Abstract
Reports of structural differences between the brains of men and women, heterosexual and homosexual men, and male-to-female transsexuals and other men have been offered as evidence that the behavioral differences between these groups are likely caused by differences in the early development of the brain. However, a possible confounding variable is the concentration of circulating hormones seen in these groups in adulthood. Evaluation of this possibility hinges on the extent to which circulating hormones can alter the size of mammalian brain regions as revealed by Nissl stains. We now report a sexual dimorphism in the volume of a brain nucleus in rats that can be completely accounted for by adult sex differences in circulating androgen. The posterodorsal nucleus of the medial amygdala (MePD) has a greater volume in male rats than in females, but adult castration of males causes the volume to shrink to female values within four weeks, whereas androgen treatment of adult females for that period enlarges the MePD to levels equivalent to normal males. This report demonstrates that adult hormone manipulations can completely reverse a sexual dimorphism in brain regional volume in a mammalian species. The sex difference and androgen responsiveness of MePD volume is reflected in the soma size of neurons there.
Structural differences between the brains of men and women (1–6), heterosexual and homosexual men (7, 8), and male-to-female transsexuals and other men (9) have been interpreted as evidence that the behavioral differences between these groups are likely caused by differences in the early, perhaps even fetal, development of the brain. However, the concentration of circulating hormones seen in these groups in adulthood sometimes varies (9, 10), and these hormones could affect the size of brain regions as defined by the standard Nissl stains used. We sought to evaluate this possibility by examining the extent to which circulating hormones can alter the size of a mammalian brain region as revealed by Nissl stains.
The medial amygdala (MeA), a component of the sexually dimorphic vomeronasal system (11), receives afferents from the accessory olfactory bulb and projects to the bed nucleus of the stria terminalis, preoptic area, hypothalamus (12, 13), and other structures in the forebrain and limbic system (13). The MeA has been implicated in reproductive behaviors, including chemosensory investigation (14, 15) and sexual arousal (16). The posterodorsal component of the medial amygdala (MePD; Fig. 1A) is 50–80% larger in male rats than in females (17, 18) and is rich in androgen and estrogen receptors (19–21). Sex differences in synaptic pattern (22) and peptide immunoreactivity (23, 24) have been reported in the MePD as well. Cytoarchitectural changes in response to castration and hormone treatment have been reported in the rat (24), but to date no studies have examined the effect of testosterone (T) treatment on the volume of the adult rat MePD.
Figure 1.
Photomicrograph of a Nissl-stained section indicating the boundaries of the MePD.
METHODS
Sixty-day-old male and female Long–Evans rats (Harlan, Blue Spruce) were kept under a 12:12 light:dark schedule and given food and water ad lib. Gonadectomies and hormone implant surgeries were performed on ketamine-anesthetized rats. Hormone capsules were made with Silastic tubing (i.d.. 0.062 inch; o.d. 0.125 inch; 40-mm long) and sealed at the ends with silicone adhesive. The capsules were either empty or filled with crystalline T and were implanted subcutaneously between the scapula of the rat’s dorsum. After postoperative recovery, all subjects participated in a water maze task, the results of which will be reported separately. Thirty days after surgery, subjects were overdosed with pentobarbital, perfused intracardially with phosphate-buffered saline followed by 10% buffered formalin for 20–30 min. The seminal vesicles of the males were dissected and weighed to confirm the efficacy of hormone treatment. Brains were postfixed in buffered formalin for approximately 1 mo before coronal sectioning through the MeA. Slides were Nissl stained with thionin, coverslipped with permount, and coded to ensure unbiased measurements. An investigator quantified the volume of the MePD, the boundaries of which were defined as in standard atlases (25, 26). The investigator selected a right or left MeA on the basis of stain and tissue quality, as a previous study had demonstrated no laterality differences in MeA volume (27).
The MePD was drawn from 60-μm sections with a camera lucida at ×4.5 magnification. Every third section was taken for further analysis. The area of the drawings was determined by digitizing them with a flatbed scanner and measuring the areas with National Institutes of Health image software. The total area of the drawn subnuclei was then multiplied by the section thickness and sampling ratio to obtain the approximate volume. The investigator also drew, with a camera lucida, approximately 25–30 neurons in the caudal half of the MePD. Only cells with a visible nucleolus and cytoplasm were selected for drawing. Mean soma size was determined by measuring the area of each drawn soma and computing the average of those somata for each subject.
