BA. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur J Immunol. 1996;26:2972–2980. doi: 10.1002/eji.1830261224. [DOI] [PubMed] [Google Scholar]
- 8.Gauchat J-F, Henchoz S, Mazzel G, Aubry J-P, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L, Thompson J, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–343. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 9.Yanagihara Y, Kajiwara K, Basaki Y, Ikizawa K, Akiyama K, Saito H. Induction of human IgE synthesis in B cells by a basophilic cell line, KU812. Clin Exp Immunol. 1997;108:295–301. doi: 10.1046/j.1365-2249.1997.d01-1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 11.Lombardero M, Heymann PW, Platts-Mills TAE, Fox JW, Chapman MD. Conformational stability of B cell epitopes on Group I and Group II Dermatophagoidesspp. allergens. J Immunol. 1990;144:1353–1360. [PubMed] [Google Scholar]
- 12.Chua KY, Stewart GA, Thomas WR, Simpson RJ, Dilworth RJ, Plozza TM, Turner KJ. Sequence analysis of cDNA coding for a major house dust mite allergen, Der pI. Homology with cysteine proteases. J Exp Med. 1988;167:175–182. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meuer SC, Hussey RE, Cantrell DA, Hodgdon JC, Schlossnan SF, Smith KA, Reinherz EL. Triggering of the T3–Ti antigen–receptor complex results in clonal T-cell proliferation through an interleukin 2–dependent autocrine pathway. Proc Natl Acad Sci USA. 1984;81:1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin LA, Kurman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, Nelson DL. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172–3177. [PubMed] [Google Scholar]
- 15.Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 16.Yssel H, Johnson KE, Schneider PV, Wideman J, Terr A, Kastelein R, de Vries JE. T cell activation-inducing epitopes of the house dust mite allergen Der p I: proliferation and lymphokine production patterns by Der p I–specific CD4+T cell clones. J Immunol. 1992;148:738–745. [PubMed] [Google Scholar]
- 17.Michael BN, Kalish RS. House dust mite–responsive human T cells require both interleukin 2 (IL-2) and interleukin 4 for optimal proliferation, whereas IL-2 alone is sufficient for proliferation of tetanus toxoid–responsive T cells. Cell Immunol. 1994;158:105–115. doi: 10.1006/cimm.1994.1260. [DOI] [PubMed] [Google Scholar]
- 18.van Neerven RJJ, Ebner C, Yssel H, Kapsenberg ML, Lamb JR. T-cell responses to allergens: epitope-specificity and clinical relevance. Immunol Today. 1996;17:526–532. doi: 10.1016/0167-5699(96)10058-x. [DOI] [PubMed] [Google Scholar]
- 19.Theze J, Alzari PM, Bertoglio J. Interleukin 2 and its receptors: recent advances and new immunological functions. Immunol Today. 1996;17:481–486. doi: 10.1016/0167-5699(96)10057-c. [DOI] [PubMed] [Google Scholar]