RESULTS
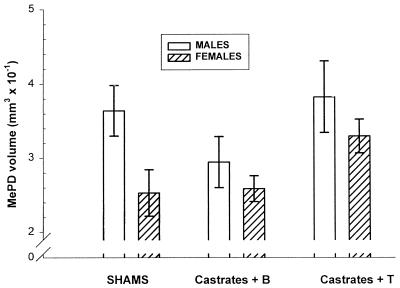
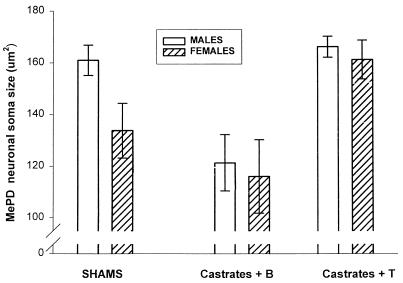
Confirming other reports, we found the MePD volume of male rats to be 65% greater than that of females (P < 0.02; two-tail t test; Fig. 2 Left). We found that treatment of females with 30 days of T increased their MePD volume to that seen in control males. Thirty days after castration, the male MePD volume was equivalent to that of control females, unless the males were given T after castration, indicating that adult androgen concentration accounts for the observed sex difference in MePD volume (Fig. 2 Right). MePD soma size of gonadally intact males was greater than that of females (P = 0.05), and either castration of males or androgen treatment of females eliminated the sex difference (Fig. 3). Seminal vesicle weights were greatly reduced in untreated castrates (0.64 ± 0.34 g) compared with sham males (2.10 ± 0.33). Castrates given T had seminal vesicle weights slightly, but not significantly, lighter than those of sham males (1.87 ± 0.15), indicating that the T capsules produced circulating levels of androgen approximately the same as intact males.
Figure 2.
Mean (± SEM) unilateral volume of MePD in rats 4 wk after sham surgery (Left) or castration followed by T treatment or no hormone (blank). Adult T treatment of castrated rats eliminated the sex difference in MePD volume. Either sex, when treated with T, displayed MePD volumes that did not differ significantly from those of control males, indicating that adult circulating androgen concentration was the sole factor determining sexual dimorphism of MePD volume. Two-way ANOVA of castrates revealed a significant main effect of treatment (F(1, 20) = 7.1, P < 0.0002), but none for sex, (F(1, 20) = 1.6, P = 0.21) or interaction.
Figure 3.
Among control animals, MePD somata were significantly larger in males (two-tail t test, P = 0.05). Gonadectomy and implantation of a blank Silastic capsule reduced the size of these neurons in males. Implantation of a capsule packed with T increased soma size in females, again eliminating the difference. Two-way ANOVA among the gonadectomized groups revealed a significant main effect of T (F(1, 18) = 21.5; P < 0.0003) but none for sex (P > 0.05) or any significant interaction.
DISCUSSION
These experiments indicate that the observed sexual dimorphism in volume of the MePD is primarily a result of adult circulating androgen. The sex difference seen between controls in this region disappeared 4 wk after adult castration. Androgen replacement maintained control MePD volumes in males and increased MePD volumes in females to those of gonadally intact males.
Other sexually dimorphic nuclei in the mammalian brain are affected by adult hormone manipulations. In male rats, the volume of the sexually dimorphic nucleus of the preoptic area is significantly reduced after adult castration (28). The volume of the sexually dimorphic area in Mongolian gerbils is reduced by 35–40% in both sexes after adult gonadectomy, and this effect can be prevented by implantation of T capsules (29). Neither of these experiments entirely reversed volumetric sex differences, however.
Sexually dimorphic nuclei in the Japanese quail and canary can be altered by hormonal manipulations. In the quail, T treatment in females causes the sexually dimorphic preoptic area to reach male volumes after 21 days, whereas male gonadectomy leads to significant reductions (30). However, it has been argued that such plasticity in birds is an adaptation to maintain low body weight for flight. The present findings in a mammalian species speak against this hypothesis. Adult androgen treatment also affects the canary song-control system (31). Cytoarchitectural and volumetric measures are modified by adult T concentrations in the male song-control nucleus (32). Adult T treatment of female canaries enlarges the brain region higher vocal center and induces the birds to produce male-like song. But even in this very plastic avian system, adult androgen manipulations do not appear capable of completely sex-reversing sexually dimorphic neural volumes (33).
Human behavior is also subject to the activational effects of androgens. Transsexuals treated with cross-sex hormones display sex reversals in their cognitive abilities, emotional tendencies, and libido (34, 35), and sex offenders are sometimes treated with antiandrogens to reduce their sex drive (36). The sociosexual changes observed in these groups most likely reflect structural and physiological plasticity in steroid-sensitive areas within the brain. The volumetric sex reversal reported here substantiates the possibility that hormones in adulthood can dramatically affect the structure of a brain region concerned with sexual behavior. Although the volumetric sexual dimorphism of the MePD is more modest than other animal models [a difference of 150% rather than 400–600% (31)], the extent of the MePD sexual dimorphism in rats in quite comparable to reported sexual dimorphisms in the human brain (1–6) and therefore supports the possibility that sexual dimorphisms of the human brain are caused solely by circulating steroids in adulthood.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant 28421.
ABBREVIATIONS
- MeA
medial amygdala
- MePD
posterodorsal component of the MeA
- T
testosterone
Footnotes
A Commentary on this article begins on page 7128.
References
- 1.Swaab D F, Fliers E. Science. 1985;228:1112–1114. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- 2.Allen L S, Gorski R A. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- 3.Witelson S F, Glezer I I, Kigar D L. J Neurosci. 1995;15:3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlaepfer T E, Harris G J, Tien A Y, Peng L, Lee S, Pearlson G D. Psychiatry Res. 1995;61:129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- 5.Giedd J N, Vaituzis A C, Hamburger S D, Lange N, Rajapakse J C, Kaysen D, Vauss Y C, Rapoport J L. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Pakkenberg B, Gundersen H J. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 7.Swaab D F, Hofman M A. Brain Res. 1990;537:141–148. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- 8.LeVay S. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J N, Hofman M A, Gooren L J, Swaab D F. Nature (London) 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- 10.Gladue B A, Green R, Hellman R E. Science. 1984;225:1496–1499. doi: 10.1126/science.6089349. [DOI] [PubMed] [Google Scholar]
- 11.Segovia S, Guillamón A. Brain Res Rev. 1993;18:51–74. doi: 10.1016/0165-0173(93)90007-m. [DOI] [PubMed] [Google Scholar]
- 12.Kevetter G A, Winans S S. J Comp Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- 13.Canteras N S, Simerly R B, Swanson L W. J Comp Neurol. 1995;360:213–245. doi: 10.1002/cne.903600203. [DOI] [PubMed] [Google Scholar]
- 14.Lehman M N, Winans S S, Powers J B. Science. 1980;210:557–559. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- 15.Coolen L M, Peters H J, Veening J G. Neuroscience. 1997;77:1151–1161. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- 16.Kondo Y, Sachs B D, Sakuma Y. Behav Brain Res. 1997;88:153–160. doi: 10.1016/s0166-4328(97)02287-0. [DOI] [PubMed] [Google Scholar]
- 17.Hines M, Allen L, Gorski R A. Brain Res. 1992;579:321–326. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- 18.Kerchner M, Malsbury C, Ward O B, Ward I L. Brain Res. 1995;672:251–260. doi: 10.1016/0006-8993(94)01378-u. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan P J. Endocrinology. 1979;104:130–136. doi: 10.1210/endo-104-1-130. [DOI] [PubMed] [Google Scholar]
- 20.Simerly R B, Chang C, Muramatsu M, Swanson L W. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 21.Yokosuka M, Okamura H, Hayashi S. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Nishizuka M, Arai Y. Brain Res. 1981;212:31–38. doi: 10.1016/0006-8993(81)90029-9. [DOI] [PubMed] [Google Scholar]
- 23.De Vries G J, Buijs R M, Sluiter A A. Brain Res. 1984;298:141–145. doi: 10.1016/0006-8993(84)91157-0. [DOI] [PubMed] [Google Scholar]
- 24.Malsbury C W, McKay K. J Neuroendocrinol. 1994;6:57–69. doi: 10.1111/j.1365-2826.1994.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1982. [DOI] [PubMed] [Google Scholar]
- 26.Swanson L W. Brain Maps: Structure of the Rat Brain. New York: Elsevier; 1992. [Google Scholar]
- 27.Cooke B M, Breedlove S M. Soc Neurosci Abstr. 1997;23:627. [Google Scholar]
- 28.Bloch G J, Gorski R A. J Comp Neurol. 1988;275:604–612. doi: 10.1002/cne.902750408. [DOI] [PubMed] [Google Scholar]
- 29.Commins D, Yahr P. J Comp Neurol. 1984;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- 30.Panzica G C, Viglietti-Panzica C, Calacagni M, Anselmetti G C, Schumacher M, Balthazart J. Brain Res. 1987;416:59–68. doi: 10.1016/0006-8993(87)91496-x. [DOI] [PubMed] [Google Scholar]
- 31.Arnold A P, Jordan C L. Frontiers in Neuroendocrinology. Vol. 10. New York: Raven; 1988. [Google Scholar]
- 32.DeVoogd T, Nottebohm F. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- 33.DeVoogd T J, Nixdorf B, Nottebohm F. Brain Res. 1985;329:304–308. doi: 10.1016/0006-8993(85)90539-6. [DOI] [PubMed] [Google Scholar]
- 34.Van Goozen S H, Cohen-Kettenis P T, Gooren L J, Frijda N H, Van de Poll N E. Neuropsychologia. 1994;32:1153–1157. doi: 10.1016/0028-3932(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 35.Van Goozen S H, Cohen-Kettenis P T, Gooren L J, Frijda N H, Van de Poll N E. Psychoneuroendocrinology. 1995;20:343–363. doi: 10.1016/0306-4530(94)00076-x. [DOI] [PubMed] [Google Scholar]
- 36.Rose R. Hormones, Behavior and Psychopathology. New York: Raven; 1976. pp. 121–124. [Google Scholar]